Key Points
Question
What are the optimum doses for relapse prevention in patients with stable schizophrenia?
Findings
In this meta-analysis of 26 studies including 4776 participants, doses higher than approximately 5-mg/d risperidone equivalent were not associated with more efficacy. However, increasing doses were associated with more adverse events.
Meaning
In this study, even low doses of antipsychotics appear to have some association with efficacy for relapse prevention in schizophrenia; however, clinicians may need to be cautious when they decrease doses at the lower dose end because further decreases of dose are accompanied by disproportionally higher relapse risk.
Abstract
Importance
The doses of antipsychotic drugs needed for relapse prevention in schizophrenia is a debated issue.
Objective
To examine dose-response findings in a meta-analysis of randomized clinical trials.
Data Sources
Studies were identified through the Cochrane Schizophrenia Group’s Study-Based Register of Trials (March 9, 2020), PubMed (January 1, 2021), and previous reviews. First authors and/or pharmaceutical companies were contacted for additional information.
Study Selection
Two reviewers independently selected randomized clinical trials that compared fixed doses of a second-generation antipsychotic, haloperidol, or fluphenazine for relapse prevention in patients with stable schizophrenia.
Data Extraction and Synthesis
Using the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline, all parameters in duplicate were extracted and frequentist dose-response random-effects meta-analyses were conducted.
Main Outcomes and Measures
Study-defined relapse (primary outcome), rehospitalization, Positive and Negative Syndrome Scale or Brief Psychiatric Rating Scale total score reduction from baseline, all-cause discontinuation, and dropouts due to adverse events.
Results
Evidence from 72 dose arms from 26 studies with 4776 participants was analyzed. The efficacy-related dose-response curves had a hyperbolic shape meaning that the probability to relapse decreased rapidly with doses of up to 5-mg/d risperidone equivalent (relative relapse risk, 0.43; 95% CI, 0.31-0.57; standardized mean difference for Positive and Negative Syndrome Scale total score reduction, −0.55; 95% CI, −0.68 to −0.41), but flattened thereafter. In contrast, dropouts due to adverse events continued to increase beyond this dose (relative risk at 5 mg/d, 1.38; 95% CI, 0.87-2.55; relative risk at 15 mg/d, 2.68; 95% CI, 1.49-4.62). In a subgroup analysis of patients in remission, a plateau was reached earlier, at approximately 2.5-mg/d risperidone equivalent.
Conclusions and Relevance
The findings of this meta-analysis suggest that doses higher than approximately 5-mg/d risperidone equivalent may provide limited additional benefit for relapse prevention but more adverse events. For patients in remission or who are receiving high-potency first-generation antipsychotics, doses as low as 2.5-mg/d risperidone equivalent may be sufficient. However, caution is needed at this low dose end when further decreases of dose may be accompanied by a disproportionally higher relapse risk. Moreover, the observations are averages, and factors such as slow or rapid metabolism, age, illness stage, comorbidities, and drug-drug interactions suggest that individual patients will often need higher or lower doses.
This meta-analysis examines dosing adjustments for prevention of relapse in patients with schizophrenia.
Introduction
Antipsychotic drugs are effective for short-term treatment of schizophrenia,1 and numerous randomized clinical trials have shown that these agents also prevent relapse.2 However, antipsychotics produce many adverse events.1 This trade-off is the reason for a debate that has accompanied these drugs since their development in the 1950s. Because patients often need to use antipsychotics for many years, adverse events, such as movement disorders and weight gain, can accumulate and result in even more severe problems, such as tardive dyskinesia3 or cardiovascular problems. Excess mortality associated with multiple causes is well documented.4 Therefore, psychiatrists need to know which doses are sufficient for maintenance treatment. If lower doses than needed for short-term treatment were sufficient, the adverse-event burden could be substantially reduced. Bollini et al5(p307) reported that “no incremental improvement was found at doses above 375-mg equivalent of chlorpromazine.” Similarly, Baldessarini and Davis6 found no significant dose effect between 100 mg/d and more than 2000 mg/d (median, 310 mg/d) chlorpromazine equivalent. Uchida et al7 reported that low doses (≥50% daily defined dose [DDD] < 1 DDD) may be as effective as standard doses. Nevertheless, these previous reviews are no longer current, and only one included a few randomized clinical trials on newer, second-generation antipsychotics.7 Moreover, these reviews did not apply the most appropriate methods to address this issue. Rather, they either assumed linear associations by applying correlations or they compared mean doses below and above more or less arbitrary cutoffs. Dose-response meta-analysis has been successfully applied to identify the optimum doses for the short-term treatment of schizophrenia.8 In contrast to the above analyses, dose-response meta-analysis does not require a specific shape of the dose-response curve and allows inclusion of more dose arms.9 We applied this method to provide guidance for clinicians on dosing in relapse prevention for schizophrenia.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for meta-analyses and was registered on Prospero (CRD42020182436) (eAppendix 1 in the Supplement).
We included all fixed-dose, randomized, blinded, or open trials of more than 3 months’ duration that compared the following drugs with placebo or at least 1 different dose of the same drug in patients with stable schizophrenia or schizoaffective disorder: amisulpride, aripiprazole (oral, Abilify Maintena and aripiprazole lauroxil), asenapine (oral or transdermal patch), brexpiprazole, cariprazine hydrochloride, clozapine, fluphenazine (oral and long-acting injectable [LAI]), haloperidol (oral and LAI), iloperidone, lumateperone tosylate, lurasidone hydrochloride, olanzapine (oral and LAI), paliperidone (oral, the monthly LAI palmitate, and the 3-monthly LAI Trevicta), quetiapine fumarate (immediate release and extended release), risperidone (oral and the LAIs Consta and Perseris), sertindole, ziprasidone, zotepine. This list comprises all second-generation antipsychotics available in the US and/or Europe. Haloperidol and fluphenazine are first-generation antipsychotics for which pivotal dose-response studies have been conducted and are reported herein. Studies that compared 2 or more dose ranges were also included. Patients had stable states (study defined) of their illness (relapse prevention studies).
The primary outcome was relapse as defined by the original authors. Secondary outcomes were rehospitalization for psychopathologic factors, the change from baseline to end point on the Positive and Negative Syndrome Scale (PANSS)10 or the Brief Psychiatric Rating Scale (BPRS),11 all-cause discontinuation as a measure of overall treatment failure, and dropout due to adverse events as a global tolerability measure. Data on drug-related adverse events vs an exacerbation of schizophrenia were preferred whenever available.
We searched the Cochrane Schizophrenia Group’s Study-Based Register of Trials (March 9, 2020) with a term combining the names of the antipsychotics in question and dosage in the pairwise comparison field of study records (eAppendix 2 in the Supplement). This register includes regular searches in multiple electronic databases, ClinicalTrials.gov, World Health Organization register of clinical trials, conference reports, and hand searches. An updated search in PubMed was made January 1, 2021 (eAppendix 2 in the Supplement). We also screened the reference lists of included studies, previous reviews,7,12,13 and a Cochrane protocol on antipsychotic dose reduction.14 We contacted all authors or pharmaceutical companies for missing data. There were no date, language, document type, or publication status limitations, except for studies from mainland China owing to frequent but usually unrecognizable quality problems.15 The data were extracted in a Microsoft Access database that allows for automatic comparison of the extractions. Risk of bias for the primary outcome was assessed with a risk of bias tool (Cochrane RoB, version 2; Cochrane Collaboration).16 Study selection, data extraction, and risk of bias assessment were made independently by 2 of 3 of us (S.L., S.B., or J.S.-T.); in case of doubt, a third reviewer (S.S. or J.M.D.) was involved.
Statistical Analysis
We conducted a 1-step, random-effects, frequentist, dose-response meta-analysis.9 The association between dose and dichotomous outcomes was measured with odds ratios and on the continuous outcome overall efficacy with standardized mean differences (Cohen d). Odds ratios have better mathematical properties than risk ratios,17 but they are more difficult to interpret. Therefore, odds ratios were also converted to relative risks and absolute numbers for illustration purposes using the meta-analytically pooled placebo rates of each outcome as baseline risks (eAppendix 5 in the Supplement). Values from the BPRS were converted to PANSS units according to Leucht et al.18 Dose-response curves were fitted with restricted cubic splines.19 In the primary analysis, we used knots located at the 25th, 50th, and 75th percentiles. Dose-response curves were estimated for all antipsychotics grouped and for each drug separately. For the pooled analysis, the doses of each antipsychotic were converted to risperidone equivalents, primarily according to the maximum effective dose method8 or, if such equivalents were not available, according to the minimum effective dose method,20,21 the mean-dose method,22,23 the DDD method,24 and based on the international consensus of antipsychotic doses”25 (fluphenazine long-acting injectable).
In post hoc analyses, we tested with linear splines up to which dose the dose-response curve still showed a significantly increasing slope (P < .10). We updated the Uchida et al7 meta-analysis by comparing standard doses (1 DDD or higher) with low doses (≥50% DDD < 1 DDD), and we compared 3- to 7-mg/d risperidone equivalent with higher doses in pairwise meta-analyses.
In sensitivity analyses, dose conversion was based on the international consensus of antipsychotic doses by Gardner et al.25 Different knot locations were used: 10%, 50%, and 90% percentiles (as previously recommended26) and 2-mg/d (the minimum effective dose for short-term treatment20), 4-mg/d, and 8-mg/d (a dose somewhat above the maximum effective dose8) risperidone equivalents (post hoc analysis). Studies that compared only a single dose of an antipsychotic with placebo (not designed to examine dose-response) were not described as double blind and were judged to be at high risk of bias (post hoc) and therefore excluded.
We planned separate analyses of children and adolescents, older patients, participants with predominantly negative symptoms, and those with a first episode of schizophrenia, but such trials were not available except for 1 single-dose and thus unanalyzable first-episode study.27 Post hoc subgroup analyses included patients with remission, oral vs LAI antipsychotics, second-generation vs first-generation antipsychotics, age (median split), and percentage men (median split).
We measured heterogeneity with the variance partition coefficient, which is a multivariate extension of the I2 value suggested by Crippa et al.9 Small-trial effects and their potential association with publication bias were explored with the contour-enhanced funnel plot28 and Egger test.29 Dose-response meta-analyses were conducted with the dosresmeta30 package and meta-analyses of the outcomes in placebo arms with meta31 in R, version 3.6.2 (R Project for Statistical Computing). With 2-sided testing, findings were considered significant at P < .05.
Results
The study selection flowchart, a description of 380 excluded studies and the following 26 included studies with 72 dose arms and 4776 participants are provided in the eFigure, eTable 1, and eTable 2 in the Supplement. The drugs evaluated included oral aripiprazole (2 studies),32,33 aripiprazole LAI (3 studies),34,35,36 fluphenazine LAI (6 studies),37,38,39,40,41,42 oral haloperidol (5 studies),43,44,45,46 haloperidol decanoate (3 studies),47,48,49 lurasidone (1 study),50 oral olanzapine (2 studies),45 olanzapine LAI (1 study),51 paliperidone LAI (1 study),52 quetiapine (2 studies),27,46 risperidone LAI (1 study),53 ziprasidone (1 study),54 and zotepine (1 study).55 Some reports included data on 2 drugs. Nineteen percent of the studies were judged to be of low overall risk of bias, 50% had some concerns of bias, and 31% were considered at high risk for bias (eAppendix 3 in the Supplement).
All but 2 studies38,44 used operationalized diagnostic criteria (Research Diagnostic Criteria, DSM-III, DSM-III-R, and DSM-IV), and all but 2 were described as double-blind.36,49 The median study duration was 48 weeks (range, 6 months to 3 years; interquartile range, 33-52 months); a single 3-year study included only 20 participants.44 In 6 studies, patients were in remission at baseline based on various criteria.27,39,40,43,44,47
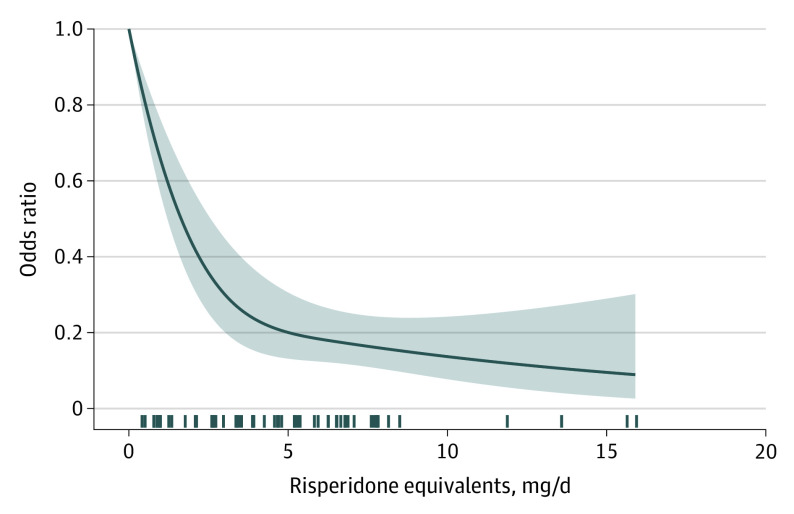
Figure 1 depicts the primary outcome.27,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 The dose-response curve of relapse, based on 71 dose arms from 26 studies27,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 (1 publication included 2 studies), initially decreased sharply, but it flattened after approximately 5-mg/d risperidone equivalent (odds ratio, 0.20; 95% CI, 0.13-0.31; relative risk, 0.43; 95% CI, 0.31-0.57) (Table 1). Figure 2 depicts secondary outcomes.27,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 The shapes of the dose-response curves of the secondary efficacy outcomes (rehospitalization, reduction in overall symptoms) and all-cause discontinuation were similar to that of relapse (Figure 2). In contrast, the curve for dropouts due to adverse events was monotonic, with higher doses always leading to more adverse events (5 mg/d: odds ratio, 1.4; 95% CI, 0.87-2.25; relative risk, 1.38; 95% CI, 0.87-2.15; 15 mg/d: odds ratio, 2.88; 95% CI, 1.52-5.45; relative risk; 2.68; 95% CI, 1.49-4.62) (Figure 2 and Table 1).
Figure 1. Relapse.
The dose-response curve for the primary outcome relapse after pooling all drugs using the primary scientific dose-equivalence method (the maximum effective dose method). The marks on the x-axis indicate for which doses data from study arms were available. A total of 26 studies with 71 individual dose arms including 4749 patients were included (1 publication reported on 2 studies).27,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 The shaded areas indicate 95% CIs for the primary outcome.
Figure 2. Rehospitalization, Overall Symptoms, All-Cause Discontinuation, and Dropout Owing to Adverse Events.
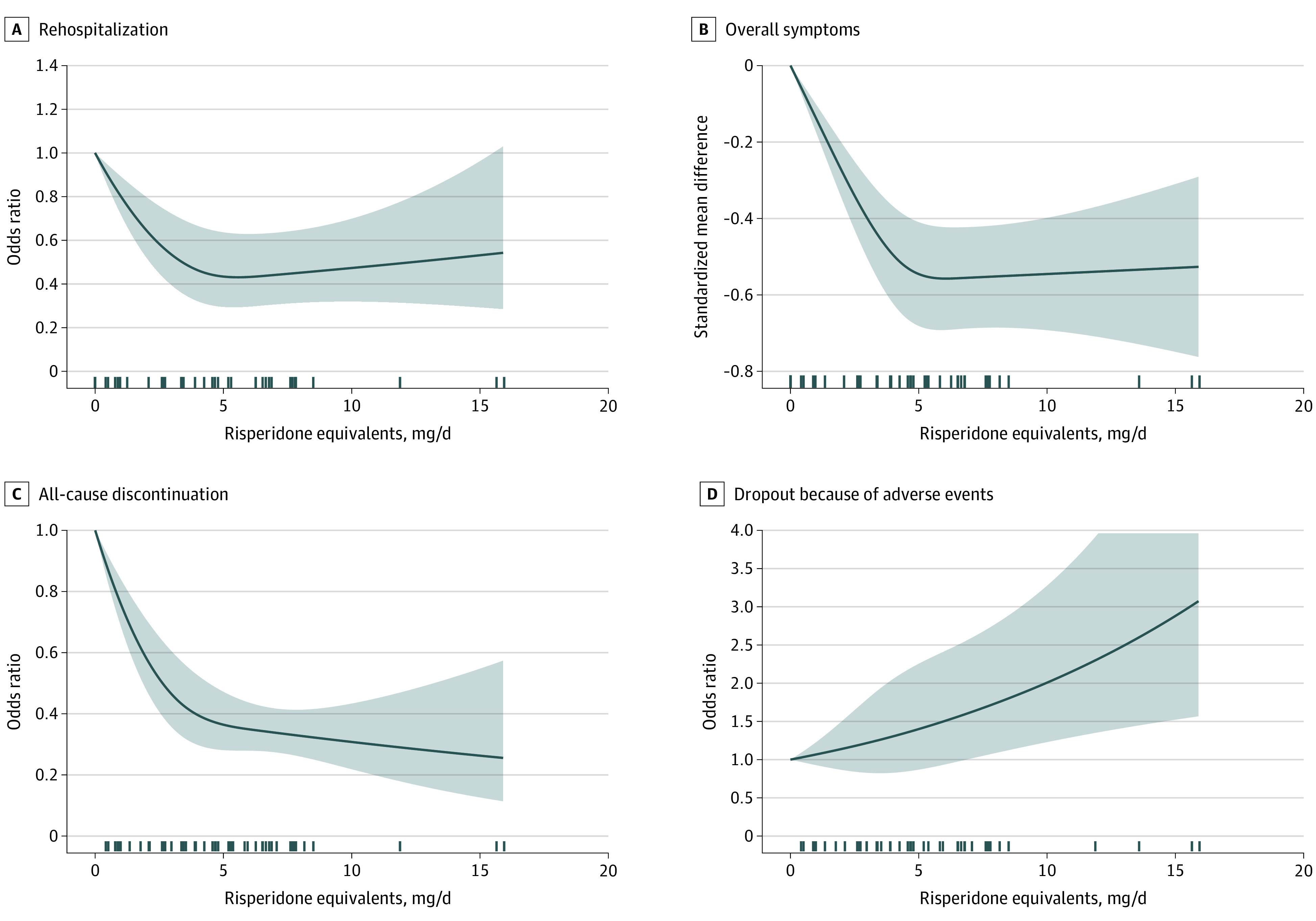
Fourteen studies with 39 arms were available for rehospitalization (A)27,34,35,37,40,41,42,45,46,49,50,51,53; 16 studies with 44 arms were available for overall symptoms, as measured by the Positive and Negative Syndrome Scale total score or the Brief Psychiatric Rating Scale total score (B)32,33,34,35,36,37,38,45,49,50,51,52,53,54,55; 23 studies with 65 dose arms were available for all-cause discontinuation (C)27,32,34,35,36,37,38,39,40,41,42,43,45,46,47,48,49,50,51,52,53,54,55; and 18 studies with 50 arms were available for dropouts due to adverse events (D).27,32,33,34,35,36,38,43,44,45,46,47,48,50,51,53,54,55 One publication45 reported on 2 studies for some of these outcomes. The marks on the x-axis indicate for which doses study arm data were available. The shaded areas indicate 95% CIs.
Table. Results per Dose After Conversion of ORs to RRs and of SMDs to PANSS Values.
| Dose, mga | Relapse | Rehospitalization | All-cause discontinuation | Dropout due to adverse events | Overall symptoms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relapse, % (95% CI) | RR (95% CI) | OR (95% CI) | Rehospitalized, % (95% CI) | RR (95% CI) | OR (95% CI) | Dropout, % (95% CI) | RR (95% CI) | OR (95% CI) | Dropout, % (95% CI) | RR (95% CI) | OR (95% CI) | SMD (95% CI) | PANSS change (95% CI)b | |
| Placebo | 67 | 1 | 1 | 18 | 1 | 1 | 75 | 1 | 1 | 4 | 1 | 1 | 0 | 9.6 |
| 0.5 | 62.1 (60.4 to 63.9) | 0.93 (0.9 to 0.95) | 0.81 (0.75 to 0.87) | 16.4 (15.7 to 17.2) | 0.91 (0.87 to 0.95) | 0.9 (0.85 to 0.94) | 72.3 (71.3 to 73.3) | 0.96 (0.95 to 0.98) | 0.87 (0.83 to 0.92) | 4.1 (3.9 to 4.4) | 1.03 (0.96 to 1.1) | 1.03 (0.96 to 1.11) | −0.07 (−0.09 to −0.05) | 8.46 (8.16 to 8.76) |
| 1.0 | 57 (53.3 to 60.7) | 0.85 (0.8 to 0.91) | 0.65 (0.56 to 0.76) | 15 (13.7 to 16.4) | 0.83 (0.76 to 0.91) | 0.8 (0.72 to 0.89) | 69.5 (67.3 to 71.6) | 0.93 (0.9 to 0.95) | 0.76 (0.69 to 0.84) | 4.3 (3.7 to 4.9) | 1.06 (0.93 to 1.22) | 1.07 (0.93 to 1.23) | −0.14 (−0.18 to −0.1) | 7.31 (6.71 to 7.92) |
| 1.5 | 51.8 (46.2 to 57.3) | 0.77 (0.69 to 0.86) | 0.53 (0.42 to 0.66) | 13.6 (11.8 to 15.6) | 0.76 (0.66 to 0.87) | 0.72 (0.61 to 0.84) | 66.5 (63.1 to 69.8) | 0.89 (0.84 to 0.93) | 0.66 (0.57 to 0.77) | 4.4 (3.6 to 5.4) | 1.1 (0.9 to 1.34) | 1.1 (0.89 to 1.36) | −0.21 (−0.26 to −0.15) | 6.17 (5.27 to 7.08) |
| 2.0 | 46.7 (39.6 to 54) | 0.7 (0.59 to 0.81) | 0.43 (0.32 to 0.58) | 12.4 (10.3 to 14.9) | 0.69 (0.57 to 0.83) | 0.65 (0.52 to 0.8) | 63.5 (58.9 to 68) | 0.85 (0.78 to 0.91) | 0.58 (0.48 to 0.71) | 4.5 (3.5 to 5.9) | 1.13 (0.87 to 1.47) | 1.14 (0.87 to 1.5) | −0.28 (−0.35 to −0.2) | 5.05 (3.85 to 6.25) |
| 2.5 | 42.1 (33.8 to 50.8) | 0.63 (0.5 to 0.76) | 0.36 (0.25 to 0.51) | 11.4 (9 to 14.2) | 0.63 (0.5 to 0.79) | 0.58 (0.45 to 0.76) | 60.7 (54.9 to 66.1) | 0.81 (0.73 to 0.88) | 0.51 (0.41 to 0.65) | 4.7 (3.4 to 6.4) | 1.17 (0.85 to 1.61) | 1.18 (0.84 to 1.65) | −0.34 (−0.43 to −0.25) | 3.98 (2.5 to 5.45) |
| 3.0 | 38 (29.2 to 47.7) | 0.57 (0.44 to 0.71) | 0.3 (0.2 to 0.45) | 10.5 (8 to 13.7) | 0.58 (0.44 to 0.76) | 0.53 (0.39 to 0.72) | 58.1 (51.5 to 64.4) | 0.77 (0.69 to 0.86) | 0.46 (0.35 to 0.6) | 4.8 (3.3 to 7) | 1.21 (0.83 to 1.74) | 1.22 (0.83 to 1.79) | −0.4 (−0.51 to −0.3) | 2.99 (1.27 to 4.72) |
| 3.5 | 34.7 (25.7 to 44.9) | 0.52 (0.38 to 0.67) | 0.26 (0.17 to 0.4) | 9.8 (7.2 to 13.2) | 0.54 (0.4 to 0.73) | 0.49 (0.35 to 0.69) | 55.9 (48.9 to 62.7) | 0.75 (0.65 to 0.84) | 0.42 (0.32 to 0.56) | 5 (3.3 to 7.4) | 1.25 (0.83 to 1.86) | 1.26 (0.82 to 1.93) | −0.45 (−0.57 to −0.34) | 2.13 (0.19 to 4.07) |
| 4.0 | 32.2 (23.4 to 42.4) | 0.48 (0.35 to 0.63) | 0.23 (0.15 to 0.36) | 9.2 (6.6 to 12.7) | 0.51 (0.37 to 0.71) | 0.46 (0.32 to 0.67) | 54.3 (47.2 to 61.2) | 0.72 (0.63 to 0.82) | 0.4 (0.3 to 0.53) | 5.2 (3.3 to 7.9) | 1.29 (0.83 to 1.97) | 1.3 (0.83 to 2.05) | −0.5 (−0.62 to −0.37) | 1.42 (−0.67 to 3.52) |
| 4.5 | 30.3 (22 to 40.2) | 0.45 (0.33 to 0.6) | 0.21 (0.14 to 0.33) | 8.9 (6.3 to 12.5) | 0.49 (0.35 to 0.69) | 0.44 (0.3 to 0.65) | 53.1 (46.2 to 59.8) | 0.71 (0.62 to 0.8) | 0.38 (0.29 to 0.5) | 5.3 (3.4 to 8.3) | 1.33 (0.85 to 2.07) | 1.35 (0.84 to 2.16) | −0.53 (−0.66 to −0.39) | 0.91 (−1.29 to 3.11) |
| 5.0 | 28.9 (21.1 to 38.3) | 0.43 (0.31 to 0.57) | 0.2 (0.13 to 0.31) | 8.7 (6.1 to 12.3) | 0.48 (0.34 to 0.68) | 0.43 (0.3 to 0.64) | 52.2 (45.7 to 58.6) | 0.7 (0.61 to 0.78) | 0.36 (0.28 to 0.47) | 5.5 (3.5 to 8.6) | 1.38 (0.87 to 2.15) | 1.4 (0.87 to 2.25) | −0.55 (−0.68 to −0.41) | 0.6 (−1.64 to 2.84) |
| 5.5 | 27.9 (20.5 to 36.7) | 0.42 (0.31 to 0.55) | 0.19 (0.13 to 0.29) | 8.6 (6.1 to 12.2) | 0.48 (0.34 to 0.68) | 0.43 (0.29 to 0.63) | 51.6 (45.6 to 57.5) | 0.69 (0.61 to 0.77) | 0.36 (0.28 to 0.45) | 5.7 (3.6 to 8.9) | 1.43 (0.9 to 2.22) | 1.45 (0.9 to 2.34) | −0.55 (−0.69 to −0.42) | 0.45 (−1.79 to 2.69) |
| 6.0 | 27.1 (20.1 to 35.5) | 0.4 (0.3 to 0.53) | 0.18 (0.12 to 0.27) | 8.7 (6.1 to 12.1) | 0.48 (0.34 to 0.67) | 0.43 (0.3 to 0.63) | 51.2 (45.6 to 56.7) | 0.68 (0.61 to 0.76) | 0.35 (0.28 to 0.44) | 5.9 (3.8 to 9.1) | 1.47 (0.94 to 2.29) | 1.5 (0.94 to 2.42) | −0.56 (−0.69 to −0.42) | 0.4 (−1.82 to 2.62) |
| 6.5 | 26.4 (19.6 to 34.5) | 0.39 (0.29 to 0.51) | 0.18 (0.12 to 0.26) | 8.7 (6.2 to 12.2) | 0.49 (0.35 to 0.68) | 0.44 (0.3 to 0.63) | 50.8 (45.5 to 56) | 0.68 (0.61 to 0.75) | 0.34 (0.28 to 0.42) | 6.1 (3.9 to 9.4) | 1.52 (0.97 to 2.36) | 1.56 (0.97 to 2.5) | −0.56 (−0.69 to −0.42) | 0.42 (−1.78 to 2.61) |
| 7.0 | 25.7 (19 to 33.7) | 0.38 (0.28 to 0.5) | 0.17 (0.12 to 0.25) | 8.8 (6.3 to 12.2) | 0.49 (0.35 to 0.68) | 0.44 (0.31 to 0.63) | 50.4 (45.1 to 55.6) | 0.67 (0.6 to 0.74) | 0.34 (0.27 to 0.42) | 6.3 (4 to 9.7) | 1.58 (1.01 to 2.43) | 1.62 (1.01 to 2.58) | −0.55 (−0.69 to −0.42) | 0.44 (−1.74 to 2.63) |
| 7.5 | 25 (18.3 to 33.2) | 0.37 (0.27 to 0.5) | 0.16 (0.11 to 0.24) | 8.9 (6.4 to 12.3) | 0.5 (0.36 to 0.68) | 0.45 (0.31 to 0.64) | 50 (44.6 to 55.4) | 0.67 (0.59 to 0.74) | 0.33 (0.27 to 0.41) | 6.5 (4.2 to 10) | 1.63 (1.05 to 2.51) | 1.68 (1.05 to 2.67) | −0.55 (−0.69 to −0.42) | 0.47 (−1.72 to 2.66) |
| 8.0 | 24.3 (17.4 to 32.9) | 0.36 (0.26 to 0.49) | 0.16 (0.1 to 0.24) | 9 (6.5 to 12.4) | 0.5 (0.36 to 0.69) | 0.45 (0.31 to 0.65) | 49.6 (43.8 to 55.3) | 0.66 (0.58 to 0.74) | 0.33 (0.26 to 0.41) | 6.8 (4.3 to 10.4) | 1.69 (1.08 to 2.59) | 1.74 (1.09 to 2.78) | −0.55 (−0.69 to −0.42) | 0.5 (−1.71 to 2.7) |
| 8.5 | 23.7 (16.5 to 32.7) | 0.35 (0.25 to 0.49) | 0.15 (0.1 to 0.24) | 9.1 (6.5 to 12.6) | 0.51 (0.36 to 0.7) | 0.46 (0.32 to 0.66) | 49.2 (42.9 to 55.5) | 0.66 (0.57 to 0.74) | 0.32 (0.25 to 0.42) | 7 (4.5 to 10.7) | 1.75 (1.12 to 2.68) | 1.8 (1.12 to 2.89) | −0.55 (−0.69 to −0.41) | 0.52 (−1.72 to 2.76) |
| 9.0 | 23 (15.6 to 32.7) | 0.34 (0.23 to 0.49) | 0.15 (0.09 to 0.24) | 9.2 (6.5 to 12.8) | 0.51 (0.36 to 0.71) | 0.46 (0.32 to 0.67) | 48.8 (41.9 to 55.7) | 0.65 (0.56 to 0.74) | 0.32 (0.24 to 0.42) | 7.2 (4.6 to 11.1) | 1.8 (1.15 to 2.78) | 1.87 (1.16 to 3.01) | −0.55 (−0.69 to −0.41) | 0.55 (−1.74 to 2.84) |
| 9.5 | 22.4 (14.6 to 32.8) | 0.33 (0.22 to 0.49) | 0.14 (0.08 to 0.24) | 9.3 (6.6 to 13) | 0.52 (0.36 to 0.72) | 0.47 (0.32 to 0.68) | 48.4 (40.8 to 56.1) | 0.65 (0.54 to 0.75) | 0.31 (0.23 to 0.43) | 7.5 (4.7 to 11.6) | 1.87 (1.19 to 2.89) | 1.94 (1.2 to 3.14) | −0.55 (−0.69 to −0.4) | 0.57 (−1.78 to 2.93) |
| 10.0 | 21.8 (13.6 to 33) | 0.32 (0.2 to 0.49) | 0.14 (0.08 to 0.24) | 9.4 (6.6 to 13.3) | 0.52 (0.36 to 0.74) | 0.47 (0.32 to 0.7) | 48 (39.6 to 56.5) | 0.64 (0.53 to 0.75) | 0.31 (0.22 to 0.43) | 7.7 (4.9 to 12) | 1.93 (1.22 to 3) | 2.01 (1.23 to 3.28) | −0.55 (−0.69 to −0.4) | 0.6 (−1.82 to 3.03) |
| 15.0 | 16.2 (6 to 37) | 0.24 (0.09 to 0.55) | 0.1 (0.03 to 0.29) | 10.4 (6.1 to 17.4) | 0.58 (0.34 to 0.97) | 0.53 (0.29 to 0.96) | 44.1 (27.5 to 62.2) | 0.59 (0.37 to 0.83) | 0.26 (0.13 to 0.55) | 10.7 (6 to 18.5) | 2.68 (1.49 to 4.62) | 2.88 (1.52 to 5.45) | −0.53 (−0.75 to −0.31) | 0.86 (−2.76 to 4.48) |
Abbreviations: OR, odds ratio; PANSS, Positive and Negative Syndrome Scale total score change from baseline to end point; RR, relative risk; SMD, standardized mean difference for PANSS change from baseline drug vs placebo.
Dose is indicated in risperidone equivalents.
Positive values indicate worsening; negative values indicate improvement.
Figure 3 depicts sensitivity analyses of the primary outcome.32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,51,52,53,54,55 The use of different knot locations (Figure 3A), the exclusion of 9 studies32,34,38,39,44,48,50,54,55 that compared a single dose with placebo (Figure 3B), exclusion of 2 studies that were not double-blind36,49 (eAppendix 4 in the Supplement), and 8 studies judged to be of high risk of bias33,36,40,41,42,43,45,52 (Figure 3C) did not produce results that were substantially different from the primary analysis. When doses were converted according to the international consensus of antipsychotic dosing25 the curve slightly bulged at the left, but generally overlapped with the primary analysis (Figure 3D).
Figure 3. Sensitivity Analyses of the Primary Outcome.
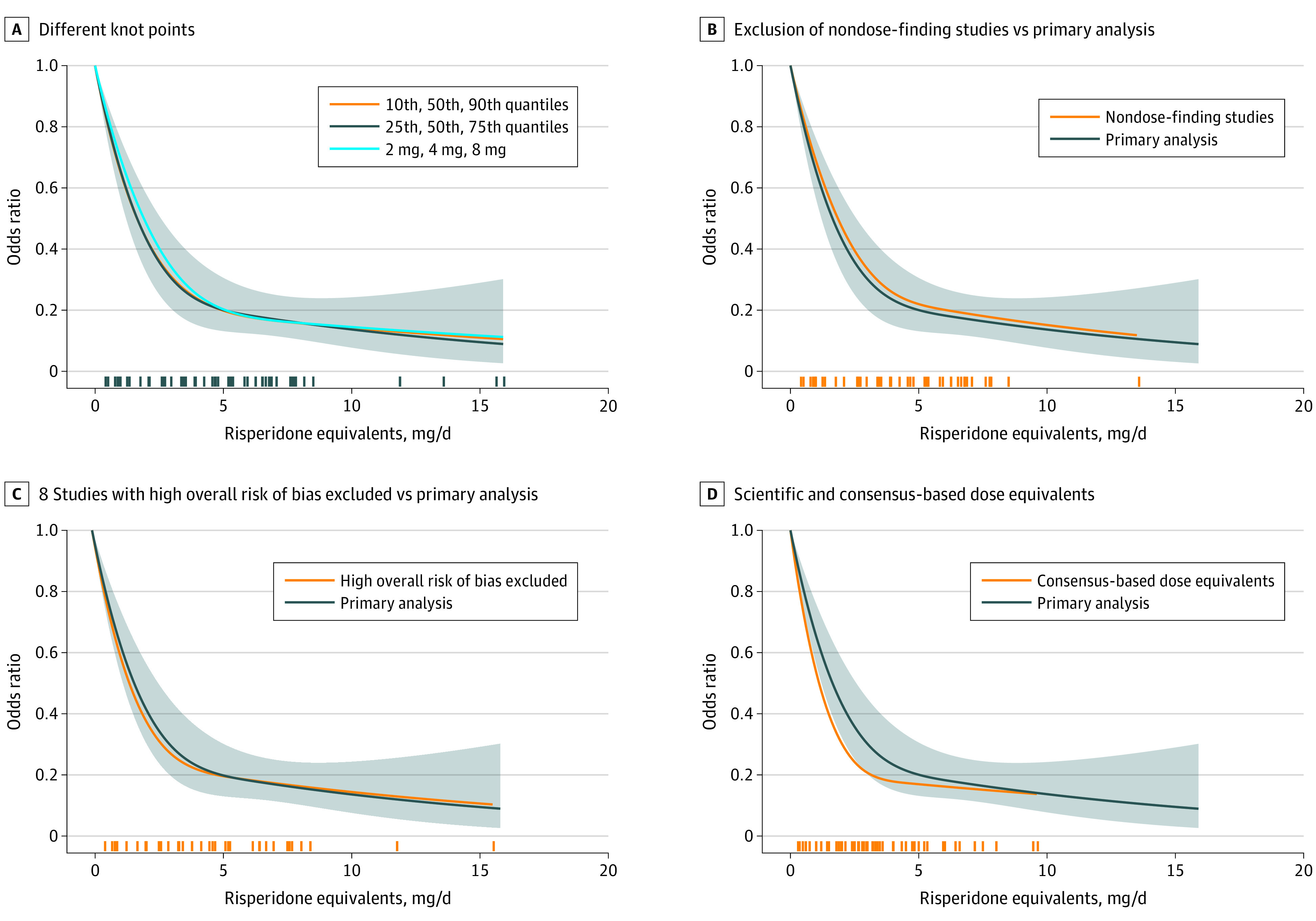
A, Different knot points were used in the statistical analysis. B, Studies that compared a single dose of an antipsychotic with placebo were excluded.32,34,38,39,44,48,50,54,55 C, The results of 8 studies judged to be at high risk of bias 33,36,40,41,42,43,45,52 were excluded and the remaining studies were compared with the overall results. D, The results of primary scientific dose-equivalence method were compared with the consensus-based dose equivalents. The marks on the x-axis indicate for which doses study-arm data were available. The shaded areas indicate 95% CIs.
Few dose arms were available for individual drugs, resulting in substantial uncertainty expressed by wide CIs (eAppendix 4 in the Supplement). With this restriction, the dose-response curves of the individual drugs approximately flattened at aripiprazole, 12.5 mg/d (similar for oral and LAI); fluphenazine LAI, 15 mg biweekly; haloperidol, 3 mg/d (similar for oral and LAI); ziprasidone, 80 mg/d; and oral olanzapine, 10 mg/d. A plateau was not reached in a single olanzapine LAI study at 300 mg every 2 weeks (approximately 20 mg/d),51 as for quetiapine doses of up to 600 mg/d. Risperidone LAI, 50 mg, biweekly was somewhat more effective than 25 mg biweekly,53 but a dose-response curve could not be constructed, just as little as in single-dose, placebo-controlled studies with lurasidone50 and zotepine.55
Figure 4 depicts subgroup analyses of the primary outcome.27,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 In patients with remission, the dose-response curve plateaued earlier (approximately 2.5-mg/d risperidone equivalent) (Figure 4A). Similarly, the curve for first-generation antipsychotics bulged earlier (approximately 3 mg/d) than that for second-generation antipsychotics, which was similar to the overall analysis (Figure 4B). Oral and LAI antipsychotics plateaued at approximately the same dose, but the odds ratio compared with placebo was lower in the LAI group, meaning the drug had greater superiority compared with placebo (Figure 4C). Although age was not an important moderator, studies with more men seemed to use lower doses; because the latter 2 analyses were based on methodologically less-sound median splits, we present them in eAppendix 4 in the Supplement.
Figure 4. Subgroup Analyses of the Primary Outcome.
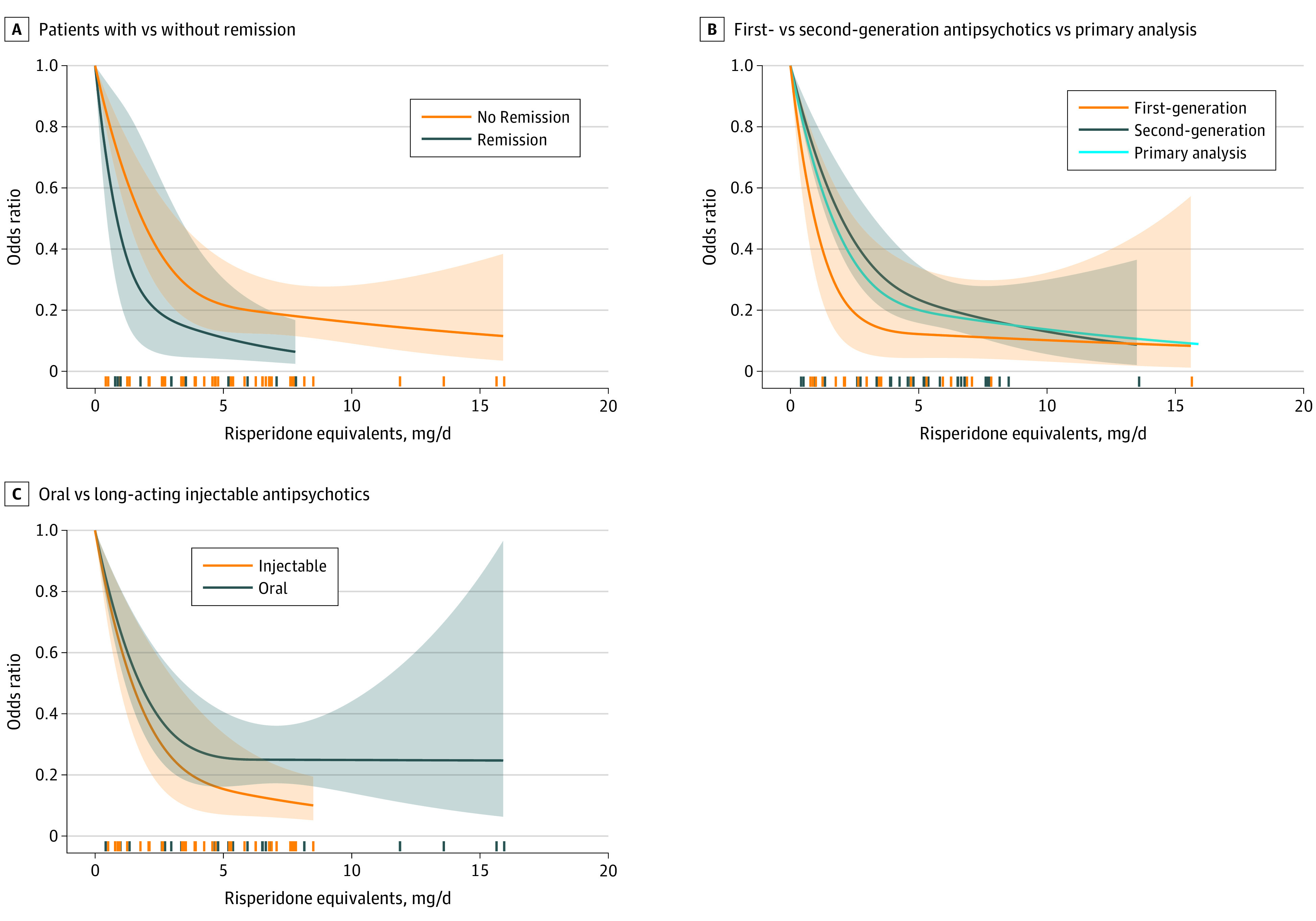
A, The results of 6 studies in patients with remission 27,39,40,43,44,47 were compared with patients without remission. B, Second-generation antipsychotics27,32,33,34,35,36,45,46,50,51,52,53,54,55 were compared with first-generation antipsychotics37,38,39,40,41,42,43,44,45,47,48,49 and the primary analysis. C, Oral formulations were compared with long-acting injectable medications.
The average percentages in the placebo groups, based on meta-analyses of the included studies, were 67% for relapse, 18% for rehospitalization, 75% for all-cause discontinuation, and 4% for dropouts due to adverse events (Table); details about their calculation are provided in eAppendix 5 in the Supplement. Compared with baseline, the PANSS total score worsened by a weighted average of 9 points in the placebo groups.
A dose of 2.5 mg/d risperidone equivalent reduced the risk to relapse from 67% to 42.1%, in relative terms, by approximately 40% (relative risk, 0.63; relative risk reduction = 1 − relative risk; thus, 1 − 0.63 = 0.37 [ie, 37%]) (Table). The mean relative risk reduction for rehospitalization was the same as that for relapse, and mean relative risk reduction for all-cause discontinuation was 25%. Dropouts due to adverse events increased by 17% (relative risk, 1.17). The standardized mean difference for overall association with efficacy compared with placebo was −0.36.
Further gains in terms of reduction of relapse risk, rehospitalization, and improvement or stabilization of symptoms were achieved by doses of approximately 5-mg/d risperidone equivalent: mean relative risk reduction of 57% for relapse, 52% for rehospitalization, 30% for all-cause discontinuation, and an effect size of −0.55 compared with placebo. Some additional gains in relapse prevention beyond 5 mg/d can be an artifact of few dose arms with very high doses being available (as indicated with marks on the x-axis in Figures 1 and 2). Rehospitalization and symptoms did not show such an association; in post hoc analyses, the slope was no longer substantially increasing above 3.5 mg/d, and very high doses were not more effective than 3- to 7-mg/d risperidone equivalent (eAppendix 7 in the Supplement). In contrast, dropouts due to adverse events continued to increase (eg, 38% relative increase compared with placebo at 5 mg/d vs 74% at 8 mg/d). The average dopamine receptor occupancies for the respective risperidone doses from another meta-analysis56 appears in eTable 3 in the Supplement, which we added for a discussion of relapse risk related to this parameter.
There were moderate levels of heterogeneity, with variance partition coefficients usually below 50% across doses and outcomes (Figures 1 and 2; eAppendix 6 in the Supplement). The Egger test for small-study effects bias was not significant, but the contour-enhanced funnel plot of the primary outcome was somewhat asymmetrical (eAppendix 6 in the Supplement).
Discussion
Our main findings were that 2.5-mg/d risperidone equivalents were associated with a relative reduction in schizophrenia relapse rates by 40%, and beyond approximately 5-mg/d risperidone equivalent there were no substantial gains in association with efficacy. In contrast, adverse events continued increasing with higher doses. The shapes of the dose-response curves were hyperbolic, meaning disproportionally higher relapse rates at the lower doses.
How do these findings compare with previous findings, which were either mainly based on first-generation antipsychotics,5,6,57 were outdated,5,6,7 were based on a small number of studies,7 and/or did not use the more appropriate method of dose-response meta-analysis?5,6,7,57 For first-generation antipsychotics, Bollini et al5 found no further gain beyond 375-mg/d chlorpromazine equivalent and Baldessarini and Davis6 found no further gain beyond 310-mg/d chlorpromazine equivalent. Risperidone, 5 mg, corresponds to chlorpromazine, 313 mg,8,58 which is similar to the previous findings. Uchida et al7 reported that standard doses (1 DDD or higher) are more effective than very low doses (<50% DDD), but not statistically significantly more than low doses (≥50% < 1 DDD). However, when we updated their meta-analysis, standard doses outperformed low doses (12 studies, odds ratio, 1.46; 95% CI, 1.04-2.04; P = .03) (eAppendix 7 in the Supplement).7 This finding suggests that low doses are more effective than placebo (Table), but less effective than standard doses (updated Uchida et al7 and Table). In addition, Tani et al57 reported that doses can be reduced as long as the remaining dose is higher than 200-mg/d chlorpromazine equivalents (shown in their figure). However, only 1 small study (31 participants) in Japanese patients, who are usually smaller and weigh less (eg, 62 kg in a large trial59) than current US or European patients, came close to 200 mg/d of chlorpromazine equivalents (Takeuchi et al,60 approximately 210 mg/d); all other studies had higher doses (mean in the dose-reduction group 450-mg/d chlorpromazine equivalents).
The relapse curve still decreased slightly above risperidone, 5 mg/d (Figure 1, Table). However, there is no reason to believe that the average dose for relapse prevention should be higher than that for short-term treatment, which is 5 mg/d, as well.8 Reviews have suggested that even in the acute phase, very high doses are not more effective than standard doses.61,62 There was no significant difference between 3- and 7-mg/d risperidone equivalent and higher doses, and the curve’s slope no longer significantly increased above 3.5 mg/d (eAppendix 7 in the Supplement). Most importantly, the dose-response curves for rehospitalization and overall symptoms clearly plateaued at 5 mg/d (Figure 2). Rehospitalization and PANSS and BPRS scores are not subject to the problem of differences in relapse definitions and therefore more robust measures. Thus, because only 4 dose arms were available above 8 mg/d, the most likely explanation is that the tail of the curve was sensitive to random extreme observations.
Patients who experienced remission at baseline plateaued earlier (approximately 2.5 mg/d risperidone). Only 6 studies in patients with remission were available,27,39,40,43,44,47 3 of which used operationalized criteria focusing on positive symptoms27,40,47; the other 3 used broad criteria, including aspects such as vocational functioning, but were not operationalized39,43,44 (eTable 1 in the Supplement). As a caveat, 3 of these studies27,43,44 were conducted in Asian patients (ie, usually smaller and lighter), and 3 used LAIs with their advantages for adherence (Figure 3). The fact that the curve for first-generation antipsychotics plateaued at approximately 3 mg is plausible because haloperidol and fluphenazine are high-potency first-generation antipsychotics, which are strong antagonists of dopamine receptors. Although the curve of LAI antipsychotics plateaued at the same dose as that of oral antipsychotics (approximately 5 mg), their difference compared with placebo was larger. Better adherence may account for this difference. Although these findings are plausible, subgroup analyses are subject to confounders and they were conducted post hoc. These results, therefore, should be interpreted with great caution.
These results can also be considered with regard to the trade-off between avoiding relapses vs adverse events and supersensitivity effects. Several studies have shown that, after a relapse, not all patients return to their psychopathologic state before the relapse.63,64,65,66,67 Relapses should therefore be avoided. Conversely, treatment with antipsychotics can lead to supersensitivity of dopamine receptors, which may make patients more prone to relapse in the long run. Supersensitivity effects have been reported in animal studies.68 In patients, the clearest proof is tardive dyskinesia.3 In view of this dilemma, the use of low doses is a pragmatic solution. However, owing to the hyperbolic shape of the curve, lower doses are associated with disproportionally higher relapse risks (Figure). This finding is also supported by the results of a meta-analysis of plasma level studies and relative dopamine receptor occupancy available as eTable 3 in the Supplement and which we added for examples to the information in the Table.56 For example, the difference in relapse risk between 7.5 mg/d risperidone equivalents and 5 mg/d risperidone equivalents is very small (28.9% − 25.0% = 3.9%), and so is the difference in dopamine receptor occupancy (81% − 76% = 6%). The difference is larger between 5 mg/d and 2.5 mg/d (absolute difference in relapse rates, 42.1% − 28.9% = 13.3%, receptor occupancy 76% − 64% = 13.2%). This variance means that it should be safe to reduce excessive doses, but caution needs to be taken at the low dose end.
Based on similar, but theoretical, considerations, Horowitz et al69 suggested reducing doses by 10 percentage points of the drug’s D2-blockade every 3 to 6 months (an early consensus guideline made similar recommendations70), with even smaller steps for the lower the dose owing to the hyperbolic dose-response function. Our data, however, suggest that antipsychotic drugs dosages should not be completely withdrawn and the safest procedure would be to stay around 5 mg/d, in particular, if there are no important adverse events. At 2 mg/d, receptor occupancy falls below the 65% threshold, which is considered to be the minimum of a postulated 65% to 80% range. Because a systematic review found only 12 studies with 70 patients, this threshold has a weak foundation.71 Moreover, if other factors, such as stress or irregular lifestyle intervene, relapses may result despite 65% dopamine occupancy.
Strengths and Limitations
Strengths of the analysis are the use of dose-response meta-analysis. Previous reviews only compared the means of higher and lower doses or applied linear regression,5,6,7,57 but our results suggest that dose-response associations are not linear. Our results were consistent across various measures of efficacy and effectiveness, and they were robust when a consensus method rather than a method based on empirical data for dose equivalence was applied. Although we consider the maximum effective dose method to be the most appropriate one, all these methods have limitations as has been discussed elsewhere.8,20,21,22,23,24
Limitations of the meta-analysis are that dose equivalence could be avoided if several dose-finding studies were available for each antipsychotic, but these are unlikely to be available in the foreseeable future. A discussion of the results of individual antipsychotic drugs is presented in eAppendix 4 in the Supplement. We included only the first-generation antipsychotics haloperidol and fluphenazine, but according to Uchida et al,7 additional data would have been available only for propericyazine (24 patients) and pimozide (24 patients),43,44 and the Cochrane review on chlorpromazine dose identified only 1 study, which was a mix of an acute- and maintenance-phase study. Due to the expectable lack of data,1 old drugs were not included in our dose-equivalence analyses,8,20,21,22,23,24 and change of trial methods72 would have made the comparison with newer drugs difficult. Relapses can occur with a delay of several months. Therefore, the difference between drug and placebo may increase over time, making the median trial duration of 1 year a limitation. Publication bias is possible. In addition, only severe adverse events led to study discontinuation so that the dropouts due to adverse events reported in the Table underestimate the true adverse event burden.
Conclusions
The results of our meta-analysis may provide some guidance based on average patients with chronic disease. The dose-response associations in specific populations are likely to be different. For example, doses might be lower for patients with a first episode of schizophrenia and higher for treatment-resistant patients. Moreover, the substantial interindividual variability in all these outcomes is important to consider. Individual dosing decisions should be guided by patient wishes. For many patients, adverse events may be a priority, and for many others, avoidance of relapse may be more important. Several factors should be considered in dosing of antipsychotic drugs to prevent relapse. These include patient characteristics, such as degree of residual symptoms; physical and psychiatric comorbidities, such as kidney damage or substance abuse; slow or ultrarapid metabolism due to polymorphisms of cytochrome enzymes; doses that were effective in the acute phase and/or for relapse prevention in the past; the severity of previous relapses (higher doses may be recommendable if relapses could be disastrous); the properties of each drug (eg, pharmacodynamic and pharmacokinetic properties, adverse event profile), and concomitant treatment with psychiatric and nonpsychiatric drugs which, by interaction, can affect drug plasma levels.73
eAppendix 1. Dose Response Meta-analysis of Antipsychotic Drugs for Relapse Prevention in Schizophrenia (protocol)
eAppendix 2. Description of the Search Strategy
eFigure. PRISMA Diagram of the Search
eTable 1. Characteristics of Included Studies
eTable 2. Characteristics of Excluded Studies
eReferences
eAppendix 3. Assessment With the Cochrane Risk of Bias Tool, Version 2
eAppendix 4. Individual Drugs and Additional Sensitivity/Subgroup Analyses
eAppendix 5. Conversion to Absolute Rates, Relative Risks, and PANSS Units
eAppendix 6. Heterogeneity and Small Trial/Publication Bias
eAppendix 7. Update of the Meta-analysis of Uchida et al (2011) and Additional Analyses of Doses Higher Than Standard Doses (≥5mg Risperidone Equivalent per Day)
eTable 3. Average Dopamine Receptor Occupancies for Risperidone Doses
References
- 1.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951. doi: 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063-2071. doi: 10.1016/S0140-6736(12)60239-6 [DOI] [PubMed] [Google Scholar]
- 3.Carbon M, Kane JM, Leucht S, Correll CU. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340. doi: 10.1002/wps.20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131. doi: 10.1001/archpsyc.64.10.1123 [DOI] [PubMed] [Google Scholar]
- 5.Bollini P, Pampallona S, Orza MJ, Adams ME, Chalmers TC. Antipsychotic drugs: is more worse? a meta-analysis of the published randomized control trials. Psychol Med. 1994;24(2):307-316. doi: 10.1017/S003329170002729X [DOI] [PubMed] [Google Scholar]
- 6.Baldessarini RJ, Davis JM. What is the best maintenance dose of neuroleptics in schizophrenia? Psychiatry Res. 1980;3(2):115-122. doi: 10.1016/0165-1781(80)90028-1 [DOI] [PubMed] [Google Scholar]
- 7.Uchida H, Suzuki T, Takeuchi H, Arenovich T, Mamo DC. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr Bull. 2011;37(4):788-799. doi: 10.1093/schbul/sbp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry. 2020;177(4):342-353. doi: 10.1176/appi.ajp.2019.19010034 [DOI] [PubMed] [Google Scholar]
- 9.Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579-1596. doi: 10.1177/0962280218773122 [DOI] [PubMed] [Google Scholar]
- 10.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 11.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. PsycholRep. 1962;10:790-812. [Google Scholar]
- 12.Ceraso A, Lin JJ, Schneider-Thoma J, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2020;8:CD008016. doi: 10.1002/14651858.CD008016.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tani H, Uchida H, Suzuki T, Fujii Y, Mimura M. Interventions to reduce antipsychotic polypharmacy: a systematic review. Schizophr Res. 2013;143(1):215-220. doi: 10.1016/j.schres.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 14.Bighelli I, Samara MT, Rodolico A, Hansen W-P, Leucht S. Antipsychotic dose reduction compared to dose continuation for people with schizophrenia. Cochrane Database Syst Rev. 2021. Accessed July 17, 2021 doi: 10.1002/14651858.CD014384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong Z, Li F, Ogawa Y, Watanabe N, Furukawa TA. Quality of randomized controlled trials of new generation antidepressants and antipsychotics identified in the China National Knowledge Infrastructure (CNKI): a literature and telephone interview study. BMC Med Res Methodol. 2018;18(1):96. doi: 10.1186/s12874-018-0554-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, version 6.1. September 2020. Accessed November 15, 2020. http://www.training.cochrane.org/handbook
- 17.Bakbergenuly I, Hoaglin DC, Kulinskaya E. Pitfalls of using the risk ratio in meta-analysis. Res Synth Methods. 2019;10(3):398-419. doi: 10.1002/jrsm.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leucht S, Rothe P, Davis JM, Engel RR. Equipercentile linking of the BPRS and the PANSS. Eur Neuropsychopharmacol. 2013;23(8):956-959. doi: 10.1016/j.euroneuro.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 19.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551-561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 20.Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40(2):314-326. doi: 10.1093/schbul/sbu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothe PH, Heres S, Leucht S. Dose equivalents for second generation long-acting injectable antipsychotics: the minimum effective dose method. Schizophr Res. 2018;193:23-28. doi: 10.1016/j.schres.2017.07.033 [DOI] [PubMed] [Google Scholar]
- 22.Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397-1402. doi: 10.1093/schbul/sbv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis JM. Dose equivalence of the antipsychotic drugs. J Psychiatr Res. 1974;11:65-69. doi: 10.1016/0022-3956(74)90071-5 [DOI] [PubMed] [Google Scholar]
- 24.Leucht S, Samara M, Heres S, Davis JM. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42(suppl 1):S90-S94. doi: 10.1093/schbul/sbv167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693. doi: 10.1176/appi.ajp.2009.09060802 [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer International Publishing; 2015. doi: 10.1007/978-3-319-19425-7 [DOI] [Google Scholar]
- 27.Chen EYH, Hui CLM, Lam MML, et al. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ. 2010;341:c4024. doi: 10.1136/bmj.c4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991-996. doi: 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crippa A, Orisini N.. Multivariate dose-response meta-analysis: the dosresmeta R package. J Statistical Software. 2016;72(1):1-15. [Google Scholar]
- 31.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG; Aripiprazole Study Group . Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048-1056. doi: 10.4088/JCP.v64n0910 [DOI] [PubMed] [Google Scholar]
- 33.McEvoy JP, Daniel DG, Carson WH Jr, McQuade RD, Marcus RN. A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res. 2007;41(11):895-905. doi: 10.1016/j.jpsychires.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 34.Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617-624. doi: 10.4088/JCP.11m07530 [DOI] [PubMed] [Google Scholar]
- 35.Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135-144. doi: 10.1192/bjp.bp.113.134213 [DOI] [PubMed] [Google Scholar]
- 36.Mallikaarjun S, Kane JM, Bricmont P, et al. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose study. Schizophr Res. 2013;150(1):281-288. doi: 10.1016/j.schres.2013.06.041 [DOI] [PubMed] [Google Scholar]
- 37.Carpenter WT Jr, Buchanan RW, Kirkpatrick B, Lann HD, Breier AF, Summerfelt AT. Comparative effectiveness of fluphenazine decanoate injections every 2 weeks versus every 6 weeks. Am J Psychiatry. 1999;156(3):412-418. [DOI] [PubMed] [Google Scholar]
- 38.Dotti A, Bersani G, Rubino IA, Eliseo C. Double-blind trial of fluphenazine decanoate against placebo in ambulant maintenance treatment of chronic schizophrenics. Rivista di Psichiatria. 1979;14:374-383. [Google Scholar]
- 39.Kane JM, Rifkin A, Quitkin F, et al. Low dose fluphenazine decanoate in maintenance treatment of schizophrenia. Psychiatry Res. 1979;1(3):341-348. doi: 10.1016/0165-1781(79)90016-7 [DOI] [PubMed] [Google Scholar]
- 40.Kane JM, Rifkin A, Woerner M, et al. Low-dose neuroleptic treatment of outpatient schizophrenics—I: preliminary results for relapse rates. Arch Gen Psychiatry. 1983;40(8):893-896. doi: 10.1001/archpsyc.1983.01790070083010 [DOI] [PubMed] [Google Scholar]
- 41.Marder SR, Van Putten T, Mintz J, et al. Costs and benefits of two doses of fluphenazine. Arch Gen Psychiatry. 1984;41(11):1025-1029. doi: 10.1001/archpsyc.1983.01790220015002 [DOI] [PubMed] [Google Scholar]
- 42.Schooler NR, Keith SJ, Severe JB, et al. Relapse and rehospitalization during maintenance treatment of schizophrenia: the effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54(5):453-463. doi: 10.1001/archpsyc.1997.01830170079011 [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa T, Tsuda A, Tanaka M, Hoaki Y, Koga I, Uchida Y. Prophylactic effect of neuroleptics in symptom-free schizophrenics: a comparative dose-response study of haloperidol and propericiazine. Psychopharmacology (Berl). 1984;82(3):153-156. doi: 10.1007/BF00427763 [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa T, Tsuda A, Tanaka M, Koga I, Uchida Y. Prophylactic effect of neuroleptics in symptom-free schizophrenics. Psychopharmacology (Berl). 1982;77(4):301-304. doi: 10.1007/BF00432759 [DOI] [PubMed] [Google Scholar]
- 45.Dellva MA, Tran P, Tollefson GD, Wentley AL, Beasley CM Jr. Standard olanzapine versus placebo and ineffective-dose olanzapine in the maintenance treatment of schizophrenia. Psychiatr Serv. 1997;48(12):1571-1577. doi: 10.1176/ps.48.12.1571 [DOI] [PubMed] [Google Scholar]
- 46.Velligan DI, Newcomer J, Pultz J, et al. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res. 2002;53(3):239-248. doi: 10.1016/S0920-9964(01)00268-7 [DOI] [PubMed] [Google Scholar]
- 47.Kane JM, Davis JM, Schooler N, et al. A multidose study of haloperidol decanoate in the maintenance treatment of schizophrenia. Am J Psychiatry. 2002;159(4):554-560. doi: 10.1176/appi.ajp.159.4.554 [DOI] [PubMed] [Google Scholar]
- 48.Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;14(suppl 2):S7-S12. [PubMed] [Google Scholar]
- 49.Huttunen MO, Piepponen T, Rantanen H, Larmo I, Nyholm R, Raitasuo V. Risperidone versus zuclopenthixol in the treatment of acute schizophrenic episodes: a double-blind parallel-group trial. Acta Psychiatr Scand. 1995;91(4):271-277. doi: 10.1111/j.1600-0447.1995.tb09781.x [DOI] [PubMed] [Google Scholar]
- 50.Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. 2016;30(1):69-77. doi: 10.1177/0269881115620460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kane JM, Detke HC, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry. 2010;167(2):181-189. doi: 10.1176/appi.ajp.2009.07081221 [DOI] [PubMed] [Google Scholar]
- 52.Hough D, Lindenmayer JP, Gopal S, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(6):1022-1031. doi: 10.1016/j.pnpbp.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 53.Simpson GM, Mahmoud RA, Lasser RA, et al. A 1-year double-blind study of 2 doses of long-acting risperidone in stable patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2006;67(8):1194-1203. doi: 10.4088/JCP.v67n0804 [DOI] [PubMed] [Google Scholar]
- 54.Arato M, O’Connor R, Meltzer HY; ZEUS Study Group . A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17(5):207-215. doi: 10.1097/00004850-200209000-00001 [DOI] [PubMed] [Google Scholar]
- 55.Cooper SJ, Butler A, Tweed J, Welch C, Raniwalla J. Zotepine in the prevention of recurrence: a randomised, double-blind, placebo-controlled study for chronic schizophrenia. Psychopharmacology (Berl). 2000;150(3):237-243. doi: 10.1007/s002130000452 [DOI] [PubMed] [Google Scholar]
- 56.Lako IM, van den Heuvel ER, Knegtering H, Bruggeman R, Taxis K. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33(5):675-681. doi: 10.1097/JCP.0b013e3182983ffa [DOI] [PubMed] [Google Scholar]
- 57.Tani H, Takasu S, Uchida H, Suzuki T, Mimura M, Takeuchi H. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology. 2020;45(5):887-901. doi: 10.1038/s41386-019-0573-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis JM. Comparative doses and costs of antipsychotic medication. Arch Gen Psychiatry. 1976;33(7):858-861. doi: 10.1001/archpsyc.1976.01770070088010 [DOI] [PubMed] [Google Scholar]
- 59.Ishigooka J, Iwashita S, Tadori Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: a 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2018;72(9):692-700. doi: 10.1111/pcn.12682 [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi H, Suzuki T, Remington G, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophr Bull. 2013;39(5):993-998. doi: 10.1093/schbul/sbt090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samara MT, Klupp E, Helfer B, Rothe PH, Schneider-Thoma J, Leucht S. Increasing antipsychotic dose for non response in schizophrenia. Cochrane Database Syst Rev. 2018;5:CD011883. doi: 10.1002/14651858.CD011883.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psychiatry. 1988;45(1):79-91. doi: 10.1001/archpsyc.1988.01800250095013 [DOI] [PubMed] [Google Scholar]
- 63.Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042. doi: 10.1038/s41386-018-0278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emsley R, Nuamah I, Hough D, Gopal S. Treatment response after relapse in a placebo-controlled maintenance trial in schizophrenia. Schizophr Res. 2012;138(1):29-34. doi: 10.1016/j.schres.2012.02.030 [DOI] [PubMed] [Google Scholar]
- 65.Emsley R, Chiliza B, Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr Res. 2013;148(1-3):117-121. doi: 10.1016/j.schres.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 66.Emsley R, Oosthuizen P, Koen L, Niehaus D, Martinez L. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83. doi: 10.1097/JCP.0b013e31827bfcc1 [DOI] [PubMed] [Google Scholar]
- 67.Pollack S, Lieberman JA, Fleischhacker WW, et al. A comparison of European and American dosing regimens of schizophrenic patients on clozapine: efficacy and side effects. Psychopharmacol Bull. 1995;31(2):315-320. [PubMed] [Google Scholar]
- 68.Joyce JN. D2 but not D3 receptors are elevated after 9 or 11 months chronic haloperidol treatment: influence of withdrawal period. Synapse. 2001;40(2):137-144. doi: 10.1002/syn.1035 [DOI] [PubMed] [Google Scholar]
- 69.Horowitz MA, Murray RM, Taylor D. Tapering antipsychotic treatment. JAMA Psychiatry. 2021;78(2):125-126. doi: 10.1001/jamapsychiatry.2020.2166 [DOI] [PubMed] [Google Scholar]
- 70.Kissling W, Kane JM, Barnes TR, et al. Guidelines for neuroleptic relapse prevention in schizophrenia: towards consensus view. In: Kissling W, ed. Guidelines for Neuroleptic Relapse Prevention in Schizophrenia. Springer; 1991:155-163. doi: 10.1007/978-3-642-86922-8_19 [DOI] [Google Scholar]
- 71.Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31(4):497-502. doi: 10.1097/JCP.0b013e3182214aad [DOI] [PubMed] [Google Scholar]
- 72.Brunoni AR, Tadini L, Fregni F. Changes in clinical trials methodology over time: a systematic review of six decades of research in psychopharmacology. PLoS One. 2010;5(3):e9479. doi: 10.1371/journal.pone.0009479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi: 10.1055/s-0043-116492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Dose Response Meta-analysis of Antipsychotic Drugs for Relapse Prevention in Schizophrenia (protocol)
eAppendix 2. Description of the Search Strategy
eFigure. PRISMA Diagram of the Search
eTable 1. Characteristics of Included Studies
eTable 2. Characteristics of Excluded Studies
eReferences
eAppendix 3. Assessment With the Cochrane Risk of Bias Tool, Version 2
eAppendix 4. Individual Drugs and Additional Sensitivity/Subgroup Analyses
eAppendix 5. Conversion to Absolute Rates, Relative Risks, and PANSS Units
eAppendix 6. Heterogeneity and Small Trial/Publication Bias
eAppendix 7. Update of the Meta-analysis of Uchida et al (2011) and Additional Analyses of Doses Higher Than Standard Doses (≥5mg Risperidone Equivalent per Day)
eTable 3. Average Dopamine Receptor Occupancies for Risperidone Doses