Abstract
Background:
The treatment for men diagnosed with localized prostate cancer has changed over time given the increased attention to the harms associated with over-diagnosis and the development of protocols for active surveillance.
Methods:
We examined trends in the treatment of men diagnosed with localized prostate cancer between 2004 and 2015, using the most recently available data from Surveillance, Epidemiology, and End Results Program (SEER)-Medicare. Patients were stratified by Gleason score, age, and race groups.
Results:
The use of active surveillance increased from 22% in 2004–2005 to 50% in 2014–2015 for patients with a Gleason score of 6 or below and increased from 9% in 2004–2005 to 13% in 2014–2015 for patients with a Gleason score of 7 or above. Patients with a Gleason score of 7 or above had increased use of intensity-modulated radiation therapy and prostatectomy, especially among patients aged 75 years and older. Among patients with a Gleason score of 6 or below non-Hispanic black men were less likely to undergo active surveillance than non-Hispanic white men.
Conclusions:
There has been a large increase in the use of active surveillance among men with a Gleason score of 6 or below. However, non-Hispanic black men with a Gleason score of 6 or below are less likely to receive active surveillance.
Keywords: Prostate cancer, Treatment trends, Active surveillance, New treatment methods, Treatment disparities
1. Introduction
Prostate cancer accounts for about 20% of newly reported cancer cases (not including nonmelanoma skin cancer) among US men in 2017 [1] and 90% of prostate cancers are diagnosed at local or regional stage [2]. Treatment options for localized prostate cancer include prostatectomy, intensity-modulated radiation therapy (IMRT), brachytherapy, 3-dimensional conformal radiation therapy, and conservative management that include active surveillance [3]. Treatments differ in terms of their impact on disease progression [4], side effects [5], and costs [6], but much of the data quantifying these tradeoffs is based on observational studies or randomized trials conducted before the era of widespread prostate-specific antigen (PSA) screening. There is considerable uncertainty about which treatment option offers the best balance of quality of life and survival [7]. The introduction of protocols for active surveillance [3] has expanded options for patients but has made patients’ decisions even more complex.
We undertook this analysis to describe trends in the management of men diagnosed with localized stage prostate cancer using the most recently-available data from Surveillance, Epidemiology, and End Results Program (SEER)-Medicare [8]. In light of the growing awareness of overdiagnosis [9], the development of active surveillance protocols and practice guidelines [3], and the release of professional recommendations advising against the routine use of active treatment [10], we hypothesized that the share of men undergoing active surveillance would increase over time. In addition, given the growing attention to personalization of treatment decisions, we expected that increases in the use of conservative management strategies would be particularly large among men with low-risk disease and those aged 75 years and older. We further hypothesized that there would be slower uptake of active surveillance among low-risk non-Hispanic black (NHB) men. Previous research has found that the introduction of new technologies, such as drugs and procedures, often expand social inequalities [11].
2. Methods
2.1. Data source and patient cohort
We evaluated trends in disease management using the SEER-Medicare database, which is a population-based database containing Medicare claims for cancer patients residing within one of the 18 SEER registry-regions. These regions contain 34.6% of the U.S. population [8]. We identified 547,383 men newly diagnosed with prostate cancer between 2004 and 2015. We excluded patients who were younger than age 66 years at diagnosis (n = 187,528), as well as those who did not have continuous Medicare Part A and Part B coverage (n = 48,826) and those who enrolled in Medicare Advantage (n = 96,299) 12 months before and 12 months after diagnosis (or until death for patients who died within 12 months). We excluded patients whose diagnosis reporting source was hospice/nursing home, autopsy report, or death certificate, as well as patients whose diagnosis date was after the date of death (n = 4,643). We further excluded patients whose cancer was not “localized/regional” as defined by the “SEER Historic Stage A” variable (n = 25,198) and patients with missing Gleason score (n = 6,592). Our final study population consisted of 178,297 patients.
To analyze the treatment trends among different patient groups, we stratified the sample by Gleason score (6 or below, 7 or above). We used the Gleason score from needle core biopsy/transurethral resection of prostate. Race was categorized as non-Hispanic white (NHW), NHB, and other race/ethnicity (included Asian, Hispanic and other race/ethnicity groups). We calculated patients’ Klabunde comorbidity index [12] using Medicare claims in the window 12 months before the diagnosis month. We used the scoring method designed by Roux et al. [13] as a proxy for patients’ socioeconomic status at zip-code level.
2.2. Outcome measures and statistical analysis
We identified patients undergoing active treatment, which included prostatectomy, IMRT, and other treatments (external radiotherapy other than IMRT, brachytherapy, androgen deprivation therapy [ADT], and cryotherapy), based on Medicare claims up to 183 days after diagnosis date, following previous work [14]. For patients who had 2 or more treatments among office, outpatient, and inpatient claims, we categorized treatment based on the treatment billed first to determine the definitive treatment (e.g., surgery or radiation therapy).
Among patients who did not undergo any active treatment, we categorized patients as undergoing active surveillance if they had at least one PSA or prostate biopsy test between the second and the 12 month after the diagnosis month, following validated algorithms [15] and no subsequent medical claim for any active treatment. We used this algorithm because it provided the highest specificity (99%), and relatively high negative predictive values (84%) and positive predictive values (86%) [15]. Patients who did not undergo any active treatment or active surveillance were grouped into a “no treatment” category (which included watchful waiting). The Healthcare Common Procedure Coding System and ICD-9/ICD-10 Procedure Codes used to define each treatment are listed in Supplementary Table 1.
We described trends in the percent of patients undergoing each type of treatment by year of diagnosis, and trends were stratified by Gleason score, age, and race (NHB vs. NHW). There were only a small number of patients with races other than NHW or NHB, and their treatment patterns as a group were similar to those of NHW patients. We fit multinomial logistic regression models to estimate the differences in the distribution of treatment types by age and by race groups. Multinomial logistic models are a generalization of the logistic regression model that can accommodate multiple, mutually exclusive binary outcomes. In our case, each patient was associated with multiple treatment options, only one of which took the value of “1”, corresponding to the patient’s actual treatment choice. We also fit a logistic regression to estimate the differences between NHB and NHW men with a Gleason score 7 or above in the probabilities of undergoing active surveillance or no treatment. We included “other race/ethnicity” as a separate category along with NHB and NHW in summary statics and regression analyses; however, we did not report results of “other race/ethnicity” on Fig. 4, Table 2, and Supplementary Table 3. In addition to stratifying patients by Gleason score (Gleason score of 6 or below VS Gleason score of 7 or above), we also analyzed the treatment trends for patients by National Comprehensive Cancer Network (NCCN) criteria of high risk (i.e., clinical stage T2c or greater, or Gleason score of 8 or above, or PSA > 19.9 ng/ml), low risk (i.e., clinical stage T1 to T2a, Gleason score of 6 or below, and PSA < 10.0 ng/ml), and intermediate risk (all other patients). The results are reported in Supplementary Table 4, Supplementary Table 5, and Supplementary Table 6.
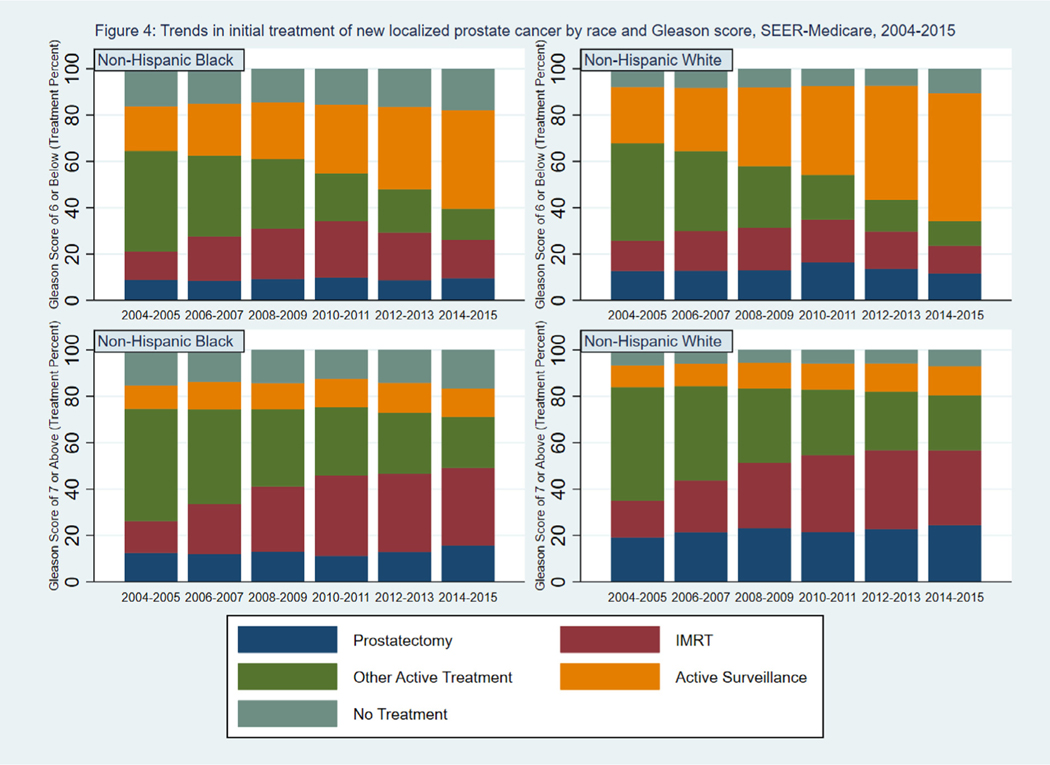
Fig. 4.
Trends in initial treatment of new localized prostate cancer by race and Gleason score, SEER-Medicare, 2004–2015. Percent of patients undergoing prostatectomy, IMRT, other active treatment, active surveillance, or no treatment by treatment year, race (non-Hispanic white, non-Hispanic black), and Gleason score (6 and below, 7 and above). IMRT = intensity-modulated radiation therapy, SEER = Surveillance, Epidemiology, and End Results.
Table 2.
Estimated probabilities of treatments for localized prostate cancer by age and race group
| Gleason score of 6 or below | Gleason Score of 7 or Above | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Prostatectomy | IMRT | AS | No treatment | Prostatectomy | IMRT | AS | No Treatment | ||
|
| |||||||||
| Age group 66–74 | 2004–2005 | 0.184[0.18,0.19] | 0.134[0.13,0.14] | 0.193[0.19,0.20] | 0.078[0.07,0.08] | 0.313[0.30,0.32] | 0.151[0.15,0.16] | 0.073[0.07,0.08] | 0.072[0.07,0.08] |
| 2006–2007 | 0.182[0.18,0.19] | 0.179[0.17,0.19] | 0.221[0.21,0.23] | 0.080[0.08,0.08] | 0.334[0.33,0.34] | 0.210[0.20,0.22] | 0.074[0.07,0.08] | 0.064[0.06,0.07] | |
| 2008–2009 | 0.177[0.17,0.19] | 0.194[0.19,0.20] | 0.278[0.27,0.29] | 0.081[0.08,0.08] | 0.335[0.33,0.34] | 0.262[0.26,0.27] | 0.084[0.08,0.09] | 0.063[0.06,0.07] | |
| 2010–2011 | 0.211[0.20,0.22] | 0.197[0.19,0.20] | 0.323[0.31,0.33] | 0.078[0.07,0.08] | 0.315[0.31,0.32] | 0.312[0.30,0.32] | 0.086[0.08,0.09] | 0.062[0.06,0.07] | |
| 2012–2013 | 0.176[0.17,0.19] | 0.174[0.17,0.18] | 0.425[0.41,0.44] | 0.080[0.07,0.08] | 0.322[0.31,0.33] | 0.316[0.31,0.32] | 0.094[0.09,0.10] | 0.064[0.06,0.07] | |
| 2014–2015 | 0.149[0.14,0.16] | 0.135[0.13,0.14] | 0.494[0.48,0.51] | 0.110[0.10,0.12] | 0.350[0.34,0.36] | 0.297[0.29,0.30] | 0.094[0.09,0.10] | 0.077[0.07,0.08] | |
| Age group 75+ | 2004–2005 | 0.038[0.04,0.04] | 0.130[0.12,0.14] | 0.292[0.28,0.30] | 0.106[0.10,0.11] | 0.056[0.05,0.06] | 0.163[0.16,0.17] | 0.116[0.11,0.12] | 0.081[0.08,0.08] |
| 2006–2007 | 0.037[0.03,0.04] | 0.172[0.17,0.18] | 0.330[0.32,0.34] | 0.107[0.10,0.11] | 0.063[0.06,0.07] | 0.239[0.23,0.24] | 0.124[0.12,0.13] | 0.075[0.07,0.08] | |
| 2008–2009 | 0.035[0.03,0.04] | 0.182[0.17,0.19] | 0.403[0.39,0.41] | 0.106[0.10,0.11] | 0.065[0.06,0.07] | 0.305[0.30,0.31] | 0.143[0.14,0.15] | 0.075[0.07,0.08] | |
| 2010–2011 | 0.042[0.04,0.05] | 0.186[0.18,0.19] | 0.473[0.46,0.48] | 0.103[0.10,0.11] | 0.061[0.06,0.06] | 0.360[0.35,0.37] | 0.146[0.14,0.15] | 0.074[0.07,0.08] | |
| 2012–2013 | 0.033[0.03,0.04] | 0.153[0.14,0.16] | 0.578[0.57,0.59] | 0.098[0.09,0.10] | 0.063[0.06,0.07] | 0.370[0.36,0.38] | 0.163[0.16,0.17] | 0.076[0.07,0.08] | |
| 2014–2015 | 0.026[0.02,0.03] | 0.112[0.10,0.12] | 0.632[0.62,0.64] | 0.130[0.12,0.14] | 0.071[0.07,0.07] | 0.360[0.35,0.37] | 0.168[0.16,0.18] | 0.093[0.09,0.10] | |
| Number of cases | 73435 | 104862 | |||||||
| Non-Hispanic Black | 2004–2005 | 0.095[0.09,0.10] | 0.137[0.13,0.15] | 0.209[0.20,0.22] | 0.128[0.12,0.14] | 0.127[0.12,0.13] | 0.157[0.15,0.16] | 0.107[0.10,0.11] | 0.119[0.11,0.13] |
| 2006–2007 | 0.095[0.09,0.10] | 0.183[0.17,0.19] | 0.237[0.23,0.25] | 0.131[0.12,0.14] | 0.142[0.13,0.15] | 0.226[0.22,0.24] | 0.113[0.11,0.12] | 0.110[0.10,0.12] | |
| 2008–2009 | 0.098[0.09,0.11] | 0.198[0.19,0.21] | 0.292[0.28,0.30] | 0.131[0.12,0.14] | 0.153[0.15,0.16] | 0.284[0.27,0.29] | 0.126[0.12,0.13] | 0.107[0.10,0.11] | |
| 2010–2011 | 0.125[0.12,0.14] | 0.206[0.19,0.22] | 0.341[0.33,0.36] | 0.125[0.12,0.13] | 0.141[0.13,0.15] | 0.336[0.32,0.35] | 0.129[0.12,0.14] | 0.106[0.10,0.11] | |
| 2012–2013 | 0.106[0.10,0.12] | 0.180[0.17,0.19] | 0.438[0.42,0.45] | 0.123[0.11,0.13] | 0.151[0.14,0.16] | 0.342[0.33,0.35] | 0.141[0.13,0.15] | 0.107[0.10,0.11] | |
| 2014–2015 | 0.090[0.08,0.10] | 0.136[0.13,0.15] | 0.493[0.48,0.51] | 0.166[0.15,0.18] | 0.166[0.16,0.17] | 0.326[0.31,0.34] | 0.143[0.13,0.15] | 0.127[0.12,0.13] | |
| Non–Hispanic White | 2004–2005 | 0.127[0.12,0.13] | 0.129[0.12,0.13] | 0.240[0.23,0.25] | 0.083[0.08,0.09] | 0.191[0.19,0.20] | 0.157[0.15,0.16] | 0.093[0.09,0.10] | 0.069[0.07,0.07] |
| 2006–2007 | 0.126[0.12,0.13] | 0.173[0.17,0.18] | 0.271[0.26,0.28] | 0.084[0.08,0.09] | 0.211[0.21,0.22] | 0.224[0.22,0.23] | 0.097[0.09,0.10] | 0.063[0.06,0.07] | |
| 2008–2009 | 0.129[0.12,0.13] | 0.185[0.18,0.19] | 0.331[0.32,0.34] | 0.083[0.08,0.09] | 0.226[0.22,0.23] | 0.281[0.27,0.29] | 0.108[0.10,0.11] | 0.061[0.06,0.06] | |
| 2010–2011 | 0.162[0.16,0.17] | 0.189[0.18,0.20] | 0.379[0.37,0.39] | 0.078[0.07,0.08] | 0.210[0.20,0.22] | 0.334[0.33,0.34] | 0.111[0.11,0.12] | 0.061[0.06,0.06] | |
| 2012–2013 | 0.136[0.13,0.14] | 0.162[0.15,0.17] | 0.481[0.47,0.49] | 0.078[0.07,0.08] | 0.224[0.22,0.23] | 0.338[0.33,0.35] | 0.121[0.12,0.13] | 0.062[0.06,0.07] | |
| 2014–2015 | 0.117[0.11,0.12] | 0.124[0.12,0.13] | 0.545[0.53,0.56] | 0.106[0.10,0.11] | 0.245[0.24,0.25] | 0.322[0.31,0.33] | 0.123[0.12,0.13] | 0.075[0.07,0.08] | |
| Number of cases | 68759 | 97366 | |||||||
Note: 95% Confidence interval in Bracket; IMRT = intensity-modulated radiation therapy; AS = active surveillance; Estimated probabilities were calculated by the coefficients from the multinomial logistic regression models described in the Outcome Measures and Statistical Analysis section. The original coefficients are in Supplement Table 2.
Models were adjusted for patients’ comorbidity, socioeconomic status, year of diagnosis, SEER region, Medicaid coverage, and residence in a rural or urban area. The coefficients from multinomial logistic regression models are difficult to interpret, so we used the coefficient estimates to calculate the estimated probabilities that patients would undergo each type of treatment. All statistical analyses were conducted in SAS version 9.4 and STATA version 15.1. The Emory Institutional Review Board exempted this study from human subjects review.
3. Results
Of the 178,297 patients who met the study inclusion criteria, 73,435 had a Gleason score 6 or below and 104,862 had a Gleason score or 7 or above. Table 1 shows patients’ characteristics by year of diagnosis. The number of patients in the sample declined from 36,244 in 2004–2005 to 22,074 in 2014–2015, with a decreasing percent of patients aged 66 to 74 and who had a Gleason score of 6 or below. We reported the percent of patients with a Gleason score 6 or below by year and stratified by age group in Supplementary Table 7.
Table 1.
Characteristics of newly diagnosed localized prostate cancer cases by year of diagnosis, SEER-Medicare, 2004–2015
| 2004–2005a | 2006–2007 | 2008–2009 | 2010–2011 | 2012–2013 | 2014–2015 | Total | |
|---|---|---|---|---|---|---|---|
| (N = 36,244) | (N = 36,827) | (N = 31,424) | (N = 29,048) | (N = 22,680) | (N = 22,074) | (N = 178,297) | |
|
| |||||||
| GS≤6a | 17,313 (47.8) | 16,240 (44.1) | 12,959 (41.2) | 11,558 (39.8) | 8,276 (36.5) | 7,089 (32.1) | 73,435 (41.2) |
| GS≥7 | 18,931 (52.2) | 20,587 (55.9) | 18,465 (58.8) | 17,490 (60.2) | 14,404 (63.5) | 14,985 (67.9) | 104,862 (58.8) |
| Age 66–74 | 19,413 (53.6) | 20,211 (54.9) | 18,483 (58.8) | 17,479 (60.2) | 14,239 (62.8) | 14,067 (63.7) | 103,892 (58.3) |
| Age 75–110 | 16,831 (46.4) | 16,616 (45.1) | 12,941 (41.2) | 11,569 (39.8) | 8,441 (37.2) | 8,007 (36.3) | 74,405 (41.7) |
| Non-Hispanic Black | 4,132 (11.4) | 3,884 (10.5) | 3,453 (11) | 3,203 (11) | 2,598 (11.5) | 2,346 (10.6) | 19,616 (11) |
| Non-Hispanic White | 29,771 (82.1) | 30,538 (82.9) | 25,838 (82.2) | 23,866 (82.2) | 18,476 (81.5) | 18,020 (81.6) | 146,509 (82.2) |
| Other Race/ethnicity | 2,341 (6.5) | 2,405 (6.5) | 2,133 (6.8) | 1,979 (6.8) | 1,606 (7.1) | 1,708 (7.7) | 12,172 (6.8) |
| Comorbidity = 0 | 21,367 (59) | 21,335 (57.9) | 17,593 (56) | 15,832 (54.5) | 12,130 (53.5) | 12,327 (55.8) | 100,584 (56.4) |
| Comorbidity > 0 and <3 | 10,463 (28.9) | 11,081 (30.1) | 9,917 (31.6) | 9,445 (32.5) | 7,379 (32.5) | 6,640 (30.1) | 54,925 (30.8) |
| Comorbidity ≥3 | 3,041 (8.4) | 3,099 (8.4) | 2,845 (9.1) | 2,768 (9.5) | 2,207 (9.7) | 1,898 (8.6) | 15,858 (8.9) |
| Comorbidity unknown | 1,373 (3.8) | 1,312 (3.6) | 1,069 (3.4) | 1,003 (3.5) | 964 (4.3) | 1,209 (5.5) | 6,930 (3.9) |
| Rural | 3,999 (11) | 4,015 (10.9) | 3,322 (10.6) | 3,000 (10.3) | 2,249 (9.9) | 2,235 (10.1) | 18,820 (10.6) |
| Urban | 32,205 (88.9) | 32,775 (89) | 28,044 (89.2) | 26,001 (89.5) | 20,394 (89.9) | 19,797 (89.7) | 159,216 (89.3) |
| Rural/urban unknown | 40 (0.1) | 37 (0.1) | 58 (0.2) | 47 (0.2) | 37 (0.2) | 42 (0.2) | 261 (0.1) |
| Not Medicaid | 33,208 (91.6) | 33,937 (92.2) | 28,872 (91.9) | 26,755 (92.1) | 20,973 (92.5) | 20,591 (93.3) | 164,336 (92.2) |
| Medicaid | 3,036 (8.4) | 2,890 (7.8) | 2,552 (8.1) | 2,293 (7.9) | 1,707 (7.5) | 1,483 (6.7) | 13,961 (7.8) |
| SES lowb | 12,207 (33.7) | 11,756 (31.9) | 10,023 (31.9) | 9,146 (31.5) | 6,920 (30.5) | 6,350 (28.8) | 56,402 (31.6) |
| SES medium | 11,860 (32.7) | 12,269 (33.3) | 10,455 (33.3) | 9,491 (32.7) | 7,483 (33) | 7,299 (33.1) | 58,857 (33) |
| SES high | 11,376 (31.4) | 11,967 (32.5) | 10,257 (32.6) | 9,732 (33.5) | 7,781 (34.3) | 7,897 (35.8) | 59,010 (33.1) |
| SES unknown | 801 (2.2) | 835 (2.3) | 689 (2.2) | 679 (2.3) | 496 (2.2) | 528 (2.4) | 4,028 (2.3) |
| Southc | 8,798 (24.3) | 9,216 (25) | 8,101 (25.8) | 7,404 (25.5) | 5,964 (26.3) | 5,711 (25.9) | 45,194 (25.3) |
| North central | 4,704 (13) | 4,488 (12.2) | 3,769 (12) | 3,576 (12.3) | 2,682 (11.8) | 2,545 (11.5) | 21,764 (12.2) |
| Northeast | 7,118 (19.6) | 7,640 (20.7) | 6,264 (19.9) | 5,754 (19.8) | 4,445 (19.6) | 4,372 (19.8) | 35,593 (20) |
| Pacific/West | 15,624 (43.1) | 15,483 (42) | 13,290 (42.3) | 12,314 (42.4) | 9,589 (42.3) | 9,446 (42.8) | 75,746 (42.5) |
Column percentage in parentheses; and GS = Gleason score. For GS, we used the Gleason score from needle core biopsy/transurethral resection of prostate (TURP)
SES for social and economic status
South: Atlanta, Rural Georgia, KY, LA, GA; North Central: Detroit, Iowa; Northeast: CT, NJ; Pacific/West: San Francisco, Hawaii, New Mexico, Seattle, Utah, Alaska, San Jose, Los Angeles, CA.
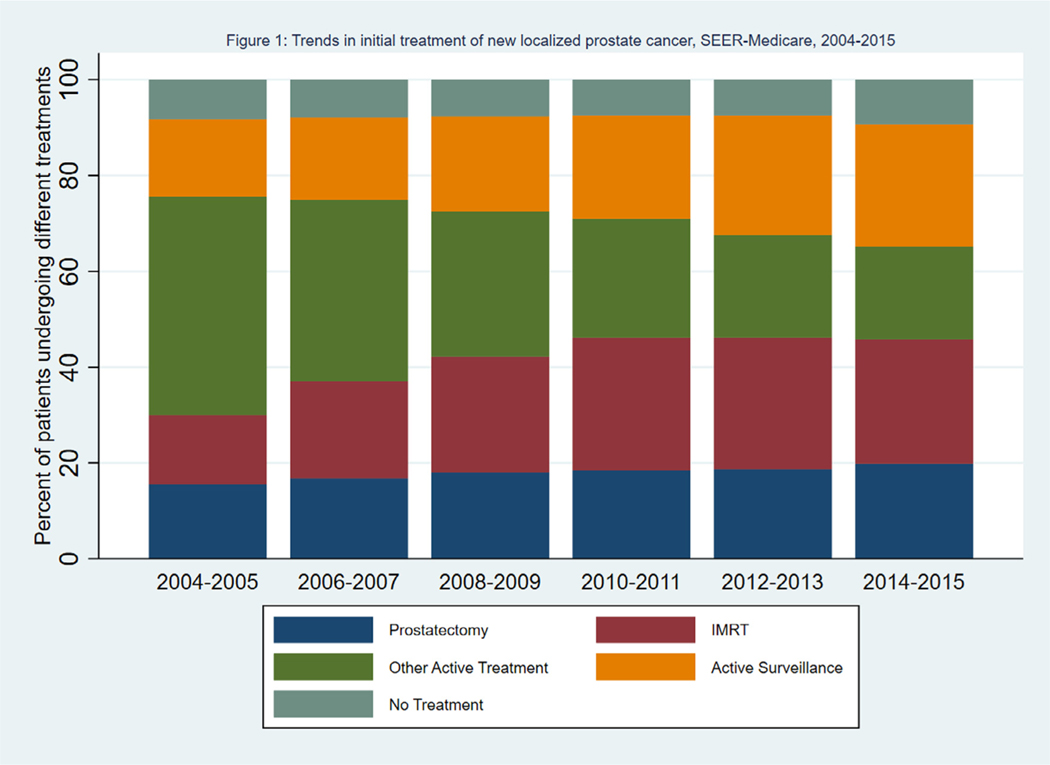
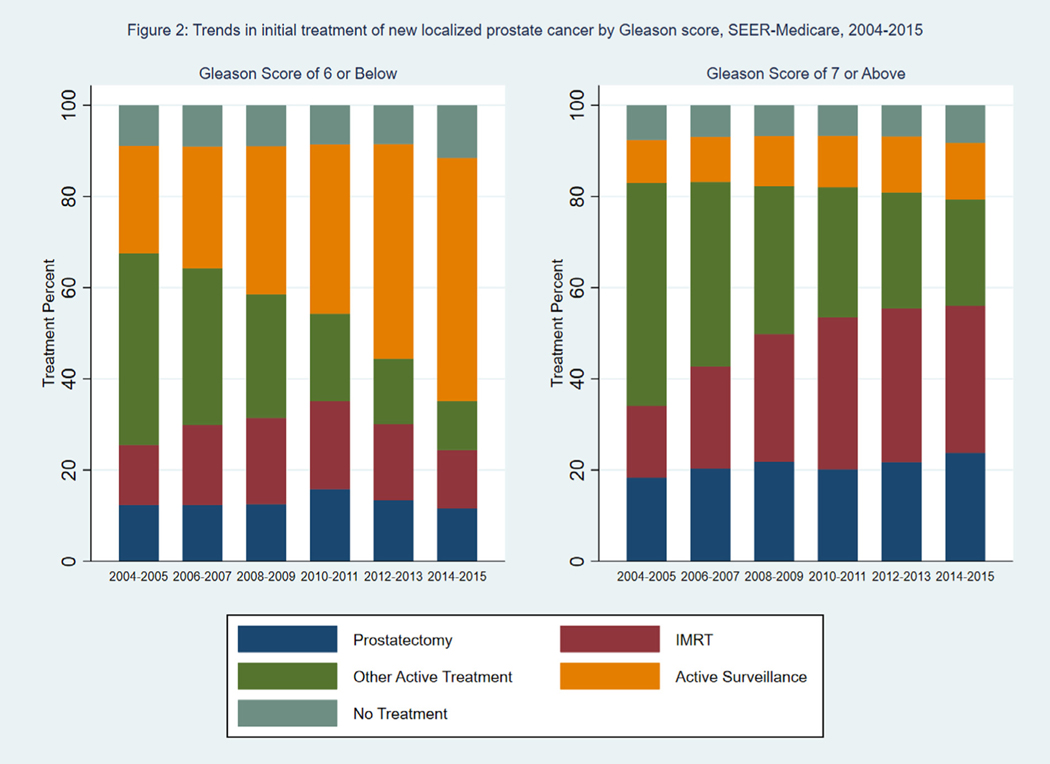
Fig. 1 shows the percent of patients receiving each type of treatment by year of diagnosis. The use of IMRT and active surveillance increased over time, with offsetting declines in the use of treatments in the “Other” category. Supplementary Figure 1 shows the trends among patients receiving each of the 3 large categories of treatments (brachytherapy, other external radiation, and ADT and cryotherapy) within the “Other” category. Between 2004 and 2015, ADT and cryotherapy ranged from 45% to 50% of other treatments, brachytherapy ranged from 25% to 30% of other treatments, and other external radiation therapy ranged from 25% to 30% of other treatments. Fig. 2 shows the percent of patients receiving each type of treatment, stratified by Gleason score. Among patients with Gleason score 6 or below, there was a large increase in the percent of patients undergoing active surveillance, from 24% in 2004–2005 to 53% in 2014–2015. There was a corresponding decrease in the percent of patients receiving treatments in the “Other” category, which included external radiotherapy other than IMRT, brachytherapy, androgen deprivation therapy, and cryotherapy. Among patients with a Gleason score 7 or above, there was a large increase in the use of IMRT, from 16% in 2004–2005 to 34% in 2014–2015, and a corresponding decrease in the use of treatments in the “Other” category. The percent of patients undergoing active surveillance increased from 9% in 2004–2005 to 12% in 2014–2015.
Fig. 1.
Trends in initial treatment of new localized prostate cancer, SEER-Medicare, 2004–2015. Percent of patients undergoing prostatectomy, IMRT, other active treatment, active surveillance, or no treatment by treatment year. IMRT = intensity-modulated radiation therapy, SEER = Surveillance, Epidemiology, and End Results.
Fig. 2.
Trends in initial treatment of new localized prostate cancer by Gleason score, SEER-Medicare, 2004–2015. Percent of patients undergoing prostatectomy, IMRT, other active treatment, active surveillance, or no treatment by treatment year and Gleason score (6 and below, 7 and above). IMRT = intensity-modulated radiation therapy, SEER = Surveillance, Epidemiology, and End Results.
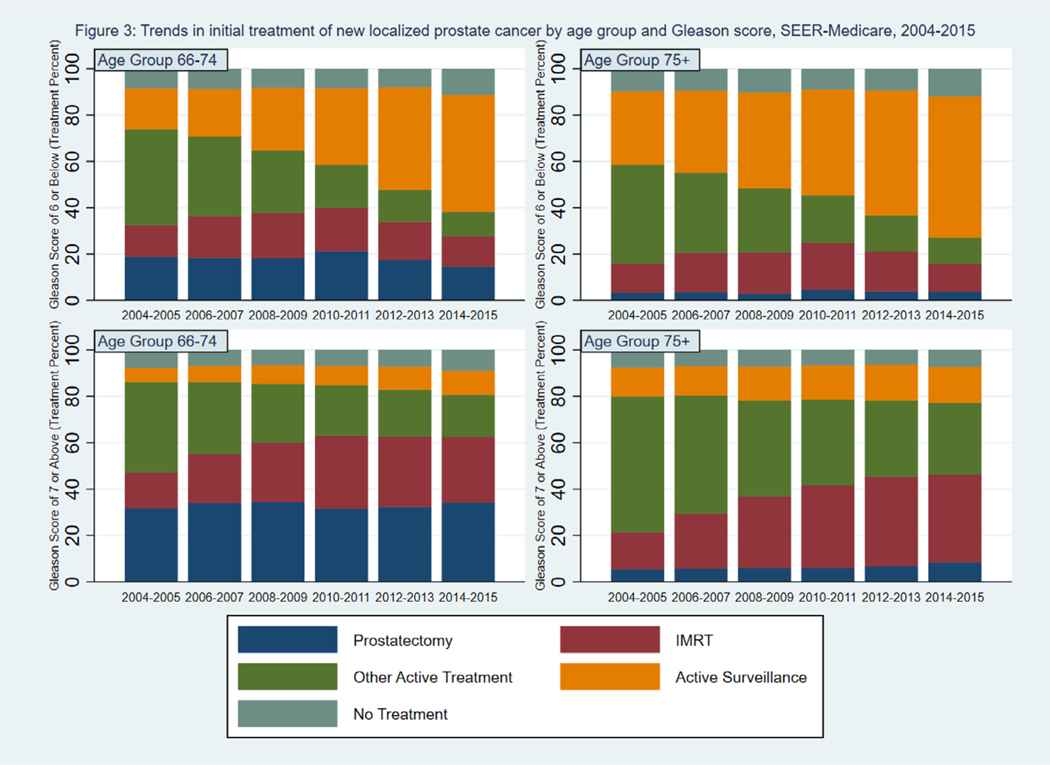
Fig. 3 shows the percent of patients undergoing prostatectomy, IMRT, active surveillance, other active treatment, and no treatment by age group and year of diagnosis, stratified by Gleason score. Among patients with a Gleason score 6 or below, the percent of patients undergoing active surveillance increased from 18% to 50% in patients aged 66 to 74 years and increased from 32% to 61% in patients aged 75 years and older. Among patients with a Gleason score 7 or above, there was a small increase (6% to 10% in patients aged 66 to 74 and 13% to 15% in patients aged 75 and older) in the percent of patients undergoing active surveillance offset by a shift away from treatments in the Other category, which included brachytherapy, toward IMRT. Among patients with a Gleason score 6 or below, those aged 75 and older were less likely to undergo active treatment compared to patients aged 66 to 74 years. Among men with a Gleason score 7 or above, men aged 75 years and older were much less likely to have a prostatectomy compared with men aged 66 to 74.
Fig. 3.
Trends in initial treatment of new localized prostate cancer by age group and Gleason score, SEER-Medicare, 2004–2015. Percent of patients undergoing prostatectomy, IMRT, other active treatment, active surveillance, or no treatment by treatment year, age (66–74 years, 75 years and above), and Gleason score (6 and below, 7 and above). IMRT = intensity-modulated radiation therapy, SEER = Surveillance, Epidemiology, and End Results.
Fig. 4 shows the percent of patients undergoing each treatment by race groups and year of diagnosis, stratified by Gleason score. Regardless of Gleason scores, a greater percent of NHW patients underwent prostatectomy compared to NHB patients. For example, among men with Gleason score 7 or above diagnosed in 2014 and 2015, 24% of NHW patients underwent prostatectomy compared to 16% of NHB patients. For patients with a Gleason score 6 or below, the percent of NHW and NHB patients undergoing active surveillance rose by equal amounts over the study period, though at any point in time NHW patients were slightly more likely to undergo surveillance. For example, for patients with a Gleason score 6 or below, a total of 42% of NHB patients vs. 55% of NHW patients underwent active surveillance in 2014 and 2015. For patients with a Gleason score 7 or above, at any point of time, NHB patients were more likely to undergo no treatment. NHB patients were also more likely to undergo active surveillance or no treatment among men with a Gleason score 7 or above. For example, among men with a Gleason score 7 or above diagnosed in 2014 and 2015, NHB men were 7.37 percentage points more likely to undergo active surveillance or no treatment than NHW (P < 0.0001). The marginal effects, standard errors, and 95% confidence intervals of the regression results are presented in Supplementary Table 3.
Table 2 shows the estimates of the probabilities of patients undergoing prostatectomy, IMRT, active surveillance, and no treatment by age, race groups, and year of diagnosis, stratified by Gleason score. These estimates were constructed using results from the multinomial logistic regression models and were adjusted for the patient characteristics described in Table 1. The original coefficients of the regression results are presented in Supplementary Table 2. The results are similar to the unadjusted share of patients undergoing each treatment in Fig. 3 and Fig. 4. Among patients with a Gleason score 6 or below, the adjusted probabilities of patients undergoing active surveillance increased from 0.193 to 0.494 in patients aged 66 to 74 years and increased from 0.292 to 0.632 in patients aged 75 and older. Among patients with a Gleason score 7 or above, the adjusted probabilities of patients undergoing active surveillance increased from 0.073 to 0.094 in patients aged 66 to 74 and increased from 0.116 to 0.168 in patients aged 75 and older. Among patients with a Gleason score 6 or below, the adjusted probabilities of patients undergoing active surveillance increased from 0.209 to 0.493 for NHB patients and increased from 0.240 to 0.545 for NHW patients. Among patients with Gleason 7 or above, NHB and NHW patients showed similarly slight increases in the probabilities of active surveillance usage over the study period (NHB 0.107 to 0.143, NHW 0.093 to 0.123).
4. Discussion
Consistent with prior studies [16], we found a large increase in the use of active surveillance over time among patients with a Gleason score 6 or below. The shift may be due to the growing awareness of the harms associated with aggressive treatment, the recent treatment guidelines that introduced active surveillance as an option [3], and the 2011 National Institutes of Health State of the Science conference consensus statement that recommended offering active surveillance to patients diagnosed with low-risk disease [17].
There is limited data with which to compare the use of active surveillance in the US and Europe. In Sweden, the use of active surveillance exceeded 90% among very low-risk prostate cancer patients and exceeded 70% among low-risk patients in 2014 [18]. Some features of the US health care system (e.g., fee for service reimbursement, medicolegal concerns, etc.) may drive patients and providers toward more aggressive treatment. We also observed an increase in the use of IMRT and prostatectomy among patients aged 75 and older with a Gleason score 7 or above. Use of definitive treatment, especially high-cost radiation therapies, among prostate cancer patients with limited life expectancy may not be cost-effective [19].
Our results build upon previous work by distinguishing between patients who received active surveillance vs. those receiving no treatment, presenting trends over a longer period, and using the most recently available data from SEER-Medicare. A closely-related recent study documented increases in the use of active surveillance using SEER registry data [20]. Our analysis incorporated information from claims, thus providing a more accurate description of treatment patterns. SEER registry data tend to underestimate active treatment for prostate cancer patients who received chemotherapy by 6 percentage points and hormone therapy by 8 percentage points [21], and may not differentiate active surveillance from no intervention [21]. In addition, we stratified patients by age and race/ethnicity groups.
NHB men diagnosed with localized prostate cancer are more likely to experience disease progression [22]. This pattern has been variously attributed to biological factors [23], social and economic status [23], and/or dietary differences [23], though more recent research suggests that socioeconomic status and treatment patterns are the largest contributors to racial differences in outcomes [24]. Hence, active surveillance is appropriate for NHB patients, though physicians may set a lower bar for treatment following an indication of progression [25]. However, our results showed that NHB patients with a Gleason score 6 or below were less likely to undergo active surveillance compared to NHW patients. Active surveillance requires routine monitoring by PSA or prostate biopsy tests. Lower active surveillance uptake rate for the NHB patients may also reflect disparities in access to regular preventive and wellness management [26] or patient preferences for active treatment [27]. Vignette studies, and/or the impact of shared decision making tools on differences in treatment may further help understanding of the reasons for treatment disparities.
Among men with a Gleason score 7 or above, our results show that NHB men were significantly less likely to undergo active treatment at any time between 2004 and 2015. Lower use of active treatment among NHB patients with a Gleason score of 7 or above may contribute to higher prostate cancer mortality rates among NHB men.
There are limitations of this analysis. When identifying treatments, we tried to account for changes to the Healthcare Common Procedure Coding System and ICD procedure codes for each treatment to produce consistent trends. However, it is possible that trends reflected changes to coding practices rather than the treatments actually received by patients.
Consistent with other studies [28], our results showed that the number of newly diagnosed localized prostate cancer cases declined. Trends in treatment may partially reflect the changing composition of the pool of patients diagnosed with localized cancer. The proportion of patients with a Gleason score 6 or below decreased from 47.8% in 2004–2005 to 32.1% in 2014–2015. For patients aged 66 to 74, the percent of patients with a Gleason score 6 or below decreased from 51.8% in 2004–2005 to 36.3% in 2014–2015. This is most likely due to the changes to the prostate cancer screening recommendations [9], and a more restrictive pathology definition of Gleason Score 6 or below [29]. Still, it is possible that patients diagnosed toward the end of our study period differed in terms of prognosis compared to patients diagnosed earlier. For example, there may be a relatively higher share of patients diagnosed based on symptoms in the years near the end of our study period compared to years in earlier period of our study. Our sample included only men aged 66 years and older. Treatment patterns, and differences in treatment patterns by Gleason score and race, may be different among men aged ≤65 years.
We selected patients with localized disease and stratified results by Gleason score, an important indicator of patients’ prognosis. We did not adjust for PSA or clinical stage, because we wanted to maximize our sample size given the number of patients missing either PSA or clinical stage data (a total of 16% of our patients lacked either PSA or clinical stage). Without these variables, we were unable to assess the suitability of active surveillance for men with a Gleason score of 7 or above [30]. Our analysis for patients within the NCCN high-risk category (Supplementary Table 4) showed that the patients in the high-risk category were less likely to use active surveillance compared to a broader patient population with a Gleason score 7 or above. Patients within the NCCN low-risk category (Supplementary Table 5) had a similar trend of active surveillance use compared to a broader patient population with a Gleason score 6 or below. The active surveillance usage for patients within the intermediate risk group (Supplementary Table 6) was between the usage for patients with a Gleason score 6 or below and the usage for patients with a Gleason score 7 or above.
At last, active surveillance was introduced in 2010 as a viable management alternative to active treatment. Active surveillance requires regular prostate-specific antigen tests, digital rectal examinations, needle biopsies, or magnetic resonance imaging at different timeframes after diagnosis for patients with different life expectancy and clinical risk [3]. Therefore, there is no standard approach yet for identifying active surveillance using claims data. Different algorithms to identify patients on an active surveillance protocol reflect the level of surveillance intensity, ranging from the most “active” surveillance by biopsy test to less “active” approach by PSA. We used the algorithms that included both biopsy test and PSA to account for the variations of active surveillance approaches used by patients and clinicians. Still, it is possible that other algorithms may show different trends of active surveillance.
5. Conclusions
Concern about the harms of use of surgery and radiotherapy for men with low-risk, localized stage prostate cancer may have influenced practice patterns: The share of men with a Gleason score 6 or below undergoing active treatment declined over time. More men underwent active surveillance, which avoids the side effects associated with active treatment and reduces the risk of progression compared to no treatment. Growing differences among men receiving active treatment in the proportion of men with a Gleason score 6 or below and those with a Gleason score 7 or above suggests that treatment is becoming increasingly personalized and that patients and clinicians may welcome the validation of additional biomarkers to differentiate between high- and low-risk diseases.
A large proportion of men, especially NHB men, did not undergo active treatment or active surveillance. It is unclear if the lack of treatment and surveillance was consistent with their preferences or reflects a lack of comfort seeking medical care, inadequate communication by clinicians, a lack of familiarity with active surveillance by their clinicians, or transportation difficulties. The fact that NHB men are historically more likely to undergo no treatment suggest that care patterns may not be entirely preference-driven.
Supplementary Material
The treatment for men with localized prostate cancer has changed over time.
There had been an increase in the use of active surveillance among men with a Gleason score of 6.
Non-Hispanic black men with a Gleason score of 6 were less likely to undergo active surveillance.
The introduction of a new treatment approach often expands social inequalities.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Funding statement: Funding support for David H. Howard was provided by the Centers for Disease Control and Prevention (CDC) (Intergovernmental Personal Act, Assignment Agreement Number 16IPA1604432) and National Institutes of Health grant R01CA208758-01A1. This work was supported by National Cancer Institute Grant R01CA208758-01A1. The funder had no role in the conduct of the study or the writing of the manuscript.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2020.11.024.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: None.
References
- 1.Amrican Cancer Society. Cancer Facts & Figures 2017. [cited 2020 November 26]; Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf
- 2.American Society of Clinical Oncology. Prostate cancer: statistics. [cited 2019 May 14]; Available from https://www.cancer.net/cancer-types/prostate-cancer/statistics
- 3.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw 2010;8(2):145. [DOI] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375(15):1415–24. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman KE, Penson DF, Zhao ZG, Huang LC, Conwill R, Laviana AA, et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. Jama-Journal of the American Medical Association 2020;323 (2):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen PL, Gu XM, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, et al. Cost Implications of the Rapid Adoption of Newer Technologies for Treating Prostate Cancer. Journal of Clinical Oncology 2011;29 (12):1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun F, Oyesanmi O, Fontanarosa J, Reston J, Guzzo T, Schoelles K. Therapies for Clinically Localized Prostate Cancer: Update of a 2008 Systematic Review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. Decedn.(Comparative Effectiveness Reviews, No. 146). [PubMed] [Google Scholar]
- 8.National Cancer Institute. SEER-Medicare: Brief Description of the SEER-Medicare Database. [cited 2019 April 22]; Available from: https://healthcaredelivery.cancer.gov/seermedicare/overview/
- 9.USPSTF. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149 (3):185–91. [DOI] [PubMed] [Google Scholar]
- 10.Hahn C, Kavanagh B, Bhatnagar A, Jacobson G, Lutz S, Patton C, et al. Choosing wisely: the American Society for Radiation Oncology’s top 5 list. Pract Radiat Oncol 2014;4(6):349–55. [DOI] [PubMed] [Google Scholar]
- 11.Weiss D, Rydland HT, Oversveen E, Jensen MR, Solhaug S, Krokstad S. Innovative technologies and social inequalities in health: A scoping review of the literature. PLoS One 2018;13(4):e0195447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology 2000;53(12):1258–67. [DOI] [PubMed] [Google Scholar]
- 13.Roux AVD, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. New England Journal of Medicine 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell JM. Urologists’ use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med 2013;369(17):1629–37. [DOI] [PubMed] [Google Scholar]
- 15.Modi PK, Kaufman SR, Qi J, Lane BR, Cher ML, Miller DC, et al. National Trends in Active Surveillance for Prostate Cancer: Validation of Medicare Claims-based Algorithms. Urology 2018;120:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler S, Muralidhar V, Chavez J, Fullerton Z, Mahal A, Nezolosky M, et al. Active Surveillance for Low-Risk Prostate Cancer in Black Patients. N Engl J Med 2019;380(21):2070–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall IJ, Richardson LC. Commentary on the State-of-the-Science Conference on the role of active surveillance in the management of men with localized prostate cancer. J Natl Cancer Inst Monogr 2012;2012 (45):135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, Stattin P. Uptake of Active Surveillance for Very-Low-Risk Prostate Cancer in Sweden. JAMA Oncol 2017;3(10):1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs BL, Zhang Y, Skolarus TA, Hollenbeck BK. Growth Of High-Cost Intensity-Modulated Radiotherapy For Prostate Cancer Raises Concerns About Overuse. Health Affairs 2012;31(4):750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahal BA, Butler S, Franco I, Spratt DE, Rebbeck TR, D’Amico AV, et al. Use of Active Surveillance or Watchful Waiting for Low-Risk Prostate Cancer and Management Trends Across Risk Groups in the United States, 2010–2015. JAMA 2019;321(7):704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER Treatment Data With Medicare Claims. Medical care 2016;54(9):e55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol 2013;31 (24):2991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate 2005;62 (3):243–52. [DOI] [PubMed] [Google Scholar]
- 24.Dess RT, Hartman HE, Mahal BA, Soni PD, Jackson WC, Cooperberg MR, et al. Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality. Jama Oncology 2019;5(7):975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooperberg MR. Re-Examining Racial Disparities in Prostate Cancer Outcomes. Journal of Clinical Oncology 2013;31(24):2979–80. [DOI] [PubMed] [Google Scholar]
- 26.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev 2000;57(Suppl 1):108–45. [DOI] [PubMed] [Google Scholar]
- 27.Hosain GM, Sanderson M, Du XL, Chan W, Strom SS. Racial/ethnic differences in treatment discussed, preferred, and received for prostate cancer in a tri-ethnic population. Am J Mens Health 2012;6(3):249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houston KA, King J, Li J, Jemal A. Trends in Prostate Cancer Incidence Rates and Prevalence of Prostate Specific Antigen Screening by Socioeconomic Status and Regions in the United States, 2004 to 2013. J Urol 2018;199(3):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner A, Etzioni R, Eggener S. Ongoing Gleason Grade Migration in Localized Prostate Cancer and Implications for Use of Active Surveillance (2004–2010). Journal of Urology 2014;191(4):E924–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aihara M, Lebovitz RM, Wheeler TM, Kinner BM, Ohori M, Scardino PT. Prostate specific antigen and gleason grade: an immunohistochemical study of prostate cancer. J Urol 1994;151(6):1558–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.