Abstract
Coronavirus disease-associated mucormycosis (CAM) is an established clinical entity in India. In the past 4 months, there has been a sharp upsurge in the number of CAM cases in most parts of the country. Early diagnosis can be lifesaving. Magnetic resonance imaging (MRI) imaging remains the corner stone of management in patients with ROCM. This review discussed the utility of MRI imaging in ROCM with an emphasis on the ideal MRI protocol in a suspected case of ROCM, the pathways of spread of infection, the classic diagnostic features, MRI for staging of the disease, MRI for prognostication, MRI for follow up, and imaging features of common differentials in ROCM. The pit falls of MRI imaging and a comparison of CT and MRI imaging in ROCM are discussed. The clinical interpretation of areas of contrast uptake and those of necrosis and its relevance to treatment are discussed. This review aims to familiarize every member of the multidisciplinary team involved in managing these patients to be able to interpret the findings on MRI in ROCM.
Keywords: COVID-19, magnetic resonance imaging (MRI), mucormycosis
Mucormycosis is a severe opportunistic fungal infection that results from a fungus of the order mucorales.[1] India is currently facing a huge surge in the number of Coronavirus disease (COVID-19)-associated mucormycosis.[2,3] This fungal infection can rapidly progress in individuals who are immunologically or metabolically compromised such as patients who have developed COVID-19 infection in the recent past.[3] Early suspicion, rapid diagnosis, and initiation of treatment are the most important factors that determine prognosis in the management of mucormycosis.[4] Imaging forms the cornerstone of management in patients with rhino-orbital-cerebral mucormycosis (ROCM). Imaging studies are readily available and rapidly give corroborative evidence when the disease is clinically suspected. In patients with clinical suspicion and imaging evidence of ROCM, empirical antifungal therapy can be started even before confirmation of the diagnosis by microbiology or histopathology.[5] In patients where biopsy is planned, imaging can be used to help guide the site for biopsy to ensure maximum diagnostic yield. In patients with proven ROCM, imaging plays an important role in determining the extent of disease, which is critical in making a decision about further line of management. It is thus imperative for every member of the multidisciplinary team involved in managing these patients to be familiar with interpretation of the magnetic resonance imaging (MRI) in ROCM. In the current communication we elaborate on the role of MRI in ROCM.
Ideal MRI protocol in a suspected case of ROCM
Optimal evaluation of ROCM needs images that depict the sinonasal structures, face, orbits, suprahyoid neck spaces, skull base, vascular structures, and intracranial compartment. It requires more anatomical coverage than conventional orbit MRI studies. The suggested protocol is listed in Table 1.
Table 1.
Ideal MRI protocol in a suspected case of ROCM
| Acquisition Details | |
|---|---|
| Preferred field strength | 1.5 or 3.0 Tesla MRI |
| Preferred planes of imaging | Axial and coronal planes |
| Preferred sequences | 2D Spin echo or fast spin echo sequences with T1W, T2W, STIR or fat saturated T2W, and fat saturated postcontrast T1W images Diffusion imaging, MR angiogram |
| Coverage | Axial images: Teeth to top of frontal sinus |
| Coronal images: Nasal cartilages to pons | |
| Preferred Slice Thickness | 2-3 mm |
|
| |
| Role of Individual Pulse Sequences | |
|
| |
| T2 and T1 weighted in axial and coronal plane | Delineate soft tissue and bone involvement |
| Short tau inversion recovery (STIR)/Fat-saturated T2W images | Most sensitive sequence to demonstrate pathology - Should be acquired at least in one plane |
| Fat-saturated T1W images with intravenous gadolinium | Best for delineating the extent of pathology and areas of avascular necrosis |
| MR angiogram | To look for angioinvasion in cases with skull base or cavernous sinus involvement |
| Diffusion imaging | To detect areas of cerebral and optic nerve infarction |
Pathways for ROCM spread
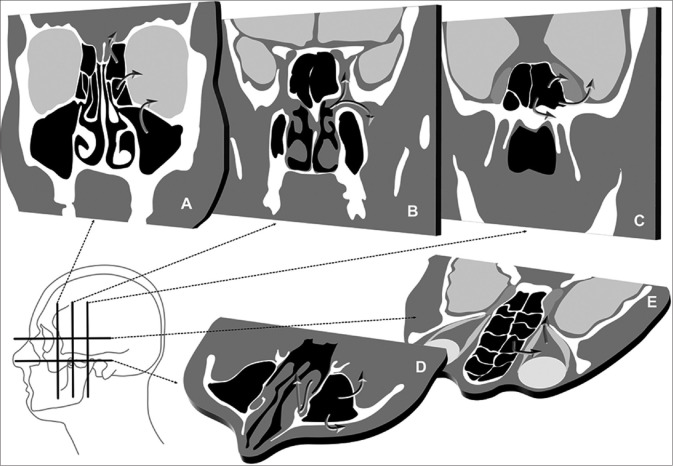
Mucormycosis spreads predominantly by direct tissue invasion. It may extend across anatomic spaces by bone destruction, by spreading across natural bony defects, along natural pathways such as the nasolacrimal ducts, lymphatics, and neurovascular bundles. Anatomical pathways involved in the spread of ROCM are summarized in Fig. 1. Careful attention to all these anatomic regions on MRI is needed for a comprehensive assessment of the extent of the disease.
Figure 1.
Pathways of spread in rhinoorbital cerebral mucormycosis. Direct spread from the ethmoid and maxillary sinuses into the orbit or intracranial compartment (a). Pterygopalatine fossa is a cross roads at the skull base, where nasal pathology may spread into the infratemporal fossa and cavernous sinus (b). Sphenoid sinus disease may extend into the cavernous sinus, brain, and skull base (c). Maxillary sinus disease may extend into the facial and retroantral soft tissue and along the nasolacrimal duct (d). Intraorbital disease may spread into the orbital apex and cavernous sinus (e)
MR imaging features of ROCM
Sinonasal involvement
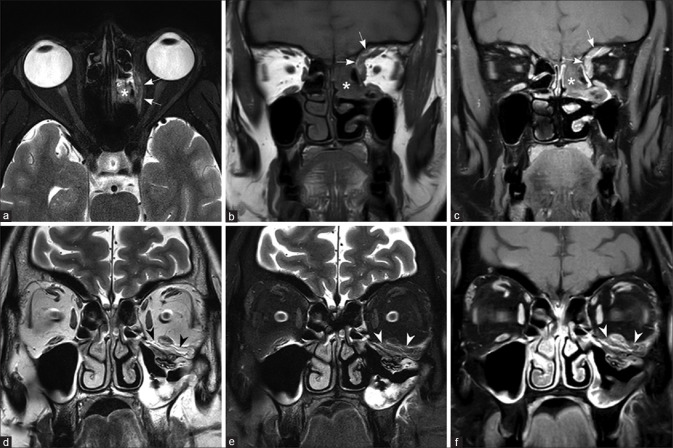
Normal paranasal sinuses are air-filled structures which are hypointense on all sequences. [Fig. 2] The normal MRI imaging findings of sinuses, orbit, and brain are listed in Table 2. The common imaging features of ROCM are listed in Table 3. In fungal sinusitis, opacification of the sinuses by soft tissue is seen. Multiple sinus involvement is seen in about half the cases of ROCM.[5] The contents of the sinuses have varying signal characteristics on MRI.[6] The T2W signal intensity is determined by the extent of necrosis (causing hyperintensity) and the presence of paramagnetic elements such as iron and manganese within the fungal hyphae (causing hypointensity)[7] [Fig. 3]. The findings on diffusion-weighted imaging are variable with one series reporting restricted diffusion.[8] On postcontrast scans, the contents of the sinuses may show a variety of appearances ranging from: 1. intense homogenous enhancement, 2. variable enhancing and nonenhancing areas, and 3. complete central nonenhancement with or without a thin irregular rim of peripheral enhancement. A characteristic imaging feature of invasive fungal sinusitis on postcontrast T1W images is the absence of enhancement in areas that normally enhance. This finding is secondary to the angioinvasive nature of the fungus, causing microthrombosis and tissue necrosis in the affected regions. [Fig. 3] This appearance – termed as the “Black Turbinate sign” – is the imaging counterpart of the necrotic eschar seen on clinical or rhinoscopic examination. Recognition of this sign may aid in early diagnosis of ROCM.[9,10]
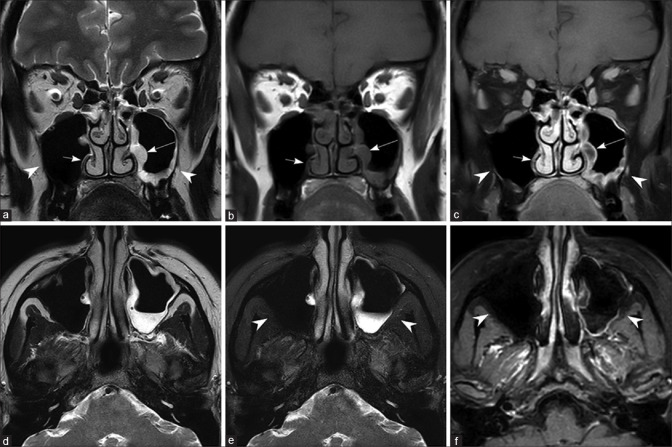
Figure 2.
Normal maxillary sinus on the right side and chronic noninvasive maxillary sinusitis on the left side Coronal T2W (a), T1W (b), postcontrast T1W image with fat saturation (c), axial T2W (d), T2W with fat saturation (e), and postcontrast T1W image with fat saturation (f). Note the normal mucosa in the right maxillary sinus (short arrow) and thickened, peripherally enhancing mucosa in the left maxillary sinus (long arrow). Note the absence of edema/enhancement in the bony structures and periantral fat (arrow heads)
Table 2.
Imaging appearances of normal structures on MRI
| Anatomical structure | T1W images | T2W images | Fat-Sat T2W images | Postcontrast T1W images |
|---|---|---|---|---|
| Nasal Cavity and Paranasal Sinuses | ||||
|
| ||||
| Mucosal lining | Isointense | Thin, linear hyperintensity | Thin, linear hyperintensity | Thin, smooth enhancement |
| Air within sinuses | Absent signal (signal void) | Absent signal (signal void) | Absent signal (signal void) | - |
|
| ||||
| Bony Structures | ||||
|
| ||||
| Cortical bone | Hypointense | Hypointense | Hypointense | No enhancement |
| Bone marrow | Hyperintense | Intermediate signal | Hypointense | Mild heterogeneous enhancement |
|
| ||||
| Orbit | ||||
|
| ||||
| Aqueous and vitreous of globe | Hypointense - similar to CSF | Hyperintense - similar to CSF | Hyperintense- similar to CSF | - |
| Coats of the globe | Intermediate signal | Hypointense | Hypointense | Thin linear enhancement of the choroid |
| Retroorbital fat | Hyperintense | Hyperintense | Hypointense | No enhancement |
| Extraocular muscles | Intermediate signal | Intermediate signal | Intermediate signal | Intense homogenous enhancement |
| Optic nerve | Isointense to cerebral white matter | Isointense to cerebral white matter | Isointense to cerebral white matter | No enhancement |
|
| ||||
| Other Structures | ||||
|
| ||||
| Periantral fat | Hyperintense | Hyperintense | Shows suppression - hypointense | No enhancement |
| Muscles | Intermediate signal | Intermediate signal | Intermediate signal | Mild homogenous enhancement |
| Cavernous sinus | Isointense | Variable signal based on blood flow | Variable signal based on blood flow | Homogenous enhancement |
| Patent blood vessels | Absent signal (flow voids) | Absent signal (flow voids) | Absent signal (flow voids) | Variable enhancement |
Table 3.
Imaging findings in ROCM
| Imaging finding | Best sequence to visualize the finding | Importance of recognition |
|---|---|---|
| Mucosal thickening with T2 hypointense components | T2W images | Suspicion of fungal etiology |
| Nonenhancement of involved mucosa/soft tissue | Post contrast T1W images | Suspicion of fungal etiology |
| Marrow edema and enhancement of adjacent bones and skull base | Fat- saturated T2W and postcontrast T1W images | Bony invasion |
| Edema and enhancement of fat planes surrounding the maxillary antrum | T1W, Fat-saturated T2W and postcontrast T1W images | Periantral soft tissue invasion |
| Edema of retroorbital fat and enhancing soft tissue in the orbit with or without involvement of the extraocular muscles | T1W, Fat-saturated T2W and postcontrast T1W images | Orbital extension |
| Diffusion restriction within the optic nerve | Diffusion-weighted images | Optic nerve infarction |
| Edema and enhancing soft tissue within the orbital apex and pterygopalatine fossa | T1W, Fat-saturated T2W and postcontrast T1W images | High risk of cavernous sinus involvement and intracranial extension |
| Internal carotid artery narrowing without or with arterial wall enhancement | MR angiogram and postcontrast T1W images | Arterial wall invasion |
| Meningeal enhancement, cerebral parenchymal signal changes with peripheral enhancement | T2W, diffusion-weighted and postcontrast T1W images | Cerebral parenchymal invasion/abscess formation |
| Cerebral parenchymal signal changes with diffusion restriction | T2W and diffusion-weighted images | Acute infarction |
Figure 3.
Sinonasal disease in ROCM.T1W (a), fat-saturated T2W (b-e) and T2W (d) images showing mucosal thickening in the left nasal cavity and ethmoid sinus, with T2 hypointense contents. The abnormal mucosa does not show enhancement on postcontrast T1W images (c-f) – “black turbinate sign” (asterisk)
However, focal nonenhancement of sinonasal soft tissues is not specific for invasive fungal sinusitis. It may be seen as a physiological variation in normal individuals (see discussion on Pitfalls in Imaging below). Thus, the significance of this finding must be interpreted in view of the clinical presentation.
Extrasinus extension
Extension beyond the sinuses is one of the most important indicators suggesting fungal etiology. Therakathu et al.[8] showed that the orbit was the most common site of extrasinus involvement followed by the face. Other sites of involvement include the masticator space, palate, skull base, orbital apex, pterygopalatine fossa, cavernous sinus, cranial nerves, internal carotid artery, and the brain. Middlebrooks et al.[11] described a CT-based seven-variable diagnostic model to predict acute invasive fungal sinusitis. The presence of any one of the seven variables (extension of disease into the periantral fat, orbits, pterygopalatine fossa, sphenopalatine foramen, nasolacrimal duct, lacrimal sac, and bone dehiscence) had a 95% sensitivity and 86% specificity for fungal etiology. The presence of any two variables gave 88% sensitivity, 100% specificity, and 100% positive predictive value for the diagnosis of invasive fungal sinusitis.
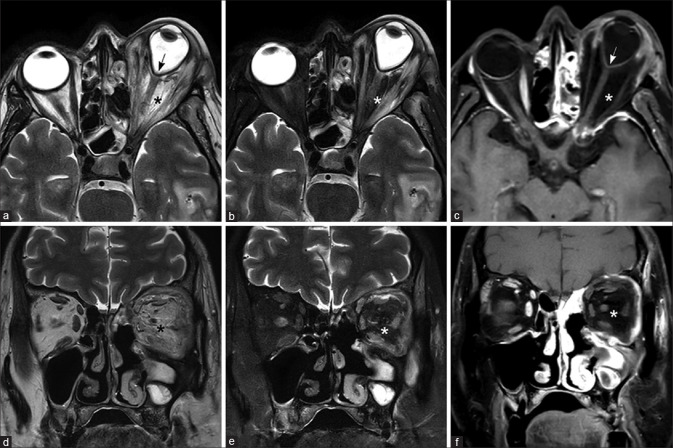
The exquisite sensitivity of MRI to soft tissue pathology permits early detection of extrasinus extension, even before bone destruction is apparent on CT scan. The pattern of extension of infection beyond the margins of the sinuses is best delineated on fat-suppressed T2W and fat-suppressed postcontrast T1W images, as edema and enhancement of bony walls. On MRI, extension into the periantral fat is seen as signal changes and enhancement within the premaxillary and retroantral fat. Further extension into the infratemporal fossa is seen as edema and enhancement within the muscles of mastication [Fig. 4]. Extension of the pathology into the pterygopalatine fossa is seen as replacement of normal fat signal surrounding the branches of the internal maxillary artery and presence of enhancing soft tissue.
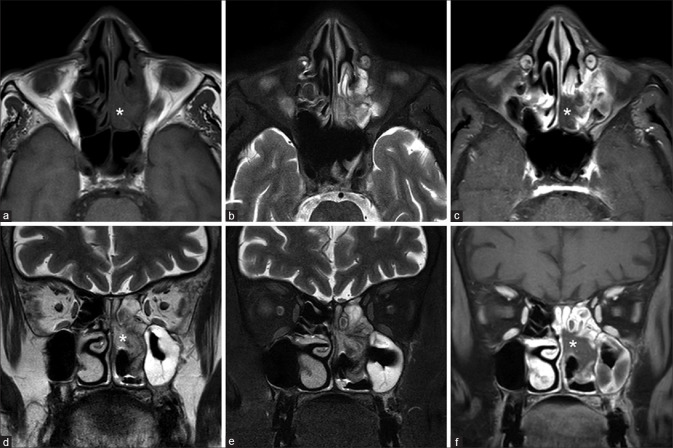
Figure 4.
Early (a-c) and advanced extrasinus extension (d-f) T1W (a), T2W (b), and fat-saturated T2W images (c) show early extrasinus extension into the retroantral fat and pterygoid muscles (arrows). T2W (d), fat-saturated T2W (e), and fat-saturated postcontrast T1W images (f) in another patient show more advanced disease with the left maxillary sinusitis and peripherally enhancing soft tissue component in the left masticator space (asterisk)
Orbital involvement
It is worthwhile to review the imaging characteristics of normal orbital tissues on MRI.[12,13] This detail is presented in Table 2. Orbital invasion in mucormycosis commonly occurs through pathways of least resistance, which include lamina papyracea, nasolacrimal duct, ethmoid foramina, and perforations of the medial orbital walls by vascular channels.[14] Rarely, in aggressive disease, destruction of the bony walls of the orbits can cause spread of infection from the maxillary sinus through the orbital floor.
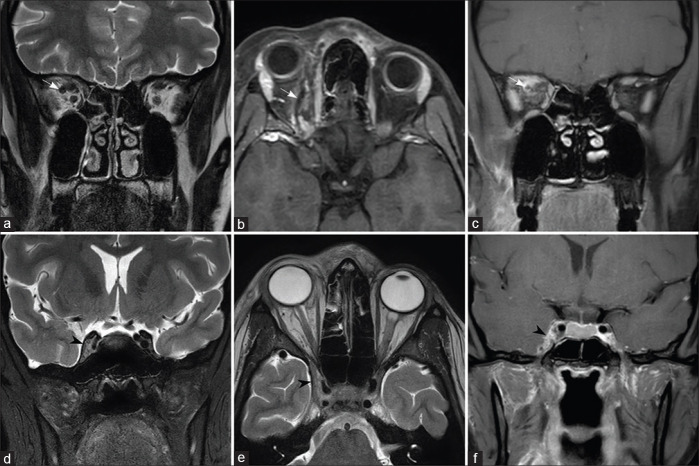
Early orbital infection shows soft tissue infiltration and edema of the retroorbital fat around the extraocular muscles. Infiltration of the retroorbital fat is best appreciated on fat-saturated T2W sequences. As orbital invasion commonly occurs through the medial wall, inflammatory tissue or abscess formation may be seen along the medial aspect of the orbit with lateral displacement and edema of the medial rectus muscle [Fig. 5].
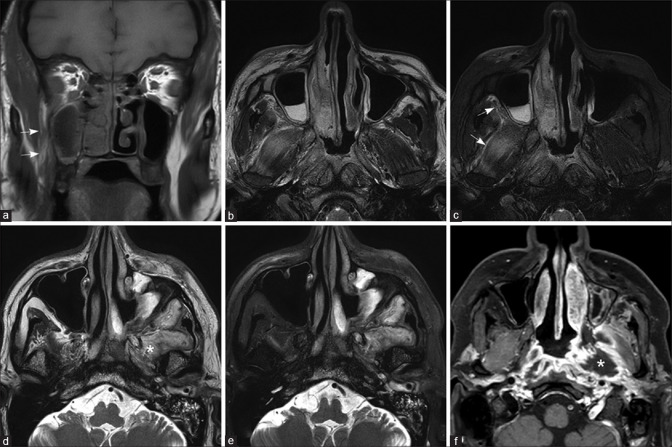
Figure 5.
Orbital extension from ethmoid sinus (a-c) and maxillary sinus (d-f) Fat-saturated T2W (a), T1W (b), and fat-saturated post-contrast T1W images (c) in a patient with early orbital extension shows the left ethmoid sinusitis (asterisk), with a thin enhancing extraconal soft tissue, involving the medial rectus, superior rectus, and superior oblique muscles (arrows). T2W (d), fat-saturated T2W (e), and fat-saturated postcontrast T1W images (f) in another patient show the left maxillary sinusitis with extension along the floor of the left orbit, involving the inferior rectus muscle (arrowheads)
Optic nerve involvement may be seen.[15] Sudden onset of blindness can be due to central retinal artery or ophthalmic artery occlusion, optic nerve infarction, or direct infiltration of the optic nerve.[16] Optic nerve infarction is seen as high-signal intensity of the nerve on diffusion-weighted imaging[17] [Fig. 5]. Direct invasion of the optic nerve can cause increased caliber of the nerve with signal intensity changes within it. Isolated involvement of the optic nerve suggests spread of infection through branches of the ophthalmic artery, which is an indication for initiation of aggressive treatment.[18]
Diffuse orbital infection may present with severe proptosis and tenting of the globe [Fig. 6]. Though rare, involvement of the globe may be seen as thickening and enhancement of the ocular coats.
Figure 6.
Globe (a-c) and optic nerve (d-f) involvement. T2W (a), fat-saturated T2W (b), and fat-saturated postcontrast T1W images (c) in a patient with orbital extension show thickening and enhancement of the Tenon’s capsule along the right globe (arrows). Fat-saturated T2W (d) and fat-saturated postcontrast T1W images (e) in another patient with right orbital extension showing extensive edema and enhancement of intraconal and extraconal fat and extraocular muscles (black arrowheads). The right optic nerve shows perineural enhancement (e) and diffusion restriction (f) suggesting inflammation and infarction (arrows)
Orbital apex involvement: Enhancing soft tissue at the orbital apex extending into both the optic canal and superior orbital fissure may present as orbital apex syndrome. When imaging findings of sinusitis are associated with orbital apex syndrome suspicion of fungal etiology must be raised.[19] Infection can spread from the orbital apex posteriorly through the superior orbital fissure into the cavernous sinus and through the inferior orbital fissure across the pterygopalatine fossa into the infratemporal fossa [Fig. 7].[20]
Figure 7.
Orbital involvement with nonenhancing necrotic soft tissue.T2W (a-d), fat-saturated T2W (b-e), and fat-saturated postcontrast T1W (c-f) images in a patient with gross proptosis and globe tenting (arrow). The intraconal and extraconal fat as well as the extraocular muscles in the left orbit show edema. The striking feature is the absence of enhancement of the involved retroorbital fat and extraocular muscles (asterisk). This is thought to represent devascularized tissue secondary to angioinvasion and microthrombosis
Cavernous sinus and major arterial involvement
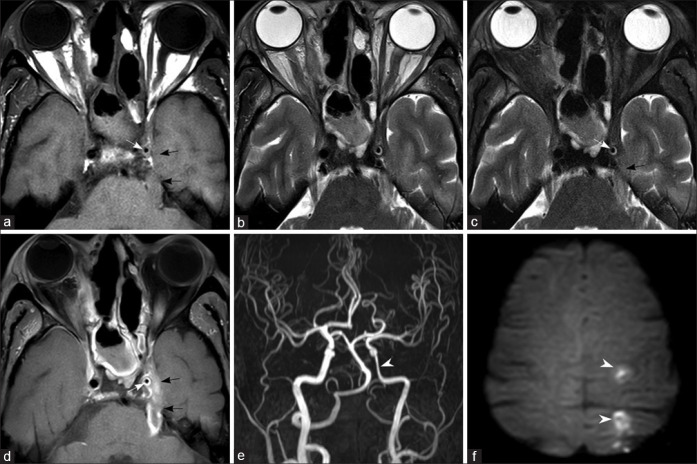
In ROCM, heterogenously enhancing soft tissue may be seen extending from the superior orbital fissure to involve the cavernous sinus. The lateral walls of the sinuses are normally concave laterally or straight on coronal and axial images. Loss of concavity of the cavernous sinus is a sign of involvement. In early stages, bulky cavernous sinus with convexity of the lateral wall is seen [Fig. 8]. Postcontrast images show filling defects within the sinus. Occlusion of the superior ophthalmic vein may occur due to extension of pathology along the vein or due to soft tissue compression at the orbital apex. On imaging, the thrombosed vein is seen as a dilated cord-like structure superior to the optic nerve, crossing from the medial to lateral side. The lumen of the vein shows loss of normal flow void and filling defects on postcontrast images [Fig. 8].[16] Cavernous segment of internal carotid artery may be encased by the soft tissue or thrombus in the cavernous sinus causing narrowing of its lumen. Alternatively, the fungus can invade the arterial wall, causing occlusion of its lumen. Areas of arterial wall invasion may be demonstrated as wall enhancement on vessel wall imaging.[21] Vascular invasion and occlusion of the cavernous segment of the internal carotid artery are the most common causes leading to cerebral infarcts. [Fig. 9]
Figure 8.
Orbital apex and pterygopalatine fossa involvement. Fat-saturated T2W (a) and fat-saturated postcontrast T1W (b and c) images through the orbit show left ethmoidal sinusitis (asterisk). There is retroorbital fat stranding and heterogeneously enhancing soft tissue at the orbital apex, extending into the anterior cavernous sinus (arrows). T2W (d) and fat-saturated postcontrast T1W (e and f) images through the pterygopalatine fossa show enhancing soft tissue on the left side (arrowheads)
Figure 9.
Involvement of the superior ophthalmic vein and cavernous sinus. The right superior ophthalmic vein shows increased caliber with retroorbital fat stranding on T2W image (a) and filling defects on fat-saturated postcontrast T1W (b and c) images (arrows). T2W (d and e) sections through the cavernous sinuses show bulky right cavernous sinus with convex lateral wall (arrowheads) and filling defects on postcontrast T1W image (f)
Intracranial extension
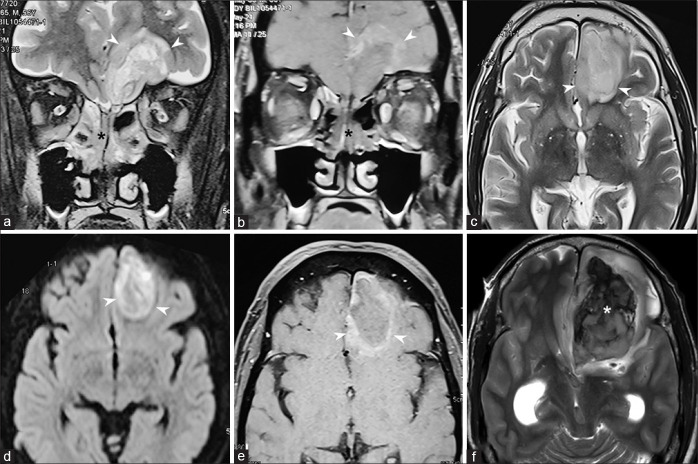
Intracranial involvement in mucormycosis commonly occurs by direct spread across the cribriform plate, walls of the ethmoid, and frontal sinuses. Extensions into the middle cranial fossa from the pterygopalatine fossa and along internal carotid artery are also seen.[16] Perineural spread from the cavernous sinus, along the trigeminal nerve, can lead to predominant posterior fossa involvement. Early intracranial spread is better appreciated on contrast-enhanced T1W images when there is meningeal enhancement.[14] Other intracranial manifestations include abscesses and infarcts. Fungal invasion of the brain parenchyma appears as ill-defined areas of altered signal intensity, usually T2 hyperintensity, in nonvascular distribution. Minimal perilesional edema and variable peripheral enhancement are present. [Fig. 10]. Development of a well-delineated mass with liquified central T2 hyperintense core showing diffusion restriction indicates abscess formation [Fig. 11]. Abscesses in ROCM may not show the characteristic well-defined rim enhancement seen in bacterial abscesses because of poor immunogenic response by a compromised host.[22]
Figure 10.
Perineural spread to the cerebral parenchyma and arteritis. T1W (a), T2W (b) images, fat-saturated T2W (c), and fat-saturated postcontrast T1W (d) images show perineural extension from the left cavernous sinus, along the trigeminal nerve up to the pons (black arrows). Thickening and enhancement of the wall of the left internal carotid artery is seen (white arrows). Arterial narrowing on MR angiogram (e) sand left cerebral watershed infarcts (f) (arrowheads) are seen
Figure 11.
Cerebral parenchymal invasion Fat-saturated T2W (a) and postcontrast T1W (b) images show extension of disease from the ethmoid sinuses (black asterisk), across the cribriform plate into the left frontal lobe (arrowheads). The parenchymal lesion is hyperintense on T2W image (c), with restricted diffusion (d), and peripheral rim of enhancement on postcontrast T1 (b-e) images. T2W image performed 1 day after the initial MRI showed hemorrhage within the lesion (white asterisk) (f)
Skull base involvement
Skull base osteomyelitis is a rare complication, usually seen in the late stages of the disease.[23,24] Bone involvement occurs relatively late in the course of the disease because angioinvasive nature of the fungus facilitates extensive spread of infection into the deep soft tissues through the perivascular channels even before bone destruction.[24] Early involvement of the bone marrow can be picked up on T1W images, which show loss of normal fat signal. The marrow appears hypointense on T1W images and hyperintense on STIR images with postcontrast images showing heterogenous enhancement.[25]
In advanced stage, there is extensive heterogeneously enhancing soft tissue with infiltration into the bones. Obliteration of normal adjacent fat planes with T2 hyperintense soft tissue edema, perineural, and intracranial spread may be seen. Abscess formation can occur appearing as fluid signal intensity area with central diffusion restriction and peripheral rim enhancement.[26]
MRI for staging of the disease and planning treatment
Honavar SG[5] proposed a four-stage system to determine the anatomical extent and severity of ROCM. It includes clinical symptoms and signs, as well as results of diagnostic tools, such as imaging and nasal endoscopy. It divides ROCM into stage I (disease limited to the nasal mucosa), stage II (extending into paranasal sinuses), stage III (involving the orbit), and stage IV (involvement of the central nervous system). However, there may be a small subset of patients where the orbital involvement occurs as a result of spread of infection from the pterygopalatine fossa to the infraorbital fissure and the orbit without significant involvement of the sinuses.
In patients with predominant sinonasal involvement and limited orbital extension, extensive debridement of the sinuses with or without resection of the medial orbital wall can be done. In patients with predominant orbital involvement, early orbital exenteration is done in addition to extensive sinonasal debridement. When patients have extensive cerebral involvement with or without bilateral orbital involvement, conservative management with antifungals and debridement of necrotic tissue is preferred.[27]
As a general principle in management of ROCM, infected tissues which are avascular represent an unsalvageable nidus of fungal elements. During surgery, these regions are usually removed and debridement is halted once bleeding tissue is encountered at the margins. On imaging, enhancement is taken as an indicator of preserved vascularity, and thus, it has been proposed that contrast-enhanced MRI can be used to guide the extent of resection.[27] MRI can also help in objective evaluation of the therapeutic response in patients with different anatomical locations and stages of the disease, and thus may contribute to optimizing treatment protocols.
Surgical options for management of advanced ROCM include potentially mutilating surgeries such as maxillectomy, palatal resection, and orbital exenteration. Imaging studies can be used to plan procedures so that maximal disease clearance can be achieved with minimal disfigurement. In selected patients who are offered a trial of conservative therapy before considering more aggressive surgical options, follow-up imaging studies permit early detection of progression of the disease, before more ominous clinical signs develop.
MRI for prognostication
Literature is divided about the prognostic value of imaging findings in ROCM. In general, the presence of imaging features of late or severe disease such as involvement of brain or orbit, increased number of involved sinuses, involvement of sphenoid or frontal sinuses, bilateral sinus involvement, and involvement of the palate has been associated with poor prognosis.[28] While some series (including the largest study on mucormycosis) did not find orbital involvement to worsen the survival,[29] several other studies found that orbital invasion was a factor predicting poor survival.[30] A few recent studies[31] found that loss of contrast enhancement in infected soft tissues was the sole imaging feature that predicted poor survival. On the other hand, a study using CT for documentation of extent of involvement[11] showed that none of the imaging findings had any prognostic significance. These authors cautioned against predicting a time course or outcome based on the presence of “advanced” disease on imaging. More prospective studies that correlate the clinical outcome (survival and visual outcome), with imaging features documented using a structured reporting format, are needed to confirm the role of imaging in prognostication of ROCM.
MRI for differential diagnosis of ROCM
Infective lesions that originate in paranasal sinuses such as acute bacterial sinusitis, allergic fungal sinusitis, malignancies of the sinonasal cavities, bony pathology such as osteoma, fibrous dysplasia, ossifying fibroma, and systemic inflammatory disorders such as IgG4-related disease, Wegener’s granulomatosis, and sarcoidosis can mimic ROCM. A detailed review of the imaging features of these entities is beyond the scope of this article and can be found elsewhere.[32,33]
The clinical and imaging findings of orbital involvement in ROCM are similar to those in bacterial orbital cellulitis. Differentiation between the two entities is of prime importance considering the radically different treatment pathways involved in their management. Apart from the clinical setting, imaging findings such as T2 hypointense and nonenhancing soft tissues may point to a fungal etiology. Similarly, involvement of the extraocular muscles without eyelid swelling or mucosal thickening in the paranasal are clues to a possible fungal etiology.[33] On similar lines, it is important to differentiate inflammatory pathologies such as IgG4-related disease, sarcoidosis, and Wegener’s granulomatosis from ROCM, considering that they are treated with immunosuppressive therapy. Careful attention to the clinical scenario, nasal endoscopy, and prompt biopsy of the lesion is important to prevent potential disastrous consequences of misdiagnosis.
MRI for follow up
MR imaging also plays an important role in follow up of patients who are on treatment. In patients with clinical suspicion of ROCM (possible ROCM), where nasal endoscopy and initial MR imaging studies are noncontributory, it is recommended to perform follow-up imaging after 72 h. The progression of the disease and development of more classic imaging findings provide more evidence for fungal etiology (probable ROCM).
MR imaging is also used in follow up of patients with early disease, who are treated with conservative measures such as limited sinus surgery and transcutaneous retrobulbar amphotericin injections. Early recognition of disease progression helps in prompt institution of more radical life-saving procedures such as orbital exenteration. While worsening radiological features represent disease progression, favorable clinical response to treatment is not always apparent on imaging. It should be remembered that imaging findings may take longer to resolve than clinical signs.[27]
Pitfalls of MR imaging
As with CT, MRI may be normal or may show nonspecific abnormalities early in the course of the disease. Thus, high-risk patients who have noncontributory imaging studies need follow-up imaging, and there should be a low threshold for performing surgical exploration and biopsy of suspicious areas in high-risk individuals.[34,35] It should be remembered that MR images may contain several artefacts that may mimic pathology. The most important among them are “susceptibility artefacts,” which occur in the soft tissues surrounding the paranasal sinuses, especially in fat-saturated sequences. Air contained within the sinuses and the presence of metallic dental work disturb the magnetic field and contribute to the artifacts in these locations. These artifacts are usually seen as areas of bright signal along the floor of the orbit, at the orbital apex and in infratemporal fossa. To the unwary eye, these may simulate pathology.[36]
Mucosal thickening and sinus opacification are nonspecific findings that may be seen in chronic noninvasive sinusitis as well as in invasive fungal sinusitis. The absence of enhancement of the involved turbinate (black turbinate sign) has been described as an early sign of fungal etiology, with moderate specificity.[9,10] However, this finding may also be seen in normal individuals as a physiological variation. This has been described as benign black turbinate by Han et al.[37] They found nonenhancing turbinates in 30% of patients who were unlikely to have invasive fungal sinusitis. In fungal sinusitis, nonenhancement is usually seen in the middle turbinate and extends beyond anatomic boundaries into the contiguous structures such as fat, muscle, and bone.[37] The involved regions show variable signal intensity on T2-weighted images. In contrast, benign black turbinate more commonly involves the posterior and mid portions of the inferior turbinate, without extension into adjacent structures. It shows central nonenhancement, with thin internal septa and thin peripheral rim of preserved mucosal enhancement. The involved structure may be intermediate or hyperintense on T2-weighted images, but never shows hypointensity. On delayed scans, the non-enhancement persists in fungal sinusitis, while benign black turbinate shows progressive enhancement.[38]
One additional pitfall of MRI is its inability to differentiate between microbiological subtypes of invasive fungal sinusitis. Apart from Mucorales (Mucor, Rhizopus, and Absidia), Aspergillus species can also lead to acute invasive fungal sinusitis. Moreover, mixed infections are also known to occur.[38] Differentiation between these subgroups has therapeutic and prognostic implications.
CT vs MRI in the management of ROCM
CT is a widely available, fast, effective, and relatively inexpensive imaging modality. Shorter acquisition times make it a more feasible imaging option in sick and uncooperative patients. In patients with ROCM, CT is useful in preoperative planning for determining sinonasal anatomy, extent of disease, and in directing the surgical approach. It is also useful for intraoperative image-guided navigation. MRI is an inherently multiplanar imaging modality, which has excellent soft tissue contrast resolution. As it does not involve exposure to ionizing radiation, MRI is a safer imaging modality in patients requiring multiple follow-up scans. However, limited accessibility, longer acquisition times, and motion degradation are its disadvantages.
In patients with suspected ROCM, rapid and accurate diagnosis of fungal etiology is the cornerstone of successful treatment. The most important imaging features that indicate a possible fungal cause are the demonstration of spread beyond the sinus walls and presence of angioinvasion. The presence of necrosis in the involved structures can be elegantly demonstrated on contrast-enhanced MRI. It provides better visualization of periantral and infratemporal fossa extension, orbital soft tissue involvement, and skull base invasion. The wide range of contrast mechanisms available on MRI permit detection of imaging signs, which are difficult or even impossible to demonstrate on CT. Examples of such features include cavernous sinus involvement, vascular invasion, perineural spread, optic nerve infarction, and differentiation of cerebral parenchymal invasion from infarction. In addition, MRI is better suited than CT in differentiating ROCM from other diseases with similar clinical presentation – such as bacterial and allergic fungal sinusitis, bacterial orbital cellulitis, nasal and paranasal tumors, inflammatory disease, and carotid-cavernous fistula.[17]
Conclusion
In conclusion, the above-outlined guidelines can be a useful template for uniform imaging and interpretation in cases of ROCM. This would serve as an appropriate stepping stone to prudent clinical decision making and patient management.
Financial support and sponsorship
This study has been funded by the Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gelston CD, Durairaj VD, Simoes EA. Rhino-orbital mucormycosis causing cavernous sinus and internal carotid thrombosis treated with posaconazole. Arch Ophthalmol. 2007;125:848–9. doi: 10.1001/archopht.125.6.848. [DOI] [PubMed] [Google Scholar]
- 2.Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM):Case report and systematic review of literature. Mycopathologia. 2021;186:289–98. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix:Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–8. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karadeniz Uğurlu Ş, Selim S, Kopar A, Songu M. Rhino-orbital mucormycosis:Clinical findings and treatment outcomes of four cases. Turk J Ophthalmol. 2015;45:169–74. doi: 10.4274/tjo.82474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honavar SG. Code mucor:Guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69:1361–5. doi: 10.4103/ijo.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatterpekar GM, Delman BN, Som PM. Imaging the paranasal sinuses:Where we are and where we are going. Anat Rec (Hoboken) 2008;291:1564–72. doi: 10.1002/ar.20773. [DOI] [PubMed] [Google Scholar]
- 7.Aribandi M, McCoy VA, Bazan C., 3rd Imaging features of invasive and noninvasive fungal sinusitis:A review. Radiographics. 2007;27:1283–96. doi: 10.1148/rg.275065189. [DOI] [PubMed] [Google Scholar]
- 8.Therakathu J, Prabhu S, Irodi A, Sudhakar SV, Yadav VK, Rupa V. Imaging features of rhinocerebral mucormycosis:A study of 43 patients. Egypt J Radiol Nucl Med. 2018;49:447–52. [Google Scholar]
- 9.Safder S, Carpenter JS, Roberts TD, Bailey N. The “Black Turbinate“sign:An early MR imaging finding of nasal mucormycosis. AJNR Am J Neuroradiol. 2010;31:771–4. doi: 10.3174/ajnr.A1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor AM, Vasan K, Wong EH, Singh N, Smith M, Riffat F, et al. Black Turbinate sign:MRI finding in acute invasive fungal sinusitis. Otolaryngol Case Rep. 2020;17 doi:10.1016/j.xocr. 2020.100222. [Google Scholar]
- 11.Middlebrooks EH, Frost CJ, De Jesus RO, Massini TC, Schmalfuss IM, Mancuso AA. Acute invasive fungal rhinosinusitis:A comprehensive update of CT findings and design of an effective diagnostic imaging model. AJNR Am J Neuroradiol. 2015;36:1529–35. doi: 10.3174/ajnr.A4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayak Bk, Desai SD, Maheshwari S. Interpretation of magnetic resonance imaging of orbit:Simplified for ophthalmologists (Part I) J Clin Ophthalmol Res. 2013;1:29–35. [Google Scholar]
- 13.Ettl A, Salomonowitz E, Koornneef L, Zonneveld FW. High-resolution MR imaging anatomy of the orbit. Correlation with comparative cryosectional anatomy. Radiol Clin North Am. 1998;36:1021–45. doi: 10.1016/s0033-8389(05)70229-3. [DOI] [PubMed] [Google Scholar]
- 14.Herrera DA, Dublin AB, Ormsby EL, Aminpour S, Howell LP. Imaging findings of rhinocerebral mucormycosis. Skull Base. 2009;19:117–25. doi: 10.1055/s-0028-1096209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang N, Zhao G, Yang S, Lin J, Hu L, Che C, et al. A retrospective analysis of eleven cases of invasive rhino-orbito-cerebral mucormycosis presented with orbital apex syndrome initially. BMC Ophthalmol. 2016;16:10. doi: 10.1186/s12886-016-0189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghuman MS, Kaur S, Bhandal SK, Ahluwalia A, Saggar K. Bilateral optic nerve infarction in rhino-cerebral mucormycosis:A rare magnetic resonance imaging finding. J Neurosci Rural Pract. 2015;6:403–4. doi: 10.4103/0976-3147.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur S, Karimi A, Mafee MF. Acute optic nerve infarction demonstrated by diffusion-weighted imaging in a case of rhinocerebral mucormycosis. AJNR Am J Neuroradiol. 2007;28:489–90. [PMC free article] [PubMed] [Google Scholar]
- 18.Alsuhaibani AH, Al-Thubaiti G, Al Badr FB. Optic nerve thickening and infarction as the first evidence of orbital involvement with mucormycosis. Middle East Afr J Ophthalmol. 2012;19:340–2. doi: 10.4103/0974-9233.97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anders UM, Taylor EJ, Martel JR, Martel JB. Acute orbital apex syndrome and rhino-orbito-cerebral mucormycosis. Int Med Case Rep J. 2015;17(8):93–6. doi: 10.2147/IMCRJ.S83036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotoudeh H, Shafaat O, Aboueldahab N, Vaphiades M, Sotoudeh E, Bernstock J. Superior ophthalmic vein thrombosis:What radiologist and clinician must know. Eur J Radiol Open. 2019;6:258–64. doi: 10.1016/j.ejro.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Moura Feitoza L, Altemani A, Adolfo da Silva N, Jr, Reis F. Teaching NeuroImages:Mucormycosis-associated vasculitis:A new sequence to show an old invasive infection. Neurology. 2019;92:e1796–7. doi: 10.1212/WNL.0000000000007275. [DOI] [PubMed] [Google Scholar]
- 22.Gamba JL, Woodruff WW, Djang WT, Yeates AE. Craniofacial mucormycosis:assessment with CT. Radiology. 1986;160:207–12. doi: 10.1148/radiology.160.1.3715034. [DOI] [PubMed] [Google Scholar]
- 23.Devireddy SK, Kishore Kumar RV, Gali R. Mucormycotic skull base osteomyelitis:A case report. J Oral Maxillofacial Surg Med Pathol. 2014;26:336–9. [Google Scholar]
- 24.Chan LL, Singh S, Jones D, Diaz EM, Jr, Ginsberg LE. Imaging of mucormycosis skull base osteomyelitis. AJNR Am J Neuroradiol. 2000;21:828–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman PR, Choudhary G, Singhal A. Skull base osteomyelitis:A comprehensive imaging review. AJNR Am J Neuroradiol. 2021;42:404–13. doi: 10.3174/ajnr.A7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Álvarez Jáñez F, Barriga LQ, Iñigo TR, Roldán Lora F. Diagnosis of skull base osteomyelitis. Radiographics. 2021;41:156–74. doi: 10.1148/rg.2021200046. [DOI] [PubMed] [Google Scholar]
- 27.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land:A tale of two pathogens. Indian J Ophthalmol. 2021;69:244–52. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashkouli MB, Abdolalizadeh P, Oghazian M, Hadi Y, Karimi N, Ghazizadeh M. Outcomes and factors affecting them in patients with rhino-orbito-cerebral mucormycosis. Br J Ophthalmol. 2019;103:1460–5. doi: 10.1136/bjophthalmol-2018-312688. [DOI] [PubMed] [Google Scholar]
- 29.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis:A review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 30.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, et al. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670–4. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YR, Kim JH, Min HS, Won JK, Kim HJ, Yoo RE, et al. Acute invasive fungal rhinosinusitis:MR imaging features and their impact on prognosis. Neuroradiology. 2018;60:715–23. doi: 10.1007/s00234-018-2034-0. [DOI] [PubMed] [Google Scholar]
- 32.Son JH, Lim HB, Lee SH, Yang JW, Lee SB. Early differential diagnosis of rhino-orbito-cerebral mucormycosis and bacterial orbital cellulitis:Based on computed tomography findings. PLoS One. 2016;11:e0160897. doi: 10.1371/journal.pone.0160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal M, Michel MA. Sino-orbital pathologies:An approach to diagnosis and identifying complications. Appl Radiol. 2017;46:8–20. [Google Scholar]
- 34.Rapidis AD. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment:perspectives from a maxillofacial surgeon. Clin Microbiol Infect. 2009;15(Suppl 5):98–102. doi: 10.1111/j.1469-0691.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 35.Groppo ER, El-Sayed IH, Aiken AH, Glastonbury CM. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2011;137:1005–10. doi: 10.1001/archoto.2011.170. [DOI] [PubMed] [Google Scholar]
- 36.Varma DR, Ponnaganti S, Dandu RV. Beware of artifacts in orbital magnetic resonance imaging. Indian J Ophthalmol. 2020;68:2516–8. doi: 10.4103/ijo.IJO_640_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Q, Escott EJ. The black turbinate sign, a potential diagnostic pitfall:Evaluation of the normal enhancement patterns of the nasal turbinates. AJNR Am J Neuroradiol. 2019;40:855–61. doi: 10.3174/ajnr.A6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zayet S, Zaghdoudi A, Ammari L, Kilani B, Tiouiri Benaissa H. Cerebro-rhino-orbital mucormycosis and aspergillosis coinfection in a patient with diabetes mellitus:A case report. ID Cases. 2020;23:e01022. doi: 10.1016/j.idcr.2020.e01022. [DOI] [PMC free article] [PubMed] [Google Scholar]