Abstract
Purpose:
COVID-19-associated rhino-orbital-cerebral mucormycosis (ROCM) has reached epidemic proportion during India’s second wave of COVID-19 pandemic, with several risk factors being implicated in its pathogenesis. This study aimed to determine the patient demographics, risk factors including comorbidities, and medications used to treat COVID-19, presenting symptoms and signs, and the outcome of management.
Methods:
This was a retrospective, observational study of patients with COVID-19-associated ROCM managed or co-managed by ophthalmologists in India from January 1, 2020 to May 26, 2021.
Results:
Of the 2826 patients, the states of Gujarat (22%) and Maharashtra (21%) reported the highest number of ROCM. The mean age of patients was 51.9 years with a male preponderance (71%). While 57% of the patients needed oxygen support for COVID-19 infection, 87% of the patients were treated with corticosteroids, (21% for > 10 days). Diabetes mellitus (DM) was present in 78% of all patients. Most of the cases showed onset of symptoms of ROCM between day 10 and day 15 from the diagnosis of COVID-19, 56% developed within 14 days after COVID-19 diagnosis, while 44% had delayed onset beyond 14 days. Orbit was involved in 72% of patients, with stage 3c forming the bulk (27%). Overall treatment included intravenous amphotericin B in 73%, functional endoscopic sinus surgery (FESS)/paranasal sinus (PNS) debridement in 56%, orbital exenteration in 15%, and both FESS/PNS debridement and orbital exenteration in 17%. Intraorbital injection of amphotericin B was administered in 22%. At final follow-up, mortality was 14%. Disease stage >3b had poorer prognosis. Paranasal sinus debridement and orbital exenteration reduced the mortality rate from 52% to 39% in patients with stage 4 disease with intracranial extension (p < 0.05).
Conclusion:
Corticosteroids and DM are the most important predisposing factors in the development of COVID-19-associated ROCM. COVID-19 patients must be followed up beyond recovery. Awareness of red flag symptoms and signs, high index of clinical suspicion, prompt diagnosis, and early initiation of treatment with amphotericin B, aggressive surgical debridement of the PNS, and orbital exenteration, where indicated, are essential for successful outcome.
Keywords: Corticosteroids, COVID-19, COVID-19-associated ROCM, diabetes mellitus, mucormycosis, orbital exenteration, paransal sinus debridement, rhino-orbital-cerebral mucormycosis, staging of rhino-orbital-cerebral mucormycosis
India is currently tackling a furious second wave of COVID-19. Only 3% of the population is fully vaccinated, and a third wave has been predicted.[1] Under these circumstances, the country has also been gripped by a formerly obscure, but notoriously fatal disease, rhino-orbital-cerebral mucormycosis (ROCM). Review of existing literature shows that India contributed to 81% of the cases of COVID-19-associated ROCM.[2] Very few case reports of COVID-19-associated ROCM were published during the first wave of the pandemic. The first series in India was reported in February 2021.[3] Since then, there has been an exponential increase in incidence in India, along with the soaring second wave of COVID-19.[4,5]
No study has been undertaken to determine the epidemiology, disease characteristics, and outcome on a large scale across the nation. There exists no formal staging system for the disease and no evidence-based protocol for the management of this condition, leaving medical teams grappling in an unchartered territory. A collaborative effort is essential to obtain real-world information and baseline epidemiological data, to identify the patients at risk, myriad presentations, the investigations at various stages of the disease, and management outcomes.
This multicentric collaborative study was undertaken with the aim to determine the patient demographics and population at risk, presenting symptoms and signs, the role of comorbidities and medications used to treat COVID-19, and the outcomes of management. The information provided by such a study may help medical professionals to recognize the early clinical features of ROCM, have a high index of suspicion in the presence of typical symptoms and signs, appropriately triage patients with possible ROCM to confirm the diagnosis, establish staging, and initiate early protocol-based, multidisciplinary management. On a national level, this study could help policymakers in healthcare to estimate the magnitude of the problem, optimize COVID-19-care guidelines to minimize risk exposure, establish post-COVID-19 follow-up protocols, set-up regional multispecialty hubs and teams for ROCM-care, and augment the availability of antifungal drugs.
Methods
We performed a retrospective, multicentric, non-interventional, observational study of patients with ROCM and concurrent or past history of COVID-19 infection. The study was approved by the Ethics Committee. Ophthalmologists across the country were invited to participate in the study and to enter their patient-anonymized data of ROCM managed or co-managed by them between January 1, 2020 and May 26, 2021 in a common database (Annexure 1, online content). The diagnosis of COVID-19 was based on any one of the following: reverse transcription polymerase chain reaction (RT-PCR) test on nasopharyngeal or oropharyngeal swabs, rapid antigen test, or computed tomography (CT) chest scores in the absence of a positive RT-PCR test in a clinically symptomatic case. A patient with symptoms and signs of ROCM, in the clinical setting of concurrent or recently treated COVID-19, was considered as possible ROCM. If clinical features were supported by diagnostic nasal endoscopy findings, contrast-enhanced magnetic resonance imaging (MRI), or CT scan, probable ROCM was diagnosed. Proven ROCM was defined as clinico-radiological features along with microbiological confirmation on direct microscopy and/or culture or histopathology with special stains or molecular diagnostics. [Table 1][6] Patients with non-COVID-19-associated ROCM or those with proven non-mucor fungal infections were excluded from the study. Patients were defined as recovered from COVID-19 if they were tested negative on a repeat RT-PCR or if two weeks had elapsed since the diagnosis.
Table 1.
Classification of COVID-19-associated rhino-orbital-cerebral mucormycosis (ROCM) as possible, probable, and proven
| Terminology | Definition |
|---|---|
| Possible ROCM | Typical symptoms and signs of ROCM |
| Clinical setting of concurrent or recently treated COVID-19 | |
| Probable ROCM | Clinical features suggestive of ROCM |
| Supportive diagnostic nasal endoscopy findings and/or | |
| Supportive radiological signs on contrast-enhanced magnetic resonance imaging or computed tomography scan | |
| Proven ROCM | Clinico-radiological features suggestive of ROCM |
| Microbiological confirmation on direct microscopy and/or | |
| Culture and/ or | |
| Histopathology with special stains and/or | |
| Molecular diagnostics |
(Modified from Honavar SG. Code Mucor: Guidelines for the Diagnosis, Staging and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19. Indian Journal of Ophthalmology. 2021;69:1361-5.)[6]
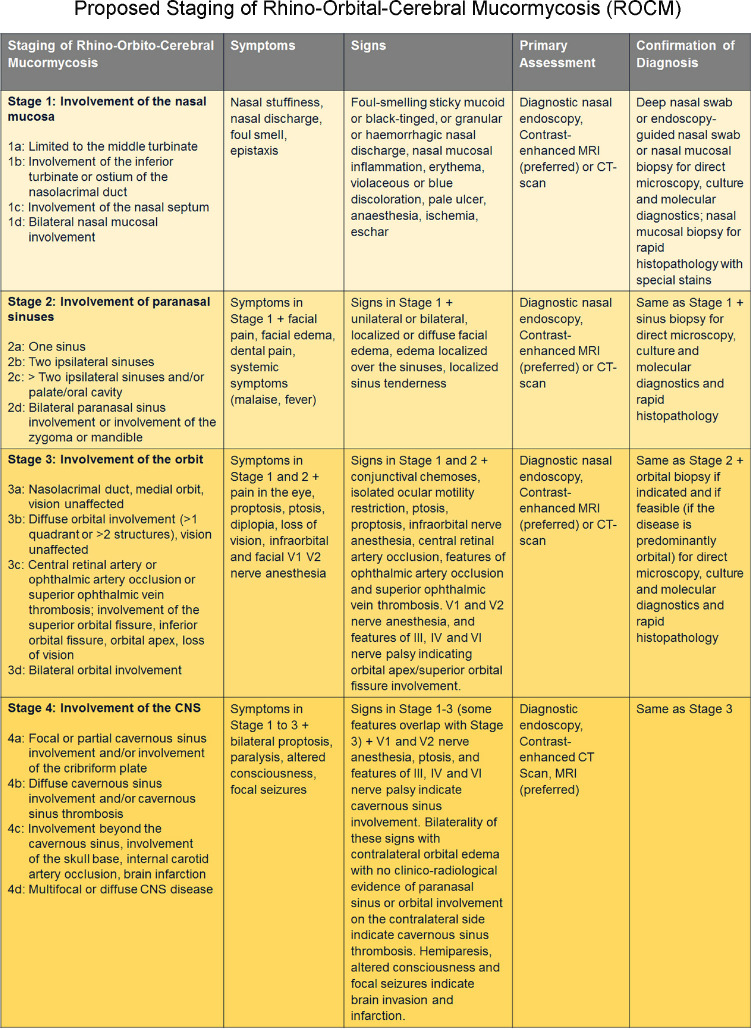
A working staging system has been proposed to help triage these patients and customize their care [Fig. 1].[6] The system is simple and follows the general anatomical progression of ROCM from the point of entry (nasal mucosa) on to the paranasal sinus (PNS), orbit, and central nervous system (CNS), and severity in each of these anatomical locations. There is an attempt to list the symptoms, signs, and preferred diagnostic tools for each of these stages.[6] In this study, all the patients were retrospectively classified into the proposed staging system. The data was analyzed using SPSS (IBM SPSS Statistics 20, SPSS Inc., Chicago, IL, USA) and Microsoft Excel (Version 16.49). The Chi-square and Fisher’s exact tests were used to compare outcomes. For all tests, p values ≤ 0.05 was defined as statistically significant.
Figure 1.
Proposed staging system for COVID-19 associated rhino-orbital-cerebral mucormycosis (Reproduced with permission from Honavar SG. Code Mucor: Guidelines for the Diagnosis, Staging and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19. Indian Journal of Ophthalmology. 2021;69:1361-5)[6]
Results
Demographics
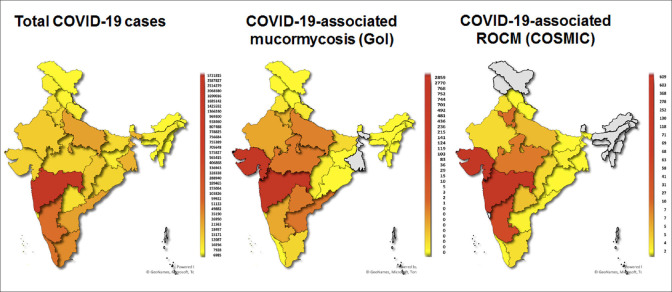
Data of 2826 patients of COVID-19-associated ROCM from 102 treatment centers, 22 states, and union territories of India was analyzed. The percentage and number of cases from each state are shown in Table 2. Gujarat, closely followed by Maharashtra, contributed to the bulk of cases, 22% (609) and 21% (603), respectively. Fig. 2 shows the distribution of COVID-19 and ROCM mapped by each state and union territory based on our data and the data on COVID-19 assocated mucormycosis released by the Government of India.[7] The mean age was 51.9 (range, 12–88) years with a male preponderance (1993, 71%).
Table 2.
Rhino-orbital-cerebral mucormycosis in 2826 patients: Patient demographics and systemic comorbidities
| Features | n=2826 patients (%) |
|---|---|
| Age | |
| Mean, years | 51.9 |
| Median (range), years | 53 (12-88) |
| Gender | |
| Male | 1993 (71) |
| Female | 833 (29) |
| State (Mucormycosis cases under treatment in the national registry)[7] | |
| Gujarat (2859) | 609 (22) |
| Maharashtra (2770) | 603 (21) |
| Karnataka (481) | 368 (13) |
| Madhya Pradesh (752) | 278 (9.8) |
| Haryana (436) | 252 (8.9) |
| Uttar Pradesh (701) | 130 (4.6) |
| Telangana (744) | 118 (4.2) |
| Rajasthan (492) | 71 (2.5) |
| New Delhi (119) | 69 (2.4) |
| Chhattisgarh (103) | 68 (2.4) |
| Tamil Nadu (236) | 63 (2.3) |
| Punjab (141) | 58 (2.1) |
| Puducherry (2) | 41 (1.5) |
| Jharkhand (29) | 31 (1.1) |
| Andhra Pradesh (768) | 27 (1.0) |
| Chandigarh (83) | 10 (0.4) |
| Kerala (36) | 7 (0.2) |
| Odisha (15) | 7 (0.2) |
| Bihar (215) | 5 (0.2) |
| Uttarakhand (124) | 5 (0.2) |
| West Bengal (NA) | 4 (0.1) |
| Himachal Pradesh (3) | 2 (0.1) |
| Diabetes Mellitus, n=2825 | |
| Yes | 2194 (78) |
| No | 631 (22) |
| Control of Diabetes Mellitus, n=2192 | |
| Controlled with oral hypoglycaemics | 593 (27) |
| Controlled with insulin | 627 (29) |
| Uncontrolled | 893 (41) |
| Diabetic ketoacidosis | 79 (3.6) |
| HbA1c, n=466 | |
| Mean, % | 9.8 |
| Median (range) | 9.6 (4.8-17.1) |
| Other comorbidities, n=859* | |
| Hypertension | 690 (80) |
| Renal diseases | 88 (10) |
| Chronic sinusitis/otitis media | 18 (2.1) |
| Bronchial asthma | 17 (2) |
| Cardiovascular disorder | 16 (1.9) |
| Cerebrovascular disease | 8 (0.9) |
| Immunosuppressive drugs | 4 (0.5) |
| Thyroid disorder | 4 (0.5) |
| Human immunodeficiency virus | 4 (0.5) |
| Organ transplant | 4 (0.5) |
| Malignancy | 2 (0.2) |
| Obesity | 2 (0.2) |
| Hepatitis B/Hepatitis C virus | 2 (0.2) |
| Rheumatoid arthritis | 2 (0.2) |
| Liver disease | 2 (0.2) |
| Pancreatitis | 2 (0.2) |
| Multiple myeloma | 1 (0.1) |
*The sum of numbers in the column is greater than 859 as many individuals had more than one comorbidity
Figure 2.
State-wise distribution of COVID-19-positive cases in India (as on June 2, 2021) (left) compared with state-wise hot-spots of COVID-19-associated rhino-orbital-cerebral mucormycosis (ROCM) cases, national data on COVID-19-associated mucormycosis (centre)[7] and COSMIC group data (right). Gujarat, Madhya Pradesh, Haryana and Telangana seem to have disproportionately more ROCM cases, while Kerala, Tamil Nadu, Andhra Pradesh and West Bengal seem to have relatively less ROCM cases as compared to the cases of COVID-19 being reported. (The COSMIC data includes only those cases that have been submitted for the study and may not be representative of the actual incidence).
COVID-19 illness and oxygen requirement
The mean cycle-threshold values on RT-PCR and high-resolution CT chest scores were 15.9 ± 6.5 (n = 286) and 12.2 ± 5 (n = 893), respectively. Table 3 shows the details of the severity of COVID-19 and its management. While 2% (54) patients were asymptomatic and home-cared and 26% (735) were symptomatic and home-cared, 72% (2029) needed hospitalization. Of those hospitalized, 79% (1602) patients required oxygen support. Overall, only 57% (1602) of all patients required oxygen support. Remdesivir was used in 10% (285) of the patients.
Table 3.
Rhino-orbital-cerebral mucormycosis in 2826 patients: COVID-19 and its management
| Features | n=2826 patients (%) |
|---|---|
| Maximum severity of COVID-19, n=2818 | |
| Asymptomatic | 54 (1.9) |
| Home care (ambulatory/assistance necessary) | 735 (26) |
| Hospitalised (no oxygen/oxygen/noninvasive ventilation/ventilator) | 2029 (72) |
| Corticosteroid administration (oral or intravenous), n=2371 | |
| Yes | 2073 (87) |
| No | 298 (13) |
| Oral corticosteroids, n=1865 | 1185 (64) |
| Mean duration (median, range), days, n=968 | 9.3 (8, 1-40) |
| Intravenous corticosteroids, n=1902 | 1476 (78) |
| Mean duration (median, range), days, n=1144 | 7 (6, 1-43) |
| Remdesivir administration, n=2816 | |
| Yes | 285 (10) |
| No | 2531 (90) |
| Mean duration (median, range), days, n=252 | 5 (5, 1-11) |
| Tocilizumab administration, n=2817 | |
| Yes | 58 (2.1) |
| No | 2759 (98) |
| Mean duration (median, range), days, n=58 | 2.3 (2, 1-7) |
| Oxygen administration, n=1602 | |
| Mask/prongs | 1249 (78) |
| Mean duration (median, range), days, n=1027 | 7.1 (12, 1-32) |
| High flow/non-invasive ventilation | 239 (15) |
| Mean duration (median, range), days, n=207 | 6.3 (5, 1-28) |
| Mechanical ventilation | 114 (7.1) |
| Mean duration (median, range), days, n=103 | 5.9 (5, 1-27) |
Risk factors for COVID-19-associated ROCM
Systemic corticosteroids (either oral or intravenous or both) were used in 87% (2073 of 2371) patients, while 13% (298) did not receive corticosteroids in any form. [Table 3] Intravenous corticosteroids were given to 78% (1476 of 1902) patients, intravenous methylprednisolone 51% (749) and dexamethasone 48% (704) being the most common types used for a median duration of six days. Oral corticosteroids were used in 64% (1185 of 1865) for a median duration of eight days. While 42% (426 of 1014) patients received only oral corticosteroids, the rest received intravenous corticosteroids as well. Table 4 shows that use of corticosteroids was the commonest risk factor and their use increased in proportion to the severity of COVID-19. Of 789 (28%) patients who were home-cared, 73% (361 of 492) received corticosteroids. Among 2029 who were hospitalized, 80% (314 of 393) of those who did not need oxygen, 93% (1061 of 1143) of those who needed oxygen by prongs/mask, 99% (226 of 229) of those who needed high-pressure non-invasive ventilation, and 97% (108 of 111) of those on mechanical ventilation received corticosteroids. Of 631 non-diabetic patients, 89% (393 of 440) received corticosteroids. Of the 174 patients who were non-diabetics and did not receive oxygen, 81% (141) received corticosteroids. Tocilizumab was used in only 2% (58).
Table 4.
Rhino-orbital-cerebral mucormycosis in 2826 patients: Risk factors with respect to the COVID-19 severity
| COVID-19 Severity | Corticosteroids | Diabetes Mellitus | No risk | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Intravenous corticosteroids | Oral corticosteroids | Any corticosteroid | Total | Uncontrolled | Diabetic ketoacidosis | |||
| Hospitalization, n=2029 | ||||||||
| No oxygen needed | 221 (60%) n=367 | 158 (44%) n=358 | 314 (80%) n=393 | 353 (82%) n=427 | 118 (33%) n=353 | 8 (2.2%) n=353 | 10 (2.5%) n=393 | 427 (100%) |
| Oxygen by prongs/mask | 826 (77%) n=1078 | 595 (56%) n=1058 | 1061 (93%) n=1143 | 995 (80%) n=1249 | 426 (43%) n=995 | 27 (2.7%) n=995 | 13 (1.1%) n=1143 | 1249 (100%) |
| Non-invasive ventilation | 198 (92%) n=216 | 106 (52%) n=205 | 226 (99%) n=229 | 195 (82%) n=239 | 66 (34%) n=195 | 16 (8.2%) n=195 | 1 (0.4%) n=229 | 239 (100%) |
| Mechanical ventilation | 105 (96%) n=109 | 22 (24%) n=91 | 108 (97%) n=111 | 102 (89%) n=114 | 39 (38%) n=102 | 13 (13%) n=102 | 0 | 114 (100%) |
| No hospitalization, n=789 | ||||||||
| Ambulatory | 93 (26%) n=354 | 229 (51%) n=450 | 275 (71%) n=388 | 454 (67%) n=677 | 208 (46%) n=454 | 10 (2.2%) n=454 | 22 (5.7%) n=388 | 677 (100%) |
| Needed assistance | 32 (34%) n=93 | 73 (69.5%) n=105 | 86 (83%) n=104 | 89 (79%) n=112 | 32 (36%) n=89 | 5 (5.6%) n=89 | 1 (0.9%) n=104 | 112 (100%) |
| Total | 1475 (66.5%) n=2217 | 1183 (52%) n=2267 | 2070 (87%) n=2368 | 2188 (78%) n=2818 | 889 (41%) n=2188 | 79 (3.6%) n=2188 | 47 (2%) n=2368 | 2818* |
*Data from eight patients was not available for analysis
Among systemic comorbidities, DM status, irrespective of control, emerged as the risk factor. While 2194 patients (78%) had DM, 972 (44%) were uncontrolled or had diabetic ketoacidosis (DKA). Of the 859 patients with other comorbidities, 80% (690) had hypertension and 10% (88) had acute or chronic renal failure [Table 2].
The proportion of patients who developed ROCM but were neither diabetic nor received corticosteroids was 2% (47). In the ambulatory, home isolation group, 6% (22) had no underlying risk factor.
Clinical presentation of COVID-19-associated ROCM
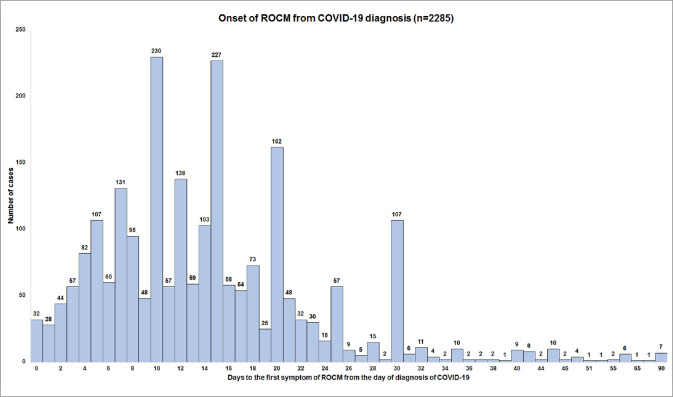
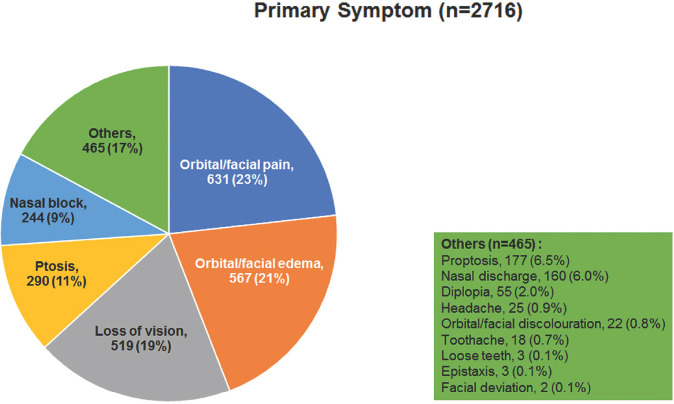
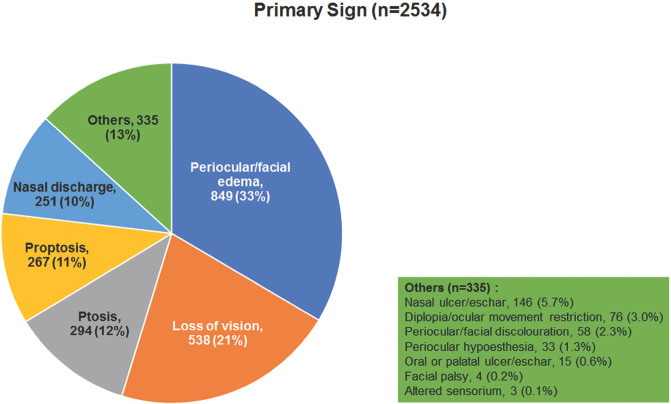
Fig. 3 shows the timeline of the onset of symptoms of ROCM from the diagnosis of COVID-19. The mean interval was 14.5 ± 10 days (n = 2285, median 13, 0–90 days) with 56% of the patients developing within 14 days. Delayed manifestation after 14 days was seen in 44%. Fig. 4 shows the frequency of the most common primary symptoms of ROCM – orbital/facial pain (23%), orbital/facial edema (21%), loss of vision (19%), ptosis (11%), and nasal block (9%). Other symptoms included proptosis, nasal discharge, diplopia, headache, orbital and facial discoloration, toothache, loose teeth, epistaxis, and facial deviation. Fig. 5 shows the frequency of the most common primary signs of ROCM – periocular/facial edema (33%), loss of vision (21%), ptosis (12%), proptosis (11%), and nasal discharge (10%). Other signs included nasal ulcer or eschar, diplopia/ocular movement restriction, periocular or facial discoloration, periocular hypesthesia, oral/palatal ulcer/eschar, facial palsy, and altered sensorium. Table 5 shows the cumulative incidence of clinical manifestations of ROCM. Loss of vision was the most common sign (63%) followed by periocular or facial edema (61%) and ptosis (54%). Proptosis was seen in 38%. Mean measured proptosis was 3.1 (range 1–8) mm in 397 patients in whom exophthalmometry measurements were available.
Figure 3.
Frequency of cases with onset of rhino-orbital-cerebral mucormycosis symptoms from the day of diagnosis of COVID-19 (Day 0)
Figure 4.
Frequency of primary symptoms in patients with COVID-19-associated rhino-orbital-cerebral mucormycosis
Figure 5.
Frequency of primary signs in patients with COVID-19-associated rhino-orbital-cerebral mucormycosis
Table 5.
Rhino-orbital-cerebral mucormycosis in 2826 patients: Clinico-radiological features
| Features | n=2826 patients (%) |
|---|---|
| Stage, n=2669 | |
| 1a | 5 (0.2) |
| 1b | 10 (0.4) |
| 1c | 16 (0.6) |
| 1d | 3 (0.1) |
| 2a | 63 (2.4) |
| 2b | 196 (7.3) |
| 2c | 247 (9.3) |
| 2d | 212 (7.9) |
| 3a | 234 (8.8) |
| 3b | 316 (12) |
| 3c | 724 (27) |
| 3d | 70 (2.6) |
| 4a | 161 (6.0) |
| 4b | 99 (3.7) |
| 4c | 237 (8.9) |
| 4d | 76 (2.8) |
| Laterality of paranasal sinus involvement, n=2669 | |
| No involvement | 34 (1.3) |
| Unilateral | 1585 (59) |
| Bilateral | 1050 (40) |
| Predominant paranasal sinus involvement (radiological), n=2428* | |
| Maxilla | 767 (32) |
| Ethmoid | 512 (21) |
| Sphenoid | 85 (3.5) |
| Frontal | 13 (0.5) |
| Diffuse | 1413 (58%) |
| Laterality of orbital involvement, n=2669 | |
| No involvement | 752 (28) |
| Unilateral | 1687 (63) |
| Bilateral | 230 (8.6) |
| Predominant orbital involvement (radiological), n=1731** | |
| Medial | 469 (27) |
| Superior | 86 (5) |
| Inferior | 221 (13) |
| Apical | 371 (21) |
| Diffuse | 674 (40) |
| Laterality of central nervous system involvement, n=2669 | |
| No involvement | 2096 (79) |
| Unilateral | 440 (16) |
| Bilateral | 133 (5.0) |
| Predominant central nervous system involvement (radiological), n=539# | |
| Cavernous sinus thrombosis/invasion | 285 (53) |
| Internal carotid artery stenosis/occlusion | 95 (18) |
| Temporal lobe abscess | 66 (12) |
| Frontal lobe abscess | 15 (2.8) |
| Skull base osteomyelitis | 38 (7.1) |
| Route of central nervous system involvement (radiological), n=430## | |
| Cavernous sinus | 299 (70) |
| Cribriform plate | 93 (22) |
| Pterygopalatine fossa | 53 (12) |
| Nasal ulcer/eschar, n=2826 | 1348 (48) |
| Nasal discharge, n=2826 | 1022 (36) |
| Periocular/facial edema, n=2826 | 1731 (61) |
| Periocular/facial discoloration, n=2826 | 526 (19) |
| Periocular hypaesthesia,n=2826 | 561 (20) |
| Ptosis, n=2826 | 1519 (54) |
| Ophthalmoplegia, n=2826 | 1459 (52) |
| Involvement of other cranial nerves, n=1720 | 391 (23) |
| Proptosis, n=2826 | 1081 (38) |
| Mean amount of proptosis (median, range), mm | 3.1 (3, 1-8) |
| Loss of vision, n=2826 | 1779 (63) |
*The sum of numbers in the column is greater than 2428 as many individuals had more than one paranasal sinus involvement. **The sum of numbers in the column is greater than 1731 as many individuals had more than one quadrant involvement. #The sum of numbers in the column is greater than 539 as many individuals had more than one central nervous system structure involvement. ##The sum of numbers in the column is greater than 430 as many individuals had more than one route of central nervous system spread
Diagnosis of COVID-19-associated ROCM
Diagnostic nasal endoscopy was performed for 79% (1921 of 2445) patients. Of the 752 patients with stage 1 and stage 2 disease, it was performed in 85% (569 of 672) patients. For diagnostic evaluation, 43% (1016 of 2362) had a deep nasal swab, while 48% (1131 of 2362) samples were collected at sinus debridement. Microbiological evidence was available for 2175 patients – direct microscopy with KOH/calcofluor white in 89% (1931), smear in 6% (121), and culture in 19% (432) cases. Rapid histopathological diagnosis was utilized in 239 cases for early diagnosis in the form of frozen section in 54% (130) and squash or imprint techniques in 46% (109) patients. At the time of analysis, histopathological confirmation was available for 39% (1090 of 2795) patients. CT scan was done for 27% (670 of 2533) and MRI for 58% (1472), while 15% (391) underwent both CT and MRI.
Anatomical and radiological involvement and staging of ROCM
Clinical and radiological involvement of the PNS, orbit, and CNS are shown in Table 5. Diffuse PNS involvement was seen in 58% (1413 of 2428) and bilateral PNS involvement was seen in 40% (1050 of 2669). In the orbit, diffuse involvement predominated in 40% (674 of 1731) followed by involvement of the medial orbit in 27% (469). Orbital apex was involved in 21% (371) patients. In the CNS, cavernous sinus was most commonly involved in 53% (285 of 539). Bilateral CNS involvement was documented in 5% (133 of 2669) cases with cavernous sinus being the most common route of spread (70%, 299 of 430).
Staging of ROCM was performed based on the available information in 2669 patients. It was not possible for 157 patients. The categorization of the patients as per the proposed staging system for ROCM[6] [Fig. 1] showed that 49% (1302) of the patients had disease severity stage 3b or less and 27% (724) had stage 3c disease [Figs. 6-17].
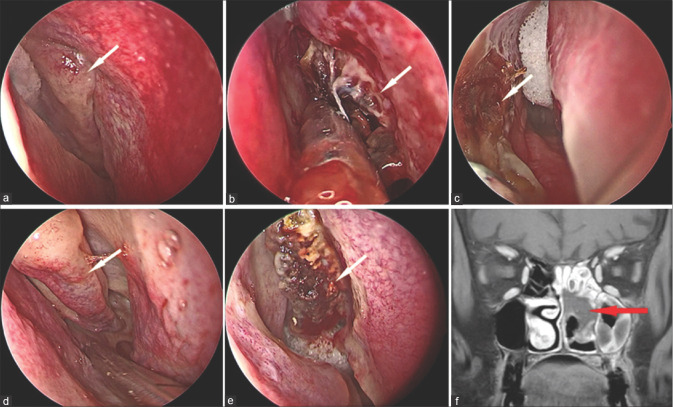
Figure 6.
Stage 1 rhino-orbital-cerebral mucormycosis. Nasal endoscopy pictures showing (a) Stage 1a: Isolated involvement of the left middle turbinate (white arrow) (b) Stage 1b: Involvement of the left inferior turbinate (white arrow) (c) Stage 1c: Involvement of the left nasal septum (white arrow) (d, e) Stage 1d: Bilateral nasal mucosal involvement (right and left side respectively) (white arrow) (f) Stage 2c rhino-orbital-cerebral mucormycosis. Coronal MRI (Post contrast T1 image with fat saturation) orbit and paranasal sinuses showing the non-enhancing inferior and middle turbinate – the “black turbinate sign” (red arrow). The “black turbinate sign” is one of the earliest MRI signs of rhino-orbital-cerebral mucormycosis, and can be detected in stage 1. (Endoscopy images provided by Sandeep Karmarkar, MRI image provided by Ravi Varma)
Figure 17.
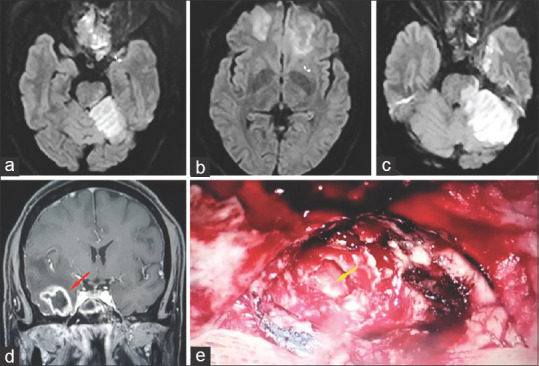
Stage 4d rhino-orbital-cerebral mucormycosis (a, b, c) MRI (Diffusion imaging) showing multifocal hyperintense areas indicating diffuse cerebral parenchymal involvement. (d) Coronal post contrast T1 MRI (T1) showing temporal lobe abscess (red arrow) (e) Endoscopic picture showing right sided temporal abscess cavity (yellow arrow). (MRI image (d) and endoscopic image provided by Sandeep Karmarkar)
Figure 7.
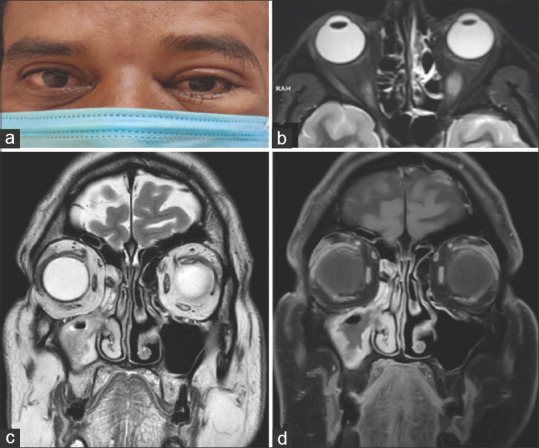
Stage 2a rhino-orbital-cerebral mucormycosis (a) Clinical photograph showing left periocular edema with mild ptosis, conjunctival congestion and chemosis. (b) Axial MRI (T2 with fat saturation) showing mucosal thickening in the left ethmoid sinus. Stage 2b rhino-orbitalcerebral mucormycosis (c) Coronal MRI (T2) of the orbit and paranasal sinuses showing mucosal thickening in the right maxillary and ethmoid sinuses (d). Coronal post-contrast (T1) MRI showing enhancement of the mucosa limited to the ipsilateral maxillary and ethmoid sinuses (Clinical image provided by Chinmayee T, MRI images by Ravi Varma)
Figure 8.
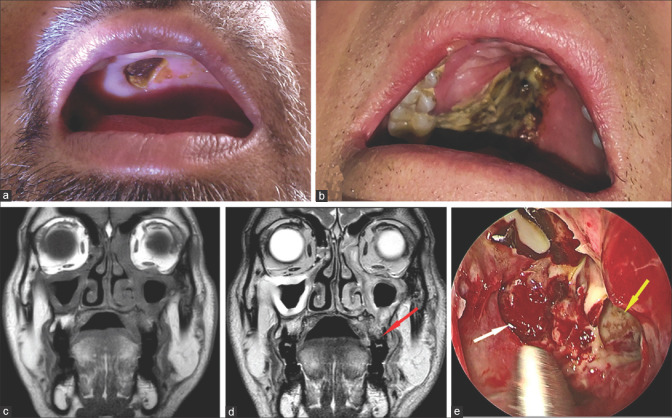
Stage 2c rhino-orbital-cerebral mucormycosis (a, b) Clinical photographs showing palatal involvement with a visible black eschar (c) Coronal MRI (T1) and (d) Coronal MRI (T2) of the orbit and paranasal sinuses showing mucosal thickening in >2 ipsilateral sinuses along with palatal involvement (red arrow) (e) Endoscopy picture showing necrosed tissue in left sphenoid sinus (white arrow) and left maxillary sinus (yellow arrow). (Clinical images provided by Chinmayee T, MRI images by Ravi Varma, endoscopy image by Sandeep Karmarkar)
Figure 9.
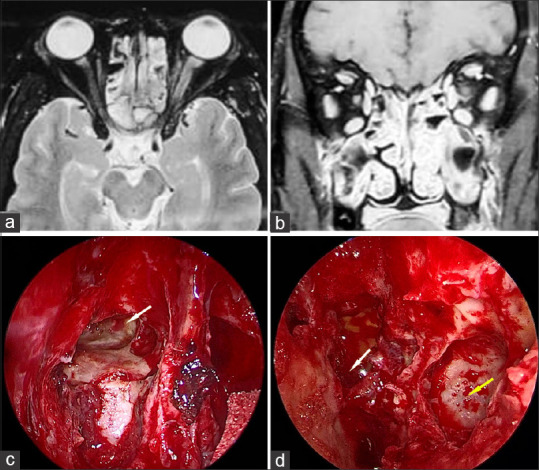
Stage 2d rhino-orbital-cerebral mucormycosis (a) Axial MRI (T2) and Coronal post contrast T1 with fat saturation (b) of the orbit and paranasal sinuses showing mucosal thickening and enhancement in bilateral ethmoid and maxillary sinuses (c) Endoscopic picture showing right frontal sinus involvement with necrotic mucosa (white arrow) along with (d) left sided involvement of sphenoid (white arrow) and maxillary sinus (yellow arrow). (Endoscopy images provided by Sandeep Karmarkar)
Figure 10.

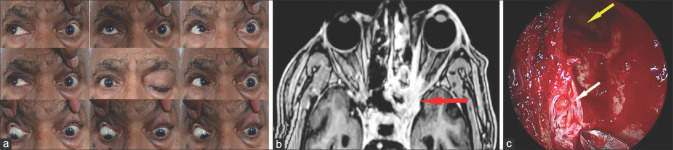
Stage 3a rhino-orbital-cerebral mucormycosis (a) Clinical picture showing left periocular edema, ptosis and proptosis (b) Endoscopy picture showing necrosed left periorbita (white arrow) (c, d) Coronal and axial T2 weighted MR images showing involvement of the right nasolacrimal duct (red arrows), extending into the medial orbit. (Endoscopy image provided by Sandeep Karmarkar, MRI images by Ravi Varma)
Figure 11.
Stage 3b rhino-orbital-cerebral mucormycosis (a) Clinical pictures showing the right eye proptosis with restriction of ocular movements in all gazes. (b) Axial contrast-enhanced MRI (T1) of the orbit, paranasal sinuses, and brain showing involvement of the medial orbit and abnormal intensity of the orbital fat in the posterior orbit along with involvement of the right ethmoid sinus
Figure 12.
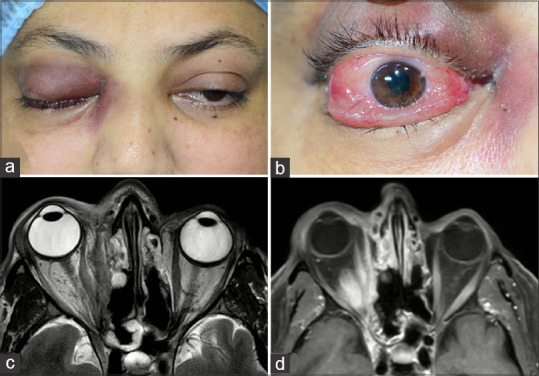
Stage 3c rhino-orbital-cerebral mucormycosis (a) Clinical picture showing right eye ptosis, periocular edema and discolouration (b) severe conjunctival congestion and chemosis (c) Axial MRI (T2) and axial post contrast (T1) with fat saturation showing right ethmoid sinus and diffuse orbital involvement extending to the orbital apex (MRI images provided by Ravi Varma)
Figure 13.
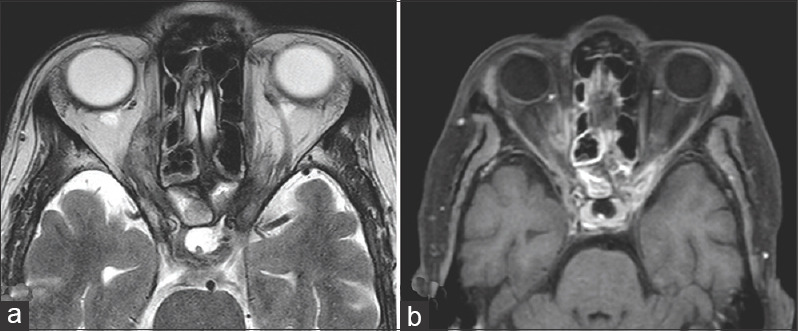
Stage 3d rhino-orbital-cerebral mucormycosis (a) Axial MRI (T2) and (b) contrast enhanced (T1) of the orbit and paranasal sinuses showing bilateral orbital apical involvement, more extensive on the right side. (Images provided by Ravi Varma)
Figure 14.
Stage 4a rhino-orbital-cerebral mucormycosis (a) Clinical photograph showing restriction of ocular movements in all gazes in the left eye. (b) Axial MRI (T1) of the orbit and brain showing diffuse orbital involvement along with focal cavernous sinus involvement (red arrow) (c) Endoscopy picture showing eroded left cribriform plate (white arrow) with dural defect (yellow arrow) (Endoscopy image provided by Sandeep Karmarkar)
Figure 15.
Stage 4b rhino-orbital-cerebral mucormycosis (a) Clinical picture showing no significant ocular manifestation, however, (b) Axial and (c) coronal T1 MRI with fat saturation of the orbit and paranasal sinuses and brain showed involvement of the sphenoid sinus with extension into the cavernous sinus. (MRI images provided by Ravi Varma)
Figure 16.
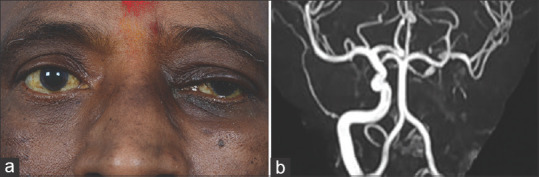
Stage 4c rhino-orbital-cerebral mucormycosis (a) Primary gaze pictures showing ptosis of the right eye along with periocular edema (b) MR angiogram showing left internal carotid artery occlusion (MR Angiography image provided by Chinmayee T)
Management of COVID-19-associated ROCM
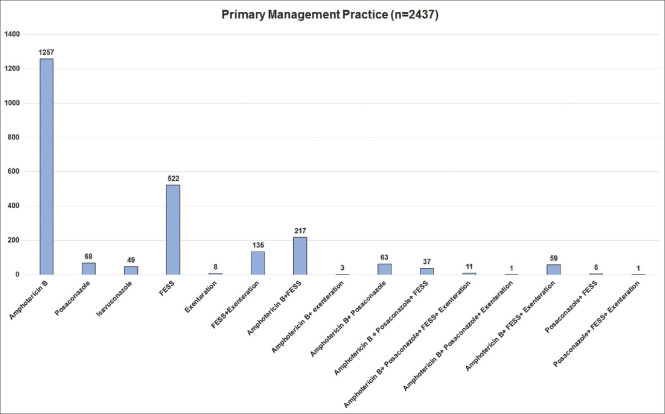
Fig. 18 shows the primary management performed for COVID-19-associated ROCM. Primary initiation of medical management with amphotericin B was preferred in 52% (1257 of 2437), primary functional endoscopic sinus surgery (FESS)/PNS debridement was performed in 21% (522) and both concurrently in 9% (217). Table 6 shows the data on the overall management of the patients till the date of analysis. Of 2066 (73%) patients who received intravenous amphotericin B for a mean duration of 9.1 (range, 1–60) days, liposomal amphotericin B was provided in 73% (1512), amphotericin B deoxycholate in 25% (516), and both in 2% (38). Combination therapy with amphotericin B and posaconazole or isavuconazole was provided in 23% (657). Step-down therapy (following cessation of intravenous amphotericin B) with oral posaconazole or isavuconazole was administered in 26% (732). In all, 67% (1585 of 2358) of patients underwent FESS/PNS debridement, of whom 27% (346 of 1286) underwent multiple sessions to clear the residual/recurrent disease, orbital exenteration was performed in 15% (339 of 2327), both FESS/PNS debridement and orbital exenteration in 17% (367 of 2186), and intraorbital amphotericin B injection was provided in 22% (511 of 2332) for a median of two (range, 1–9) injections.
Figure 18.
Primary management of patients with COVID-19-associated rhino-orbital-cerebral mucormycosis
Table 6.
Rhino-orbital-cerebral mucormycosis in 2826 patients: Management
| Features | n=2826 patients (%) |
|---|---|
| Intravenous Amphotericin B, n=2066 | |
| Deoxycholate | 516 (25) |
| Liposomal | 1512 (73) |
| Both | 38 (1.8) |
| Number of days of intravenous amphotericin B, n=1920 | |
| Mean (median, range) | 9.1 (7, 1-60) |
| Combination therapy, n=657 | |
| Intravenous amphotericin B + posaconazole | 633 (96) |
| Intravenous amphotericin B + isavuconazole | 24 (3.7) |
| Stepdown therapy, n=732 | |
| Oral posaconazole | 698 (95) |
| Oral isavuconazole | 34 (4.6) |
| FESS/Paranasal sinus debridement, n=2358 | |
| Yes | 1585 (67) |
| No | 773 (33) |
| Orbital exenteration, n=2327 | |
| Yes | 339 (15) |
| No | 1988 (85) |
| FESS/Paranasal sinus debridement+Orbital exenteration, n=2186 | |
| Yes | 367 (17) |
| No | 1819 (83) |
| Number of paranasal sinus debridement necessary, n=1531 | |
| Mean (median, range) | 1.3 (1, 1-5) |
| Paranasal sinus irrigation with amphotericin B, n=2190 | |
| Yes | 980 (45) |
| No | 1210 (55) |
| Intraorbital amphotericin B, n=2332 | |
| Yes | 511 (22) |
| No | 1821 (78) |
| Number of intraorbital amphotericin B injections given, n=511 | |
| Mean (median, range) | 2.4 (2, 1-9) |
*The sum of numbers in the column is >2175 as some of the patients underwent more than one type of microbiological test
Outcome of COVID-19-associated ROCM
Of 2218 patients for whom we had outcome data till the day of data collection with a mean follow-up of 14.4 ± 21.3 days (n = 872, range, 1–-270), 41% (909) were alive and well with regression of ROCM, 32% (717) were alive with clinico-radiologically stable ROCM, 13% (287) had progressive ROCM on treatment and 14% (305) had expired. Among the ones alive, ocular outcome was available for 1838 patients, 16% (289) had orbital exenteration and in 84% (1549), the eye could be salvaged. Of these, vision salvage (vision better than 20/200) was reported in 55% (845). [Table 7] Of 511 who received intraorbital amphotericin B, 88% (377 of 381 alive and for whom outcome was available) had eye salvage and 38% (126 of 330) had vision salvage. Eye salvage was achieved in 100% (50 of 50) in stage 3a, 98% (81 of 83) in 3b, 83% (97 of 117) in 3c, 77% (10 of 13) in 3d, 71% (24 of 34) in 4a, 79% (11 of 14) in 4b, 82% (22 of 27) in 4c, and 67% (8 of 12) in 4d.
Table 7.
Rhino-orbital-cerebral mucormycosis in 2826 patients: Outcome
| Features | n=2826 patients (%) |
|---|---|
| Final systemic outcome, n=2218 | |
| Alive with regression | 909 (41) |
| Alive with stable residual | 717 (32) |
| Alive with progression | 287 (13) |
| Death | 305 (14) |
| Final ocular outcome, n=1838 | |
| Orbital exenteration | 289 (16) |
| Eye salvage | 1549 (84) |
| Final visual acuity, n=1426 | |
| PL negative | 298 (21) |
| PR inaccurate | 88 (6.2) |
| ≤20/200 | 195 (14) |
| 20/100-20/40 | 317 (22) |
| ≥20/40 | 528 (37) |
Table 8 shows the outcome of patients at the time of last follow up based on the stage of the disease and surgical intervention. For stages 1 and 2, of the 382 patients who underwent PNS debridement, stable residual or regression was seen in 366 (96%) compared to those who did not undergo any surgery (p < 0.05). Orbital exenteration was performed in 25 of 375 patients with ROCM stages 3a and 3b, and of these, 84% (21) had stable residual or regressed disease, similar to the 370 patients for whom orbital exenteration was not done (p = 0.96). PNS debridement in patients with stages 3a and 3b ROCM reduced disease progression and mortality from 35 to 11% (p < 0.05). Prognosis was poor once the disease advanced to stage 3c or worse with mortality and disease progression seen in 39% (451 of 1145) of the patients compared to 12% (119 of 1027) in those with stage 3b or better (p < 0.05). For diffuse sino-orbital disease (stages 3c, 3d), both orbital exenteration and PNS debridement appeared to be beneficial. Mortality and disease progression was seen in 47% (74 of 158) of the patients who did not undergo PNS debridement as compared to 22% (90 of 410) of those who did (p < 0.05). Mortality and disease progression was also significantly less following orbital exenteration, 22% (36 of 164) vs 33% (134 of 405) without orbital exenteration (p = 0.008). For stage 4, surgical intervention improved the prognosis. Mortality and disease progression reduced from 67% (96 of 143) to 39% (115 of 297) following PNS debridement and from 52% (164 of 314) to 39% (48 of 124) following orbital exenteration.
Table 8.
Rhino-orbital-cerebral mucormycosis in 2826 patients: Overall stage-based outcome
| ROCM stage and surgical management (n) | Death or disease progression (%) | Stable residual or regressed disease (%) | p |
|---|---|---|---|
| ROCM Stage 1, n=29 | |||
| Paranasal sinus debridement (6) | 2 (33) | 4 (67) | p=0.18 |
| No paranasal sinus debridement (23) | 1 (4.3) | 22 (96) | |
| ROCM Stage 2, n=502 | |||
| Paranasal sinus debridement (376) | 14 (3.7) | 362 (96) | p<0.05 |
| No paranasal sinus debridement (126) | 22 (18) | 104 (82) | |
| ROCM Stage 1 and 2, n=531 | |||
| Paranasal sinus debridement (382) | 16 (4.2) | 366 (96) | p<0.05 |
| No paranasal sinus debridement (149) | 23 (15) | 126 (85) | |
| ROCM Stage 3a, 3b, n=418 | |||
| Paranasal sinus debridement (347) | 38 (11) | 309 (89) | p<0.05 |
| No paranasal sinus debridement (71) | 25 (35) | 46 (65) | |
| ROCM Stage 3a, 3b, n=375 | |||
| Orbital Exenteration (25) | 4 (16) | 21 (84) | p=0.965 |
| No orbital exenteration (370) | 58 (16) | 312 (84) | |
| ROCM Stage 3c, 3d, n=568 | |||
| Paranasal sinus debridement (410) | 90 (22) | 320 (78) | p<0.05 |
| No paranasal sinus debridement (158) | 74 (47) | 84 (53) | |
| ROCM Stage 3c, 3d, n=569 | |||
| Orbital Exenteration (164) | 36 (22) | 128 (78) | p=0.008 |
| No orbital exenteration (405) | 134 (33) | 271 (67) | |
| ROCM Stage <4, n=1517 | |||
| Paranasal sinus surgery (1139) | 144 (12.6) | 995 (87.3) | p<0.05 |
| No paranasal sinus surgery (378) | 122 (32.3) | 256 (68) | |
| ROCM Stage 4, n=440 | |||
| Paranasal sinus surgery (297) | 115 (39) | 182 (61) | p<0.05 |
| No paranasal sinus surgery (143) | 96 (67) | 47 (33) | |
| ROCM Stage 4, n=438 | |||
| Orbital Exenteration (124) | 48 (39) | 76 (61) | p=0.01 |
| No orbital exenteration (314) | 164 (52) | 150 (48) | |
| ROCM Stage, n=2172 | |||
| ≤3b (1027) | 119 (11.6) | 908 (88.4) | p<0.05 |
| > 3b (1145) | 451 (39) | 694 (61) |
In all, 137 patients had a follow-up of more than 3 weeks (mean 45.6 days, 21–270 days). Of these, 6% (8) had expired, besides which, disease progression was seen in 6% (8), residual disease was stable in 24% (32), and regressed in 65% (88). Eye salvage was achieved in 66% (83 of 125) and vision > 20/200 was present in 67% (56 of 83). Table 9 shows the stage-based outcome of these patients. Of the 20 patients with stage 2 disease, 17 underwent PNS debridement and all had regressed or stable lesion. PNS debridement was performed for all 30 patients with stages 3a and 3b and orbital exenteration was done in two cases. Mortality and disease progression was seen in 17% (15 of 86) of patients with stage 3c or worse as compared to 2% (1 of 50) of patients with stage 3b or better (p = <0.05). Orbital exenteration did not significantly alter the outcome in patients with disease stages 3c and 3d (p = 0.24). PNS debridement for stages 3c and 3d reduced disease progression and mortality from 33% (1 of 3) to 13% (5 of 38), but the results were not statistically significant. All 16 patients with stage 4 disease, who were treated with orbital exenteration, survived with stable or regressed lesion (p = 0.03).
Table 9.
Rhino-orbital-cerebral mucormycosis in 2826 patients: Stage-based outcome in patients with a follow-up of more than >3 weeks
| ROCM stage and surgical management (n) | Death or disease progression (%) | Stable residual or regressed disease (%) | p |
|---|---|---|---|
| ROCM Stage 1, n=0 | NA | NA | NA |
| ROCM Stage 2, n=20 | |||
| Paranasal sinus debridement (17) | 0 | 17 (100) | 1* |
| No paranasal sinus debridement (3) | 0 | 3 (100) | |
| ROCM Stage 3a, 3b, n=30 | |||
| Paranasal sinus debridement (30) | 1 (3.3) | 29 (97) | 1* |
| No paranasal sinus debridement (0) | |||
| ROCM Stage 3a, 3b, n=29 | |||
| Orbital exenteration (2) | 0 | 2 (100) | 1* |
| No orbital exenteration (27) | 1 (3.7) | 26 (96.3) | |
| ROCM Stage 3c, 3d, n=41 | |||
| Paranasal sinus debridement (38) | 5 (13) | 33 (87) | p=0.34 |
| No paranasal sinus debridement (3) | 1 (33) | 2 (67) | |
| ROCM Stage 3c, 3d, n=42 | |||
| Orbital exenteration (19) | 5 (26) | 14 (74) | p=0.24 |
| No orbital exenteration (23) | 2 (8.6) | 21 (91) | |
| ROCM Stage <4, n=91 | |||
| Paranasal sinus surgery (85) | 6 (7.1) | 79 (93) | p=0.74 |
| No paranasal sinus surgery (6) | 1 (17) | 5 (83) | |
| ROCM Stage <4 (3a-d), n=71 | |||
| Orbital exenteration (21) | 5 (24) | 16 (76) | p=0.03 |
| No orbital exenteration (50) | 3 (6.0) | 47 (94) | |
| ROCM Stage 4, n=41 | |||
| Paranasal sinus surgery (41) | 7 (17) | 34 (83) | 1* |
| No paranasal sinus surgery (0) | |||
| ROCM Stage 4, n=42 | |||
| Orbital exenteration (16) | 0 | 16 (100) | p=0.03 |
| No orbital exenteration (26) | 7 (2.7) | 19 (97) | |
| ROCM Stage, n=136 | |||
| ≤3b (50) | 1 (2.0) | 49 (98) | p=0.007 |
| >3b (86) | 15 (17.4) | 71 (82.6) |
*Fisher exact test statistical value. Value not significant at P<0.05
Discussion
Mucormycosis is an opportunistic, potentially lethal, angioinvasive fungal infection predisposed by uncontrolled DM, corticosteroids, immunosuppressive therapy, primary or secondary immunodeficiency, hematological malignancies, hematological stem cell transplantation, solid organ malignancies, solid organ transplantation, and iron overload.[3] Other less common risk factors include intravenous drug use, human immunodeficiency virus infection, renal failure, liver diseases, and chronic alcoholism, and malnutrition and low birth weight in the pediatric population. Post-pulmonary tuberculosis and chronic kidney disease have been found to be emerging risk factors based on studies in the Indian population.[8,9] It can affect the nose, sinus, orbit, CNS, lung, gastrointestinal tract, skin, jaw bones, heart, kidney, and mediastinum. ROCM is the most common presentation, contributing to about two-thirds of all cases of mucormycosis.[10,11] The spores are inhaled into the nasopharynx and tissue invasion, thrombosis, and necrosis progresses from the nose, to the PNS, orbit, and CNS. The prevalence has been estimated to be 0.005–1.7 per million population worldwide before the pandemic. The prevalence in India has always been much higher, nearly 80 times that in other parts of the world, 0.14 per 1000.[2,12,13] India also occupies the second position in the world as far as the number of diabetics is concerned.[14]
With COVID-19, the incidence of secondary bacterial or fungal infections is 8%, with aspergillosis and candida being the most common fungi reported.[15,16] The current wave of COVID-19 has seen a surge of mucormycosis. COVID-19 produces a hypoxic environment with high glucose levels, high levels of ferritin, and attenuated phagocytic activity of leukocytes due to immunosuppression by the virus itself and the corticosteroids used in the management. This setting is highly conducive for the fungal spores to germinate and proliferate.[2] Unhygienic practices, prolonged hospital stay with possibility of nosocomial infection, use of immunosuppressants like tocilizumab, and associated comorbidities are other risk factors attributed to the increasing incidence of COVID-19-associated ROCM.[2] This study was done with the goal of improving the knowledge about ROCM in COVID-19 patients in order to ultimately improve the management and outcome.
Epidemiology of COVID-19-associated ROCM
In the Indian population, the mean age of COVID-19 patients admitted to a hospital was 45–50.7 years and 56–93% of the patients were males.[17,18,19] The median age of affliction of COVID-19-associated ROCM patients has been reported to be 55 years (range 10–86 years) in a review of global cases by European Confederation of Medical Mycology and International Society of Human and Animal Mycoses.[20] In the reported cases of COVID-19-associated ROCM, there was a male predilection (79%).[2] The demographic profile in our series was consistent with these studies with a mean age of 51.9 years and 71% male patients. Male gender has also been found to be associated with greater severity of COVID-19. Greater outdoor exposure and, therefore, to fungal spores may be the possible reason for this majority.
Potential risk factors for COVID-19-associated ROCM
In a study from India, of 235 patients infected with SARS-CoV-2 infection during the first wave and requiring hospitalization, 77% required oxygen support in any form, 22% with high flow nasal cannula, and 26% required invasive mechanical ventilation.[17] In a larger study from Mumbai, 11% required oxygen with mask/cannula, 0.7% required non-invasive ventilation, and only 0.2% required ventilator support.[21] In this study, oxygen requirement was observed in 57% (1602 of 2818), and of these, masks/prongs were used in 78%, non-invasive ventilation in 15%, and mechanical ventilation was necessary in 7%. Our data of COVID-19-associated ROCM patients shows that 43% (1216 of 2818) did not require oxygen support during the treatment of COVID-19. However, most of these had DM and majority received corticosteroids in some form, suggesting that contaminated oxygen may not be the driver of infection. Of 47 patients with COVID-19-associated ROCM who were non-diabetics and did not receive corticosteroids, 51% (24) required hospitalization and 30% (14) received oxygen. Since there is no large, published study on the hospitalized COVID-19 patients in India, it is difficult to understand if patients developing ROCM have a greater requirement of oxygen support as compared to those patients who do not develop ROCM. In the absence of any other discernible risk factor in 2.5% (10) patients, it could be speculated that they may have developed a nosocomial infection.
Remdesivir and tocilizumab have specific indications for use. Remdesivir has been authorized for emergency use for COVID-19, while tocilizumab is an off-label use.[22]
Tocilizumab was administered in only six reported cases of mucormycosis in literature.[20] In our series, 10% of the patients received remdesivir and only 2.1% received tocilizumab, and may not have a played a role in increasing the risk of ROCM.
Corticosteroids have been maligned for their role in increasing the susceptibility to mucormycosis and the allegation is not totally ill-founded. A cumulative dose greater than 600 mg for prednisone and 2–7 g of methylprednisolone has been found to predispose immunocompromised patients to mucormycosis.[23] Prolonged, >3 weeks of high-dose systemic corticosteroid has been implicated as a risk factor for mucormycosis in non-COVID-19 patients.[24] Corticosteroids have been used for many diseases apart from COVID-19 and have been a part of the guidelines even during the first wave. The RECOVERY trial has shown the benefits of corticosteroids in reducing mortality in patients with moderate to severe COVID-19.[25] The updated national guidelines recommend the use of intravenous methylprednisolone (or an equivalent of dexamethasone 0.1–0.2 mg/kg) at a dose of 0.5–1 mg/kg in two divided doses for moderate disease and 1–2 mg/kg in two divided doses (dexamethasone 0.2–0.4 mg/kg) for severe disease for a duration of 5–10 days.[22] According to the published literature, 76% of the patients with COVID-19-associated ROCM gave history of systemic corticosteroids.[2] In India, this fraction is higher, 88%.[26] Our data revealed that systemic corticosteroids had been used in 87% of the patients. While the mean duration of both oral and intravenous corticosteroids was within the stipulated recommendations, 21% (373 of 1775) patients received systemic corticosteroids for more than ten days. Of 789 mild cases of COVID-19 not requiring hospital admission, 73% used corticosteroids. Irrational or injudicious use of corticosteroids can be a possible cause for ROCM.
In Indian patients with COVID-19, DM was seen in 11–23% of the hospitalized patients.[17,18] New onset DM was seen in 20.6% of patients with mild to moderate COVID-19.[27] The virus is said to damage the pancreatic islet cells producing new onset DM, worsening of pre-existing DM or DKA. The cytokine storm indirectly fuels this by resulting in insulin resistance.[26,28,29] Corticosteroids also precipitate hyperglycemia and DKA. Hyperglycemia causes glycosylation of transferrin and ferrtin and reduces iron binding. By reducing the ability of transferrin to chelate iron, acidosis presents an additive effect causing an overall increase in free iron levels, allowing mucor to thrive. DM has been identified as an independent risk factor for mucormycosis.[8] In a large series of cases from India of mucormycosis in the pre-COVID-19 era, 74% of the patients were diabetics.[10] The risk of mucormycosis is 7.5 times higher in diabetics than the general population.[30] When we look at the current scenario of COVID-19-associated ROCM in India, in a series of 41 cases by John et al.,[26] 93% were diabetics. The literature review of the existing global data by Singh et al.[2] and Hoenigl et al.[20] showed that diabetics account for 80% of the cases and concomitant DKA was found in 15–41% of the patients. Of these, 90–97% of the cases were type 2 and 80.3% were uncontrolled.[2,20] Our data showed similar results, but we did not differentiate between type 1 and type 2. Of the 210 patients who did not receive corticosteroids or need oxygen, 84% (173) were diabetics.
ROCM is not known to affect healthy individuals except in rare cases. Hypertension has been noted in 19% of the cases, while no associated comorbidities were documented in only 5% of the reported cases of COVID-19-associated ROCM.[20] In our series of 2826, 24% (690) had hypertension and 3% (88) had renal disease. Similar to prior studies on ROCM and ROCM in COVID-19, in our series, 18% (506 of 2826) patients had no systemic comorbidity except for COVID-19. This is important because in patients presenting with signs and symptoms of ROCM in the background of COVID-19, one should still investigate thoroughly and have a high index of suspicion even in the absence of an underlying comorbidity. Among those who were home isolated, 5% patients required neither corticosteroids nor were they diabetics, but still developed ROCM. They did not have any known risk factors. Though the proportion is small, the absolute number, i.e. 23 is still alarming and mandates further study of virus – host interactions and search for other potential risk factors. Mucorales are known commensals in the gastrointestinal tract but are also found in the nasopharynx and PNS. A breach in the mucosal barrier may induce infection from endogenous source.[31]
The etiology of COVID-19-associated ROCM appears multifactorial. Pre-existing DM; new-onset COVID-19-related hyperglycemia; impaired mucosal immunity, mechanical breach in the nasal mucosa and mucosal necrosis, hypoxia, and increased ferritin levels all attributed to COVID-19; systemic corticosteroids; nosocomial infections in hospitalized patients, specifically those in the setting of intensive care, all seem to conjure up a vicious combination of risk factors. In a large study of 5428 hospitalized patients with COVID-19 between March 2020 to May 2021, of whom 1027 were in the intensive care unit (915 of 1027 received corticosteroids and 417 had DM), no case of ROCM was reported.[32] The authors attributed it to adherence to low dose corticosteroid protocol and strict glycemic control.[32] Future studies could address these issues, and also look at the aspect of air quality in hospitals as a possible contributing factor. Apart from all these predisposing factors, the tropical Indian climate may itself predispose vulnerable patients to ROCM.[17,18,26,31,32,33,34]
Timing of occurrence of COVID-19-associated ROCM
ROCM occurs both concomitantly with COVID-19 as well as post-recovery. Of 80 cases of ROCM, 93% were hospitalized and undergoing active treatment for COVID-19 in the review by Hoenigl et al.[20] The median time for diagnosis of mucormycosis was ten days from the day of COVID-19 diagnosis. For those who developed signs of mucormycosis after the diagnosis of COVID-19, the duration was 14.5 days.[20] The ratio is less skewed in the literature review by Singh et al.[2] with 60% of the cases seen in active and 40% in recovered COVID-19 patients. Both the series included mucormycosis at all anatomical sites and not just ROCM. In our series, the peak was seen on day ten with 10% (230 of 2285) developing symptoms of ROCM, and 56% patients developed ROCM symptoms within 14 days from the diagnosis of COVID-19. Additional spikes were seen on day 15 and day 20 with 10% (227) and 7% (162) of patients, respectively. Development of ROCM while the patient is still under active treatment for moderate or severe COVID-19 may pose significant challenges in the management – specifically termination of corticosteroids, and surgery under general anesthesia. In all, 44% of patients presented with ROCM following recovery from COVID-19 in our series. Delayed ROCM, three months post-COVID-19 was noted in seven patients. Smaller spikes are also seen in the graph around day 30, 45, and 60. This makes follow-up of COVID-19 patients necessary for a period of three months after recovery. Educating the patients and families with a checklist of symptoms, mucor helplines, and follow-up clinics for COVID-19 recovered patients are possible solutions.
Symptoms and signs and the site of involvement of COVID-19-associated ROCM
ROCM has visible signs and symptoms to allow early diagnosis as compared to mucormycosis at other anatomical sites. The red flag/warning signs should be known to all ophthalmologists. The most common signs and symptoms reported are loss of vision, orbital/facial pain, periocular/facial edema, ptosis, and nasal discharge as determined from our study. All of these are evident just on inspection and requires no additional skill or instrument.
The right and left PNS, orbit, and brain were equally affected. It is interesting to note that bilateral PNS involvement was seen in 40% of the patients. This increases the imminent risk to both the orbits unless diagnosed and controlled. Diffuse involvement of the PNS and even structures within the orbit was seen most commonly. The medial orbit was predominantly affected in 27% of the cases. This can be through the nasolacrimal duct or the lamina papyracea. The orbital apex was involved in 21% of the patients. Apical involvement may not present with fulminant signs and symptoms. While the eye may be white and proptosis may be subtle, ptosis, other cranial nerve palsies, and regional hypaesthesia and diminution of vision may be the early signs of orbital apical involvement. Early diagnosis of orbital apical involvement may help minimize the risk of progression to the cavernous sinus.
CNS involvement has been documented in 37% of the cases of COVID-19-associated ROCM.[20] In our study, 21% (573 of 2669) patients had CNS involvement. The information about predominant site of involvement was available for 539 patients and 53% (285) had focal or diffuse cavernous sinus involvement or thrombosis. PNS and brain involvement was noted in three patients without the orbit being involved. The proposed staging system allows the patients to be assigned to the most severe stage based on the anatomical location and the severity therein and, thus, can be extended to classify cases with non-contiguous involvement.
Diagnosis of COVID-19-associated ROCM
Contrast-enhanced MRI is the imaging modality of choice. It allows delineation of soft tissue involvement earlier and is better than a CT scan, especially in the setting of orbital and cerebral involvement. Contrast-enhanced CT scan is relatively faster and can be used for patients where MRI is not feasible. Mucormycosis leads to tissue necrosis, and bone erosion is not a common finding, so a CT scan may not support an early diagnosis. In our series, MRI was the preferred modality in 58%, and 27% underwent a CT scan. In patients where contrast cannot be given, even a plain CT is still necessary to evaluate the extent of the disease. PET-CT is a useful tool for detection and assessment of response to treatment limited by cost of repeated imaging.[35,36]
Diagnostic nasal endoscopy allows a quick inspection and sampling from the nasal cavity. It is a simple, bedside yet powerful tool to diagnose suspected cases in stage 1 and early stage 2 before the clinical and radiological signs are evident. It is encouraging that it was done in 78.6% of the cases. Nasal-endoscopy-guided swabs from the area of discharge, nasal mucosal inflammation, ulcer, necrosis, or eschar are likely to yield a better representative sample for direct microscopy rather than a random, blind nasal swab.
Rapid diagnosis of mucormycosis can be achieved with direct microscopy using KOH wet mounts, with or without fluorescent brighteners like blankophor and calcofluor white, and this was done in 88.8% of the cases that we analyzed. Nasal endoscopy-guided micro-biopsy from the abnormal nasal mucosa or the turbinates can be performed in the clinic or bedside and can provide a quick, representative sample for rapid diagnostic tests apart from the routine microbiology and histopathology.[37] Frozen sections, squash and imprint diagnosis can be done on any fresh tissue, and can also help in defining clear surgical margins intraoperatively. Rapid histopathological tests were performed in only 239 cases in our series, and its potential seems untapped, although further studies are required to determine the sensitivity and specificity of these tests.
Culture is required for identification of genus, species, and antifungal susceptibility. However, false-negative results may be obtained in 50% of the cases and is attributed to improper sampling, tissue handling, and ongoing antifungal therapy.[38,39] Histopathology of the biopsied tissue may be necessary for the definitive diagnosis of mucormycosis, to detect angioinvasion and to distinguish an infection from a contaminant. It can also reveal co-infection. Immunohistochemistry with monoclonal antibodies can aid, particularly in cases where cultures are negative. Matrix-assisted laser desorption ionization time of flight mass spectrometry is a newer method but requires further validation.[37] PCR is also a rapid test as compared to histopathology and culture and can be done on serum and paraffin-embedded tissue.[37,40] They can also be used to monitor treatment. In ROCM, where early diagnosis is the key to survival, these resources should be harnessed and made more widely available.
Management of COVID-19-associated ROCM
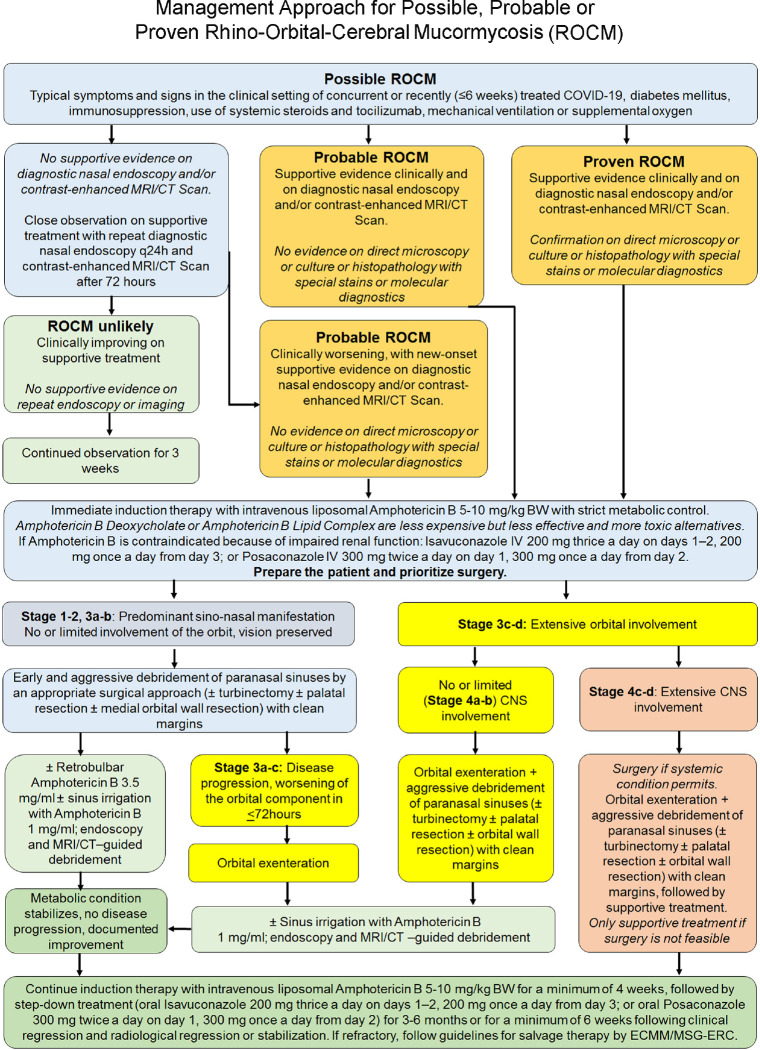
The management of mucormycosis essentially involves control of hyperglycemia or any other risk factor, optimal surgical debridement, and medical management with antifungal agents. A large review of 929 cases showed that survival was only 3% with no intervention, 57% with surgery alone, 61% with amphotericin deoxycholate, and 70% when treated with both amphotericin and surgical debridement.[11] Fig. 19 shows the proposed management guideline for COVID-19-associated ROCM.[6]
Figure 19.
Proposed management algorithm for rhino-orbital-cerebral mucormycosis (Reproduced with permission from Honavar SG. Code Mucor: Guidelines for the Diagnosis, Staging and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19. Indian Journal of Ophthalmology. 2021 Jun;69:1361-5)[6]
Amphotericin B is the antifungal drug of choice for mucormycosis. It has been used in 88% of the patients of COVID-19-associated ROCM.[20] Even in our series, at the time of analysis, 73% of the patients had received amphotericin B. Induction should be in full dose (5 mg/kg body weight for stages 1a–3d and up to 10 mg/body weight for stages 4a–4d) with liposomal amphotericin B. In situations with resource constraint, one can use amphotericin B deoxycholate or amphotericin B lipid complex. The liposomal form is preferred since it is less nephrotoxic and, therefore, higher doses may be given for a prolonged duration. Our data shows that 73% of the patients received the liposomal form. Some patients received both the liposomal and deoxycholate type because of the logistic reasons. In patients with compromised renal functions, posaconazole and isavuconazole have been found to be effective alternatives, but amphotericin B remains the treatment of choice. Some patients also received these drugs as primary management possibly because of the unavailability of amphotericin B.
Prolonged step down oral antifungal therapy is warranted for 3–6 months.[37,41,42] Isavuconazole has been used in mucormycosis either in combination with amphotericin B or as a salvage therapy and as monotherapy. Isavuconazole was approved in 2015 for the treatment of invasive aspergillosis and mucormycosis. The VITAL study showed that isavuconazole was non-inferior to amphotericin B against mucormycosis, as primary treatment, in refractory cases and in patients with toxicity to other antifungals. The safety profile is better, and it can be given both orally and as intravenous injections (loading dose 200 mg TDS day 1 and 2, followed by 200 mg daily). Although the hepatotoxicity is less than other azoles, liver functions should be monitored. It is also tolerated well for extended use, over six months.[43,44] Twenty-six percent of the patients received step-down treatment, 95% with posaconazole. These numbers are expected to rise with a longer follow up.
A study from India has shown posaconazole to be highly effective as salvage therapy for ROCM with life salvage and complete resolution in 67% of the patients.[45] However, there is no data supporting the use of combination therapy and has not been recommended in any of the major treatment guidelines.[37,41,42] Few retrospective clinical studies have concluded the superiority of combination therapy with an Echinocandin (Caspofungin) and Polyene (liposomal amphotericin B).[46] However, phase III randomized placebo-controlled trials are required to establish the benefit.[47] In our series, 23% of the patients received combination therapy with posaconazole being the preferred drug added to amphotericin B.
Intraorbital injection of amphotericin B deoxycholate in a concentration of 3.5 mg/mL has shown to be effective for life and eye salvage in certain case reports.[48,49,50] There is paucity of data with regard to its safety and potential for vision salvage. Nevertheless, for a disease with such a high mortality rate, where vision is of relatively minor concern, it is being increasingly used as an adjunct to medical therapy and surgical debridement.
PNS debridement as a primary management was performed in 21% of the cases in our series. This serves both diagnostic and therapeutic purpose. PNS debridement was performed in 67% overall. Orbital exenteration was done in 15% patients, and simultaneous PNS debridement and orbital exenteration was performed in 17%. In a review of 80 published cases of ROCM in COVID-19, 37% required an orbital exenteration. Orbital exenteration is conventionally done in cases with no visual potential, with diffuse orbital involvement, but with the disease limited to the orbit without or minimal extension to the cavernous sinus. The decision lies with the treating physician because there is no firm consensus regarding the indications and timing of orbital exenteration. No significant difference has been found in survival with or without orbital exenteration.[51,52] Several case reports have illustrated the management of sino-orbital mucormycosis without orbital exenteration. Some series have even found orbital exenteration to be detrimental to survival and allowing further dissemination of the disease. A retrospective case series showed that for limited sino-nasal disease, surgical sino-nasal debridement achieved success in 94% of the cases. On the other hand, patients with the rhino-orbital disease treated with orbital exenteration along with sinus debridement had a treatment failure with progression/mortality in 89% of the cases albeit the worse systemic and more severe disease.[51,52,53,54] Analysis of our data showed that in patients with limited sino-orbital disease (3a, 3b) orbital exenteration does not alter the outcome significantly. However, when it advances to stages 3c and worse, orbital exenteration seems to help in improving the outcome. Mortality and disease progression was less in patients with stages 3c and 3d ROCM treated with orbital exenteration (33%) vs those without orbital exenteration (22%) (p = 0.008). In patients with involvement of the CNS, contrary to standard understanding, our study found that orbital exenteration is beneficial. Mortality and disease progression was seen in 39% of the patients treated with orbital exenteration as compared to 52% in patients without orbital exenteration. Orbital exenteration is ideally performed following confirmation of diagnosis by histopathology. However, in the absence of prior histopathological confirmation, but with clinical–radiological–microbiological evidence supporting the diagnosis of ROCM with documented disease progression, the treating multispecialty medical team and the family could take a decision for orbital exenteration to prioritize life salvage. A good counselling about the necessity and availability of cosmetically sound rehabilitation may help patients in accepting this radical, but potentially life-saving procedure.
Literature has shown that ROCM patients without CNS involvement had better outcome with surgical intervention than with medical management alone (mortality 14% vs 63%, p = 0.01). Surgery did not have an impact on the survival of patients with CNS involvement (mortality 71% vs 57%, p = 0.66).[20] The first part is supported by our data where mortality and disease progression decreased from 32% to 13% in patients without CNS involvement treated with PNS debridement. However, the analysis of patients with CNS involvement showed that PNS debridement was associated with better outcome than with no surgical intervention at all. Death and disease progression reduced from 67 to 39% with surgery. In patients with a follow-up of at least 3 weeks, 100% of the patients with stage 4 ROCM who had undergone orbital exenteration had stable residual or regressed lesion (p = 0.03). Thus, surgery may not be a contraindication in patients with CNS involvement and may indeed improve survival. These findings suggest that orbital exenteration may play a significant role in advanced disease while a more conservative approach may be preferred in patients with disease stage 3b or better. Readers are cautioned that these are preliminary results and the follow up of patients are not sufficient to provide convincing evidence.
Prognosis of COVID-19-associated ROCM
ROCM is a rapidly progressive disease, with 30–90% mortality rate in cases with cerebral involvement.[20,33] For cases associated with COVID-19, the overall mortality has been estimated to be 31%.[2] The median time before succumbing to the disease was 75 days.[20] Results from our series show that overall, the mortality with COVID-19-associated ROCM is 14% and disease progression is seen in 13% of the cases. These results are likely to change over time as the patients are followed up. At 3 weeks follow up, 88% (120 of 136) of the patients had stable/regressed disease. Based on our results, it is clear that the proposed staging corresponds to the severity of the disease as well as the survival outcome. While prospective studies are required to validate it, this is a breakthrough for a disease that had no previous logical classification or the staging system based on the anatomical progression and disease severity in each anatomical location.
Limitations
The data includes only those cases that were submitted for the study and may not be representative of the actual incidence of COVID-19-associated ROCM in India. The follow up of patients is limited and majority are still under active treatment. The outcome analysis is, therefore, to be interpreted with caution. However, we believe that there is an imminent need for this data for triaging and guiding the management. Further study of this data over time will give a better picture of the true survival outcome. There is no large-scale data on COVID-19 patients who did not develop ROCM to serve as a control group, limiting the scope of determining risk factors. Majority of the contributors were ophthalmic institutions. Since this data was retrospectively collected, and most cases were co-managed by different departments, either in the same institution or separately, all information for every patient was not available at the time of collection. However, in future, it will allow the treating teams to collect information in a more uniform manner based on the understanding of the factors which are important. With information changing at a very rapid pace, this is the most holistic data that we have in hand at present. Information about vaccination, RT-PCR status at the time of diagnosis of mucormycosis, additional surgical procedures like pterygopalatine fossa clearance, maxillectomy, palatal resection and medial orbital wall resection, and timing of intervention was not collected and may be important for future studies.
Conclusion
COVID-19-associated ROCM predominantly affects middle-aged and older males with majority of the patients developing onset of ROCM symptoms between day 10 and day 15 from the diagnosis of COVID-19. Delayed presentation can occur up to three months. Post-COVID-19 follow-up for a period of three months is recommended, possibly in the setting of a formal post-COVID-19 follow-up clinic. DM and corticosteroids are consistent, important, and independent risk factors for COVID-19-associated ROCM. Glycemic control is of paramount importance in a patient with COVID-19. Corticosteroids are part of the armamentarium against COVID-19 but must be used judiciously in only patients with moderate to severe disease as per the dose and duration recommended. In the absence of DM, corticosteroids, and hospitalization, the risk of acquiring ROCM is rare. Future studies should address the issue of healthcare-associated ROCM, including breach in aseptic precautions during hospitalization and air quality control in hospitals as potential risk factors.
Periorbital and facial pain and edema, nasal discharge, ptosis, and loss of vision are the common symptoms and signs. A majority of patients are diagnosed at stage 3, when the orbit is already involved. The common clinical symptoms and signs should be recognized promptly, followed by an expedited diagnosis by diagnostic nasal endoscopy, an endoscopy-guided nasal swab for microbiological evaluation and nasal micro-biopsy for rapid histopathology. Contrast-enhanced MRI is the imaging modality of choice, in the absence of which a CT scan is suggested. ROCM should be staged, triaged, and managed by a team of intensivists, infectious diseases specialists, ophthalmologists/oculoplasty surgeons, otorhinolaryngologists, maxillofacial surgeons, neurosurgeons, radiologists, microbiologists, and pathologists.
Antifungal medications should be initiated empirically upon clinical or clinical–radiological correlation in a symptomatic patient in a COVID-19 setting while awaiting culture and histopathology confirmation. Liposomal amphotericin B is the drug of choice and all efforts must be made to ensure its availability. PNS debridement should be radical and may be repeated as required. In patients with limited orbital involvement (stages 3a and b), intraorbital injection of amphotericin B may be a promising option. Patients with diffuse orbital involvement will need orbital exenteration. Patients with CNS involvement seem to fare better when PNS debridement and orbital exenteration are included in their management. A longer follow-up is essential to determine the prognosis conclusively, but the analysis of our largest series of real-world data does provide some insightful information that may help plan the management of COVID-19-associated ROCM.
COVID-19-associated ROCM needs to be tackled as aggressively as the disease itself with a concerted effort from multidisciplinary medical teams and the government. Accepting the facts that Indians inherently have a higher prevalence of DM, the tropical climate predisposes to mucormycosis, and moderate to severe cases of COVID-19 will need corticosteroids for life salvage, we can expect to continue to see ROCM in the days to come. Logistical preparedness to ensure adequate supply of amphotericin B and creation of well-equipped, dedicated regional hubs of multidisciplinary ROCM-management centres, each connected to spokes of COVID-19-treatment facilities, may help salvage the life and eyes of these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1. [Last accessed on 2021 May 31]. Available from: https://www.nytimes.com/interactive/2021/world/india-covid-cases.html .
- 2.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19:A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021 doi: 10.1016/j.dsx.2021.05.019. doi:10.1016/j.dsx. 2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land:A tale of two pathogens. Indian J Ophthalmol. 2021;69:244–52. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix:Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–8. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–4. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honavar SG. Code Mucor:Guidelines for the Diagnosis, Staging and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19. Indian Journal of Ophthalmology. 2021;69:1361–5. doi: 10.4103/ijo.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. [Last accessed on 2021 Jun 3]. Available from: https://twitter.com/DVSadanandGowda/status/1397441659458125825 .
- 8.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DC, et al. The epidemiology and clinical manifestations of mucormycosis:A systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Prakash H, Ghosh AK, Rudramurthy SM, Singh P, Xess I, Savio J, et al. A prospective multicenter study on mucormycosis in India:Epidemiology, diagnosis, and treatment. Medical Mycol. 2019;57:395–402. doi: 10.1093/mmy/myy060. [DOI] [PubMed] [Google Scholar]
- 10.Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26:944–e9. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis:A review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 12.Chander J, Kaur M, Singla N, Punia RP, Singhal SK, Attri AK, et al. Mucormycosis:Battle with the deadly enemy over a five-year period in India. J Fungi. 2018;4:46. doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5:26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Diabetes Federation. Idf Diabetes Atlas. 2019. [Last accessed on 2021 May 27]. Available from: https://diabetesatlas.org/en/resources/
- 15.Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus:A rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–68. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic:A clinical and diagnostic perspective from China. Mycopathologia. 2020;31:1–8. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayina CA, Haritha D, Soni L, Behera S, Nair PR, Gouri M, et al. Epidemiological and clinical characteristics and early outcome of COVID-19 patients in a tertiary care teaching hospital in India:A preliminary analysis. Indian J Med Res. 2020;152:100–4. doi: 10.4103/ijmr.IJMR_2890_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan A, Tiwari P, Bhatnagar S, Patel A, Maurya A, Dar L, et al. Clinico-demographic profile and hospital outcomes of COVID-19 patients admitted at a tertiary care centre in north India. Indian J Med Res. 2020;152:61–9. doi: 10.4103/ijmr.IJMR_1788_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marimuthu Y, Kunnavil R, Anil NS, Nagaraja SB, Satyanarayana N, Kumar J, et al. Clinical profile and risk factors for mortality among COVID-19 inpatients at a tertiary care centre in Bengaluru, India. Monaldi Arch Chest Dis. 2021 doi: 10.4081/monaldi.2021.1724. doi:10.4081/monaldi. 2021.1724. [DOI] [PubMed] [Google Scholar]
- 20.Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, et al. The Emergence of COVID-19 Associated Mucormycosis:Analysis of Cases From 18 Countries. [Last accessed on 2021 Jun 02]. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3844587 . [DOI] [PMC free article] [PubMed]
- 21.de Souza R, Mhatre S, Qayyumi B, Chitkara G, Madke T, Joshi M, et al. Clinical course and outcome of patients with COVID-19 in Mumbai City:An observational study. BMJ Open. 2021;11:e042943. doi: 10.1136/bmjopen-2020-042943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Last accessed on 2021 May 27]. Available from: https://covid.aiims.edu/clinical-guidance-for- management-of-adult-covid-19-patient s/
- 23.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–38. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 24.Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood. 2011;118:1216–24. doi: 10.1182/blood-2011-03-316430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge:The perfect storm for mucormycosis. J Fungi. 2021;7:298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathish T, Anton MC. Newly diagnosed diabetes in patients with mild to moderate COVID-19. Diabetes Metab Syndr. 2021;15:569–71. doi: 10.1016/j.dsx.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–9. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kothandaraman N, Rengaraj A, Xue B, Yew WS, Velan SS, Karnani N, et al. COVID-19 endocrinopathy with hindsight from SARS. Am J Physiol Endocrinol Metab. 2021;320:E139–50. doi: 10.1152/ajpendo.00480.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bala K, Chander J, Handa U, Punia RS, Attri AK. A prospective study of mucormycosis in north India:Experience from a tertiary care hospital. Med Mycol. 2015;53:248–57. doi: 10.1093/mmy/myu086. [DOI] [PubMed] [Google Scholar]
- 31.Limon JJ, Skalski JH, Underhill DM. Commensal fungi in health and disease. Cell Host Microbe. 2017;22:156–65. doi: 10.1016/j.chom.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulakavalupil B, Vaity C, Joshi S, Misra A, Pandit RA. Absence of Case of Mucormycosis (March 2020–May 2021) under strict protocol driven management care in a COVID-19 specific tertiary care intensive care unit, Diabetes &Metabolic Syndrome:Clinical Research &Reviews (2021) doi: 10.1016/j.dsx.2021.06.006. doi:https://doi.org/10.1016/j.dsx.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9:523. doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S44–54. doi: 10.1093/cid/cir867. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Wu H, Huang F, Fan Z, Xu B. Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clin Nucl Med. 2013;38:e370–1. doi: 10.1097/RLU.0b013e3182867d13. [DOI] [PubMed] [Google Scholar]
- 36.Altini C, Ferrari C, Rubini D, Dicuonzo F, Rubini G. (18) F-FDG PET/CT contribution to diagnosis and treatment response of rhino-orbital-cerebral mucormycosis. Hell J Nucl Med. 2015;18:68–70. doi: 10.1967/s002449910167. [DOI] [PubMed] [Google Scholar]
- 37.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis:An update. J Fungi. 2020;64:265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis) Clin Infect Dis. 2012;54(Suppl 1):S55–60. doi: 10.1093/cid/cir868. [DOI] [PubMed] [Google Scholar]
- 39.Lackner M, Caramalho R, Lass-Flörl C. Laboratory diagnosis of mucormycosis:Current status and future perspectives. Future Microbiol. 2014;9:683–95. doi: 10.2217/fmb.14.23. [DOI] [PubMed] [Google Scholar]
- 40.Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18:845–54. doi: 10.1080/14737159.2018.1522250. [DOI] [PubMed] [Google Scholar]
- 41.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B. Global guideline for the diagnosis and management of mucormycosis:An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–21. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi. 2018;4:90. doi: 10.3390/jof4030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR, 3rd, et al. Isavuconazole treatment for mucormycosis:A single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–37. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 44.Ellsworth M, Ostrosky-Zeichner L. Isavuconazole:Mechanism of action, clinical efficacy, and resistance. J Fungi. 2020;6:324. doi: 10.3390/jof6040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manesh A, John AO, Mathew B, Varghese L, Rupa V, Zachariah A, et al. Posaconazole:An emerging therapeutic option for invasive rhino-orbito-cerebral mucormycosis. Mycoses. 2016;59:765–72. doi: 10.1111/myc.12529. [DOI] [PubMed] [Google Scholar]
- 46.Reed C, Bryant R, Ibrahim AS, Edwards J, Jr, Filler SG, Goldberg R, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47:364–71. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, et al. Combination therapy for mucormycosis:Why, what, and how? Clin Infect Dis. 2012;54(Suppl 1):S73–8. doi: 10.1093/cid/cir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee AS, Lee PW, Allworth A, Smith T, Sullivan TJ. Orbital mycoses in an adult subtropical population. Eye. 2020;34:1640–7. doi: 10.1038/s41433-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirabayashi KE, Kalin-Hajdu E, Brodie FL, Kersten RC, Russell MS, Vagefi MR. Retrobulbar injection of amphotericin B for orbital mucormycosis. Ophthalmic Plast Reconstr Surg. 2017;33:e94–7. doi: 10.1097/IOP.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 50.Safi M, Ang MJ, Patel P, Silkiss RZ. Rhino-orbital-cerebral mucormycosis (ROCM) and associated cerebritis treated with adjuvant retrobulbar amphotericin B. Am J Ophthalmol Case Rep. 2020;19:100771. doi: 10.1016/j.ajoc.2020.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Songu M, Unlu HH, Gunhan K, Ilker SS, Nese N. Orbital exenteration:A dilemma in mucormycosis presented with orbital apex syndrome. Am J Rhinol. 2008;22:98–103. doi: 10.2500/ajr.2008.22.3121. [DOI] [PubMed] [Google Scholar]
- 52.Pelton RW, Peterson EA, Patel BC, Davis K. Successful treatment of rhino-orbital mucormycosis without exenteration:The use of multiple treatment modalities. Ophthalmic Plast Reconstr Surg. 2001;17:62–6. doi: 10.1097/00002341-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Hargrove RN, Wesley RE, Klippenstein KA, Fleming JC, Haik BG. Indications for orbital exenteration in mucormycosis. Ophthalmic Plast Reconstr Surg. 2006;22:286–91. doi: 10.1097/01.iop.0000225418.50441.ee. [DOI] [PubMed] [Google Scholar]
- 54.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D'Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol. 2003;51:231–6. [PubMed] [Google Scholar]