Abstract
To date, the Endophthalmitis Vitrectomy Study (EVS) has remained the hallmark of evidence-based management of acute bacterial endophthalmitis after cataract surgery with an intraocular lens. In the last quarter-century since its publication, several studies have reported that the microbiological spectrum of endophthalmitis is not the same across the world; there is emerging antibiotic resistance of gram-negative microorganisms to the EVS recommended antibiotics; there are newer molecules that could cross the blood-retinal barrier; the advances in vitreous surgery have become safer than before, and there are newer methods of microbiological evaluation. One of the often-mentioned drawbacks of the EVS was not recruiting grossly infected eyes with poor visibility of the iris and vitreous. Keeping these factors in mind, a new prospective multi-centered randomized study, the Endophthalmitis Management Study (EMS), is designed. The EMS will recruit all post-cataract surgery endophthalmitis patients irrespective of severity (including suspected fungal infection); the EMS will use quantifiable inflammatory score instead of the presenting vision to allocate for surgery, randomize the eyes to two different combinations of intravitreal antibiotics and use the newer microbiological diagnostic techniques. We believe the EMS findings will complement the EVS recommendations.
Keywords: Cataract surgery, endophthalmitis, inflammatory score, intravitreal antibiotic, vitrectomy
Postoperative endophthalmitis is a dreaded complication. It could occur after any intraocular surgery, though, by sheer number, endophthalmitis after cataract surgery and intravitreal injection occurs more often. The current treatment strategy is based on the recommendations of the Endophthalmitis Vitrectomy Study (EVS).[1] Although the EVS studied only acute post-cataract surgery/secondary intraocular lens (IOL) bacterial endophthalmitis occurring within six weeks of the primary surgical event, this strategy is also used in other postoperative intraocular surgery and sometimes, even in chronic endophthalmitis. A quarter-century has elapsed since the first publication of the EVS. Many new pieces of evidence have emerged in the world literature on the efficacy and deviations of the EVS protocol, on the regional spectrum of causative microorganisms, antibiotic susceptibility, and advances in vitreous surgery.[2] Probably it is time to revisit the EVS protocol with another prospective study of a similar dimension.
We propose a prospective multi-center study, the Endophthalmitis Management Study (EMS), to determine the changes required in the strategic management of endophthalmitis. The EMS has the objectives to determine the most suitable intravitreal antibiotic for a gram-negative infection, an alternative to ceftazidime, and to develop guidelines to perform vitrectomy based on the inflammatory score at presentation instead of the presenting visual acuity.
Study Protocol
The EMS is a prospective randomized multi-center study of post-cataract surgery acute endophthalmitis with IOL. The study has obtained institutional ethical clearance and is registered in the Clinical Trial Registry of India (CTRI/2019/02/017876). Patients would be recruited from four large tertiary centers at Bhubaneswar, Hyderabad, Vishakhapatnam, and Vijayawada, located in 3 adjoining states of India. The primary intervention is an inflammatory score-based decision for either intravitreal injection alone or vitrectomy. The primary intravitreal antibiotics would be randomized to two different combinations: vancomycin + ceftazidime or vancomycin + imipenem. This randomization will be computer-generated for the entire cohort, irrespective of the study location.
Patient selection
Inclusion criteria: Clinical signs and/or symptoms of endophthalmitis with special reference to four ocular tissues (cornea, anterior chamber, iris, and vitreous; Table 1); cataract surgery with IOL implantation within 6 weeks of the diagnosis; visual acuity from <6/18 to light perception; and written signed consent. A certified optometrist would test the vision; a comprehensive ophthalmologist/retina fellow would do the clinical examination under the supervision of a fellowship-trained retina specialist.
Table 1.
Guidelines for Inflammatory Score
| Tissue | Response | Points | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 1 | 2 | 3 | 4 | ||
| Cornea | Clarity | Clear | Mild | Moderate (iris visible) | Severe (Iris bare details) | Opaque (no iris view) |
| Abscess | None | < 1 mm | 1-2 mm | 3-4 mm | > 5 mm | |
| Anterior Chamber | Flare/Cells | None | Trace | Mild | Moderate | Severe |
| Fibrin/ Hypopyon | None | Mild <25% | Moderate >25% | Severe <75% | No iris view | |
| Iris | Blood Vessels dilated | None | Mild | Moderate | Severe | NVI |
| Exudates over iris | None | Mild <25% | Moderate <50% | Severe <75% | Pupil occluded | |
| Vitreous | Flare | None | Trace | Mild | Moderate | Severe |
| Opacities | None | Cells | Clumps | Red reflex | Opaque | |
Additional scoring: Cornea opaque, add 20; AC opaque, add 15; Pupil fully covered with exudates, add 10; vitreous opaque, add 5
Exclusion criteria: Patients younger than 18 years; one-eyed individual; unfit for surgery; retinal detachment at presentation confirmed by indirect ophthalmoscopy/ultrasonography; and unwilling to sign the consent.
Inflammation scoring [Table 1]
Inflammatory score (IS) will be measured on a scale of 0 (not affected) to 4 (worst affected) in four cardinal ocular tissues afflicted in endophthalmitis- the cornea, anterior chamber, iris, and vitreous, with two cardinal signs. There will be provision for additional scoring when the tissue on evaluation does not allow further assessment of the remaining ocular tissues.[3] The following examples are illustrative:
Example One: (1) Corneal abscess >5 mm (IS-4) and total corneal opacity precluding view of iris and other ocular structure behind (IS- 4), then it will attract additional scoring of 20; thus total IS = 4+ 4 + 20 = 28. Example Two: Mild corneal edema (IS-1) and no corneal abscess (IS-0), mild AC flare (IS-2) and hypopyon <25% (IS-1); no dilated iris vessels (IS-0) and <50% exudates over the iris (IS- 2); Severe vitreous flare or vitreous opacities no view of the disc (IS-4) will attract additional scoring of 5; thus total IS = 1 + 2 + 1 + 2 + 4+ 5 = 15
Treatment [Fig. 1]
Figure 1.
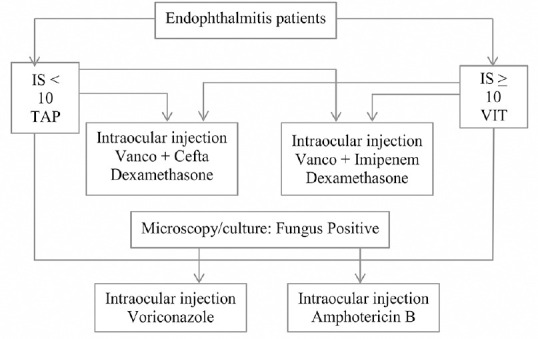
EMS randomization. Cefta- ceftazidime; TAP- vitreous tap (aspiration); Vanco- vancomycin; VIT- vitrectomy
Primary intervention, will be based on the IS as follows:
IS < 10: Vitreous TAP + Intravitreal antibiotics, further randomized to vancomycin + ceftazidime, and vancomycin + imipenem;
IS ≥ 10: Vitreous surgery + Intravitreal antibiotics, further randomized to vancomycin + ceftazidime, and vancomycin + imipenem.
All patients will receive oral ciprofloxacin and intravitreal dexamethasone.
Antifungal agents would be used in microbiology confirmed fungal endophthalmitis; in such a case, the patients would be randomized to intravitreal amphotericin B and voriconazole; all confirmed fungal endophthalmitis patients will also receive oral itraconazole.
EMS medications [Table 2]
Table 2.
Guidelines for EMS medications
| Drugs | Intravitreal (0.1 ml) | Systemic | Topical |
|---|---|---|---|
| Vancomycin | 1.0 mg | - | - |
| Ceftazidime | 2.25 mg | - | - |
| Imipenem | 50 µg | - | - |
| Amphotericin B | 5 µg | - | - |
| Voriconazole | 100 µg | - | - |
| Ciprofloxacin | - | Oral 750 mg q12h x 7 d | 0.3% q 2h x 3 d q 4h x 11d |
| Itraconazole | - | Oral 100 mg q12h x 21d | - |
| Natamycin 5% suspension | - | - | q 2h x 7 d q 4h x 14 d |
| Prednisolone acetate 1% suspension | - | - | q 2h x 7 d q 4 h x 14 d |
| Cyclopentolate 1% | - | - | q 8hx 21d |
The EMS will use drugs via three routes- intravitreal, systemic, and topical. The primary intravitreal injection would be two antibiotics where vancomycin is common, and the patients would be randomized to intravitreal ceftazidime and imipenem. Intravitreal antifungal agents will be injected only in microbiologically proven cases (smear and/or culture positive for fungus). Systemic therapy would be confined to oral route only- ciprofloxacin to begin with and switch over to itraconazole in confirmed fungal infection.
Study sample size and statistical analysis
In calculating the study sample size, two assumptions were made. These were (1) considering IS at presentation for the selection of primary therapy is superior to considering the presenting vision, and (2) near-complete vitrectomy is better than vitreous Tap in improving the outcome of treatment. The following formula was used.
Test of superiority:
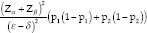
Where: a = 0.05 (5% level of significance); power = 0.8 (80% power); p1 = 0.65 (only Injection); p2 = 0.85 (Injection + Surgery); e = 0.15 (clinically meaningful difference between the two groups); d = 0.05 superiority margin.
Descriptive statistics and exploratory data analysis, two-sample proportion test, and/or Fisher exact test and test of association would be used for data analysis. To obtain 80% of the power with α error of 0.05, the total sample size will be 436 patients.
Microbiology
Four microbiological tests, two traditional and two molecular, would be performed on the vitreous fluid from each patient. These are:
Traditional- Direct microscopy and Culture-Antibiotic Susceptibility;
Molecular- Polymerase Chain Reaction (PCR) + Sanger sequencing and Next Generation Sequencing (NGS).
Approximately 500 ml – 700 ml of undiluted vitreous will be required for microbiological processing; 200 ml of vitreous fluid will be transferred to a microcentrifuge tube and kept at 4°C for molecular analysis; remaining 300-500 ml vitreous sample will be used for microscopy and culture for bacteria and fungus.
Direct Microscopy- Gram stain for bacteria and fungus, and calcofluor white for fungus
Culture-Culture media: 5% sheep blood agar (aerobic and anaerobic), 5% sheep blood chocolate agar, thioglycollate broth, brain-heart infusion broth, and Roberson’s cooked meat broth- all incubated at 37°C for 7 days; Sabouraud dextrose agar and Potato dextrose agar incubated at 25-27°C for 14 days.
Species identification- Bacteria- Vitek 2 Compact (bioMérieux, France) automated identification system for positive bacterial cultures and, if required, DNA sequencing; Fungus- Lactophenol cotton blue staining (LPCB) and DNA sequencing
Antibiotic Susceptibility Testing- Bacteria- E-Test/Vitek for minimum inhibitory concentration (MIC); Fungus- Microbroth Dilution method. The susceptibility tests will be done for the following antibiotics- Gram-positive bacteria: amikacin, gentamicin, tobramycin, chloramphenicol, ciprofloxacin, levofloxacin, ofloxacin, moxifloxacin gatifloxacin, cefuroxime, linezolid, tetracycline, and vancomycin; Gram-negative bacteria: amikacin, gentamicin, chloramphenicol, ciprofloxacin, ceftazidime, gatifloxacin, moxifloxacin, ofloxacin, meropenem, imipenem, colistin, and piperacillin/tazobactam; Fungi: amphotericin B, caspofungin, ketoconazole, natamycin, posaconazole, and voriconazole,
PCR-based DNA sequencing- DNA would be extracted using Qiagen DNA Mini kit from a 200 ml sample. Eubacterial and pan fungal PCR would be carried out for the 16S rDNA region for bacteria[4] and the internal transcribed spacers (ITS), including 5.8S rDNA (ITS1-5.8S rDNA-ITS2) region for fungi, respectively. The PCR product would be purified and sequenced (Sanger method) for species identification.
Next-Generation Sequencing- Next-generation sequencing would be done after amplifying for V3-V4 region of the 16S rRNA gene (DNA extraction by Qiagen DNA Mini kit) for the prokaryotes and the internal transcribed spacers (ITS), including 5.8S rDNA (ITS1-5.8S rDNA-ITS2) region for the eukaryotes and then subjected to Hi-Seq sequencing on the Illumina platform.[5]
Surgical interventions
Vitreous Tap (TAP): Under aseptic conditions, a pars plana stab incision with a 23- gauge needle attached to a 2-ml syringe will be made in the superotemporal quadrant of the eye, and undiluted mid vitreous will be aspirated using mechanical suction. About 0.7-1.0 ml of the vitreous sample would be collected for microbiologic evaluation. AC TAP, when required, would be done using a 26 G needle under aseptic precaution. The needle would be kept with bevel down over the iris, and aqueous would be aspirated into an attached tuberculin syringe. The syringe hub would be fixed with a silicon stopper to prevent air entry and sent under a sterile condition to the microbiology laboratory.
Vitrectomy (VIT): All cases allocated to the vitrectomy group will undergo a standard 3-port pars plana vitrectomy. Three ports will be made 3.5 mm behind the limbus, and infusion cannula will be secured inferotemporally. Using a 23G/25G system, pars plana vitrectomy would be performed. The aim would be to debulk the vitreous, the endpoint being visibility of the optic disc and the first-order vessels of the retina. Vitrectomy would be extended up to mid periphery, up to the vortex vein. Posterior vitreous detachment would not be actively induced. A complete vitrectomy would be done if a retinal detachment occurs during surgery, followed by an endolaser and silicone oil injection as deemed appropriate.
Endoscopic vitrectomy will be done in cases with poor corneal clarity, using the 20/23G endoscope (E2 Laser and Endoscopy System; EndoOptiks, Inc) with light and video dual function.[6]
Intravitreal injection: All antibiotic injections will be freshly constituted at the time of injection after the vitreous TAP or VIT procedure. Under the aseptic condition, antibiotics would be injected from the pars plana region 3.5 mm behind the limbus. Each antimicrobial agent would be loaded in different syringes. The EMS intravitreal antibiotic preparation schedule is shown in Table 3. All patients would be initially randomized for intravitreal antibiotics. On microbiology confirmation of fungal infection, the patients would be randomized to receive intravitreal amphotericin B and voriconazole [Fig. 1].
Table 3.
Intravitreal antibiotic preparation of EMS drugs
| Molecule | Volume | Add DW | Take | Add RL | The final dose in 0.1 ml |
|---|---|---|---|---|---|
| Ceftazidime | 250 mg | 1 ml | 0.1 ml | 0.9 ml | 2.25 mg |
| Vancomycin | 500 mg | 10 ml | 0.2 ml | 0.8 ml | 1 mg |
| Imipenem | 500 mg | 10 ml* | 0.1 ml | 0.9 ml double dilution | 50 µg |
| Amphotericin B | 50 mg | 10 ml | 0.1 ml | 0.9 ml double dilution | 5 µg |
| Voriconazole | 200 mg | 20 ml | 0.1 ml | 0.9 ml | 100 µg |
*NS - normal saline; DW - distilled water; RL - Ringer’s lactate. Double dilution- the first dilution method is repeated
Repeat intervention
Repeat intervention will be directed as per microbiological work up- culture and antibiotic susceptibility. Unless resistant to the specific antibiotic, the eyes with gram-positive cocci infection will receive repeat intravitreal vancomycin + dexamethasone. The eyes with gram-negative bacilli infection will receive intravitreal ceftazidime/imipenem + dexamethasone as per the initial randomization. Another suitable antibiotic will be injected if the organism is resistant to vancomycin (gram-positive cocci) or ceftazidime/imipenem (gram-negative bacilli). In cases of fungal infection, intravitreal amphotericin B shall be given.
The need for repeat intervention will be determined every 48-72 hours. For the vitreous TAP group, repeat intravitreal injections shall be given at 48-72 hours till disc and first-order vessels are visible or till minimal echoes persist on the B scan ultrasound (on a comparable gain setting) in cases with poor anterior segment visibility. For the VIT group, repeat intravitreal injections along with a lavage procedure, if required, shall be done at 48-72 hours till disc and first-order vessels are visible or till minimal echoes persist on the B scan ultrasound (on a comparable gain setting) in cases with poor anterior segment visibility.
The TAP group of eyes would be converted to the VIT group if a second intravitreal injection fails, judged by an Inflammatory score (>10). An inflammatory score of 4 or less will be considered as a sign of resolution of endophthalmitis.
The treatment protocol is illustrated in Fig. 2.
Figure 2.
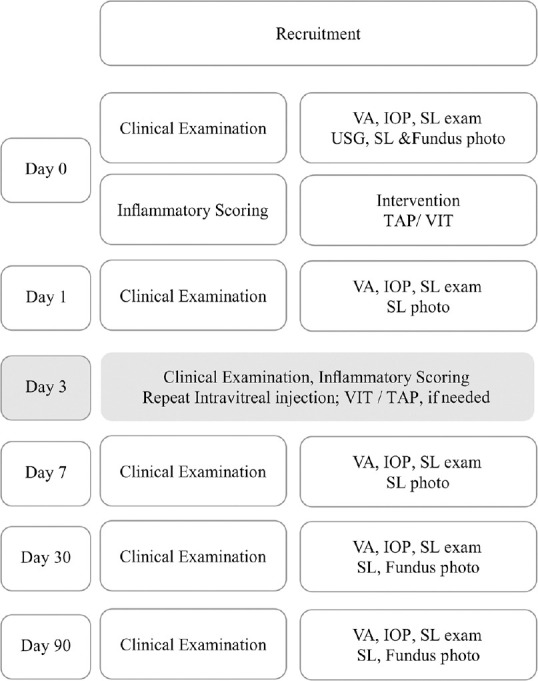
EMS treatment protocol. IOP- intraocular pressure; SL- Slitlamp; VA- visual acuity. Day 3 (shaded boxes)- Repeat intervention, when considered
Ophthalmic examinations
Visual Acuity will be measured using a standardized chart (ComPlog)[7] placed at 4 meters, failing which at 1 meter, failing which at closer distances. Refraction and subjective correction will be tried at day 30 and day 90 visits. The fellow eye would be occluded during vision testing.
Testing of count Finger Vision. Examiner’s fingers (1, 2, or 5) will be viewed at a distance of 2 feet. The fellow eye will be closed. The lamp used for near vision testing will be directed to the hand and fingers from behind the patient’s head.
Testing of Hand motions (HM) vision. Examiner’s hand, with all the fingers extended and separated, will be viewed at a distance of 1 foot. The fellow eye will be closed. The lamp used for near vision testing should be directed to the hand and fingers from behind the patient’s head.
Testing Light Perception (LP). An indirect ophthalmoscope at the highest illumination will be used as the light source. The fellow eye closed, the light will be placed at 3 feet away and directed in and out of the eye from 4 different directions, up and down, right and left.
Intraocular pressure (IOP) will be measured in both eyes using Goldmann applanation tonometer (GAT). It will be estimated in the unaffected eye first. When GAT is not possible, the pressure will be measured digitally using the index fingers of both hands.
Clinical examination of the anterior segment will be done using a slit lamp. Records would be made for the conjunctiva (injection, wound dehiscence/leak and for any bleb), the cornea (clarity and abscess), anterior chamber (flare-cells, and fibrin-hypopyon), iris (dilated vessels and exudative membrane), and vitreous (flare and opacities). In the events of total corneal opacity precluding further examination of the eye, the presence/absence of a red glow will be elicited.
B-scan Ultrasonography will be done when the ocular media prevent evaluation of the vitreous and retina. The test will be done with a 10 MHz probe placed over the closed lids, the patient either in sitting or in a semi-supine position. The contact gel will be placed on the probe, and the indicator of the probe will be placed superiorly. The first image will be taken with the optic nerve in the center, and after that, the probe will be angled in four cardinal directions. Subsequently, the indicator of the probe will be sequentially positioned nasally, temporally, and inferiorly sequentially to map the entire vitreous and retina. The images will be stored in the electronic medical record system.
Photo documentation of the anterior segment will be done with a slit lamp camera; At least two images will be taken- slit view and diffuse illumination view. Dilated fundus photographs will be taken by a 45° retinal camera; 4 areas will be imaged and coalesced [Fig. 3].
Figure 3.
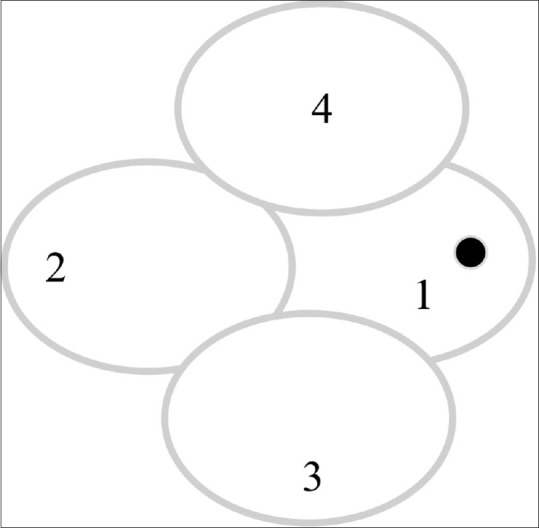
Fundus photograph scheme (Right eye). 1- Disc nasally; 2- Temporal to macula; 3- Inferior to macula; 4- Superior to macula. (mirror image for the left eye)
Outcome measures
Primary: VA at one and three months: ≥20/200.
Secondary Outcome: (1) Decrease in Inflammatory score; (2) Retinal detachment at any time within 3 months; (3) Hypotony <5 mm Hg between 1- 3 months.
Discussion
Over two decades, the EVS has remained the seminal study in post-cataract surgery bacterial endophthalmitis. It was the first evidence-based treatment strategy and has benefitted patients and care providers for over a quarter of a century. Despite this legacy, there are limitations.[8] These are:
The EVS recommendations on the choice of treatment strategy based on the presenting vision may not always reflect the severity of inflammation.
The EVS recommended core vitrectomy only in the eye with presenting vision of LP or worse. Over the years, with the advent of newer technology of small gauge vitrectomy and superior fluidics, there is greater safety of vitreous surgery than when the EVS was designed. The benefit of vitrectomy could be higher in more virulent infections because of the mechanical removal of bacteria and toxins from the eye irrespective of the presenting vision.
The EVS restricted the choice of systemic antibiotics to only amikacin and ceftazidime. The study did not recommend other antibiotics such as systemic fluoroquinolones that have good intraocular penetration in an inflamed eye.
The EVS used oral corticosteroid one day after the intravitreal antibiotic injections but did not use intravitreal corticosteroid (dexamethasone) and thus denied the possibility of early control of inflammation associated with the bacterial infection.
The rationale of the EMS
Inflammatory- score-based treatment choice. Inflammation is the basic pathologic response to the vitreous infection in endophthalmitis. The inflammatory cascade activated by the specific toxic effects of the pathogens ultimately determines the final anatomical and visual outcome.[9] It is unclear whether the most severe damage to the visual function is caused by the infectious process or by the host immune response. In EMS, we have chosen to use an inflammatory score, quantified and used by us earlier.[3]
Vitrectomy. The Complete and Early Vitrectomy (CEVE) study proposed that if the eye with good red reflex or with some retinal visibility does not benefit from intravitreal therapy (antibiotics and corticosteroid) in 24 hours, it should receive a complete vitrectomy regardless of visual acuity.[10] A complete vitrectomy includes separation of posterior hyaloid in the posterior pole and staying short of the periphery. The rationale of complete vitrectomy is that it reduces the inflammatory debris in the vitreous cavity and reduces macular complications. Technically, the 20G vitrectomy available during the EVS study period (1990- 1995) was not as safe as the currently used 23G and 25G vitrectomy system.[11] Aided with the wide-angle viewing system, vitrectomy is possible more often today than recommended in the EVS and is likely to be more advantageous. In the EMS, we have chosen to perform vitrectomy till the mid periphery and not actively induce posterior vitreous detachment. We would also perform an endoscopic vitrectomy when indicated.[6]
Systemic Antibiotics. The good bioavailability of fluoroquinolone (ciprofloxacin 1500 mg, moxifloxacin 800 mg, gatifloxacin 800 mg, daily in 2 divided doses) achieves intravitreal drug concentrations exceeding the minimum inhibitory concentration (MIC90) for most bacteria responsible for endophthalmitis.[12,13,14] In the EMS, we have chosen to administer oral ciprofloxacin (750 mg twice a day for 7 days) in all patients. In microbiologically proven cases of fungal endophthalmitis, the systemic antibiotic will be shifted to oral itraconazole (100 mg twice a day for 21 days, after baseline renal function tests).
Intravitreal dexamethasone. In a prospective randomized study, we have shown that intravitreal dexamethasone reduces the inflammatory component of postoperative endophthalmitis without adversely impacting the final visual outcome at 12 weeks.[3] Other studies have confirmed the benefits of concurrent intravitreal antibiotics and corticosteroid (dexamethasone and triamcinolone) injection in endophthalmitis, though one study did not confirm such benefit.[15,16,17,18,19] In the EMS, all patients will receive intravitreal dexamethasone (400 μg) as the primary therapy. It will be repeated, if indicated, except in microbiologically proven fungal infection (switched to antifungal agents and randomized to amphotericin B and voriconazole)
Microbiology and Intravitreal Antibiotics. The spectrum of infecting microorganisms in endophthalmitis has not changed much in the last quarter-century. The gram-positive cocci (particularly coagulase-negative Staphylococcus) is the predominant organism, though, proportionately, it is not as high in Asia and India as reported from Europe and the USA.[2] In the last quarter-century, antibiotic susceptibility has changed for gram-negative bacteria, from ceftazidime to imipenem.[20,21,22] Ciprofloxacin, a second-generation fluoroquinolone, possesses excellent activity against gram-negative bacteria. But, we did not consider this antibiotic because of its short half-life (t1/2) in vitreous and the need to repeat every 12 hours.[23]
The technology of detecting microorganisms and testing antibiotic susceptibility has changed from traditional biochemical tests/culture (of the vitreous fluid), and antibiotic susceptibility testing has been upgraded from Kirby Bauer technique to MIC determination using E-test/Vitek.[5] The EMS would use these modern microbiology methods in the study (including NGS), which promise to provide greater insight into the type of organisms associated with postoperative endophthalmitis.
Conclusion
Compared to EVS, the EMS is a study closer to the real-world situation by virtue of its recruitment design. The EVS recruited patients with sufficient clarity of cornea and anterior chamber and excluded eyes with a strong suspicion of fungal infection.[1] Thus it is intuitive to believe that many eyes with more virulent (gram-negative) and fungal infection were not recruited. We and others have shown a sufficiently large load of gram-negative bacteria and fungi in postoperative endophthalmitis in India and Asia.[2] We believe that the EMS findings will answer some of the unanswered questions of the EVS and complement the EVS recommendations.
EMS Working Group
Bhubaneswar LVPEI- Umesh Behera, Hyderabad LVPEI- Md. Hasnat Ali, Taraprasad Das (PI), Anthony Vipin Das, Vivek P Dave (PI), CP Deepa, Avantika Dogra, Subhadra Jalali, Joveeta Joseph, Raja Narayanan, Savitri Sharma, Vijayawada LVPEI- Sameera Nayak, Visakhapatnam LVPEI- Prabhjot K Multani.
Financial support and sponsorship
Hyderabad Eye Research Foundation, Hyderabad, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Endophthalmitis Vitrectomy Study Group. Results of the endophthalmitis vitrectomy study:A randomized trial of immediate vitrectomy and intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–96. [PubMed] [Google Scholar]
- 2.Das T. Redefining evidence in the management of acute post-cataract surgery endophthalmitis in India-The 2014 Adenwalla Oration, All India Ophthalmological Society. Indian J Ophthalmol. 2017;65:1403–6. doi: 10.4103/ijo.IJO_755_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das T, Jalali S, Gothwal VK, Sharma S, Naduvilath TJ. Intravitreal dexamethasone in exogenous bacterial endophthalmitis:Results of a prospective randomized study. Br J Ophthalmol. 1999;83:1050–5. doi: 10.1136/bjo.83.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy GSN, Agarwal RK, Matsumoto GI, Shivaji S. Arthrobacter flavus sp. nov., a psychrotropic bacterium isolated from a pond in Mc Murdo Dry Valley, Antarctica. Int J Syst Evol Microbiol. 2000;50:1553–61. doi: 10.1099/00207713-50-4-1553. [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh D, Joseph J, Chakrabarti M, Sharma S, Jayasudha R, Sama KC, et al. New insights into culture negative endophthalmitis by unbiased next generation sequencing. Sci Rep. 2019;9:844. doi: 10.1038/s41598-018-37502-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dave VP, Pappuru RR, Tyagi M, Pathengay A, Das T. Endoscopic vitrectomy in endophthalmitis:Initial experience of 33 cases at a tertiary eye care center. Clin Ophthalmol. 2019;13:243–51. doi: 10.2147/OPTH.S185716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laidlaw DA, Tailor V, Shah N, Atamian S, Harcourt C. Validation of a computerized logMAR visual acuity measurement system (COMPlog):Comparison with ETDRS and electronic ETDRS testing algorithm in adults and amblyopic children. Br J Ophthalmol. 2008;92:241–4. doi: 10.1136/bjo.2007.121715. [DOI] [PubMed] [Google Scholar]
- 8.Flynn HW, Scott IU. Legacy of the endophthalmitis vitrectomy study. Arch Ophthalmol. 2008;126:59–61. doi: 10.1001/archopht.126.4.559. [DOI] [PubMed] [Google Scholar]
- 9.Vallejo- Garcia JL, Asencio-Duran M, Pastora-Salvador N, Vinciguerra P, Romano MR. Role of inflammation in endophthalmitis. Mediators Inflamm. 2012;2012:196094. doi: 10.1155/2012/196094. doi:10.1155/2012/196094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn F, Gianpaolo G. Essentials in Ophthalmology. Spinger; 2007. Complete and early vitrectomy for endophthalmitis (CEVE) as today's alternative to endophthalmitis vitrectomy study .in Vitreo retinal surgery; pp. 53–68. [Google Scholar]
- 11.Mohamed S, Claes C, Tsang CW. Review of small gauge vitrectomy:Progress and innovations. J Ophthalmol. 2017;2017:6285869. doi: 10.1155/2017/6285869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariprasad SM, Shah GK, Mieler WF, Feiner L, Blinder KJ, Holecamp NM, et al. Vitreous and aqueous penetration of orally administered moxiflooxacin in humans. Arch Ophthalmol. 2006;124:178–82. doi: 10.1001/archopht.124.2.178. [DOI] [PubMed] [Google Scholar]
- 13.Rajpal, Srinivas A, Azad RV, Sharma YR, Kumar A, Satapathy G, et al. Evaluation of vitreous levels of gatifloxacin after systemic administration in inflamed and non-inflamed eyes. Acta Ophthalmol. 2009;87:648–52. doi: 10.1111/j.1755-3768.2008.01323.x. [DOI] [PubMed] [Google Scholar]
- 14.El Baba FZ, Trousdale MD, Gauderman WJ, Wagner DJ, Liggertt PE. Intravitreal penetration of oral ciprofloxacin in humans. Ophthalmology. 1992;99:483–6. doi: 10.1016/s0161-6420(92)31943-8. [DOI] [PubMed] [Google Scholar]
- 15.Gan IM, Ugahary LC, van Dissel JT, Feron E, Peperkamp E, Veckeneer M, et al. Intravitreal dexamethasone as adjuvant in the treatment of postoperative endophthalmitis:A prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2005;243:1200–5. doi: 10.1007/s00417-005-0133-1. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht E, Richards JC, Pollock T, Cook C, Myers L. Adjunctive use of intravitreal dexamethasone in presumed bacterial endophthalmitis:A randomised trial. Br J Ophthalmol. 2011;95:1385–8. doi: 10.1136/bjo.2010.187963. [DOI] [PubMed] [Google Scholar]
- 17.Falk NS, Beer PM, Peters GB., III Role of intravitreal triamcinolone acetonide in the treatment of postoperative endophthalmitis. Retina. 2006;26:545–8. doi: 10.1097/00006982-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Pathengay A, Shah GY, Das T, Sharma S. Intravitreal triamcinolone acetonide in the management of exogenous bacterial endophthalmitis. Am J Ophthalmol. 2006;141:938–40. doi: 10.1016/j.ajo.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 19.Shah GK, Stein JD, Sharma S, Sivalingam A, Benson WE, Regillo CD, et al. Visual outcomes following the use of intravitreal steroids in the treatment of postoperative endophthalmitis. Ophthalmology. 2000;107:486–9. doi: 10.1016/s0161-6420(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 20.Ambiya V, Das T, Sharma S, Chhablani J, Dave V, Jalali S, et al. Comparison of clinico-microbiological profile and treatment outcome of in-house and referred post cataract surgery endophthalmitis in a teaching tertiary care center in south India. J Ophthalmic Inflamm Infect. 2016;6:45. doi: 10.1186/s12348-016-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave VP, Pathengay A, Nishant K, Pappuru RR, Sharma S, Narayanan R, et al. Clinical presentations, risk factors, and outcome of ceftazidime- resistant gram-negative endophthalmitis. Clin Exp Ophthalmol. 2017;45:254–60. doi: 10.1111/ceo.12833. [DOI] [PubMed] [Google Scholar]
- 22.Joseph J, Sontam B, Madhuri G, Sharma S, Tyagi M, Dave V, et al. Trends in microbiological spectrum of endophthalmitis in India:A review of 25 years. Eye. 2019;33:1990–5. doi: 10.1038/s41433-019-0380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radhika M, Mithal K, Bawdekar A, Dave V, Jindal A, Relhan N, et al. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J Ophthalmic Inflamm Infect. 2014;4:22. doi: 10.1186/s12348-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


