Abstract
Purpose:
This study aimed to examine the corneal endothelial morphology and thickness in patients with Type 2 diabetes mellitus (T2DM) and compare them with age and sex-matched nondiabetic controls.
Methods:
This hospital-based cross-sectional observational study was conducted in the ophthalmology department of a tertiary hospital consisting of 262 patients (131 with T2DM as cases and 131 without diabetes who served as controls). All patients underwent a comprehensive ocular examination including visual acuity, slit-lamp biomicroscopy, intraocular pressure measurement. Central corneal thickness (CCT), endothelial cell density (ECD), coefficient of variance (CV), and percentage of hexagonal cells (HEX) were compared between the cases and controls. Predictors of corneal endothelial dysfunctions were analyzed. Data analysis was done by Statistical Package for the Social Sciences (SPSS) version 17.0. Chi-square test, Fisher’s exact test, and Spearman’s rho correlation analysis were used as appropriate.
Results:
Patients with T2DM showed poorer visual acuity and higher intraocular pressure. As compared to controls, patients with T2DM had thicker CCT, lesser ECD, decreased HEX, and higher CV but the differences were statistically nonsignificant. HbA1c levels showed a significant positive correlation with CCT and CV and a negative correlation with ECD. Macroalbuminuria and higher albumin creatinine ratio was associated with an increase in CV in patients with T2DM.
Conclusion:
Our study showed that poorly controlled patients with T2DM and those with macroalbuminuria have corneal endothelial abnormalities.
Keywords: Corneal endothelium, macroalbuminuria, specular microscopy, type 2 diabetes
Type-2 diabetes mellitus (T2DM) is a major public health problem worldwide and is fast gaining the status of a potential epidemic in India.[1] Various studies have reported structural, functional, and biochemical alteration in endothelial cells due to diabetes mellitis (DM).[2,3,4,5] These changes are known to cause endothelial dysfunction which is well known in type-1 diabetes mellitus (T1DM), whereas studies on corneal dysfunction in T2DM have reported variable results.[6,7,8,9,10,11] Corneal endothelial cells are critical to maintain the hydration and clarity of the cornea. These cells are known to decrease with age at a rate of 0.5% per year.[12] As patients with T2DM are usually more than 40 years of age, there may also be an additional effect of age-related corneal dysfunction. Therefore, in interpreting the corneal endothelial dysfunctions in T2DM, age must be taken into consideration.[13] Moreover, it has been emphasized that many elderly patients with T2DM who undergo cataract surgery may be at further risk of endothelial damage.[14,15,16,17] Hence, exploring the role of corneal endothelial functions in patients with T2DM is needed. Further, complications of T2DM such as diabetic retinopathy (DR) and diabetic nephropathy (DN) can also have an impact on corneal endothelial functions. Only a handful of studies have looked into these associations.[18,19,20,21] Therefore, we devised this study to find out corneal endothelial dysfunctions in a large cohort of patients with T2DM and compared them with normal controls. We also studied the impact of complications of T2DM such as DR and DN on the health of corneal endothelium in these patients.
Methods
This was a hospital-based, cross-sectional, observational study conducted from July 2018 to June 2019 in a tertiary care center. One hundred and ninety-four consecutive diabetes patients and 154 nondiabetic patients visiting the ophthalmology outpatient clinic were screened and those fulfilling the inclusion and exclusion criteria were included in the study.
Inclusion criteria for cases
Patients aged more than 40 years with an established diagnosis of T2DM based on ADA criteria[22] were included in the study.
Inclusion criteria for controls
The control group comprised of age and sex-matched non-DM patients visiting the eye OPD for correction of refractive error.
Exclusion criteria for both cases and controls
Patients with active and previous ocular infection or inflammation, previous ocular surgery or trauma, retinal photocoagulation, glaucoma, underlying corneal disease, ocular surface disorders, adnexal diseases, contact lens wear, and regular usage of any eye drops or known tear interfering systemic drugs, such as hormone replacement and antihistamines were excluded. Patients with a history of hypertension, systemic illness known to impair tear function and kidney function such as rheumatoid arthritis and systemic lupus erythematosus was also excluded.
Basic demographic features such as age and sex of cases and controls were noted. All the patients underwent blood pressure measurement. Baseline biochemical tests included blood sugar (fasting and postprandial), glycosylated hemoglobin (HbA1c), and serum creatinine and urine albumin creatinine ratio (ACR) was done in all patients. Because of the inconvenience of 24-h urine collection, the spot ACR was adopted to estimate urine albumin excretion rates. The value of ACR used to grade the albuminuria was as follows: Normal ≤30 mg/mg, microalbuminuria (30-300 mg/mg), and macroalbuminuria (>300 mg/mg).[23]
Ophthalmological examination
All patients underwent a comprehensive ocular examination including visual acuity, slit-lamp examination, intraocular pressure measurement using Goldman applanation tonometer, dilated fundus examination with a direct ophthalmoscope and 90D lens. Slit-lamp examination was performed with particular attention to any evidence of dry eye, anterior segment infection, and inflammation. Early treatment diabetic retinopathy study (ETDRS) classification was followed to grade the retinopathy.[24] Specular microscopy was done prior to applanation tonometry. Corneal endothelial structure and central corneal thickness (CCT) were examined by noncontact specular microscopy using Topcon Specular Microscope (SP-1P model). The endothelial morphologic parameters studied were endothelial cell density (ECD), coefficient of variance (CV), percentage of hexagonal cells (HEX). Right eye findings were considered for statistical calculation in both groups to maintain uniformity and avoid confusion. Institutional ethical clearance was taken for the study. Written informed consent was taken from all the patients included in the study.
Statistical analysis
Statistical testing was conducted with the Statistical Package for the Social Sciences (SPSS) version 17.0. Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as absolute numbers and percentages. The comparison of normally distributed continuous variables between the groups was performed using Student’s t test. Nominal categorical data between the groups were compared using the Chi-square test or Fisher’s exact test as appropriate. Spearman’s rho correlation test was applied to find the relationship between corneal changes and duration of diabetes mellitus, HbA1c % (glycemic status), DN, and severity of DR. A value of P ≤ 0.05 was considered statistically significant.
Results
One hundred and ninety-four consecutive diabetes patients and 154 nondiabetic patients were screened for the study from the ophthalmology outpatient clinic of a tertiary hospital. Sixty-three diabetes patients and 33 nondiabetic cases were excluded due to various reasons and finally a total of 262 patients were recruited for the study consisting of 131 patients with T2DM and 131 healthy patients without DM as controls [Fig. 1].
Figure 1.
Flowchart of recruitment of participants of the study
Comparisons between cases and controls
The demography, clinical features, and baseline biochemical test results are shown and compared in Table 1. Both the cases and the controls were matched for age, sex (P = 0.621 and P = 0.387). The best-corrected visual acuity (BCVA) was significantly lower (P = 0.002) and the mean intraocular pressure was significantly higher in the cases as compared to controls (P < 0.001).
Table 1.
Demography and baseline investigations in cases and controls
| Parameters | Case (mean±standard deviation) | Control (mean±standard deviation) | P |
|---|---|---|---|
| Age (years) | 53.26±6.24 | 53.66.± 6.73 | 0.621 |
| Sex (F/M) | 62/69 | 62/69 | 0.387 |
| Visual acuity (decimal) | 0.55±0.29 | 0.63±0.24 | 0.007 |
| IOP (mm of Hg) | 17.17±3.26 | 14.30.± 2.70 | <0.001 |
| Blood sugar Fasting (mg/dl) | 112.98±17.59 | 91.52±10.23 | <0.001 |
| Blood sugar Post prandial (mg/dl) | 185.09±54.46 | 135.24.± 5.56 | <0.001 |
| HbA1c (%) | 6.97±0.99 | 5.58±0.42 | <0.001 |
| Duration of DM (years) | 7.29±6.00 | - | - |
| S creatinine (mg/dl) | 1.33±0.504 | - | - |
| Albumin- creatinine ratio (ACR) (mg/dl) | 380.51±172.26 | - | - |
Differences in mean values of CCT, CV, ECD, and HEX between the cases and controls were not statistically significant [Table 2]. However, cases had higher mean CCT (514.54 ± 38.17 μm vs. 511.65 ± 32.64 μm), higher mean CV (31.60 ± 3.51% vs. 30.85 ± 6.68%), lower mean ECD (2762.08 ± 320.21 cells/mm2 vs. 2771.46 ± 336.7 cells/mm2) and lower mean percentage of HEX (55.34 ± 5.82% vs. 56.94 ± 7.00%) as compared to the controls. Representative photographs of corneal morphology of cases and controls are shown in Figs. 2 and 3.
Table 2.
Corneal endothelial measures in cases and controls
| Corneal endothelial measures | Cases Mean±SD | Controls Mean±SD | P |
|---|---|---|---|
| CCT (µm) | 514.54±38.17 | 511.65±32.64 | 0.510 |
| CV (%) | 31.60±3.51 | 30.85±6.68 | 0.256 |
| ECD (cells/mm2) | 2762.08±320.21 | 2771.46±336.7 | 0.818 |
| HEX (%) | 55.34±5.82 | 56.94.± 7.00 | 0.145 |
Figure 2.
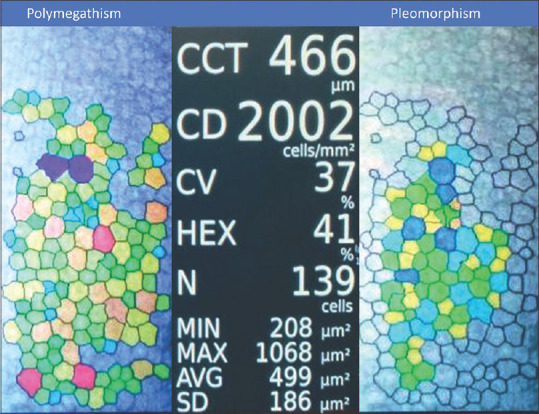
Specular microscopy findings in a 65-year-old female with type-2 diabetes
Figure 3.

Specular microscopy findings in a 60-year-old female without diabetes
Assessment of corneal endothelial morphology within the cases
Based on disease duration
We assessed the duration of T2DM in cases and studied its impact on the corneal endothelium. For this purpose, we divided them into groups, namely those who had T2DM for less than or up to 10 years and those with disease duration of more than 10 years. One hundred and two (77.9%) patients had disease duration of less than or equal to 10 years whereas 29 patients had more than 10 years of diabetes. These two groups did not have any statistically significant difference in terms of CCT, CV, ECD, and HEX [Table 3].
Table 3.
Comparison of corneal endothelial measures based on duration of DM
| Corneal endothelial measures | T2DM duration≤10 yrs (n=102) Mean±SD | T2DM duration >10 yrs (n=29) Mean±SD | P |
|---|---|---|---|
| CCT (µm) | 513.06±38.95 | 519.76±35.46 | 0.406 |
| CV (%) | 31.49±3.52 | 31.97±3.48 | 0.521 |
| ECD (cell/mm2) | 2762.82±316.48 | 2759.48±338.75 | 0.961 |
| HEX (%) | 55.64±5.76 | 54.31±6.01 | 0.280 |
Based on diabetic complications
Among the cases, DR was seen in 47/131 (35.9%) patients of which 33 (70.21%) patients had nonproliferative diabetic retinopathy (NPDR), and 14 (29.79%) had proliferative diabetic retinopathy (PDR). In the NPDR group, mild NPDR was seen in 21 patients, moderate NPDR was seen in 5 patients, severe NPDR in 5, and very severe NPDR in 2 patients. We categorized patients with T2DM as no DR, nonproliferative DR, and proliferative DR and compared the endothelial functions among these three groups. Again, no statistically significant difference was observed between the three groups [Table 4].
Table 4.
Comparison of corneal endothelial measures based on severity of diabetic retinopathy
| Corneal endothelial measures | No DR (n=84) Mean±SD | NPDR (n=33) Mean±SD | PDR (n=14) Mean±SD | P |
|---|---|---|---|---|
| CCT (µm) | 516.83±38.34 | 509.76±37.55 | 512.07±40.21 | 0.648 |
| CV (%) | 31.16±3.37 | 31.19±3.61 | 31.21±4.15 | 0.521 |
| ECD (cell/mm2) | 2779.9±327.27 | 2718.15±298.2 | 2758.71±340.08 | 0.647 |
| HEX (%) | 55.95±5.83 | 54.36±5.30 | 55.09±6.99 | 0.536 |
DN was categorized into two groups, namely microalbuminuria and macroalbuminuria. Thirty-three patients had micro-albuminuria and 98 patients had macro- albuminuria. ECD, HEX, and CCT did not differ between the two groups; however, the mean CV was significantly higher in the group with macroalbuminuria (P = 0.012) [Table 5].
Table 5.
Comparison of corneal endothelial measures based on urinary ACR level
| Corneal endothelial measures | Urinary ACR level (30-300) µg/mg (n=33) | Urinary ACR level (>300) µg/mg (n=98) | P |
|---|---|---|---|
|
|
|
||
| Mean±SD | Mean±SD | ||
| CCT (µm) | 523.03±32.78 | 530.27±3.26 | 0.140 |
| CV (%) | 30.27±3.26 | 32.04±3.49 | 0.012 |
| ECD (cell/mm2) | 2748.94±409.45 | 2766.51±286.41 | 0.786 |
| HEX (%) | 56.67±8.00 | 54.90±4.84 | 0.131 |
Predictors of corneal endothelial dysfunctions in cases with T2DM
Spearman’s rho correlation analysis suggested no significant correlation between corneal morphological parameters with age, duration of DM and severity of DR. However, the HbA1c levels showed significant correlation with ECD (r = –.243; P = 0.005), CV (r = 0.319, P < 0.001) and CCT (r = 0.326; P < 0.001) [Table 6]. Endothelial cell hexagonality correlated significantly with ACR (r = –.196; P = 0.025).
Table 6.
Correlation of corneal endothelial measures with HbA1c, duration of diabetes, diabetic retinopathy and nephropathy
| Corneal endothelial measures | CCT | CV | ECD | HEX | |
|---|---|---|---|---|---|
| HbA1c (%) | r | 0.326 | 0.319 | -0.243 | -0.106 |
| p | <0.001 | <0.001 | 0.005 | 0.227 | |
| DOD (yrs) | r | 0.072 | 0.047 | 0.071 | -0.073 |
| p | 0.412 | 0.596 | 0.421 | 0.41 | |
| ACR | r | 0.402 | 0.039 | -0.066 | -0.196 |
| p | 0.091 | 0.662 | 0.457 | 0.025 | |
| DR | r | 0.081 | 0.08 | -0.071 | -0.087 |
| p | 0.359 | 0.362 | 0.420 | 0.320 | |
Discussion
This study was conducted to examine the corneal endothelium in patients with T2DM and to find the factors that correlate with endothelial changes. We found no significant difference in the corneal morphology and central corneal thickness between the T2DM group and control group, though there was a nonsignificant trend toward increased central corneal thickness, decreased cell density and increased polymegathism and pleomorphism in patients with T2DM. The duration of diabetes and presence and grade of retinopathy did not affect the corneal endothelium. However, patients with T2DM having macroalbuminuria had significant cell variance. The control status of blood sugar correlated significantly with corneal endothelial morphology and ACR correlated with cell hexagonality. Our results are broadly consistent with several previously published studies,[9,10,18,25,26] and showed some novel findings also.
Hexagonality and cell variance
We did not find any difference in cell hexagonality and cell variance between cases and controls which is consistent with some previous studies.[18,25,26] The effect of diabetes on corneal morphology apparently differs depending on the type of diabetes. Studies done on T1DM have concluded that corneal morphology and thickness are altered significantly from nondiabetic controls.[8,9,27] The changes observed in these eyes were similar to that occurring with age namely an increase of CV and decrease in HEX%.[13] Age may be a confounder in patients with T2DM as most of these patients are above the age of 40 years and hence the difference between the cases and age-matched controls may be masked as suggested by some authors.[9,11]
Endothelial cell density
ECD reflects the endothelial health and function and is maximum at birth and decreases with age.[12] These cells do not regenerate but are replaced by cell migration and expansion. We did not find any difference in ECD in patients with T2DM and the controls which is similar to some of the previous studies reported in the literature.[9,10,25,28,29] However, contrarily, lower ECD has been reported in patients with T2DM in some population-based and some clinic-based studies[7,18,30,31] though the age stratification of cases and controls was not clear in some of these studies.
Central corneal thickness
CCT reflects the functioning of corneal endothelial cells. Stromal hydration is maintained by the endothelial Na + K+ pump and the tight junctions present between the corneal endothelial cells. There is large evidence in the literature indicating the presence of a thicker cornea in TIDM as compared to controls[6,8,9,10] but the same is not true for T2DM. Although some have found an increase in CCT in T2DM[7,25] others like our study did not find any difference in CCT in T2DM from controls.[3,9,10,26,31] It is likely that diabetes alone may not affect the corneal thickness but other factors may also contribute significantly such as age, duration of DM, and its control. Taken together all these factors may adversely affect the functioning of the endothelium.
Duration of diabetes
T2DM is a chronic disease and the effect of duration of the disease on corneal endothelium is variably reported in the literature. Lee et al. found significantly higher CCT and CV but no statistically significant difference in ECD and hexagonality between those having DM for over 10 years and those less than 10 years duration.[8] A study by Leelawongtawun et al. found decreased hexagonality and increased CV in all diabetic groups when compared to control eyes but found no significant differences between the duration stratified diabetic groups.[27] In our study, no statistically significant difference was detected between the mean values of CCT, ECD, CV, and HEX in patients with T2DM based on a cut-off duration of 10 years which is in agreement with the study by El-Agamy et al.[30]
Associations with retinopathy
Retinopathy is a major complication of DM due to microangiopathy. It is thought that a correlation between DR and corneal endothelial cell loss may be expected due to common pathophysiological mechanisms of endothelial damage such as the accumulation of advanced glycation end products and increased oxidative stress. However, here again, the jury is still out as conflicting results have been published. There are several studies that found no difference in the corneal endothelial morphology and thickness of diabetes patients with and without retinopathy and no correlation of corneal morphology with the severity of retinopathy.[9,18,30] We too did not find any difference in the corneal endothelium between those having DR and those who did not. Some recent studies, however, have reported association between endothelial changes and DR and found that the endothelial changes were more marked in higher grades of retinopathy.[19,20]
Associations with nephropathy
Only a handful of studies have explored the relation between DN with corneal endothelium although corneal endothelial changes associated with chronic renal failure have been well documented.[32,33] A study investigating the relationship between the corneal endothelium and microalbuminuria in patients with DM without DR found that patients with micro-albuminuria had similar corneal endothelial measurements when compared with microalbuminuria negative patients and non-DM patients.[21] Our study showed higher mean CCT, CV, and ECD values, and lesser mean HEX in T2DM patients with macroalbuminuria. However, only the CV values reached the level of statistical significance when compared to T2DM patients without macroalbuminuria. Several factors are known to alter the corneal endothelial morphology in patients of chronic renal failure. Some of these factors are increased levels of toxic compounds present in blood and plasma and elevated levels of oxidized glutathione due to dysfunction in the glutathione system.[34] We believe that similar factors may operate in T2DM patients with macroalbuminuria as well which can cause the corneal endothelial morphological changes seen in our study.
Correlations and associations
We did not find a significant correlation of the duration of diabetes, presence, and severity of retinopathy with parameters of endothelial cell morphology. HbA1C was the single most important parameter that was found to correlate with endothelial cell morphology, whereas urinary ACR level correlated with cell hexagonality. There was a positive correlation between HbA1C and central corneal thickness (P < 0.001), cell variance (P < 0.001), and a negative correlation with ECD (P < 0.005). Modis et al.[10] reported an inverse correlation of HbA1c with ECD, whereas Shenoy et al.[19] found that uncontrolled diabetes was a risk factor of compromised corneal endothelium in DR patients. Toprac et al.[21] found that patients with HbA1c <7% had lower hexagonality ratio than in patients with HbA1c >7%. However, the matter remains still inconclusive as a few other studies found no association of corneal endothelial changes with HbA1c.[9,18,26,30]
There are only a few studies highlighting the association of nephropathy and corneal endothelium. Ohguro et al.[32] found polymegathism and pleomorphism significantly more in the eyes of the patients undergoing dialysis, whereas Diaz et al.[33] reported a significant decrease in cell density in patients with nephropathy. We found that there was a significant correlation of cell hexagonality with urinary ACR (r = –.196; P = 0.025). Several studies have reported the importance of reduced glutathione in the maintenance of corneal endothelial morphology.[34,35] It is postulated that due to the increased oxidative stress and a reduction in the level of reduced glutathione in aqueous in diabetes patients with kidney disease, there may be a decrease in HEX and an increase in pleomorphism.
There are a few limitations of our study. This was a cross-sectional study and measurements were made at one point in time. A longitudinal prospective study would have provided a clearer picture. Also, the duration of DM was less than 10 years in 78% of the patients with T2DM and hence long-term effects of DM on corneal endothelial functions could have been missed. However, despite these limitations, our results show that rather than the duration of DM, its effective control is probably a more important determinant of corneal endothelial functions in T2DM. In contrast to one previous study which found a correlation of HbA1c with HEX only, we found that HbA1c correlated with ECD, CV, and CCT. Also unlike any other previous study, we could show that the presence of macroalbuminuria was associated with an increase in CV in patients with T2DM and increased ACR correlated with a decrease in HEX values. Therefore, besides age, renal function is another factor that can impair corneal endothelial functions in patients with T2DM. The comparison of our study with previously published studies that looked into the endothelial functions in T2DM is shown in Table 7.
Table 7.
Comparisons of studies assessing corneal endothelial functions in patients with T2DM with our study
| Parameters Studies | El-gamy et al.[30] (2017) | Storr - Paulsen et al.[25] (2013) | Choo et al.[31] (2010) | Sudhir et al.[18] (2012) | Inoue et al.[26] (2002) | Durukan et al.[20] (2019) | Toprack et al.[21] (2019) | Our study | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Demography | |||||||||
| Number of patients cases/control | 57/54 | 107/128 | 100/100 | 1191/120 | 129/1265 | 120/112 | 100/92 | 131/131 | |
| Nature of study | Clinic based | Clinic based | Clinic based | Population based | Clinic based | Clinic based | Clinic based | Clinic based | |
| Gender (male: Female) in cases | 27/30 | 51/56 | - | 637/554 | - | 67/53 | 41/59 | 62/69 | |
| Mean age±standard deviation (in years) | 57.08±8.37 | 72.1±11 | Patients above 50 years were included | 54.8±9.5 | 67.8±8.1 | 59.5±8.1 | 53.3±10.4 | 53.26±6.24 | |
| Duration of diabetes | 12.87±8.03 | - | - | - | - | 11.5±6.2 | 9±5.6 | 7.29±6.00 | |
| Mean HbA1C | 8.57% ± 2.09 | 7.3±0.2 | - | - | - | 9.2±11.5 | 7.1±1.2 | 6.97±0.99 | |
|
| |||||||||
| Corneal endothelial measures | |||||||||
|
| |||||||||
| ECD (cell/µm) | Case | 2,491.98±261.08 | 2578±77 | 2541.6±516.4 | 2550.96±326.17 | 2.565±271 | 2,295±311 | 2,413.4±296.8 | 2762.08±320.21 |
| Control | 2,629.68±293.45 | 2605±66 | 2660.1±515.5 | 2634.44±256.00 | 2.540±252 | 2,501±302 | 2,454.8±303.4 | 2771.46±336.7 | |
| P | 0.014 | 0.60 | <0.01 | 0.001 | 0.24 | 0.003 | 0.341 | 0.818 | |
| HEX (%) | Case | 33.24±10.25 | 58.0±1.0 | 41.1% ± 19.6% | 56.57±7.26 | 37.4±7.5% | 49.1±10.2 | 69.2±6.4 | 55.34±5.82 |
| Control | 34.24% ± 8.73% | 58.5±1.1 | 45.2±20.6. | 57.08±6.80 | 38.0±7.0% | 55.2±10.0 | 69.1±6.1 | 56.94.± 7.00 | |
| P | 0.603 | 0.67 | <0.01 | 0.437 | 0.34 | 0.04 | 0.944 | 0.145 | |
| CV | Case | 0.41±0.07 | 31.9±1.0 | 67.2±47.2 | 35.56±5.00 | 0.64±0.11 | 36.0±6.2 | 30.8±6.2 | 31.60±3.51 |
| Control | 0.37±0.08 | 31.9±0.9 | 58.2±43.0 | 34.97±5.03 | 0.64±0.10 | 36.0±6.2 | 30.2±5.3 | 30.85±6.68 | |
| P | 0.008 | 0.9 | <0.01 | 0.720 | 0.74. | 0.344 | 0.522 | 0.256 | |
| CCT (/µm) | Case | 545.61±30.39 µm) | 538±7 | 517.3±53.4 | 524.75±34.52 | - | 544.2±38.0 | 541.3±55.5 | 514.54±38.17 |
| Control | 539.42±29.22 µm), | 546±5 | 510.8±71.9, | 526.06±33.38 | - | 521.5±31.5 | 535.9±34.8 | 511.65±32.64 | |
| P | 0.301 | <0.05 | 0.149 | 0.685 | - | 0.005 | 0.421 | 0.510 | |
|
| |||||||||
| Associations and correlations | |||||||||
|
| |||||||||
| Correlations of endothelial parameters with clinical parameters (duration of DM, HbA1C, diabetic retinopathy,) | No correlation | - | No correlation | Age and diabetes status correlated with ECD | Age correlated with ECD, CV, HEX. | DR correlated with endothelial changes. The corneal endothelial changes were more prominent in eyes with increased stage of DR | Significant associations between HbA1c((≤ 7 per cent versus > 7 per cent) versus HEX | HbA1c correlated with ECD, CV, CCT. | |
| ACR with HEX | |||||||||
Conclusion
Our study shows that the poorly controlled patients with T2DM and those with macroalbuminuria have corneal endothelial abnormalities. Therefore we advocate routine specular microscopy, HbA1c estimation, and urine examination for albuminuria prior to intraocular surgery in all patients with T2DM. Also, necessary precautions such as the use of dispersive viscoelastic devices, minimum phaco power, and quicker operating time should be undertaken during cataract surgery in patients with T2DM to reduce the risk of endothelial damage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7:45–8. doi: 10.4066/AMJ.2013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrich BT, Schlötzer-Schrehardt U, Skeie JM, Burckart KA, Schmidt GA, Reed CR, et al. Mitochondrial and morphologic alterations in native human corneal endothelial cells associated with diabetes mellitus. Invest Ophthalmol Vis Sci. 2017;58:2130–8. doi: 10.1167/iovs.16-21094. [DOI] [PubMed] [Google Scholar]
- 3.Hugod M, Storr-Paulsen A, Norregaard JC, Nicolini J, Larsen AB, Thulesen J. Corneal endothelial cell changes associated with cataract surgery in patients with type 2 diabetes mellitus. Cornea. 2011;30:749–53. doi: 10.1097/ICO.0b013e31820142d9. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Kim C-S, Sohn E, Jeong I-H, Kim H, Kim JS. Involvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:529–36. doi: 10.1007/s00417-010-1573-9. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki J, Tsubota K, Yoshida A, Tornheim K, Laing RA. Changes of corneal redox state in diabetic animal models. Cornea. 1995;14:196–201. [PubMed] [Google Scholar]
- 6.Anbar M, Ammar H, Mahmoud RA. Corneal endothelial morphology in children with type 1 diabetes. J Diabetes Res. 2016;2016:7319047. doi: 10.1155/2016/7319047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roszkowska AM, Tringali CG, Colosi P, Squeri CA, Ferreri G. Corneal endothelium evaluation in type I and type II diabetes mellitus. Ophthalmologica. 1999;213:258–61. doi: 10.1159/000027431. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Oum BS, Choi HY, Lee JE, Cho BM. Differences in corneal thickness and corneal endothelium related to duration in diabetes. Eye. 2006;20:315–8. doi: 10.1038/sj.eye.6701868. [DOI] [PubMed] [Google Scholar]
- 9.Larsson LI, Bourne WM, Pach JM, Brubaker RF. Structure and function of the corneal endothelium in diabetes mellitus type I and type II. Arch Ophthalmol. 1996;114:9–14. doi: 10.1001/archopht.1996.01100130007001. [DOI] [PubMed] [Google Scholar]
- 10.Módis L, Jr, Szalai E, Kertész K, Kemény-Beke A, Kettesy B, Berta A. Evaluation of the corneal endothelium in patients with diabetes mellitus type I and II. Histol Histopathol. 2010;25:1531–7. doi: 10.14670/HH-25.1531. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein AS, Janson BJ, Skeie JM, Ling JJ, Greiner MA. The effects of diabetes mellitus on the corneal endothelium:A review. Surv Ophthalmol. 2020;65:438–50. doi: 10.1016/j.survophthal.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Tuft SJ, Coster DJ. The corneal endothelium. Eye (Lond) 1990;4:389–424. doi: 10.1038/eye.1990.53. [DOI] [PubMed] [Google Scholar]
- 13.Carlson KH, Bourne WM, McLaren JW, Brubaker RF. Variations in human corneal endothelial cell morphology and permeability to fluorescein with age. Exp Eye Res. 1988;47:27–41. doi: 10.1016/0014-4835(88)90021-8. [DOI] [PubMed] [Google Scholar]
- 14.Mathew PT, David S, Thomas N. Endothelial cell loss and central corneal thickness in patients with and without diabetes after manual small incision cataract surgery. Cornea. 2011;30:424–8. doi: 10.1097/ICO.0b013e3181eadb4b. [DOI] [PubMed] [Google Scholar]
- 15.Ventura A, Walti R, Bohnke M. Corneal thickness and endothelial density before and after cataract surgery. Br J Ophthalmol. 2001;85:18–20. doi: 10.1136/bjo.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikubo S, Takamura Y, Kubo E, Tsuzuki S, Akagi Y. Corneal changes after small-incision cataract surgery in patients with diabetes mellitus. Arch Ophthalmol. 2004;122:966–9. doi: 10.1001/archopht.122.7.966. [DOI] [PubMed] [Google Scholar]
- 17.Yang R, Sha X, Zeng M, Tan Y, Zheng Y, Fan F, et al. The influence of phacoemulsification on corneal endothelial cells at varying blood glucose levels. Eye Sci. 2011;26:91–5. doi: 10.3969/j.issn.1000-4432.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Sudhir RR, Raman R, Sharma T. Changes in the corneal endothelial cell density and morphology in patients with type 2 diabetes mellitus:A population-based study, Sankara Nethralaya diabetic retinopathy and molecular genetics study (SN-DREAMS, Report 23) Cornea. 2012;31:1119–22. doi: 10.1097/ICO.0b013e31823f8e00. [DOI] [PubMed] [Google Scholar]
- 19.Shenoy R, Khandekar R, Bialasiewicz A, Al Muniri A. Corneal endothelium in patients with diabetes mellitus:A historical cohort study. Eur J Ophthalmol. 2009;19:369–75. doi: 10.1177/112067210901900307. [DOI] [PubMed] [Google Scholar]
- 20.Durukan I. Corneal endothelial changes in type 2 diabetes mellitus relative to diabetic retinopathy. Clin Exp Optom. 2020;103:474–8. doi: 10.1111/cxo.12971. [DOI] [PubMed] [Google Scholar]
- 21.Toprak I, Fenkci SM, Yaylali GF, Martin C, Yaylali V. Effect of microalbuminuria on corneal endothelium in patients with diabetes without retinopathy. Clin Exp Optom. 2020;103:625–9. doi: 10.1111/cxo.12969. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens PE, Levin A Kidney Disease:Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease:Synopsis of the kidney disease:Improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 24.Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie House classification. ETDRS report number 10. Early treatment diabetic retinopathy study research group. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 25.Storr-Paulsen A, Singh A, Jeppesen H, Norregaard JC, Thulesen J. Corneal endothelial morphology and central thickness in patients with type II diabetes mellitus. Acta Ophthalmol. 2014;92:158–60. doi: 10.1111/aos.12064. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Tokuda Y, Inoue Y, Amano S, Oshika T, Inoue J. Corneal endothelial cell morphology in patients undergoing cataract surgery. Cornea. 2002;21:360–3. doi: 10.1097/00003226-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Leelawongtawun W, Surakiatchanukul B, Kampitak K, Leelawongtawun R. Study of corneal endothelial cells related to duration in diabetes. J Med Assoc Thai. 2016;99(Suppl 4):S182–8. [PubMed] [Google Scholar]
- 28.Schultz RO, Matsuda M, Yee RW, Edelhauser HF, Schultz KJ. Corneal endothelial changes in type I and type II diabetes mellitus. Am J Ophthalmol. 1984;98:401–10. doi: 10.1016/0002-9394(84)90120-x. [DOI] [PubMed] [Google Scholar]
- 29.Itoi M, Nakamura T, Mizobe K, Kodama Y, Nakagawa N, Itoi M. Specular microscopic studies of the corneal endothelia of Japanese diabetics. Cornea. 1989;8:2–6. [PubMed] [Google Scholar]
- 30.El-Agamy A, Alsubaie S. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin Ophthalmol. 2017;11:481–6. doi: 10.2147/OPTH.S126217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choo M, Prakash K, Samsudin A, Soong T, Ramli N, Kadir A. Corneal changes in type II diabetes mellitus in Malaysia. Int J Ophthalmol. 2010;3:234–6. doi: 10.3980/j.issn.2222-3959.2010.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohguro N, Matsuda M, Fukuda M. Corneal endothelial changes in patients with chronic renal failure. Am J Ophthalmol. 1999;128:234–6. doi: 10.1016/s0002-9394(99)00086-0. [DOI] [PubMed] [Google Scholar]
- 33.Diaz CP, Bordas FD, Garcia JR, Camps EM, Carceller A. Corneal disease in patients with chronic renal insufficiency undergoing hemodialysis. Cornea. 2001;20:695–702. doi: 10.1097/00003226-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Sati A, Jha A, Moulick PS, Shankar S, Gupta S, Khan MA, et al. Corneal endothelial alterations in chronic renal failure. Cornea. 2016;35:1320–5. doi: 10.1097/ICO.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M, Kinoshita S, Ohashi Y, Shimomura Y, Ohguro N, Okamoto H, et al. Comparison of the effects of intraocular irrigating solutions on the corneal endothelium in intraocular lens implantation. Br J Ophthalmol. 1991;75:476–9. doi: 10.1136/bjo.75.8.476. [DOI] [PMC free article] [PubMed] [Google Scholar]



