Abstract
Purpose:
The aim of this study was to evaluate the association of morphological features of subretinal hyperreflective material (SHRM) with visual acuity (VA), geographic atrophy (GA) and scar formation in eyes with neovascular age-related macular degeneration (neovascular AMD) and to compare with controls of neovascular AMD without SHRM.
Methods:
Retrospective analysis of 157 wet AMD eyes with SHRM and 50 eyes without SHRM treated with Anti-VEGF. Baseline spectral domain-OCT characteristics (SHRM location, height, width, area, reflectivity, border definition) were collected and were correlated with VA at baseline, 3, 6, 12 months and looked for development of scar and geographical atrophy (GA) and were compared to the control group.
Results:
When compared to the control, baseline parameters with a significant predictive value of 12-VA were presence of SHRM, foveal involvement of SHRM, high reflective SHRM, well-defined SHRM borders and thick SHRM. VA was decreased with greater SHRM height, width and area (P < 0.001). Decreasing reflectivity of SHRM lesions and disappearance of SHRM correlated with better VA at 12 months (P < 0.05). At 12 months, scar and GA was present more often in eyes with persistent SHRM than in eyes with SHRM that resolved and those without SHRM in the control group.
Conclusion:
SHRM can be considered as a surrogate OCT biomarker in predicting final visual outcome in neovascular age-related macular degeneration. Baseline parameters predicting poorer vision at 12-follow-up were presence of SHRM involving the fovea, well-defined SHRM borders, greater SHRM height, width and area and persistence of SHRM with Anti-VEGF therapy.
Keywords: Anti-VEGF therapy, geographic atrophy, neovascular age-related macular degeneration, scar formation, subretinal hyper reflective material, visual acuity
The first revolutionary leap in AMD treatment occurred a little over a decade ago, with the introduction of anti-VEGF and the same have reduced the incidence of legal blindness by more than 50%.[1,2] However, there are concerns regarding the maintenance and consistency of efficacy of anti-VEGF drugs in different patients. This dilemma can be solved by identification of valid biomarkers relevant for visual function, disease activity and prognosis, which can provide solid guidance for therapeutic management.
With the advent of spectral-domain optical coherence tomography (SD-OCT), an increasingly profound understanding of the wet AMD has emerged. Morphological features such as intraretinal fluid (IRF), intra-retinal cavitations (IRC), subretinal fluid (SRF), and fibrovascular pigment epithelial detachment (FVPED) have been proposed as potential biomarkers for monitoring the effect.[3] Degenerative IRC and FVPED at baseline were associated with both poor baseline visual acuity (VA) and lower visual improvement, while SRF at baseline was associated with better VA gain. One of the newer biomarkers in AMD is the recently described subretinal hyperreflective material (SHRM).[4,5] This SD-OCT feature is identified as hyperreflective material located between neurosensory retina and retinal pigment epithelium (RPE).
SHRM likely consists of fluid, fibrin, blood, scar or the fibrovascular tissue and this composition may change with time and with anti VEGF therapy.[6,7] It has been documented that SHRM lesion size correlates with VA, and SHRM decreases in size with anti-VEGF therapy.[4] The morphological features of SHRM have been studied previously.[8,9] It is important to characterize SHRM morphologic features and their functional consequences which will enable the treating physician to tailor treatment to provide adequate disease control, minimize recurrence and neurosensory damage, and limit the number of invasive and costly interventions. The purpose of the study was to evaluate the association of morphological features of SHRM with visual acuity, geographic atrophy (GA) and scar formation in eyes with neovascular age-related macular degeneration (neovascular AMD) and to compare with controls of neovascular AMD without SHRM.
Methods
Study design
This was a retrospective review of SD-OCT images of 157 eyes diagnosed as neovascular AMD with SHRM and 50 age matched controls of neovascular AMD without SHRM done at a tertiary eye center in Southern India. Local ethics committee approval was obtained prior to the initiation of the study. The research followed the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Study procedures
We reviewed the electronic medical records and obtained the follow-up information on all consecutive patients diagnosed with neovascular AMD between January 2014 and February 2020. Eyes with a minimum follow-up of 1 year were included in the study. The initial diagnosis of neovascular AMD was made based on fundoscopy and confirmed by SD-OCT and Fundus Fluorescein Angiography (FFA) imaging on Spectralis Heidelberg Engineering, Germany. The scan pattern used for patients included macular dense scan (area 20°x20°, 49 B scans at an interscan distance of 120 mm). Masked readers (S.I and D.A) graded the morphology of SHRM in the study eye at 4 time points: baseline, 3 months, 6 months and 12 months. Poor quality images which obscured the SHRM morphology and patients who lost for review visits were excluded from the study.
On subgroup analysis, eyes with SHRM were subdivided based on location: at the fovea and outside the central 1-mm2 subfield. The dimensions including height, width, area was measured at the baseline and the change in dimensions were recorded at 3,6 and 12 months. SHRM height was measured from the inner border of SHRM to the inner border of the RPE layer with the help of inbuilt caliper. When the SHRM-RPE border could not be identified, height was measured from the inner SHRM border to Bruch’s membrane regardless of whether there was associated RPE atrophy. Categories below and above the median were created for SHRM width, height, and area measured at baseline and were compared to the visual outcomes. The hyperreflectivity of SHRM was documented as high (similar to RPE reflectivity) and low (similar to outer plexiform reflectivity). The SHRM borders were identified as being well defined or poorly defined. The integrity of outer retinal layers was studied and the loss of structures where documented and measured. All the morphological parameters of SHRM were compared to VA, scar and GA progression at 3 follow-up visits. The intraclass correlation coefficient between the readers agreement for presence and location of SHRM and for other morphological features was excellent (>0.90).
Statistical analysis
Descriptive analysis of the population’s characteristics was carried out. Results of continuous variables are reported as mean (SD), median (SD) and that of categorical variables are reported as counts and percentages. The differences between quantitative variables were analyzed using Paired t test and the nonparametric alternative Wilcoxon signed rank test. The differences between categorical variables were analyzed using the nonparametric tests (Chi-square test or Fisher’s exact test). Kruskal–Wallis test and Mann–Whitney U test was used to compare the BCVA between the groups at different time points. To determine the baseline variables that predicted baseline BCVA, stepwise backward multivariate logistic regression analysis was used. P value < 0.05 is considered as significant for all the comparisons. Data were analyzed using IBM SPSS 26.0 version.
Results
A total of 157 eyes of 157 patients diagnosed as neovascular AMD with SHRM and 50 age matched controls of neovascular AMD without SHRM were included in the study. Male to female ratio (1:1.9) and the mean age (72.5 + 11.3) were comparable in both the groups. The mean BCVA at baseline was 0.68 + 0.41 cent for eyes with SHRM and 0.46 + 0.38 logMAR for those without SHRM (P = 0.001). At 6 and 12 months, the BCVA did not differ significantly between patients with SHRM and without SHRM. The mean BCVA at 6 months was 0.50 + 0.41 logMAR for eyes with SHRM and 0.35 + 0.30 logMAR for those without SHRM (P = 0.17). The mean BCVA at 12 months was 0.52 + 0.43 logMAR for eyes with SHRM and 0.45 + 0.42 logMAR for those without SHRM (P = 0.58).
Correlation of SHRM location with visual acuity
The presence of SHRM was associated with worse VA, at all locations, regardless of height or width and when compared to the control group (P = 0.001). In the subgroup analysis, foveal involvement of SHRM had a worse visual acuity at baseline, 3,6 and 12 months when compared to eyes with absence of SHRM in the central 1 mm2 of fovea. There was a significant correlation between both VA and SHRM dimensions such as height, width and area. Worst VA at the baseline occurred when SHRM was located at the fovea with an area exceeding 0.24 mm2 (logMAR 0.83 + 0.31) as compared with no SHRM at the fovea (logMAR 0.46 + 0.34; P = 0.03). VA was worse when SHRM was involving the fovea and the baseline width was more than 1500 u (logMAR 0.77 + 0.41) as compared with no SHRM at the fovea ((logMAR 0.43 + 0.22; P = 0.02). When the foveal involving SHRM exceeded a height more than 175 u the baseline VA was poor (logMAR 0.78 + 0.45) as compared with no SHRM at the fovea (logMAR 0.41 + 0.29; P = 0.02). The disappearance of SHRM from baseline to 3 months correlated with better VA (P = 0.001) but did not show statistically significant improvement at 6 and 12 months.
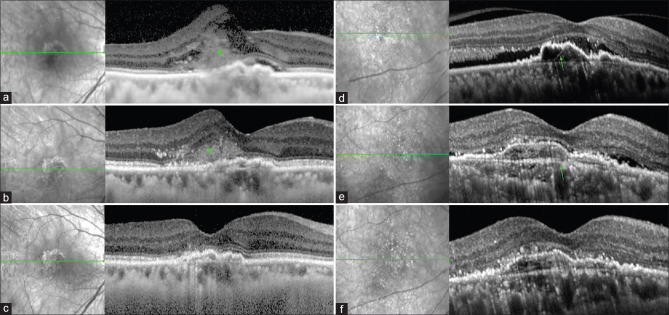
To know whether baseline VA is a poor predictor of final visual outcome, study eyes were stratified into three groups; group 1 with good baseline vision (>logMAR 0.3 n = 24;15.2%), group 2 with intermediate baseline vision (0.3–0.8 n = 97; 61.8%) and group 3 with poor baseline vision (<0.9 n = 36,22.9%). At final visit (12 month), all the 3 groups had a positive impact on VA. Around 78% of eyes remained stable or improved in VA at 1-year follow-up. Gain in vision was most pronounced in group 2 with SHRM and group 3 in controls without SHRM [Fig. 1].
Figure 1.
(a) Baseline SD-OCT image of neovascular AMD with SHRM (green asterisk) above RPE (b) Partial resolution of SHRM at 3 months after anti-VEGF therapy (c) Complete resolution of SHRM at 12-follow-up. (d) Baseline SD-OCT image of neovascular AMD with fibrovascular PED (green arrow) without SHRM (e) Partial resolution of SRF at 3 months after anti-VEGF therapy (f) Complete resolution of SRF with persistence of FVPED at 12-follow-up
Correlation of SHRM morphology with visual acuity
When SHRM was present, the median height at the foveal was 175 + 52 mm, the median width at the fovea was 1500 + 451 mm and the median area anywhere within the scan was 0.24 + 0.14 mm2. When the baseline SHRM width, height and area within fovea was greater than the median width, the corresponding VA was significantly worse at 3, 6, and 12 months when compared with the control group (P = 0.001). Ellipsoid zone (EZ) loss was noted more often in eyes with underlying foveal SHRM compared with eyes without foveal SHRM.
Hyperreflective SHRM at baseline was found to have worse baseline and final VA (P = 0.047). As the reflectivity of SHRM decreases, the VA was better, but not statistically significant (P = 0.59). Hyperreflective SHRM was associated more with the persistence of SHRM at 6 and 12 months (P = 0.004).
Well defined SHRM borders at baseline were associated with poor baseline VA. If the anterior border was well defined in terms of VA at 3, 6, 12 months showed worse VA (logMAR 0.67,0.69.0.73, respectively) when compared to the poorly defined SHRM border group (logMAR 0.46,0.44.0.50, respectively, at 3, 6, 12 months, P = 0.001). Similarly, there was significant difference in VA whether posterior border was well defined (logMAR 0.57,0.61.0.67, respectively, at 3, 6, 12 months) when compared to the poorly defined SHRM border group (logMAR 0.40,0.44.0.39, respectively, at 3,6,12 months, P = 0.002). Persistence of SHRM at 12 months was significantly correlated to the well-defined anterior and posterior borders at baseline. The presence of IRF was associated with poorer BCVA at baseline and the final visual outcome at 12 months relative to the absence of this morphological feature (P = 0.001) [Tables 1 and 2].
Table 1.
Univariate analysis of baseline dimensions of SHRM with BCVA
| Baseline BCVA | BCVA 3 Months | BCVA 6 Months | BCVA 12 Months | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mean (SD) | P | Mean (SD) | P | Mean (SD) | P | Mean (SD) | P | |
| SHRM Height (>500) | ||||||||
| Yes | 1.12 (0.670) | 0.086 | 1.02 (0.505) | 0.020** | 0.76 (0.461) | 0.043** | 0.83 (0.404) | 0.171** |
| No | 0.67 (0.401) | 0.49 (0.407) | 0.49 (0.397) | 0.53 (0.437) | ||||
| SHRM Width (>1500) | ||||||||
| Yes | 0.76 (0.429) | 0.045** | 0.61 (0.443) | 0.003** | 0.60 (0.426) | 0.040** | 0.64 (0.444) | 0.033** |
| No | 0.61 (0.388) | 0.40 (0.367) | 0.40 (0.345) | 0.42 (0.401) | ||||
| SHRM Area (>0.15) | ||||||||
| Yes | 0.73 (0.432) | 0.041** | 0.54 (0.433) | 0.049** | 0.53 (0.412) | 0.043** | 0.58 (0.454) | 0.054 |
| No | 0.56 (0.334) | 0.41 (0.360) | 0.40 (0.344) | 0.39 (0.314) | ||||
Table 2.
Multivariate regression analysis of baseline parameters significantly associated with BCVA
| Dependent Variable | Coefficient | F | P | |
|---|---|---|---|---|
| Visit | ||||
| Baseline | SHRM Width (>1500) | 0.074 | 8.346 | 0.004** |
| SHRM Height (>500) | -0.208--- 4.701---0.032** | |||
| 12 months | SHRM Width (>1500) | 0.071 | 8.242 | 0.005** |
| SHRM Height (>500) | 0.210 | 6.491 | 0.002** | |
GA and scar formation
In total, 42.6% of the study eyes at 6 months and 34.3% at 12 months showed persistence of SHRM. The incidence of GA and scar formation in the study group was correlated with the persistence of SHRM from baseline. At 12 months, GA was present in 28 eyes (17.83%) and scar was present in 50 eyes (31.8%) in the study group. In the control group without SHRM at 12 months, GA was noted in 14.1% and scar was present in 9.4%. Scar formation was more frequent in eyes with SHRM compared with those without SHRM (P = 0.003), but not with GA (P = 0.65). The EZ loss at the fovea was noted more often in eyes with underlying foveal SHRM compared with eyes without foveal SHRM (84% vs. 29%; P < 0.0001). Presence of EZ loss at the baseline showed a significant correlation to the development of GA (P = 0.002), but not with scar formation (P = 0.07). Hyperreflective SHRM and well-defined anterior and posterior borders at baseline were significantly associated with scar formation at 12 follow-up (P = 0.004, P = 0.001, respectively). However, there was no significant difference whether the anterior border was well or poorly defined in terms of GA at 12 months. Reflectivity of SHRM also did not correlate with GA at the final visit.
Discussion
In Type II (classic CNVM), new vessels from the choroidal neovascular complex penetrates through the Bruch’s membrane and proliferate in the subretinal space.[10,11] The neovascular membrane can be visualized in SD-OCT as a poorly defined, medium- to hyperreflective material between the neurosensory layers and RPE, termed “SHRM”. In CATT, 77% of eyes showed SHRM at the time of enrolment, with a decrease to 54% after 2 years of treatment.[6] This current study showed presence of SHRM in 75.84% of treatment-naive eyes with neovascular AMD and persistence of SHRM in 34.3% at the end of 1 year.
To augment the understanding on the prognostic value of this SD-OCT biomarker, the baseline SHRM characteristics were correlated with VA at 3 different time points and compared to the control group of neovascular AMD without SHRM. Of the baseline OCT parameters, the presence of SHRM, foveal involvement, larger baseline SHRM dimensions, increased reflectivity, well-defined SHRM borders, SHRM associated with IRF, EZ loss involving fovea was associated with worse visual outcome at 1 year after treatment. SHRM width >1500 u had the greatest adverse effect on VA, to a greater extent than height and area. In eyes with foveal SHRM, if the SHRM is not too broad, even when the central SHRM is thick, they may be able to fixate eccentrically enough to maintain a reasonable VA, whereas if the lesion is wide, it would be more difficult to fixate eccentrically. Moreover, the increased barrier between the neurosensory retina and RPE, interferes with metabolic and nutrient exchange and can damage the overlying photoreceptors.[12] This is further supported by the fact that SHRM was associated with the disruption of Ellipsoid layer. SRF involving the fovea was associated with better VA at baseline and 1-year follow-up. SRF may protect the photoreceptors from various harmful SHRM components such as hemorrhage, split products of fibrin and fibrotic tissue. This supports the evidence that SHRM may damage the overlying photoreceptors directly by a toxic effect.
The loading dose of anti-VEGF therapy (3 months) correlated with significantly decreased SHRM dimensions and the SHRM height declined more slowly after that. [Fig. 1] Anti-VEGF therapy decreases capillary endothelial permeability, thereby reducing vascular fluid leakage. The rapid decrease in SHRM thickness is caused by reduction in the fluid component of SHRM induced by anti-VEGF therapy regardless of drug and regimen.[4,6,13] Angiogenesis is the development of new capillaries from pre-existing vascular network. Anti-VEGF could block the angiogenesis, but it is less effective at decreasing the size of the already formed neovascular complex.[14,15] When the treatment continues over time and the relative amount of SHRM fluid declines, there may be an increased fibrotic component, rendering anti-VEGF therapy less effective in reducing SHRM thickness. Anti-VEGF treatment induces a maturation of the neovascular complexes towards an organized tissue in which hyperreflectivity increases over time with well-defined borders.[15,16,17]
Our study also reported that the degree to which the SHRM anterior and posterior borders were defined, correlated with worse baseline VA and follow-up VA. This is in contrast to the study reported by Kumar et al. whom couldn’t find a significant correlation with VA and the border definition.[8] But the finding was in concordance with Pokroy et al.[9] and Casalino et al.[18] who corroborated the CATT analysis, showing that well-defined SHRM borders on SD-OCT appear to represent fibrotic tissue or mature neovascular complexes. This is also supported by our own observation that well-defined SHRM borders at baseline was associated with hyperreflectivity of SHRM. SHRM persistence and scar formation at 6,12 months was significantly correlated with hyperreflectivity and well-defined borders. The latter observation is in agreement with, Charafeddin et al.[19] who reported that reduction of thickness and volume of SHRM is accompanied by increase of the mean reflectivity following anti-VEGF treatment. We propose that baseline well defined borders of SHRM and hyperreflectivity can be considered as an early tomographic biomarker for retinal fibrotic scar evolution.
The mean VA in the study and control group at 12 months was significantly better than the baseline value. The mean VA at 12 months in control group without SHRM was more than the study group, but was not statistically significant. This may be due to the underlying pathophysiological processes that continues in neovascular AMD resulting in both cellular loss and cellular structural disorganization and appearance of geographical atrophy.[14,20,21] These processes result in worsening of the macular function even though the macula is maintained in a fluid-free environment. This is further supported by the data that EZ and ELM integrity was disrupted in eyes with worse VA both in study and control group at 1-year follow-up. Incidence of GA in both study and control group was almost similar was independent on the presence of SHRM. It remains unclear whether the development of GA undergoing anti-VEGF therapy secondary to neovascular AMD results from macular drying followed by normal disease progression, or whether VEGF inhibition has a neurotoxic effect on the macula, thus causing frequent appearance and more rapid growth of GA. The irreversible nature of the structural damage to the macular retina can limit functional improvement and as the pathologic changes progress over time despite optimal treatment.
SHRM persisted in more than one third of the eyes during anti-VEGF therapy. Persistence of SHRM was associated with increased incidence of scar formation particularly when the baseline dimensions involving the fovea were larger than the median. [Fig. 2] Of the baseline OCT parameters, the presence well-defined SHRM borders and hyperreflectivity was associated with increased incidence of scar formation. The involution and contraction that occurs with anti-VEGF therapy has been likened to a wound-healing response whereby the growth stimulus to the vascular component has been removed, allowing the inflammatory and fibrotic elements to predominate. It’s a well-known fact that the type of CNV, predicted scar formation. Scars were least likely to develop in eyes with occult CNV only. When occult CNV is admixed with the classic type, the risk doubles. The risk triples when the angiographic phenotype subset is composed predominantly of classic CNV.[22] Intravitreal anti-VEGF treatment decreases scar formation in purely occult lesions without SHRM by confining the CNV to the sub-RPE space.[22] With isolation of the CNV complex within the sub-RPE compartment, the anatomical integrity of the outer retina is preserved, and mechanical disruption due to neovascular ingrowth and cicatrization in the sub neurosensory space or toxicity due to accompanying hemorrhage is averted. Our data is consistent with the CATT trial whom showed that SHRM may be a direct factor in the development of a scar. Scar can develop in areas adjacent to the pre-existing SHRM.
Figure 2.
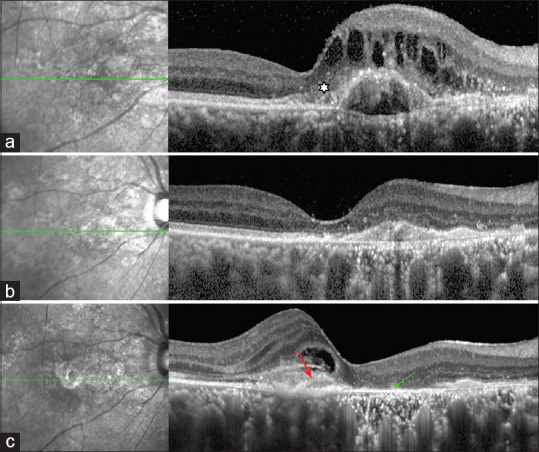
(a) Baseline SD-OCT image showing SHRM (white asterisk) above RPE with fibrovascular pigment epithelial detachment (FVPED). (b) SD-OCT image showing complete resolution of IRF with collapse of FVPED at 6- follow-up. (c) SD-OCT image at 12 months showing GA at the site of SHRM (green arrow) and scar formation temporal to macula (red arrow)
Strengths of our study are the standardized imaging approach with high resolution OCT for all included patients, qualitative and quantitative analysis of the data in a large sample size and the analysis with follow-up out to 12 and comparison with control group without SHRM. Limitations include the retrospective nature and the absence of correlation to multimodal imaging. However, such a future study would be of interest and might fill the lacunae in the knowledge of SHRM.
Conclusion
To conclude, SHRM can be considered as a surrogate OCT biomarker in predicting final visual outcome in neovascular age-related macular degeneration. Baseline parameters predicting poorer vision at 12-follow-up were presence of SHRM involving the fovea, well-defined SHRM borders, greater SHRM height, width and area and persistence of SHRM with Anti-VEGF therapy. Incidence of fibrotic scar formation was higher with the hyperreflective and well defined SHRM at baseline and persistence of SHRM after anti-VEGF therapy. Incidence of geographical atrophy was independent on the presence or absence of SHRM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Finger RP, Daien V, Eldem BM, Talks JS, Korobelnik JF, Mitchell P, et al. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration- A systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 2020;20:1–4. doi: 10.1186/s12886-020-01554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark:Year 2000 to 2010. Am J Ophthalmol. 2012;153:209–13. doi: 10.1016/j.ajo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Waldstein SM, Simader C, Staurenghi G, Chong NV, Mitchell P, Jaffe GJ, et al. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology. 2016;123:1521–9. doi: 10.1016/j.ophtha.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Keane PA, Liakopoulos S, Chang KT, Wang M, Dustin L, Walsh AC, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in neovascular age-related macular degeneration. Ophthalmology. 2008;115:2206–14. doi: 10.1016/j.ophtha.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt-Erfurth U, Waldstein SM. A paradigm shifts in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1–24. doi: 10.1016/j.preteyeres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Willoughby AS, Ying G, Toth CA, Maguire MG, Burns RE, Grunwald JE, et al. Subretinal hyperreflective material in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015;122:1846–53. doi: 10.1016/j.ophtha.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah VP, Shah SA, Mrejen S, Freund KB. Subretinal hyperreflective exudation associated with neovascular age-related macular degeneration. Retina. 2014;34:1281–8. doi: 10.1097/IAE.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 8.Kumar JB, Stinnett S, Han JI, Jaffe GJ. Correlation of subretinal hyperreflective material morphology and visual acuity in neovascular age-related macular degeneration. Retina. 2020;40:845–56. doi: 10.1097/IAE.0000000000002552. [DOI] [PubMed] [Google Scholar]
- 9.Pokroy R, Mimouni M, Barayev E, Segev F, Geffen N, Nemet AY, et al. Prognostic value of subretinal hyperreflective material in neovascular age-related macular degeneration treated with bevacizumab. Retina. 2018;38:1485–91. doi: 10.1097/IAE.0000000000001748. [DOI] [PubMed] [Google Scholar]
- 10.Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Liakopoulos S, Ongchin S, Bansal A, Msutta S, Walsh AC, Updike PG, et al. Quantitative optical coherence tomography findings in various subtypes of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:5048–54. doi: 10.1167/iovs.08-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:1860–70. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiss CG, Geitzenauer W, Simader C, Gregori G, Schmidt-Erfurth U. Evaluation of ranibizumab-induced changes in high-resolution optical coherence tomographic retinal morphology and their impact on visual function. Invest Ophthalmol Vis Sci. 2009;50:2376–83. doi: 10.1167/iovs.08-2017. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H, Yamashiro K, Tsujikawa A, Ota M, Otani A, Yoshimura N. Association between foveal photoreceptor integrity and visual outcome in neovascular age-related macular degeneration. Am J Ophthalmol. 2009;148:83–9. doi: 10.1016/j.ajo.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;8:CD005139. doi: 10.1002/14651858.CD005139.pub3. doi:10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 17.Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration:The comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–61. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casalino G, Bandello F, Chakravarthy U. Changes in neovascular lesion hyperreflectivity after anti-VEGF treatment in age related macular degeneration:An integrated multimodal imaging analysis. Invest Ophthalmol Vis Sci. 2016;57:288–98. doi: 10.1167/iovs.15-18753. [DOI] [PubMed] [Google Scholar]
- 19.Charafeddin W, Nittala MG, Oregon A, Sadda SR. Relationship between subretinal hyperreflective material reflectivity and volume in patients with neovascular age-related macular degeneration following anti-vascular endothelial growth factor treatment. Ophthalmic Surg Lasers Imaging Retina. 2015;46:523–30. doi: 10.3928/23258160-20150521-03. [DOI] [PubMed] [Google Scholar]
- 20.Ristau T, Keane PA, Walsh AC, Engin A, Mokwa N, Kirchhof B, et al. Relationship between visual acuity and spectral domain optical coherence tomography retinal parameters in neovascular age-related macular degeneration. Ophthalmologica. 2014;231:37–44. doi: 10.1159/000354551. [DOI] [PubMed] [Google Scholar]
- 21.Saxena S, Srivastav K, Cheung CM, Ng JY, Lai TY. Photoreceptor inner segment ellipsoid band integrity on spectral domain optical coherence tomography. Clin Ophthalmol. 2014;8:2507–22. doi: 10.2147/OPTH.S72132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:656–66. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]