Abstract
Purpose:
To report endogenous fungal endophthalmitis, postrecovery from severe COVID-19 infection in otherwise immunocompetent individuals, treated with prolonged systemic steroids.
Methods:
Retrospective chart review of cases with confirmed and presumed fungal endogenous endophthalmitis, following severe COVID-19 disease, treated at two tertiary care referral eye institutes in North India.
Results:
Seven eyes of five cases of endogenous fungal endophthalmitis were studied. All cases had been hospitalized for severe COVID-19 pneumonia and had received systemic steroid therapy for an average duration of 42 ± 25.1 days (range 18–80 days). All the cases initially complained of floaters with blurred vision after an average of 6 days (range 1–14 days) following discharge from hospital. They had all been misdiagnosed as noninfectious uveitis by their primary ophthalmologists. All eyes underwent pars plana vitrectomy (PPV) with intravitreal antifungal therapy. Five of the seven eyes grew fungus as the causative organism (Candida sp. in four eyes, Aspergillus sp. in one eye). Postoperatively, all eyes showed control of the infection with a marked reduction in vitreous exudates and improvement in vision.
Conclusion:
Floaters and blurred vision developed in patients after they recovered from severe COVID-19 infection. They had received prolonged corticosteroid treatment for COVID-19 as well as for suspected noninfectious uveitis. We diagnosed and treated them for endogenous fungal endophthalmitis. All eyes showed anatomical and functional improvement after PPV with antifungal therapy. It is important for ophthalmologists and physicians to be aware of this as prompt treatment could control the infection and salvage vision.
Keywords: Corticosteroid therapy, COVID-19, Endogenous endophthalmitis, Floaters, Fungal endophthalmitis, Pars plana vitrectomy
The novel coronavirus disease (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has affected a significantly high population of previously healthy individuals who developed severe pneumonia with rapid oxygen desaturation requiring urgent hospitalization for respiratory support, intensive care, intravenous drugs, fluids, and steroids all of which predispose them to secondary infections.[1]
Ophthalmic manifestations of COVID-19 have been reported with conjunctivitis being the most common.[2] Posterior segment pathology including central retinal vein occlusion,[3] central retinal artery occlusion,[4] and acute macular neuroretinitis[5] have been reported post COVID-19.
Endogenous endophthalmitis is a sight-threatening ocular infection presenting as a potential ocular emergency. It can manifest at any age and is generally due to a hematogenous spread of infection from a remote systemic location, unrelated to prior ophthalmic surgery or trauma. It is most often seen in patients with chronic debilitating disease or immunocompromised states.[6] In the past 8 months, we noticed a significant increase in the number of cases of endogenous endophthalmitis reporting to our clinics. What was unique was that all these individuals had recently recovered from severe COVID-19 infection for which they had received prolonged steroid therapy in an intensive care unit (ICU) setting. These patients had initially complained of floaters along with blurred vision and had been diagnosed as noninfectious uveitis by their primary ophthalmologists and had received further steroid therapy. However, their clinical picture, nonresponse to steroids along with deteriorating vision indicated a likely infectious etiology rather than a hypersensitivity-related entity. One very recent case series from India also described presumed fungal endophthalmitis in four cases post COVID-19.[7]
To our knowledge, this is the first study in the literature, describing cases with confirmed fungal endogenous endophthalmitis in ICU-treated patients post their complete recovery from severe COVID-19 disease.
Methods
We undertook a retrospective chart review of patients with a diagnosis of endogenous fungal endophthalmitis between May 2020 and January 2021 at two tertiary Ophthalmic institutes in North India.
The diagnosis of endogenous endophthalmitis in our study was based on clinical signs such as the presence of anterior and posterior segment inflammation, vitritis, and characteristic fundal lesions such as vitreous, subretinal exudates, and the lack of any relationship to a potential exogenous cause such as surgery and trauma.[8]
All our cases had recently recovered from confirmed (RT-PCR) severe COVID-19 disease (as per the WHO definition).[1] All cases included had been initially diagnosed as having noninfectious uveitis and were worsening despite treatment. Hence, we decided to study this subject in greater detail.
The collection of the clinical data and surgical management was done with the consent of the patients and with approval by the Institutional Review Board of our hospital. Date of Approval was 1st February 2021. Demographic details, comorbidities, duration, and treatment during hospitalization and ocular course details were complied. Blood culture was advised for all cases. All eyes underwent three-port pars plana vitrectomy (PPV). Undiluted vitreous was collected at the beginning of each surgery using a vitreous cutter. The vitreous biopsy aspirate was immediately transferred for microbiological analysis. The samples were plated directly onto chocolate agar, 5% sheep blood agar, and Sabouraud’s dextrose agar (SDA). Chocolate and blood agars were incubated at 35°C for up to 2 weeks. SDA was incubated at 35°C for 2–3 weeks. Plates were examined daily for detection of fungal growth.
In view of the strong clinical suspicion of fungal endophthalmitis, all eyes received empirical intravitreal antifungal therapy at the time of the first intervention. The use of silicone oil tamponade was an intraoperative decision based on the surgeon’s preference and perception of future risk of retinal detachment. In case of bilateral involvement, the eye with a better prognosis and more likely to be salvageable was operated on first. At the same sitting, intravitreal antifungal and intravitreal antibiotics were injected into the other eye, which was subsequently operated after 1 week.
The main outcomes measured included confirmation of the diagnosis, infection control, reduction in inflammation, and visual recovery postintervention.
Cases were categorized as “confirmed” fungal endogenous endophthalmitis on the basis of microbiology of the vitreous sample, if KOH or Gram smear showed the presence of budding yeast-like structure or fungal branching filaments or fungal colonies were grown on culture. They were considered as “presumed” fungal endophthalmitis if the clinical picture showed ocular inflammation with the presence of fluffy creamy yellowish lesions with a string of pearl appearance and responded to vitrectomy with intravitreal, topical, and systemic antifungal therapy.
Results
From May 2020 to January 2021, five cases presented to us with endogenous endophthalmitis postrecovery from severe COVID-19. In all the cases, the signs far exceeded the symptoms. Three cases were unilaterally affected, and two cases had bilateral involvement. The clinical picture and previtrectomy working diagnosis were suggestive of fungal endophthalmitis in all the cases. All seven eyes underwent a vitrectomy to salvage vision. All subjects were male. The median age of the cases was 47 years (range 30–67 years). Three of these (Case numbers 3, 4, and 5) had hypertension and two (Cases 4 and 5) had Type 2 diabetes mellitus, which were well controlled systemically on medication. The demographic details and clinical profile of each of the cases are depicted in Table 1.
Table 1.
Demographic details and clinical profile of patients who developed endogenous endophthalmitis post COVID-19
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age (years) | 30 | 62 | 47 | 44 | 67 |
| Gender | M | M | M | M | M |
| Systemic illness | Nil | Nil | HT | DM, HT | DM, HT |
| Duration of Hospitalization | 21 days | 12 days | 8 days | 30 days | 20 days |
| ICU stay | Yes | Yes | Yes | Yes | Yes |
| Duration between COVID-19 diagnosis and ocular symptoms | 2 weeks | 2 weeks | 4 weeks | 5 weeks | 3 weeks |
| Duration between discharge from hospital to onset of symptoms | 1 days | 6 days | 14 days | 8 days | 1 day |
| Duration of symptoms prior to reporting to us | 2 weeks | 8 weeks | 4 weeks | 2 weeks | 3 weeks |
| Treatment with steroids | IV methylprednisolone f/b oral methylprednisolone | Oral dexamethasone f/b oral Prednisolone | IV dexamethasone f/b oral Dexamethasone | IV dexamethasone f/b oral prednisolone | IV methylprednisolone f/b oral methylprednisolone |
| Total duration of steroid therapy | 21 days | 80 days | 18 days | 42 days | 50 days |
| Eye affected | OU | OD | OS | OS | OU |
M=Male, HT=Hypertension, DM=Diabetes Mellitus, ICU=Intensive care Unit, IV=Intravenous, OD=Right, OS=Left, and OU=Both
All cases had previously been hospitalized in ICUs for COVID-19 pneumonia with the mean duration 18.2 ± 8.56 days (range 8–30 days) of hospital stay. All cases had received intravenous antibiotics, as well as intravenous or oral antiviral therapy. They had also received systemic steroid therapy for an average duration of 42 ± 25.1 days (range 18–80 days). All cases received systemic steroids for the management of severe COVID-19 pneumonia. It is of note that Case 2 received 20 days of steroids for COVID-19, followed by 60 days of a tapering regimen of oral steroids from his treating ophthalmologist for his ocular condition. All cases required oxygen support, but none of them had been on invasive mechanical ventilation.
The average period between the diagnosis of COVID-19 infection and onset of ocular complaints was 3.2 weeks (range 3–5 weeks). The average period of onset of symptoms after discharge from the hospital was 6 days (range 1–14 days). The average time of presentation to our institutes after the onset of symptoms was 3.8 weeks (range 2–8 weeks). All patients were initially diagnosed as noninfectious uveitis. The blood culture of these cases showed no bacterial or fungal growth. Ocular features, microbiological findings, treatment administered, and outcome are shown in Table 2.
Table 2.
Ocular features, microbiological findings, treatment, and outcome for seven eyes of five cases with endogenous endophthalmitis post COVID-19
| Eye (Case) | 1 (Case 1) | 2 (Case 1) | 3 (Case 2) | 4 (Case 3) | 5 (Case 4) | 6 (Case 5) | 7 (Case 5) |
|---|---|---|---|---|---|---|---|
| Presenting Vision | 20/200 | 20/400 | HM | CFCF | 20/600 | 20/1200 | HM |
| Prior ocular Treatment for uveitis | Topical NSAIDs and prednisolone | Topical NSAIDs and prednisolone | Oral and topical prednisolone | Oral and topical prednisolone f/b PPV | Topical NSAIDs and prednisolone | Oral, topical Prednisolone, periocular triamcinolone | Oral, topical Prednisolone, periocular triamcinolone |
| Anterior segment findings | AC cells 1+, Flare 1+ | AC cells 1+, Flare 1+ | AC cells 2+, Flare 2+PSC Cataract | AC Cells 1+Flare 1+ | AC cells 3+, Flare 3+ | AC cells 3+, Flare 3+NS+PSC cataract | AC cells 3+, Flare 3+NS+PSC cataract |
| Fundus findings | Creamy-white fluffy lesion at posterior pole, break through hemorrhage. | Creamy-white fluffy lesions at posterior pole, break through hemorrhage. | Vitreous exudates with string of pearls appearance. | Vitreous exudates, granuloma nasally and at posterior pole. Total RD. | Vitreous exudates with cotton balls, small retinal abscess inferior to the ONH. | Vitreous exudates with retinal abscess inferotemporal to fovea. | Vitreous exudates with retinal abscess at macula and large subretinal abscess temporally. |
| Organism isolated on culture | Candida sp. | Candida sp. | nil | Aspergillus sp. | nil | Candida sp. | Candida sp. |
| Treatment | PPV with lV voriconazole and silicone oil injection | PPV with IV voriconazole and silicon oil injection | PPV with IV voriconazole and IV antibiotics | Re-Vitrectomy +Lensectomy +RR silicone oil+IV Voriconazole | IV voriconazole f/b PPV with IV voriconazole+IV antibiotics | PPV with IV voriconazole+IV antibiotics | PPV with IV voriconazole+IV antibiotics+silicone oil |
| Postoperative status | Vitreous cavity clear. ERM along the disc to macula | Vitreous Cavity clear. ERM over macula | Vitreous cavity clear PSC cataract + | Vitreous cavity clear, retina attached, retinal abscess resolving | Vitreous cavity clear, area of abscess getting scarred | Vitreous cavity clear, area of abscess getting scarred | Vitreous cavity clear, area of central abscess getting scarred, other abscesses resolving |
| Final Vision | 20/80 | 20/40 | 20/200 | 20/1200 | 20/40 | 20/80 | CFCF |
| Follow up | 24 weeks | 24 weeks | 16 weeks | 10 weeks | 8 weeks | 8 weeks | 8 weeks |
NSAID=Nonsteroidal antiinflammatory drugs, PPV=Pars plana vitrectomy, PSC=Posterior subcapsular, IV=Intravitreal, AC=Anterior chamber, HM=Hand movements, CFCF=Counting fingers close to face, ERM=Epiretinal membrane, RR=Relaxing retinotomy, and RD=Retinal detachment
The median preoperative visual acuity at presentation was 1.8 LogMAR (Snellen equivalent = 20/1200), Range HM (hand movements) to 20/200. All cases presented to us with anterior chamber inflammation. Fundus examination revealed creamy white posterior pole lesions along with vitreous exudates [Figs. 1-3]. Case 3 had undergone a previous PPV for suspected endophthalmitis after initial therapy elsewhere with oral and topical steroids for suspected noninfectious uveitis. He presented to us with his vitreous cavity full of exudates, total retinal detachment, and a large creamy-white granuloma nasally. We performed revitrectomy, with lensectomy and relaxing retinotomy nasally to release the traction and adequately debulk the granuloma. He also had a small subretinal abscess at the posterior pole superonasal to fixation [Fig. 2]. Case 5 presented with vitreous exudates in both eyes, with the left eye being more severely affected, having multiple subretinal abscesses [Fig. 3].
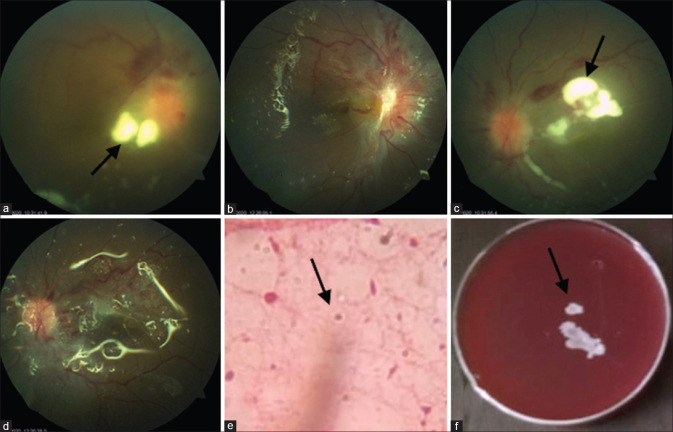
Figure 1.
Bilateral involvement in case 1. (a and c) Right and left eyes, respectively, showing whitish fluffy lesions (arrows) at the posterior pole, breakthrough preretinal hemorrhage, and mild disc hyperemia. (b and d) Postvitrectomy picture in the right and left eyes, with silicone oil in situ, clear vitreous cavity, and small fibrous proliferation over the optic disc. (c) Wet film 10% KOH mount shows double-walled yeasts-like organism. (arrow) (f) Growth on blood agar is seen as creamy white confluent colonies of Candida sp
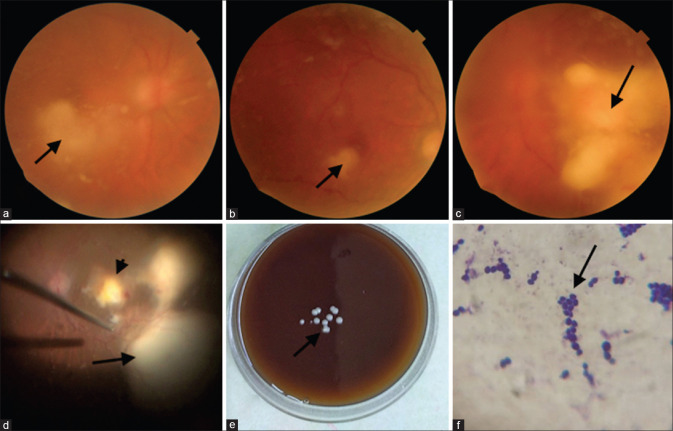
Figure 3.
Bilateral involvement in case 5. (a) Right eye showed diffuse fluffy cotton balls and a retinal abscess (arrow). (b) four weeks postvitrectomy; the vitreous cavity is clear, and the retinal abscess (arrow) is resolving. (c) the worse affected left eye showed a large clump of vitreous exudates (arrow). (d) intraoperatively a retinal abscess is seen at the posterior pole (arrowhead) along with two larger retinal abscesses (arrow). (e) Blood agar showing creamy white, smooth, discrete, and well-defined colonies of Candida sp. (arrow) (f) Gram stain showing budding gram-positive yeast cells. (arrow)
Figure 2.
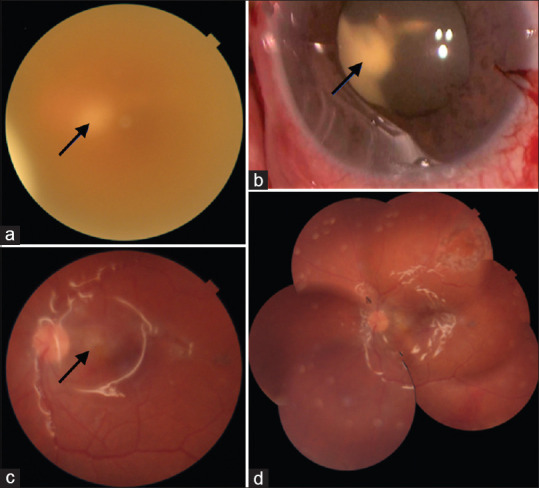
Left affected eye in case 3. (a) Fundus photograph showing whitish retinal abscess (arrow) seen hazily through the turbid vitreous. (b) Intraoperatively, a large clump of exudates (arrow) was seen in the retrolenticular space. Lensectomy and retinotomy were necessary to remove this large granuloma. (c) 1 posttreatment, the retinal view improved and the resolving retinal abscess is seen (arrow). (d) Fundus photo montage shows clear vitreous cavity, silicone oil in situ, and well-attached retina
All cases underwent three-port PPV with intravitreal injection of voriconazole at the end of the surgery. In addition, eye numbers 1, 2, 4, and 7 had silicone oil injection at the end of surgery as retinal tamponade, due to the severity of the infection. Microbiological examination of the vitreous biopsy aspirate was carried out for all the cases. This revealed a growth of Candida sp. in four eyes, Aspergillus sp. in one eye; these were categorized as confirmed fungal endophthalmitis. There was no growth in two eyes that were categorized as presumed fungal endophthalmitis cases.
All cases showed a significant improvement in terms of control of intraocular infection with a reduction in inflammation in the form of a quiet anterior chamber and clear vitreous cavity [Table 2]. The median postoperative vision achieved 0.6 LogMAR (Snellen equivalent 20/80) Range CFCF (counting fingers close to face) to 20/40. All cases received broad-spectrum antibiotics and antifungal eye drops (Topical Voriconazole) along with oral voriconazole.
Discussion
Endogenous endophthalmitis is a rare, but potentially devastating intraocular infection in which pathogens reach the eye via the bloodstream. Identified risk factors for endogenous endophthalmitis include chronic diseases (e.g., diabetes mellitus, renal failure, malignancies, and acquired immunodeficiency syndrome); immunosuppressive treatment; recent invasive surgery; intravenous drug abuse; indwelling catheters; organ transplantation; and pregnancy or postdelivery.[6]
A database review from January 2019 till May 2020 at our two tertiary care institutions revealed a total of 32 cases of endophthalmitis of which there were only two cases of endogenous fungal endophthalmitis. The sudden uptick in these cases of endogenous endophthalmitis during the past 8 months of the COVID-19 pandemic led us to conduct this study.
In our case series, otherwise immunocompetent patients with a history of recent severe COVID-19 infection with prolonged hospitalization and treatment with corticosteroids developed endogenous endophthalmitis.
COVID-19 viral infection causes a significant strain on the immune system of a patient. Yang et al.[9] have reported a significant decrease in lymphocytes especially the CD4 counts, which could predispose to opportunistic infections. The current guidelines in India recommend the use of intravenous corticosteroids as a life-saving option in severe COVID-19 disease.[10] This is further supported by the RECOVERY trial data,[11] which showed a reduction in mortality rate in patients on mechanical ventilators or on oxygen support when treated with steroids. An aggressive though necessary use of steroids could further worsen an already compromised immune system, making it susceptible to secondary infections. Widely accepted standardized protocols,[12] as well as the RECOVERY study, recommended using oral or intravenous steroids up to 10 days for severely ill patients with COVID-19, who are on supplemental oxygen or ventilatory support. In contrast, our patients received an average of 42 ± 25.1 days (range 18–80 days) of steroid therapy.
Fungal organisms are responsible for more than half of all cases of endogenous endophthalmitis, with Candida albicans being the commonest pathogen (75–80%).[13] A review of literature revealed a number of reports of serious systemic fungal infections seen in post COVID-19 patients. White et al.[14] showed an incidence of 26.7% of invasive fungal disease in ICU patients of COVID-19, most commonly with Aspergillus with the use of corticosteroid increasing the likelihood of fungal infection. From India, Mehta et al.[15] reported a severe case of Rhino orbital mucormycosis associated with COVID-19 in a 60-year-old patient, which they attributed to the extensive use of parental corticosteroids. Sen et al.[16] reported six cases of mucormycosis in patients with moderate to severe COVID-19 infection. They also found that the increased use of corticosteroids increased the risk of invasive fungal infection with mucormycosis.
Shah et al.[7] recently reported four cases of presumed fungal endogenous endophthalmitis with a similar clinical picture in post COVID-19 patients. However, a microbiological diagnosis could not be obtained in any of their cases.
In our series of patients, a fungal pathogen was identified as the causative agent for endophthalmitis in five out of the seven eyes. In the other two eyes, presumed fungal endophthalmitis was the working diagnosis. In all our cases, the signs far exceeded the symptoms, supporting a fungal etiology.
Though one attempts to identify the source of infection in endogenous endophthalmitis, it may not always be possible. In our study, the blood culture showed no bacterial or fungal growth in any of our cases. Binder et al.[17] in their 18-year review of endogenous endophthalmitis have shown that in 44% of their patients no additional infection foci other than the eye was found, and thus postulated it to a transient bacteremia or fungemia.
During the COVID-19 pandemic, a fivefold increase in intravenous line-related candidemia has been reported.[18] These studies indicate that prolonged hospitalization and treatment with steroids during the pandemic are creating an ideal setting for fungal infections to develop.
In our cases, we presume that indwelling peripheral venous catheter intravenous lines along with the immunosuppressed state, due to the prolonged steroid therapy, were the probable sources of infection.
The vision loss in our patients was gradual. This led to a delay in patients seeking consultation and reaching an appropriate diagnosis. Compounding the situation is the fact that vitritis has been reported in patients with COVID-19,[19] prompting ophthalmologists to treat such patients with topical, periocular, and oral steroids. In the past, ICU patients were predominantly treated with high-dose antimicrobial agents rather than immunosuppressive therapy. During the pandemic, we have patients receiving high-dose corticosteroids in the ICU for COVID-19 infection.
Fungal endogenous endophthalmitis usually has an indolent course. This immunosuppressed state has resulted in further masking the signs and symptoms of intraocular inflammation leading to delayed presentation and manifestations of endogenous endophthalmitis.
In fact, all our cases were initially diagnosed as noninfectious uveitis by their primary ophthalmologists and treated with steroids [Table 2].
The prognosis and outcome of endogenous endophthalmitis are generally worse than exogenous endophthalmitis because of compromised host immunity, the initial involvement of the posterior segment, and aggressive pathogens being involved. However, prompt diagnosis and treatment would improve anatomical and visual outcomes.[20] In our patients too, we were able to salvage all eyes with prompt vitrectomy along with intravitreal voriconazole injection. Postoperatively, we achieved control of infection in the form of a clear vitreous cavity, along with an improvement in vision.
We acknowledge several shortcomings in our study. The number of eyes studied is small, which could be due to the rare nature of this disease. The duration of the follow-up is short. Two of the seven eyes had a clinical diagnosis where the microbiological confirmation was lacking. However, the main purpose of our current work is to create awareness and avoid misdiagnosis of this potentially blinding infectious disease.
Our study in conjunction with the series by Shah et al.,[7] highlights the emerging issue of endogenous fungal endophthalmitis in post COVID-19 cases.
Conclusion
Our findings emphasize the importance for all caregivers to have a high index of suspicion of endogenous fungal endophthalmitis in post COVID-19 patients complaining of blurred vision with floaters. We recommend a fundus examination of patients on intensive steroid therapy for severe COVID-19, especially in those with any visual complaints.We would also like to highlight the value of judicious use of steroids, which while being a life-saving medication in patients with severe COVID-19 could predispose them to infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Clinical management of COVID-19. [Last accessed on 2021 Feb 10]. Available from: https://www.who.int/publications/i/item/clinical-management -of-covid-19 .
- 2.Kumar KK, Sampritha UC, Prakash AA, Adappa K, Chandraprabha S, Neeraja TG, et al. Ophthalmic manifestations in the COVID-19 clinical spectrum. Indian J Ophthalmol. 2021;69:691–4. doi: 10.4103/ijo.IJO_3037_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheth JU, Narayanan R, Goyal J, Goyal V. Retinal vein occlusion in COVID-19:A novel entity. Indian J Ophthalmol. 2020;68:2291–3. doi: 10.4103/ijo.IJO_2380_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumitrascu OM, Volod O, Bose S, Wang Y, Biousse V, Lyden PD. Acute ophthalmic artery occlusion in a COVID-19 patient on apixaban. J Stroke Cerebrovasc Dis. 2020;29:104982. doi: 10.1016/j.jstrokecerebrovasdis.2020.104982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2020;34:2352–3. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingappan A, Wykoff CC, Albini TA, Miller D, Pathengay A, Davis JL, et al. Endogenous fungal endophthalmitis:Causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol. 2012;153:162–6.e1. doi: 10.1016/j.ajo.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Shah KK, Venkatramani D, Majumder PD. A case series of presumed fungal endogenous endophthalmitis in post COVID-19 patients. Indian J Ophthalmol. 2021;69:1322–5. doi: 10.4103/ijo.IJO_3755_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connell PP, O'Neill EC, Fabinyi D, Islam FMA, Buttery R, McCombe M, et al. Endogenous endophthalmitis:10-year experience at a tertiary referral centre. Eye. 2011;25:66–72. doi: 10.1038/eye.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Gou J, Gao J, Huang L, Zhu Z, Ji S, et al. Immune characteristics of severe and critical COVID-19 patients. Sig Transduct Target Ther. 2020;5:179. doi: 10.1038/s41392-020-00296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical management protocol for COVID 19. [Last accessed on 2021 Jan 29]. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf .
- 11.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covid 19:Management in hospitalised adults. [Last accessed on 2021 May 18]. https://www.uptodate.com/contents/covid-19-management-in-hospitalized-adults?search=coronavirus&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2 .
- 13.Schiedler V, Scott IU, Flynn HW, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis:Clinical features and visual acuity outcomes. Am J Ophthalmol. 2004;137:725–31. doi: 10.1016/j.ajo.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 14.White PL, Dhilon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020:ciaa1298. doi: 10.1093/cid/ciaa1298. doi:10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID 19. Cureus. 2020;12:e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in viral land:A tale of two pathogens. Indian J Ophthalmol. 2021;69:244–52. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binder M, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis:An 18 year review of culture positive cases at a tertiary care center. Medicine. 2003;82:97–105. doi: 10.1097/00005792-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Clough N, Pringle E, Minakaran N, Schelenz S. Care for critically Ill patients with COVID-19:Don't forget the eyes. Eye. 2021;35:1054–5. doi: 10.1038/s41433-020-01148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zago Filho LA, Lima LH, Melo GB, Zett C, Farah ME. Vitritis and outer retinal abnormalities in a patient with Covid 19. Ocul Immunol Inflamm. 2020;28:1298–300. doi: 10.1080/09273948.2020.1821898. [DOI] [PubMed] [Google Scholar]
- 20.Keswani T, Ahuja V, Changulani M. Evaluation of outcome of various treatment methods for endogenous endophthalmitis. Indian J Med Sci. 2006;60:454–60. [PubMed] [Google Scholar]