Abstract
Purpose:
The aim of this study was to assess the systemic immune-inflammation index (SII) levels, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in patients with keratoconus (KC).
Methods:
A total of 42 patients with KC (KC group) and 42 age- and sex-matched healthy subjects (control group) were included into this cross sectional study. Complete blood count parameters were assayed. SII, NLR, red cell distribution width (RDW), and PLR values were calculated. The SII value was calculated as follows: platelet count × (neutrophil/lymphocyte).
Results:
SII, NLR, RDW, and PLR values were significantly higher in KC group compared to control group [709 ± 236 vs. 418 ± 117 (P < 0.001), 2.5 ± 0.8 vs. 1.76 ± 0.3 (P < 0.001), 14.3 ± 1.6% vs. 12.9 ± 0.54% (P < 0.001), and 143 ± 36 vs. 106 ± 23 (P < 0.001), respectively]. Using the receiver operating characteristics (ROC) curve analysis to predict KC, the highest area under the curve (AUC) was determined SII (0.846 for SII, 0.778 for NLR, and 0.796 for PLR).
Conclusion:
SII, NLR, RDW, and PLR levels were significantly increased in patients with KC. This study supports the idea that several inflammatory pathways may play important role in the pathogenesis of this disorder. SII may be much better marker than NLR and PLR for predicting the inflammatory status of the disease.
Keywords: Inflammation, keratoconus, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, red cell distribution width, systemic immune-inflammation index
Keratoconus (KC) is an ectatic corneal disorder characterized by stromal thinning, protrusion, and scar formation in the cornea and leads to irregular astigmatism, myopia, and decreased vision.[1] Pathophysiological mechanism of KC is still not fully understood yet. Many possible mechanisms have been proposed. Genetic predisposition and environmental factors play a crucial role in the pathogenesis of the disease.[2] Current reports and studies have demonstrated that pro-inflammatory markers (Interlukin-6, 1-b and Interferon-γ) are over expressed, whereas the anti-inflammatory marker (Interlukin-10) is under expressed in the tear film and of patients with KC.[3] In addition to these local inflammatory markers, systemic inflammatory markers, such as neutrophil/lymphocyte ratio (NLR), monocyte/high-density lipoprotein cholesterol ratio, platelet/lymphocyte ratio (PLR), and red blood cell distribution width (RDW) have also been shown to be elevated in patients with KC.[4,5,6] Therefore, the pathogenesis of keratoconus may involve complex chronic inflammatory pathways. In this context, classifying KC as inflammatory-related rather than a non-inflammatory disease appears to be more appropriate, and may help focus attention on the possibility of developing effective anti-inflammatory therapies for its management.
Systemic immune-inflammation index (SII), as a novel inflammatory biomarker, has been proposed as prognostic indicators in several different clinical settings, including coronary artery disease,[7] cancers.[8,9] In the field of ophthalmology a recent study has shown a significant relationship between SII and primary open-angle glaucoma,[10] and also with dry eye disease.[11] To the best of our knowledge, the importance of SII in patient with KC has not been reported to date. We hypothesized that SII, a combination of three peripheral inflammatory cells (lymphocytes, neutrophils, and platelet count), could better reflect the inflammatory status and prognosis than other inflammatory markers in KC.
In the present study, we particularly hoped to contribute to the inflammatory basis of KC. Hence, we aimed to investigate the levels of SII as well as NLR, RDW, and PLR to find out whether they may predict the development and progression of disease in patients with KC.
Methods
This cross-sectional study included 42 patients with KC (KC group) and 42 age- and sex- matched healthy individuals (Control group) who were examined at the ophthalmology clinic of a university hospital. The study was conducted in accordance with the Helsinki Declaration rules and informed consent forms of all volunteers. Ethics committee approval was received from our university hospital.
The exclusion criteria were study patients with an ocular disease other than KC, history of prior corneal surgery as refractive, cross-linking or any other ocular surgery, diabetes mellitus, hypertension, cardiovascular diseases, cerebrovascular disease, acute or chronic renal failure, acute or chronic liver disease, acute or chronic local/systemic infection, anemia, malignancy, those using steroids or anti-hyperlipidemic therapy, patients with a history of previous surgery within the last 3 months, smokers and alcohol users, pregnancy, systemic and/or ocular allergic diseases such as atopic eye disease or associated hay fever and current anti-inflammatory therapies. Additional exclusion criteria for healthy participants were spherical error >+3.00 D and >–3.00 D, corneal astigmatism >1.50 D, and clinical findings and family history of KC.
All study participants underwent detailed ophthalmologic evaluation including refractive error, visual acuity, tonometry, topography, slit-lamp and fundoscopic examination.
Diagnosis of KC was made according to a constellation of signs and symptoms including progressively worsening refractive error, scissoring reflex on retinoscopy, irregular astigmatism, slit lamp signs (Fleischer ring, Vogt’s striae, corneal thinning and scarring), and topographical evidence of corneal ectasia.[12] In this context, eyes with corneal thinning, apical protrusion, and ectasia were accepted as KC after clinical and topographic evaluations by Sirius (CSO Inc, Florence, Italy). Mean keratometry (K mean) values of KC eyes were recorded. If KC was present in one eye of the patient, that eye or KC was present in two eyes of the patient, staging was performed based on the eye with high keratometry value in our study. The most common subclinical KC definition used refers to an eye with topographic signs of KC and/or suspicious topographic findings under normal slit-lamp examination and keratoconus in the fellow eye and the most common forme fruste KC definition refers to an eye with normal topography, normal slit-lamp examination, and KC in the fellow eye.[13] Fellow KC eyes of patients with forme frustae/sub-clinical keratoconus were also included. Based on the anterior surface K mean, two KC subgroups were established as; mild/moderate group (K mean between ≤48 D and <52 D); and severe group (Kmean ≥52 D) using Amsler–Krumeich classification.[14] After a fasting period of 8-12 h, venous blood samples were collected by antecubital vein and mixed with 0.2 ml 3.8% sodium citrate solution and analyzed within 2 h after sampling with Cell-Dyn 3700 Hematology Analyzer (Cell-Dyn 3700, Abbott Diagnostics, Abbott Park, IL, USA). The NLR was calculated by dividing the neutrophil count by the lymphocyte count, the PLR was calculated by dividing the platelet count by the lymphocyte count and SII as platelet count × (neutrophil/lymphocyte).
Statistical analysis
All analyses were performed using SPSS 21.0 for Windows software. Quantitative variables were described using mean value ± standard deviation. The normal distribution of the results was checked by Kolmogorov–Smirnov test. The distribution of all variables was found to be normal. Independent Samples T test was used to compare parameters between two groups. Comparison of categorical variables was made with Chi-square test. A one-way ANOVA was used to compare the study groups. Subgroup analysis was interpreted with Bonferroni test. In order to show sensitivity and specificity of the optimal SII cutoff values in patients with KC, receiver operating characteristic (ROC) curves were created and areas under curve (AUC) were calculated. A value of P < 0.05 was considered as significant.
Results
The mean ages of the KC group and control group were 31.2 ± 9.5 and 32.2 ± 6.5 years, respectively (P = 0.569). Genders was also similar between the groups (P = 0.503). Of all 42 patients with KC, 24 (57.1%) patients were in mild/moderate subgroup, 18 (42.9%) patients were in severe subgroup. Demographics and clinical characteristics of the groups are shown in Table 1. Compared to the control group, SII [Fig. 1], NLR, RDW levels, and PLR were significantly higher in KC group [709 ± 236 vs. 418 ± 117 (P < 0.001), 2.5 ± 0.8 vs. 1.76 ± 0.3 (P < 0.001), 14.3 ± 1.6% vs. 12.9 ± 0.54% (P < 0.001), and 143 ± 36 vs. 106 ± 23 (P < 0.001), respectively]. Complete blood count data of the groups are shown in Table 2. In the severe KC subgroup, SII gradually elevated, and the difference was statistically significant (P = 0.040), Table 3). We have also showed the comparison of blood parameters among mild (Kmean ≤ 48 D), moderate, severe KC, and controls in Table 4. The differences regarding SII, NLR, and PLR were significant among the groups (P < 0.001 for all). There was also a significant difference between mild KC and control group (for SII P < 0.001, for NLR P < 0.001, and for PLR P = 0.01). However, the differences were not significant between mild and moderate KC groups (P = 1 for all).
Table 1.
Comparison of demographic characteristics between the groups
| Characteristics | Control Group | Keratoconus Group | P |
|---|---|---|---|
| Number of subjects, n | 42 | 42 | |
| Age, years (Mean±SD) (min-max) | 32.2±6.5 (21-45) | 31.2±9.5 (18-52) | 0.569 |
| Gender, n (%) | |||
| Female | 24 (57.1) | 27 (64.2) | 0.503 |
| Male | 18 (42.9) | 15 (35.8) | |
| Keratoconus severity, (n %) | |||
| Mild/moderate | 24 (57.1) | ||
| Severe | 18 (42.9 ) |
SD: Standard deviation
Figure 1.
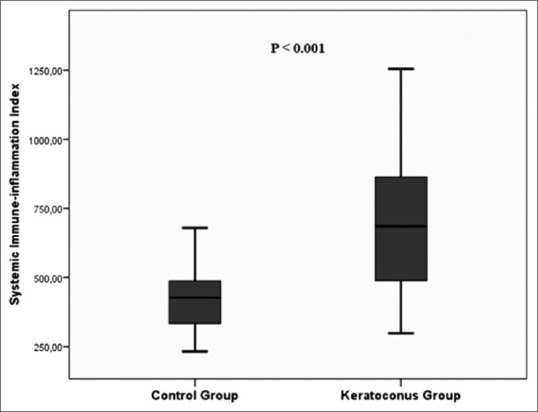
Comparison of systemic immune inflammation index levels (SII) between the kerataconus and control groups
Table 2.
Laboratory characteristics of patients with keratoconus and control subjects
| Characteristics (Mean±SD) | Study Groups | P | |
|---|---|---|---|
|
| |||
| Control group (n=42) | Keratoconus group (n=42) | ||
| White Blood Cell Count (×109/L) | 6.92±1.28 | 7.73±1.36 | 0.006 |
| Neutrophil Count (×109/L) | 3.96±0.91 | 4.96±1.1 | <0.001 |
| Lymphocyte Count (×109/L) | 2.29±0.48 | 2.12±0.56 | 0.131 |
| Platelet Count (×109/L) | 238±45 | 286±50 | <0.001 |
| Systemic immune inflammation index (min-max) | 418±117 (232-679) | 709±236 (298-1254) | <0.001 |
| Neutrophil to Lymphocyte Ratio (min-max) | 1.7±0.3 (1.28-2.59) | 2.5±0.8 (1.29-5.45) | <0.001 |
| Platelet to Lymphocyte Ratio (min-max) | 106±23 (68-165) | 143±36 (76-216) | <0.001 |
| Red Cell Distribution Width (%) | 12.9±0.5 | 14.3±1.6 | <0.001 |
| Hemoglobin (g/dL) | 13.9±1.1 | 13.7±1.8 | 0.549 |
SD: Standard deviation
Table 3.
Comparison of laboratory data of keratoconus patients according to keratoconus severity
| Characteristics (Mean±SD) | Keratoconus group (n=42) | P | |
|---|---|---|---|
|
| |||
| Mild/moderate subgroup (n=24) | Severe subgroup (n=18) | ||
| Systemic immune inflammation index | 645±210 | 795±248 | 0.040 |
| Neutrophil to Lymphocyte Ratio | 2.49±1.01 | 2.51±0.58 | 0.925 |
| Platelet to Lymphocyte Ratio | 141±41 | 146±30 | 0.640 |
SD: Standard deviation
Table 4.
Comparison of laboratory data among the groups
| Characteristics (Mean±SD) | Mild Keratoconus (n=13) | Moderate Keratoconus (n=11) | Severe Keratoconus (n=18) | Control Group (n=42) | P |
|---|---|---|---|---|---|
| Systemic immune inflammation index | 643±250 | 672±145 | 869±213 | 418±116 | <0.001 |
| Neutrophil-to-lymphocyte ratio | 2.66±1.22 | 2.28±0.70 | 2.51±0.58 | 1.76±0.37 | <0.001 |
| Platelet-to-lymphocyte ratio | 137±46 | 144±35 | 146±30 | 106±22 | <0.001 |
SD: Standard deviation
According to the ROC curve analysis, the predictive value of the SII, NLR, and PLR was evaluated by comparing the AUC area. The AUC of the SII, NLR, and PLR for KC were 0.846, 0.778, and 0.796, respectively [Fig. 2], indicating that SII is superior to other inflammatory indices. The optimal cutoff value of SII to predict KC was > 469, with 79% sensitivity and 72% specificity (95% confidence interval 0.763–0.929, P < 0.001).
Figure 2.

Receiver operator characteristic (ROC) curve of systemic immune inflammation index (SII), neutrophil/lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) for the prediction of kerataconus
Discussion
To date, this is the first study evaluating the SII levels in patients with KC. This study showed that compared to healthy controls, KC eyes had significant increased levels of SII as well as NLR, RDW and PLR. In addition, SII values were found to be significantly higher in patients with severe patients with KC.
KC is an asymmetric bilateral progressive corneal ectasia affecting life period between adolescence and fourth decades of life and may lead high rates of decreased vision.[2,3,4,5] Stromal thinning and changes in the cellular and lamellar structure of the cornea occur in KC.[15,16,17] Early diagnosis and treatment of the disease with advancing technological possibilities are more possible today. Corneal cross-linking is also gaining rapid popularity which has proven to be effective in halting the progression of KC. Although there are current methodologies to understand the mechanism of development of the disease, progression and risk factors have not been fully understood. Nearly 10%–20% of the patients may progress to advanced stages and could be candidates for corneal transplantation. Therefore, by evaluating simple and cheap routine hematological inflammation biomarkers, whether they may have a role in development of KC and predicting the progression of KC were aimed to find out. In addition to its correlation with atopy, eye rubbing, wearing contact lenses, ultraviolet irradiation or genetic component, recent studies mentioned the role of inflammation in the pathogenesis of KC.[18,19] Oxidative stress and inflammation may cause degradation of corneal extracellular matrix and modification of cellular components involving inflammatory features such as increased levels of matrix metallopeptidase 9, interleukin 6, tumor necrosis factor alpha, and hepatocyte growth factor.[20,21,22,23,24] Patients with KC were found to have significantly higher levels of interleukin-6, tumor necrosis factor-alpha, and matrix metalloproteinase 9 in their tear fluid.[23,24,25,26] Reports providing immuno-histochemical evidence of inflammation with cellular infiltration of macrophage, deposition of leukocytes and localization of dendritic Langerhans cells in keratoconic cornea were also appeared.[27,28] In addition, Loh et al.[29] investigated the cytokine profile of human keratoconic corneas. They supported the evidence for inflammatory pathway activation in KC and a possible redefinition of KC as a chronic inflammatory corneal disease.
The relation between NLR and KC investigated in the study of Karaca et al.[4] and they hound that the NLR values were higher in progressive patients with KC compared to nonprogressive group and controls. There was also a positive significant correlation between NLR and disease progression in their study. In the present study, apart from NLR, SII levels, for the first time, were also found significantly elevated in KC group. Additionally, in the severe KC subgroup SII gradually significantly elevated. Previous studies demonstrated an association between increased RDW and several ocular diseases including glaucoma,[30] retinal vein occlusion,[31] seasonal allergic conjunctivitis,[32] and pterygium.[33] In addition to these studies, in the present study increased RDW and PLR levels were demonstrated in patients with KC. Serum inflammation biomarkers in patients with KC were also studied in another study.[6] However, the authors did not find a significant difference between KC and control groups in terms of RDW levels, but they found PLR significantly higher in their KC group. In our study, both RDW and PLR levels, as well as SII and NLR, were found significantly elevated in KC group. In addition, the sample size of their KC group included 35 patients which were comparatively smaller than ours.
The mechanism underlying the association of SII with KC is not clear. Proposed mechanisms include possible roles for oxidative stress and chronic inflammation, as well as for endothelial dysfunction.[1] Tang et al.[10] reported that elevated NLR and SII might serve as readily available inflammatory predictors in primary open angle glaucoma patients. SII is one of the newly prognostic biomarker based on platelets, neutrophils, and lymphocytes. Lymphocytes are known to play a crucial role inhibiting cell proliferation and migration.[34] Lymphopenia indicates the ineffectiveness of the immune surveillance systems. High SII, consisting of high neutrophil and platelet as well as low lymphocyte counts, indicates inflammation activity that may be associated with KC. In this study, SII provided much stronger survival prediction compared to NLR. In the light of these findings, as a combination of both NLR and PLR, SII may be the most suitable biomarker for the pathogenesis and prediction of severity of KC. This study may add new information to understanding the role of inflammation in the pathophysiology of KC. In the light of our findings, most importantly, we feel that management of inflammation can be an important first step in the management and progression of KC.
This study has some limitations. The current study’s limitations include its relatively small sample size and single-center design. In addition, our study was lack of cytokines and C-reactive protein serum measurement as the other oxidative stress and inflammatory biomarkers. Inflammatory mediators in the tear film could not been studied. We have not collected the patients’ follow-up results yet. This may be considered as a subject of another study. Finally, we could not mention the process of calibration and validation of the automatic blood cell analyzer.
Conclusion
In conclusion, this study supports the idea that inflammation may play an important role in the pathogenesis of KC. The levels of SII, indicator of inflammation, as well as NLR, RDW and PLR, were found to be statistically significantly higher in patients with KC. SII, as a novel biomarker studied for the first time in patients with KC, may also be useful to grade the severity of disease, follow up the patients, and monitor the treatment. Moreover, SII may be much better than NLR and PLR for predicting the inflammatory status of the disease. However, further prospective, randomized controlled studies with larger series of patients are needed to obtain stronger evidence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 3.Sorkhabi R, Ghorbanihaghjo A, Taheri N, Ahoor MH. Tear film inflammatory mediators in patients with keratoconus. Int Ophthalmol. 2010;35:467–72. doi: 10.1007/s10792-014-9971-3. [DOI] [PubMed] [Google Scholar]
- 4.Karaca EE, Özmen MC, Ekici F, Yüksel E, Türkoğlu Z. Neutrophil to- lymphocyte ratio may predict progression in patients with keratoconus. Cornea. 2014;33:1168–73. doi: 10.1097/ICO.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 5.Katipoğlu Z, Mirza E, Oltulu R, Katipoglu B. May Monocyte/HDL Cholesterol ratio (MHR) and Neutrophil/Lymphocyte ratio (NLR) be an indicator of inflammation and oxidative stress in patients with Keratoconus? Ocul Immunol Inflamm. 2019;27:1–5. doi: 10.1080/09273948.2019.1611876. [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt E, Ucak T. Serum inflammation biomarkers in patients with Keratoconus. Ocul Immunol Inflamm. 2020:1–4. doi: 10.1080/09273948.2020.1741648. doi:10.1080/09273948.2020.1741648. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50:e13230. doi: 10.1111/eci.13230. [DOI] [PubMed] [Google Scholar]
- 8.Fest J, Ruiter R, Mulder M, Koerkamp BG, Ikram MA, Stricker BH, et al. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer. 2020;146:692–8. doi: 10.1002/ijc.32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, et al. Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. 2020;24:610–8. doi: 10.1007/s11605-019-04187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang B, Li S, Han J, Cao W, Sun X. Associations between blood cell profiles and primary open-angle glaucoma:A retrospective case-control study. Ophthalmic Res. 2020;63:413–22. doi: 10.1159/000504450. [DOI] [PubMed] [Google Scholar]
- 11.Ozarslan Ozcan D, Kurtul BE, Ozcan SC, Elbeyli A. Increased systemic immune- Inflammation index levels in patients with dry eye disease. Ocul Immunol Inflamm. 2020:1–5. doi: 10.1080/09273948.2020.1821899. doi:10.1080/09273948.2020.1821899. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Sahebjada S, Al-Mahrouqi HH, Moshegov S, Panchatcharam SM, Chan E, Daniell M, et al. Eye rubbing in the aetiology of keratoconus:A systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2021 doi: 10.1007/s00417-021-05081-8. doi:10.1007/s00417-021-05081-8. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Henriquez MA, Hadid M, Izquierdo L., Jr A systematic review of subclinical keratoconus and forme fruste keratoconus. J Refract Surg. 2020;36:270–9. doi: 10.3928/1081597X-20200212-03. [DOI] [PubMed] [Google Scholar]
- 14.Krumeich JH, Kezirian GM. Circular keratotomy to reduce astigmatism and improve vision in stage I and II keratoconus. J Refract Surg. 2009;25:357–65. doi: 10.3928/1081597X-20090401-07. [DOI] [PubMed] [Google Scholar]
- 15.Piñero DP, Alio JL, Barraquer RI, Michael R. Corneal biomechanics, refraction, and corneal aberrometry in keratoconus:An integrated study. Invest Ophthalmol Vis Sci. 2010;51:1948–55. doi: 10.1167/iovs.09-4177. [DOI] [PubMed] [Google Scholar]
- 16.Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948–56. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 17.Romeo-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus:A review. Cont Lens Anterior Eye. 2010;33:157–66. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Gatzioufas Z, Panos GD, Hamada S. Keratoconus:Is it a non-inflammatory disease? Med Hypothesis Discov Innov Ophthalmol. 2017;6:1–2. [PMC free article] [PubMed] [Google Scholar]
- 19.Ionescu IC, Corbu CG, Tanase C, Ionita G, Nicula C, Coviltir V, et al. Overexpression of tear inflammatory cytokines as additional finding in keratoconus patients and their first degree family members. Mediators Inflamm. 2018;2018:4285268. doi: 10.1155/2018/4285268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, Acera A. Keratoconus:An inflammatory disorder? Eye (Lond) 2015;29:843–59. doi: 10.1038/eye.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvis V, Tello A, Barrera R, Niño CA. Inflammation in keratoconus. Cornea. 2015;34:e22–3. doi: 10.1097/ICO.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 22.McMonnies CW. Inflammation and keratoconus. Optom Vis Sci. 2015;92:e35–41. doi: 10.1097/OPX.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 23.Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112:654–9. doi: 10.1016/j.ophtha.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 24.You J, Wen L, Roufas A, Hodge C, Sutton G, Madigan MC. Expression of HGF and c-Met proteins in human keratoconus corneas. J Ophthalmol. 2015;2015:852986. doi: 10.1155/2015/852986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lema I, Sobrino T, Durán JA, Brea D, Díez-Feijoo E. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. 2009;93:820–4. doi: 10.1136/bjo.2008.144253. [DOI] [PubMed] [Google Scholar]
- 26.Jun AS, Cope L, Speck C, Feng X, Lee S, Meng H, et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011;6:e16437. doi: 10.1371/journal.pone.0016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathew J, Goosey J, Burns A, Bergmanson J. Immunohistochemistry and ultrastructure of anterior stromal cells in keratoconus. Invest Ophthalmol Vis Sci. 2010;51:6230. [Google Scholar]
- 28.Mandathara PS, Stapleton FJ, Kokkinakis J, Willcox MD. A pilot study on corneal Langerhans cells in keratoconus. Contact Lens Anterior Eye. 2017;41:219–23. doi: 10.1016/j.clae.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Loh IP, Sherwin T. Is keratoconus an inflammatory disease? The implication of inflammatory pathways. 2020:1–10. doi: 10.1080/09273948.2020.1780271. doi:10.1080/09273948.2020.1780271. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Zhao B, Wang MY, Chen XY, Li D, Jiang XQ, et al. Associations between the red blood cell distribution width and primary angle-closure glaucoma:A potential for disease prediction. EPMA J. 2019;10:185–93. doi: 10.1007/s13167-019-00166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozkok A, Nesmith BLW, Schaal S. Association of red cell distribution width values with vision potential in retinal vein occlusion. Ophthalmol Retina. 2018;2:582–6. doi: 10.1016/j.oret.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Kurtul BE, Kabatas EU, Boybeyi SD, Caglar AA, Ozer PA. Increased red cell distribution width levels in children with seasonal allergic conjunctivitis. Int Ophthalmol. 2018;38:1079–84. doi: 10.1007/s10792-017-0563-x. [DOI] [PubMed] [Google Scholar]
- 33.Kurtul BE, Kabatas EU, Ozates S. The correlation of routine hematological with pterygium. Ther Adv Ophthalmol. 2019;11:2515841419848922. doi: 10.1177/2515841419848922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]


