Abstract
The purpose of the study is to describe cilioretinal artery (CILRA) occlusion that is presumed to be associated with COVID-19 without severe respiratory distress and inform ophthalmologists of unusual ocular presentations of COVID-19. Here, we present the first case of a patient with isolated CILRA occlusion and paracentral acute middle maculopathy (PAMM) after recently polymerase chain reaction-proven COVID-19. A 26-year-old female patient presented with a visual field defect in her left eye for 2 days and decreased vision compared to her right eye. It was learned that the patient had a laboratory-proven COVID-19 infection with mild respiratory symptoms that did not require hospitalization 2 weeks ago. Fundus examination revealed retinal edema in the left eye area supplied by the CILRA. Spectral-domain optical coherence tomography revealed a prominent hyperreflective band at the inner nuclear layer level. These findings led us to the diagnosis of isolated CILRA occlusion and PAMM associated with recent COVID-19. CILRA occlusion and PAMM could be associated with the inflammatory and procoagulant condition caused by the SARS-CoV-2 infection.
Keywords: Cilioretinal artery occlusion, COVID-19, ophthalmology, paracentral acute middle maculopathy retina, SARS-CoV-2
Cilioretinal arteries (CILRAs) are a congenital variant of the retinal circulation that can be detected by clinical examination and fluorescein angiography (FA) and occur in approximately 20– 32% of normal eyes.[1] CILRAs originate from the peripapillary choroid or one of the short posterior ciliary arteries and typically provide vascularization to the inner retina within the papillomacular area.[2] Cilioretinal artery occlusion (CILRAO) accounts for approximately 5% of retinal artery occlusions. While the symptoms and signs of CILRAO vary according to the etiology, they are categorized as one of three separate clinical presentations: (1) isolated CILRAO, (2) CILRAO associated with central retinal vein occlusion, and (3) CILRAO with giant cell arteritis.[3]
SARS-CoV-2 infection contributes to clotting abnormalities by disrupting factors released by endothelial cells that keep blood vessels in an antithrombotic state. The molecular interaction of SARS-CoV-2 with the ACE2 receptor located on the endothelial cell surface leads to early impairment of endothelial function and subsequent vascular inflammation and thrombosis of peripheral blood vessels.[4,5] The effects of the SARS-CoV-2 procoagulant state over the choroidal vascular system have not been investigated yet.
We report the first patient who developed an isolated CILRAO after SARS-CoV-2 infection to the best of our knowledge.
Case Report
A 26-year-old female complained of a central visual field defect in her left eye (LE), which had started 2 days before presentation. The patient realized a decrease in visual function when she covered the right eye (RE). Her medical history was not significant in any disease or drug use history other than SARS-CoV-2 infection 2 weeks ago. When she applied to the hospital 2 weeks ago with fever and dry cough complaints, nasopharyngeal samples were tested positive for SARS-CoV-2 infection in the polymerase chain reaction molecular test; she was diagnosed with COVID-19 [Table 1].
Table 1.
The patient’s clinic, coagulation and inflammatory markers during COVID-19 infection
| Age | 26 |
|---|---|
| Height | 168 cm |
| Weight | 51 kg |
| Thrombin time | 17 sn |
| Activated partial thromboplastin time | 39 sn |
| D-dimer | 611.4 ng/ml |
| Fibrinogen | 4.8 g/l |
| Platelet count | 178 g/l |
| C-reactive protein | 38.4 |
| Ferritin | 912 |
| Procalcitonin | 0.85 |
At presentation, the best-corrected visual acuity (BCVA) Snellen equivalent was 20/20 in her RE and 20/25 in her LE. Her refractive status was -0.75/-0.50 axis 100 in her RE and -0.75/-0.25 axis 60 in her LE. Anterior segment examination was unremarkable. Intraocular pressure was 14 mmHg in both eyes. Dilated fundus examination of the LE revealed a focal area of well-demarcated retinal whitening over the distribution of a CILRA in the superior papillomacular bundle region [Fig. 1a]. In FA, the slow filling of the involved CILRA was prolonged to the late venous phase; however, there was no clinically visible embolus. Spectral Domain-Optical Coherence Tomography (SD-OCT) demonstrated hyperreflectivity and thickening of all inner retinal layers in the area of involvement consistent with paracentral acute middle maculopathy (PAMM) [Fig. 1b]. Standard automated perimetry (SAP) (Humphrey Field Analyzer 24-2 SITA-standard test) revealed paracentral scotoma in her LE [Fig. 1c]. FA showed a filling delay in the CILRA region [Fig. 1d]. We diagnosed the disease as isolated CILRAO. After applying the dorzolamide/timolol fixed combination, topical bimatoprost and oral 250 mg acetazolamide, ocular massage was performed, and hyperbaric oxygen (HBO) therapy was initiated 48 h after the onset of symptoms.
Figure 1.
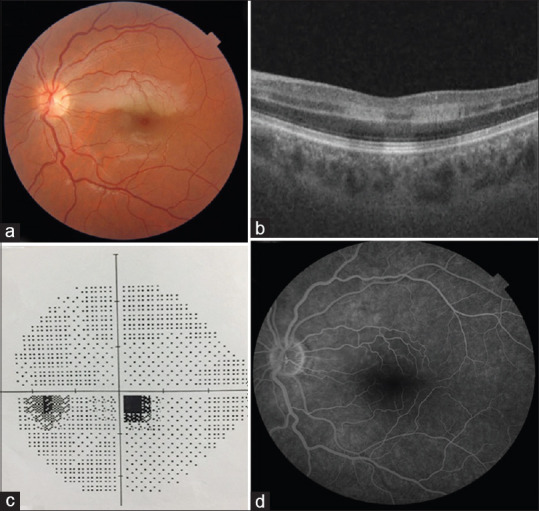
(a) Color fundus photograph of the isolated cilioretinal artery occlusion at the time of admission. (b) Paracentral acute middle maculopathy image on optical coherence tomography. (c) Paracentral scotoma on standard automated perimetry. (d) Filling delay in the cilioretinal artery in fluorescein angiography. The filling of the cilioretinal artery started at 21 s
The patient underwent 20 HBO therapy sessions (2.5 atm, 2 h daily, total 40 h). Three weeks after HBO treatment, the retinal edema significantly resolved [Fig. 2a], but the visual acuity level remained unchanged, which had a BCVA of 20/25 in her LE. The patient reported persistence of the inferior paracentral scotoma in her LE. In line with the patient’s statement, it was observed that the paracentral scotoma area expanded further in SAP [Fig. 2b]. On the third week after the end of treatment, SD-OCT revealed no significant change in the PAMM lesion [Fig. 2c], and it was observed that perfusion delay continued in the CILRA area in FA [Fig. 2d]. Her blood laboratory findings, systemic physical and cardiological examination, electrocardiogram, echocardiogram, neck Doppler ultrasonography, and chest X-ray imaging were unremarkable. No significant pathology was found in the studies on thrombophilia parameters (antithrombin III, protein C, protein S activities, and prothrombin G20210A mutation) and autoimmune antibodies (lupus anticoagulant, anticardiolipin antibodies) that predispose to thromboembolic pathologies. Also, there was no history of oral contraceptive pill use. In the examination performed 4 months after the onset of symptoms, it was observed that dysregulation developed in the inner layers of the retina; however, the BCVA of LE increased to 20/20, and the lower paracentral scotoma in the visual field shrunk [Fig. 3].
Figure 2.
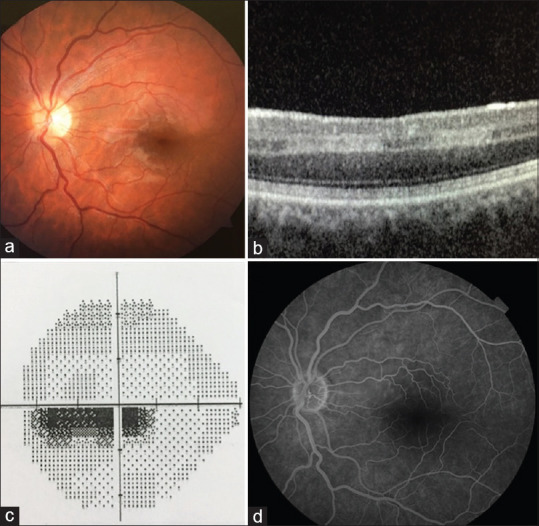
(a) Retinal edema significantly regressed in the color fundus photograph of the isolated cilioretinal artery occlusion 3 weeks after the patient’s admission. (b) It is seen that the paracentral acute middle maculopathy image in optical coherence tomography continues with a slight decrease. (c) Standard automated perimetry shows the deepening of the paracentral scotoma. (d) Fluorescein angiography shows that the cilioretinal artery filling delay continues
Figure 3.
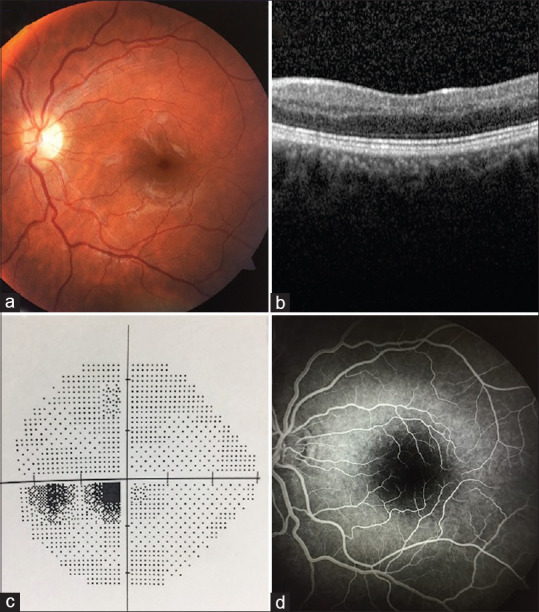
(a) At the final examination performed 4 months later, it was observed that retinal edema significantly regressed in the color fundus photograph of the isolated cilioretinal artery occlusion. (b) On optical coherence tomography image, it was observed that the paracentral acute middle maculopathy image regressed and some irregularities occurred in the inner retinal layers. (c) Standard automated perimetry shows the improvement of the paracentral scotoma. (d) In fluorescein angiography, the cilioretinal artery filling delay continues, but no obvious filling defect is observed
Discussion
The CILRA supplies a portion of the papillomacular bundle and a part of the fovea. Unlike the central retinal artery, CILRA does not have protective mechanisms against blood flow changes[6] and SD-OCT of most eyes with isolated acute CILRAO shows hyperreflectivity, mostly limited to the inner nuclear layer (INL), in a model known as PAMM, which was described by Sarraf et al.[7] Recently, Pichi et al.[3] reported the presence of PAMM lesions in all eyes with isolated CILRAO, which has been attributed to hypoperfusion of the deep vascular plexus and ischemic insult of the middle retinal layers, especially INL.[8] INL is more sensitive to low oxygen levels due to its deeper location in the watershed area between the retina and choroidal circulation.[9] In accordance with the literature, PAMM lesions’ presence in our case with isolated CILRAO was remarkable.
There are several potential causes of CILRAO, including hypertension, hypercholesterolemia, cardiac arrhythmia, hemoglobinopathies, vasculitis, causes of thrombophilia, migraine, pregnancy, embolism from an atherosclerotic lesion, or hypercoagulable state such as COVID-19. With the help of systemic and cardiovascular evaluation, laboratory and genetic findings, all possible causes except hypercoagulation state were ruled out in our case. With the patient’s age, the absence of any disease and drug history, and the exclusion of genetic causes of predisposition to hypercoagulability through extensive research, it was concluded that the most likely cause was COVID-19, a significant cause of acquired hypercoagulability.
It has been reported that COVID-19 disease causes inflammation-induced homeostasis changes and susceptibility to thrombosis in both venous and arterial circulation. SARS-CoV-2 directly affects the endothelial cells, causing diffuse endothelial inflammation (endotheliitis) and disruption of factors released by endothelial cells, contributing to maintaining blood vessels into an antithrombotic state, which leads to endothelial dysfunction and thrombosis.[10,11] Structural analyzes have been made that the SARS-CoV-2 spike protein interacts with subdomain I of ACE 2 receptor, which has also been detected in retinal tissue. However, once the connection between SARS-CoV-2 and ACE2 is established, the outer part of ACE2 is split and released. With downregulation of receptor expression, dysregulation also occurs in the renin–angiotensin system, which regulates the vasoconstriction, proliferation, and proinflammatory status of the vascular tree. It activates vascular endothelial cell dysfunction and triggers the prothrombotic cascade, vasoconstriction, and inflammation.[12] Also, endothelial cell dysfunction includes downregulation of endothelial nitric oxide synthase levels, altered expression of a vascular endothelial growth factor that induces a cytokine storm, recruits macrophages, and causes inflammatory reactions similar to those of vasculitis.[13,14]
Significantly prolonged prothrombin time and high D-dimer levels have been reported in studies investigating the risk of thromboembolic events during and after COVID-19 infection.[4] There are increasing reports in the literature of both venous and arterial thromboembolic complications caused by COVID-19. One of these reported that approximately 8% of hospitalized COVID-19 patients developed thromboembolic complications despite receiving anticoagulant prophylaxis. It is known that a significant percentage of individuals infected with SARS-CoV-2 survive the infection with mild symptoms or asymptomatic, and the available clinical and histopathological data on these cases are limited. Therefore, the idea is that thromboembolic complications in COVID-19 patients are a large-scale problem that should be considered during and after infection.
We report a patient who developed isolated CILRAO after a moderate COVID-19 disease, which was proven 2 weeks ago. In detailed clinical and laboratory studies, congenital and/or acquired pathology that could lead to the development of CILRAO was not encountered. Also, prolonged prothrombin time, high D-dimer levels, and high levels of inflammation biomarkers such as CRP and ferritin were detected in the patient during COVID-19 infection. Besides, it was found that she did not receive anticoagulant prophylaxis treatment. In light of these data, we assumed that the prothrombotic vascular endothelial microenvironment induced by SARS-CoV-2 might have represented a catalyst for the development of CILRAO. Our case was isolated CILRAO which suggested that the primary pathology was ischemia developing on the thromboembolic background. Although she did not apply within the first 24 h, it was thought that she could benefit from HBO treatment. In the literature, it has been reported that in the isolated CILRAO case, vision increased from 20/200 to 20/20 with HBO treatment initiated 22 h after the visual symptoms.[15] HBO therapy, which was initiated between 6 h and 6 days from the onset of central retinal artery occlusion, was reported to show significant improvement in vision level and retinal functions.[16] In a meta-analysis, it was reported that patients who received oxygen therapy showed a 5.61-fold probability of visual improvement compared to the control group who did not receive oxygen therapy, and it was reported that over 9 h of treatment length increased the therapeutic effect.[17] The inner retinal layers may receive sufficient oxygen through the diffusion of the choroidal circulation to sustain viability, and HBO can reduce the risk of retinal infarction, resolution of retinal edema by increasing tissue oxygen saturation. Although it cannot be started in the first 24 h of the onset of symptoms, in correlation with the positive results in the literature, we think that HBO treatment may contribute to the increase of the visual level to 20/20 and the improvement of paracentral scotoma in the visual field.
An antiinflammatory treatment was not considered in this case because the patient applied after the active SARS-CoV-2 infection was resolved. However, since endothelial inflammation predisposes to thromboembolic events, the use of systemic steroids and other antiinflammatory agents may be in question, especially in the acute phase in severe COVID-19 cases with high cytokine load. Further research can be designed to determine whether antiinflammatory therapy will reduce the incidence of thromboembolic events.
To the best of our knowledge, there are very few case reports of developing retinal artery occlusion associated with COVID-19, and there are additional systemic conditions such as hypertension, diabetes mellitus, and sickle cell anemia that may contribute to the development of thromboembolic events.[18,19] However, there is no case in the literature reporting isolated CILRAO in a young patient with no other systemic disease and no significant medical history. Although it is impossible to argue with absolute certainty, we think that the relationship between CILRAO and COVID-19 disease is reasonable. We must consider that the inflammatory and procoagulant condition secondary to COVID-19 can trigger retinal vascular occlusion even in young patients without another disease.
Conclusion
In conclusion, the relationship between microvasculitis and COVID-19 is becoming clearer day by day. We think that the development of retinal vascular occlusion is an important observation in a young patient who does not have any risk factors in terms of thromboembolic and vascular event development after COVID-19 with mild to the moderate clinical course as in our case. Considering the inflammatory and procoagulation status associated with COVID-19, we think that planning prophylactic antithrombotic (antiplatelet or anticoagulant) therapy may reduce potential adverse effects on the retinal and systemic vascular structure, especially in the middle and elderly population with systemic risk factors.
Ethics statement
Ethics committee approval was received from Kayseri City Training and Research Hospital (Approval number: 137319932). A scientific research application was made to the Ministry of Health of the Republic of Turkey on the COVID-19 19 case (2021-02-11T14_17_59).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Patel PS, Sadda SR. Retinal Artery occlusions. In: Wilkinson CP, Hinton DR, Sadda SR, Wiedemann P, editors. Ryan vs Retina. Sixth Edition. Philadelphia (PA): Elsevier; 2018. pp. 1136–50. [Google Scholar]
- 2.Hayreh S, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008;28:581–94. doi: 10.1097/IAE.0b013e31815ec29b. [DOI] [PubMed] [Google Scholar]
- 3.Picchi F, Fraggiota S, Freund KB, Au A, Lembo A, Nucci P, et al. Cilioretinal artery hypoperfusion and its association with paracentral acute middle maculopathy. Br J Ophthalmol. 2019;103:1137–45. doi: 10.1136/bjophthalmol-2018-312774. [DOI] [PubMed] [Google Scholar]
- 4.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–40. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–40. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30:359–94. doi: 10.1016/j.preteyeres.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarraf D, Rahimy E, Fawzi AA, Sohn E, Barbazetto I, Zacks DN, et al. Paracentral acute middle maculopathy:A new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131:1275–87. doi: 10.1001/jamaophthalmol.2013.4056. [DOI] [PubMed] [Google Scholar]
- 8.Nemiroff J, Phasukkijwatana N, Sarraf D. Optical coherence tomography angiography of deep capillary ischemia. Dev Ophthalmol. 2016;56:139–45. doi: 10.1159/000442806. [DOI] [PubMed] [Google Scholar]
- 9.Bakhoum MF, Freund KB, Dolz-Marco R, Leong BC, Baumal CR, Duker JS, et al. Paracentral acute middle maculopathy and the ischemic cascade associated with retinal vascular occlusion. Am J Ophthalmol. 2018;195:143–53. doi: 10.1016/j.ajo.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, et al. Modulation of TNF alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Nat Acad Sci USA. 2008;105:7809–14. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criado PR, Abdalla BM, de Assis IC, van Blarcum de Graaff Mello C, Caputo GC, Vieira IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not?Revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745–56. doi: 10.1007/s00011-020-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janga H, Cassidy L, Wang F, Spengler D, Oestern-Fitschen S, Krause MF, et al. Site-specific and endothelial-mediated dysfunction of the alveolar-capillary barrier in response to lipopolysaccharides. J Cell Mol Med. 2018;22:9829–98. doi: 10.1111/jcmm.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aktaş S, Uyar OM, Özer E, Aktaş H, Eltutar K. Idiopathic isolated cilioretinal artery occlusion treated with hyperbaric oxygen therapy. Turk J Ophthalmol. 2016;46:244–7. doi: 10.4274/tjo.87513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yotsukura J, Adachi-Usami E. Correlation of electroretinographic changes with visual prognosis in central retinal artery occlusion. Ophthalmologica. 1993;207:13–8. doi: 10.1159/000310400. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Chen S, Li S, Zhang J, Luan D, Zhao S, et al. Oxygen therapy in patients with retinal artery occlusion:A meta-analysis. PLoS One. 2018;13:e0202154. doi: 10.1371/journal.pone.0202154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montesel A, Bucolo C, Mouvet V, Moret E, Eandi CM. Case Report:Central retinal artery occlusion in a COVID-19 patient. Front Pharmacol. 2020;11:588384. doi: 10.3389/fphar.2020.588384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21:e00867. doi: 10.1016/j.idcr.2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]


