Gaudin et al. discuss work from the Reiter laboratory demonstrating that the DISCO complex initiates ciliogenesis by restraining centriole length and assembling distal appendages.
Abstract
Centriole maturation is essential for ciliogenesis, but which proteins and how they regulate ciliary assembly is unclear. In this issue, Kumar et al. (2021. J. Cell Biol. https://doi.org/10.1083/jcb.202011133) shed light on this process by identifying a ciliopathy complex at the distal mother centriole that restrains centriole length and supports the formation of distal appendages.
The primary cilium plays a crucial role in embryonic development by allowing the integration of a variety of inputs, including chemical and mechanical signals. Primary cilia are found on most cell types; thereby, mutations in genes encoding cilia components may perturb many cellular functions, including airway mucus clearance, mechanosensation, and cell signaling, which are central regulators of organ function and homeostasis. Numerous mutations leading to ciliary dysfunction have been identified in recent years and thus linked to human cilia-related diseases, called ciliopathies (1, 2). Some of these mutations affect components of the centrioles, which are cytoplasmic cylindrical structures composed by triplets of microtubules arranged in a ninefold symmetry.
Cilia originate from centrioles and are anchored to the cell surface. In most mammalian cells, centrioles are present within the centrosome, the main organizing center of microtubules. During G1 phase, cells have one centrosome containing two centrioles of different ages. The older mother centriole is distinguished from the younger daughter centriole by the presence of two sets of appendages organized around its circumference. The centrosome duplicates in S phase and, as a result, a new centriole is formed orthogonally to each parent centriole. The new centrioles subsequently elongate during S and G2 phases, and each daughter cell inherits a parent and a newly formed centriole after mitosis. During this transition, new centrioles become daughter centrioles, and the daughter centriole from the previous cycle acquires appendages to mature into a mother centriole. Distal appendages (DAs) are essential for anchoring the mother centriole to the plasma membrane and for the formation of a cilium (2). The formation of a mature centriole competent for ciliogenesis is therefore a complex process taking place over three successive cell cycles.
Different molecular factors required for the progressive maturation of centrioles and the assembly of DAs have been identified in the past, and perturbation of their function has been linked to ciliopathies (2, 3). However, the precise mechanism by which DAs are assembled onto centrioles remains elusive. In this issue, Kumar et al. focused their attention on CEP90, a poorly characterized protein whose mutations have been implicated in several ciliopathies (4). CEP90 is a component of centriolar satellites, which are proteinaceous granules located at the periphery of the centrosome (5, 6). Using a combination of expansion microscopy and structured illumination super-resolution microscopy techniques, the authors found that CEP90 also localized to centrioles, where it formed a discontinuous ring with a ninefold symmetry. CEP90 localized near a well-characterized DA component, CEP164, which was consistent with CEP90 being present at the base of these appendages. Then, they searched for CEP90 interactors. For that, the researchers first had to circumvent the shortcoming of discriminating between interactions that may take place at the centrosome from those occurring within centriolar satellites. To get around this, Kumar et al. used a cell line in which satellite assembly is inhibited. Among the candidates they found interacting with CEP90 at the centrosome were OFD1 and Moonraker (MNR), which are two proteins previously associated with multiple ciliopathies. OFD1 is a centriole component required to restrict centriole elongation and assemble DAs (7). MNR, also called OFIP or KIAA073, is a satellite component necessary for cilia formation (8). Making again use of super-resolution microscopy, the authors showed that all three proteins colocalized at the centriole distal end, with the MNR protein being the closest to the centriole wall, so they named this newly identified complex after DISCO (distal centriole complex).
Next, Kumar et al. elegantly demonstrated that, as previously shown for OFD1 (7), inactivating either CEP90 or MNR led to the absence of cilia in cells. In mice, deficiency of any of these proteins resulted in Hedgehog signaling inhibition and early arrest of embryonic development. As reported for OFD1-deficient cells, loss of MNR in human cells resulted in overly long centrioles. However, centriole length was normal in CEP90-deficient cells, suggesting partially distinct functions between members of the DISCO complex. The authors noted that ciliogenesis was blocked at an early stage in CEP90−/− and MNR−/− cells and, given that DAs are essential for centriole anchoring and ciliogenesis, they decided to examine DA organization in these cells (4). Indeed, they found that DA components, such as CEP83, were not recruited during centriole maturation in MNR−/− or CEP90−/− cells, and DAs were not detected by electron microscopy. These findings pointed out that CEP90 and MNR, like OFD1, were required for the assembly of DAs.
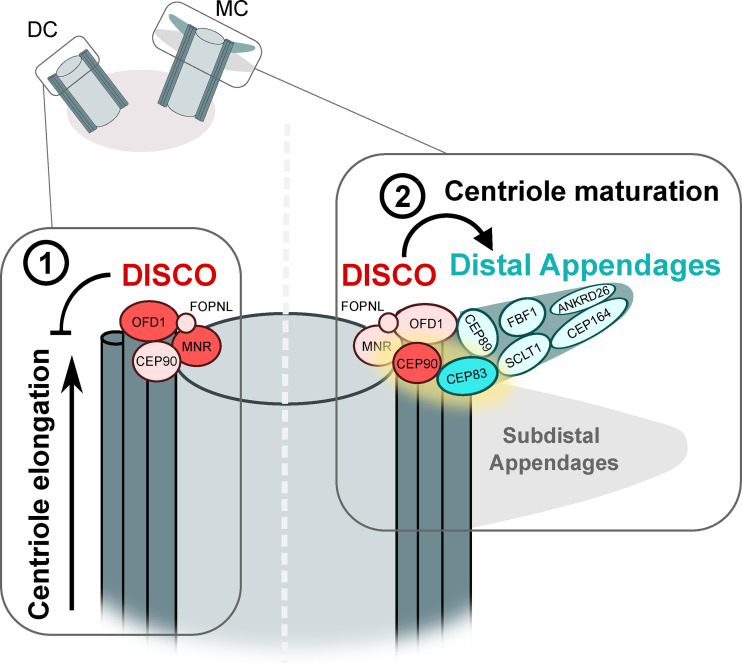
Since CEP90 is required for satellite accumulation around the centrosome, and satellites are, in turn, essential for ciliogenesis (6), one possible explanation to their results is that CEP90 might affect DA assembly indirectly via its role in satellite localization. To answer this question, the authors again used cells lacking centriolar satellites. CEP90 was correctly localized at centriole distal ends in these cells, and DAs were formed, supporting a direct requirement for the centriolar pool of CEP90 in DA assembly. Putting all their data together, Kumar et al. proposed the following model: First, MNR is recruited to elongating centrioles, which, in turn, triggers the recruitment of OFD1 to arrest elongation at the end of the first cell cycle. MNR and OFD1 then recruit CEP90, which initiates the recruitment of DA components, including CEP83, at the end of the following cell cycle (Fig. 1). Thus, the DISCO complex allows for coupling the arrest of centriole elongation to centriole maturation across successive cell cycles.
Figure 1.
The DISCO complex restrains centriole elongation and initiates DA assembly. (1) The DISCO complex member MNR is recruited first at the distal end of assembling centrioles. MNR then recruits other members of the complex, including OFD1, which inhibits centriole elongation at the end of the first cell cycle, i.e., when newly formed centrioles become daughter centrioles (DCs). Other members of the complex include CEP90 and possibly also FOPNL. (2) At the end of the following cell cycle, as the daughter centriole matures into a mother centriole (MC), CEP90 initiates the recruitment of CEP83, the most upstream component in DA assembly. A previously identified interaction between OFD1 and another DA component, CEP89, might also contribute to DA organization (10). Proteins are drawn in contact with each other when an interaction or hierarchical recruitment was described (3, 4, 8, 11).
Besides OFD1 and MNR proteins, Kumar et al. also identified a protein called FGFR1OP N-Terminal Like (FOPNL or FOR20) as a potential CEP90 interactor (4). Interestingly, this interaction was confirmed in a recent study describing that a complex containing CEP90, OFD1, and FOPNL localizes at the distal end of Paramecium centrioles and is necessary for the recruitment of DA components and centriole docking in Paramecium and human cells (9). FOPNL was previously found in complex with MNR and OFD1 and shown to facilitate their interaction (8). Together, these data suggest that the DISCO complex could also include FOPNL. The functional similarities of some of the components of the DISCO complex between Paramecium and humans strongly suggest that the role of DISCO in centriole maturation and ciliogenesis is broadly conserved across species.
Previous studies in different organisms have underpinned the relevance of ciliopathy-associated proteins to ensure normal organism development and tissue function (1, 2). Overall, the findings by Kumar et al. highlight the critical role of a ciliopathy-associated protein complex at distal centrioles in building distal appendages, thus supporting centriole maturation and ciliogenesis in rodents and human cells (4).
Acknowledgments
The authors declare no competing financial interests.
References
- 1.Braun, D.A., and Hildebrandt F.. 2017. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a028191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiter, J.F., and Leroux M.R.. 2017. Nat. Rev. Mol. Cell Biol. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanos, B.E., et al. 2013. Genes Dev. 10.1101/gad.207043.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar, D., et al. 2021. J. Cell Biol. 10.1083/jcb.202011133 [DOI] [Google Scholar]
- 5.Kim, K., and Rhee K.. 2011. J. Cell Sci. 10.1242/jcs.078329 [DOI] [PubMed] [Google Scholar]
- 6.Kim, K., et al. 2012. PLoS One. 10.1371/journal.pone.0048196 [DOI] [Google Scholar]
- 7.Singla, V., et al. 2010. Dev. Cell. 10.1016/j.devcel.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevrier, V., et al. 2016. Hum. Mol. Genet. 10.1093/hmg/ddv488 [DOI] [PubMed] [Google Scholar]
- 9.Le Borgne, P., et al. 2021. bioRxiv. 10.1101/2021.07.13.452210 [DOI]
- 10.Sillibourne, J.E., et al. 2013. Biol. Open. 10.1242/bio.20134457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowler, M., et al. 2019. Nat. Commun. 10.1038/s41467-018-08216-4 [DOI] [PMC free article] [PubMed] [Google Scholar]