Abstract
Silymarin and quercetin (SQ) are known antioxidants with substantial free radical scavenging activities. The efficacy of SQ activity is restricted due to poor absorption and availability. This study aims to increase the hepatoprotective activity of SQ by a newer delivery technique. We have optimized a technique, miniaturized scaffold (MS), for the delivery of active compounds of SQ. SQ molecules were embedded in MS and characterized by morphology, particle size, miniaturization efficiency, and functional group. Further, the hepatoprotective effects of MSQ were investigated through in vitro and in vivo methods. Hepatotoxicity was induced in rats by carbon tetrachloride (CCl4), and subsequently, hepatotoxic rats were treated with the miniaturized scaffold of SQ (MSQ) for 8 weeks. The body weight were significantly high in groups fed with MSQ. A substantial decrease in triglyceride, total cholesterol, low-density lipoprotein, alanine aminotransferase, and aspartate aminotransferase activities were observed in rats treated with MSQ. Similarly, rats treated with MSQ exhibited lower lipid accumulation in the hepatocytes. The experiments clearly demonstrated the efficacy of MSQ as a superior hepatoprotective agent against non-alcoholic fatty liver disease simulated through toxicity induced by CCl4.
1. Introduction
The liver is a vital organ that facilitates several physiological functions, primarily the metabolism of ingested molecules, especially drugs.1 Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease having a prevalence rate of 25% worldwide.2 Many drugs cause damage to hepatocytes and hepatic tissues, leading to NAFLD and has been known to cause prolonged liver disease.3 NAFLD is characterized by the accumulation of triacylglycerol inside liver cells.4 Even though it is considered a relatively preliminary form of chronic liver injury, it could lead to liver cirrhosis.5 Therefore, there is a need to explore ways to reduce the inflammation in the liver caused by NAFLD. Carbon tetrachloride is an effective hepatotoxin that is used to induce liver damage and to involve the increase of inflammatory response.6 The toxicity of CCl4 leads to the reactive oxygen species (ROS), and free radicals are produced during the metabolism.7 Many animal models are used to study liver inflammation to test the potential treatments, including the carbon tetrachloride-induced rat model; Wistar rats are a suitable model for testing the hepatoprotection.8 Hepatic damage caused by CCl4 primarily decreases the activities of antioxidant enzymes, which leads to lipid peroxidation and generation of free radicals.9 Several hepatoprotective agents, including natural substances such as bioactive compounds, have been reported to counter ROS-mediated tissue damage by their antioxidant and free radical scavenging abilities.10 Hepatic damage can be recovered by bioactive compounds such as silymarin and quercetin (SQ).11 Silymarin is a mixture of flavonolignans from milk thistle seeds and comprises seven main components: silybin A, silybin B, isosilybin A, isosilybin B, silychristin, silydianin, and taxifolin. Silymarin mainly grows in Africa, South America, Australia, and many parts of Asia.12 Silymarin has anti-inflammatory activity, antioxidant activity, anti-apoptotic activity, and hepatoprotection activity, and further, it helps in tissue repair and regeneration.13 A similar compound that benefits hepatoprotection is quercetin (3,3′,4′,5,7-pentahydroxyflavone), which is an essential dietary flavonoid found in red onions, citrus fruits, tea, apples, berries, and grapes. Besides, quercetin exhibits anti-inflammatory properties with the ability of a molecule to scavenge free radicals.14 Inhibition of lipid peroxidation and chelation are the basic mechanisms behind the antioxidant effects of quercetin, which could prevent mitochondrial oxidative damage of rat hepatocytes.15 Dietary quercetin can alleviate non-alcoholic steatohepatitis induced by a high-fat diet and has the potential to reduce the inflammatory state that occurs in the body in association with metabolic syndrome.16 Quercetin protects against NAFLD and shows a positive effect on enzymes such as glutathione reductase (GR), catalase, and superoxide dismutase (SOD) and decreases lipid peroxidation; thus, it acts as a hepatoprotectant and helps in liver regeneration.17 The therapeutic effect of SQ is restricted due to its low aqueous solubility and poor intestinal absorption.18 SQ are easily degradable because of their sensitivity to environmental factors such as light, heat, and oxygen.19 The characteristics of flavonoids may limit the dissolution rate and target delivery, hence resulting in low absorption and availability. Combining two or more bioactive compounds has better efficacy when compared to a single bioactive compound; furthermore, it could slow down the elimination rate and produce a more extended efficiency.20 Miniaturization technique is a promising and new technique, which can be designed to build diagnostically valuable systems. The study’s objective was to improve SQ’s efficacy and absorption by a novel technique of miniaturized scaffolding of bioactive molecules and can serve as effective carriers and enhance hepatoprotective activity in CCl4-induced NAFLD rats. This miniaturized scaffold containing SQ is expected to facilitate better permeability and absorption capacity. Hereby, we report a detailed study of SQ-entrapped miniaturized scaffold for its efficacy toward hepatoprotection against NAFLD.
2. Results and Discussion
2.1. Morphology and Particle Size of Microspheres
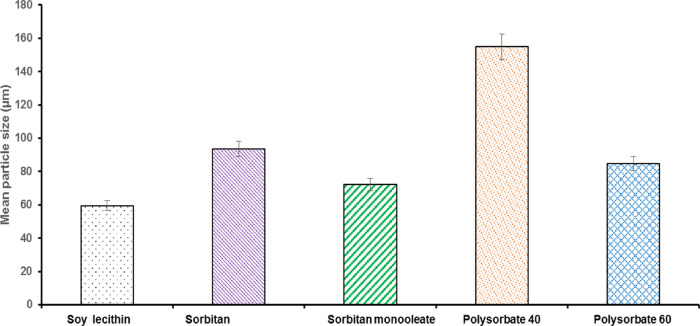
SQs were characterized using light microscopy and a particle size analyzer. A different SQ concentration with various surfactants was optimized to form stable formulations (Figure 1). Among the surfactants, polysorbates 40 and 60 showed aggregation of the microspheres. On the other hand, sorbitan monolaurate and sorbitan monooleate showed coalescence. The microspheres prepared using soy lecithin exhibited uniform droplet size distribution (Figure 1e). This is due to the surfactant’s amphiphilic nature, which offers higher stability to the oil droplets in the microsphere system.
Figure 1.
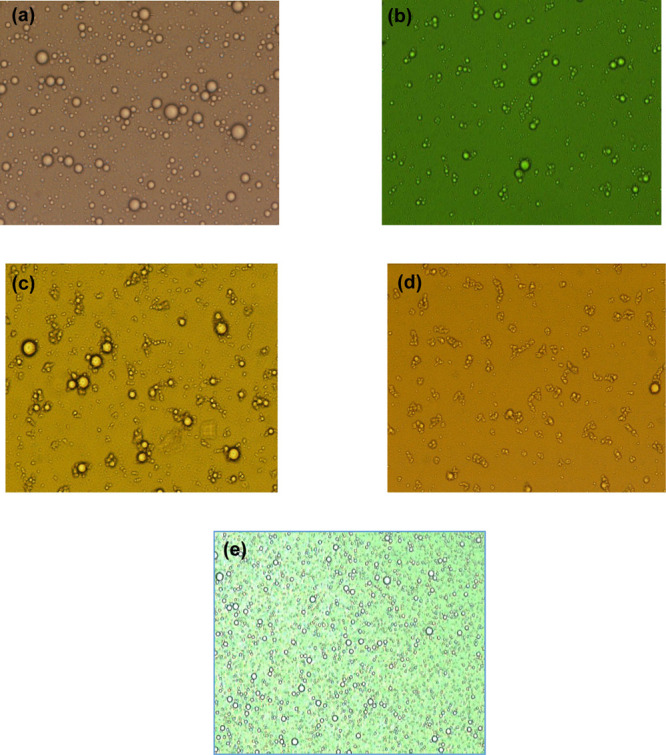
Microscopic images of microspheres with different surfactants. (a) Sorbitan monolaurate, (b) sorbitan monooleate, (c) polysorbate 40, (d) polysorbate 60, and (e) soy lecithin.
The microspheres of silymarin and quercetin with surfactants were spherical. The mean particle size of soy lecithin was 58.22 ±09.69 μm (Figure 2), which is the lowest among SQ microsphere compared with other surfactants. The small dimensions exhibited by the soy lecithin microsphere could be due to the efficient combination of the bioactive compounds with a surfactant, showing the homogeneous distribution of bioactive compounds in the miniature scaffold. The most stable formulation was seen in soy lecithin, which was stable at room temperature (28 ± 1 °C) for 30 days. The solubility capacity of the surfactant increases in the form of miniature, and the hydrophobic force locates the bioactive compounds in the scaffold.21
Figure 2.
Mean particle size (μm) of microsphere prepared by different surfactants such as soy lecithin, sorbitan monolaurate, sorbitan monooleate, polysorbate 40, and polysorbate 60.
2.2. Miniaturization Efficiency of SQ
The concentration of quercetin and silymarin after hosting in the miniaturized scaffold are shown in Table S1 was evaluated by a respective standard marker in high-performance liquid chromatography (HPLC). The concentration of quercetin was higher in SQBC (98.84 ± 0.57%). The increase in miniaturization efficiency was found in SQBC in which isosylibin A (9.90 ± 0.53 mg/g) and isosylibin B (4.99 ± 0.23 mg/g) were higher compared with other biopolymers. Similarly, the concentration of silybin B (9.15 ± 0.62 mg/g) is higher in SQBC. The result showed an increase in the efficiency of SQBC biopolymer in retaining compounds in the miniaturized matrix. β-Cyclodextrin encompasses 7-d-glucose units that are connected in conjunction with α-1, 4 linkages. Besides, the structure of β-cyclodextrin seems like a thick-walled bucket with a hydrophobic cavity combined with a hydrophilic exterior. The weak forces such as van der Waals forces, dipole–dipole interaction, and hydrogen bonding have supported them to create an inclusion complex by entrapping the guest molecule within its cavity.22,23 Therefore, the result confirmed a higher efficiency of SQ in the miniaturized matrix when β-cyclodextrin was used as a biopolymer.
2.3. Fourier Transform Infrared Spectroscopy of MSQ
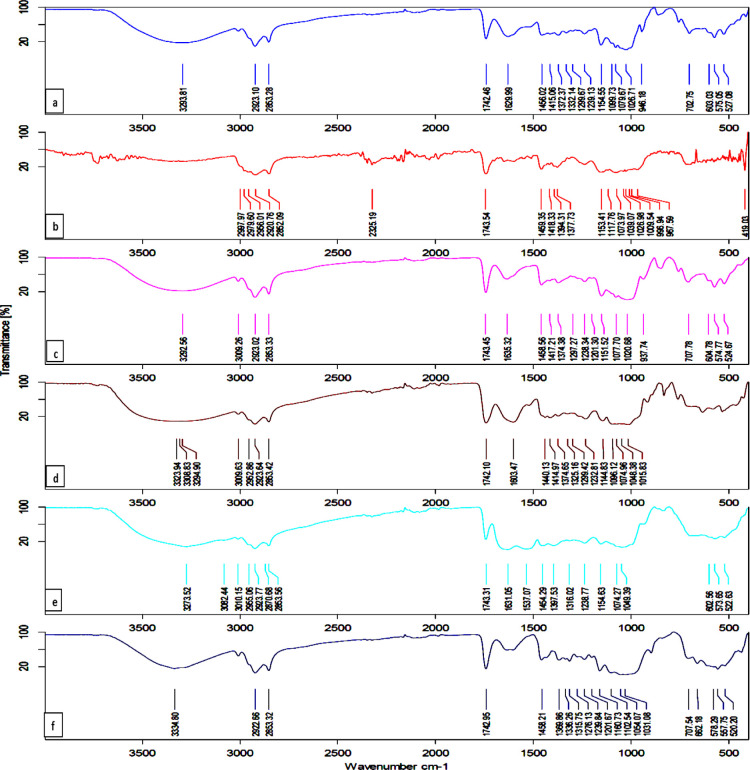
Fourier transform infrared (FTIR) analysis was carried out to identify the functional group of bioactive compounds in the miniaturized samples. The major characteristic peaks of quercetin at 1100–1600 cm–1 and OH phenolic bending at 1200–1400 cm–1 are present in the miniaturized scaffold, which is comparable with native quercetin.24Figure S1 indicates that there is no chemical interaction occurred in the miniaturized scaffold. A spectrum of silymarin showed bands at 3333 cm–1, (OH), 2926 cm–1 (CH), and 1742 cm–1 (C=O), which are comparable with native silymarin.25Figure 3 illustrates the functional groups of silymarin in SQBC at characteristic peaks of OH stretching at 3293 cm–1, C=O stretching at 1629 cm–1, C=C stretching at 1458 cm–1, and C–Cl stretching at 603 cm–1, respectively. The characteristic bands of free quercetin in all the biopolymer show the aromatic bending and stretching at around 1100 and 1600 cm–1 and then −OH phenolic bending at 1200–1400 cm–1.
Figure 3.
IR spectra of SQ with biopolymers such as (a) SQBC, (b) SQMD, (c) SQC, (d) SQP, (e) SQWP, and (f) SQGA.
Spectra of solid dispersions of SQBC, SQC, SQMD, SQP, SQWP, and SQGA have not shown any changes from the standard spectra of SQ (Figure S1). The result suggested that the miniaturization process has not affected the functional groups of the bioactive compounds. Thus, the overall study indicates that there was no chemical interaction between the bioactive compound and biopolymer in the miniaturized scaffold form.
2.4. In Vitro Evaluation of MSQ by Dissolution and Release Kinetics
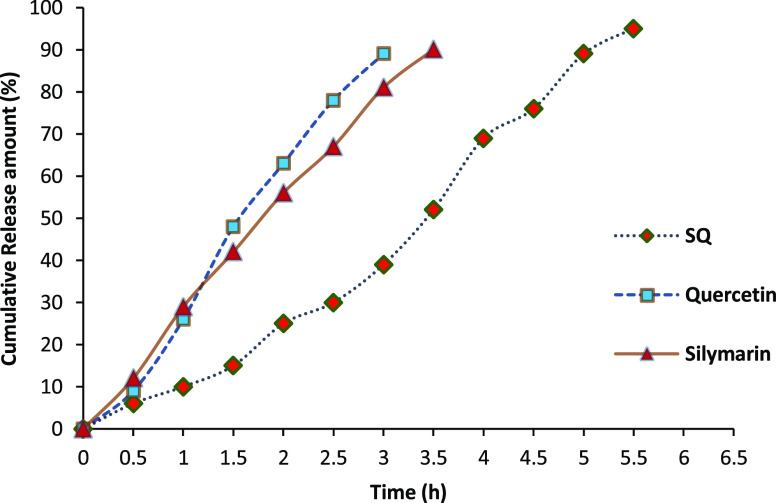
In vitro dissolution tests, using a pH change method, were carried out to investigate the influence of formulation on flavonoid release from the miniaturized scaffold. The dissolution study of silymarin, quercetin, and miniaturized scaffold of SQ (MSQ) is shown in Figure 4. Pure quercetin solution exhibited a much faster release rate with approximately 75% of quercetin dissolved at the initial 2 h. From the in vitro release study, it was found that the miniaturized form had a significant improvement in the release rate when compared with pure silymarin or quercetin. MSQ exhibited slow release of the compounds at a comparable concentration of bioactives within 4.5 h. This may be due to the absorption of the compounds in the miniaturized matrix.26 The bioactive compound in a miniaturized matrix can be retained and released slowly; the phenomenon has also contributed to increased solubility. The release rate of SQ in the miniaturized scaffold had shown a good resistance at pH 7.4. The combination of SQ in the miniaturized scaffold had shown a slow release in the simulated intestinal conditions than the native form of SQ. The efficiency of miniaturized SQ is very high (97.26%). This is because of the incorporation of bioactive compounds in the miniaturized scaffold, which may have better and sustained release characteristics of bio-actives. The slower and sustained release of SQ may be attributed to the diffusion of SQ entrapped within the miniaturized scaffold. Miniaturization technique has given satisfying efficiency for the target delivery of SQ.
Figure 4.
In vitro release profile of bioactive compound in the miniaturized scaffold.
2.5. In Vivo Evaluation of a Miniaturized Scaffold of SQs in a CCl4-Induced Hepatotoxicity Rat Model
Rats were treated with CCl4 to induce hepatotoxicity for evaluating the hepatoprotective effect of MSQ. The average, initial, and final body weights and their relative organ weights of different experimental rat groups, namely, control, CCl4-treated rat group, and those treated with native form of silymarin, quercetin, miniaturized SQ with low, medium, and high doses are shown in Table 1. In the CCl4-treated group, the rats had statistically lower body weights (256.23 ± 6.64 g BW) when compared to that of experimental control (278.06 ± 5.71 g BW) and those with HDMSQ (278.25 ± 6.26 g BW) -treated animals. Similar findings were reported such as the bodyweight of the rat group administrated with carbon tetrachloride and it had a lesser bodyweight.27 The higher dose of miniaturized SQ caused improvement in the bodyweight of rats at the end of 8 weeks of feeding. This result was comparable to the experimental control rat group.
Table 1. Effect of Oral Administration for 8 Weeks of Miniaturized SQ on Relative Organ Weight in Male Wistar Ratsa.
| groups | initial BW (g) | final BW (g) | liver (g) | kidney (g) | brain (g) |
|---|---|---|---|---|---|
| normal control | 237 ± 1.06 | 278.06 ± 5.71a | 09.82 ± 0.98b | 1.77 ± 0.013b | 1.66 ± 0.06b |
| CCl4 | 238 ± 1.19 | 256.23 ± 6.64b | 12.89 ± 0.75a | 1.99 ± 0.14a | 1.98 ± 0.07a |
| silymarin | 239 ± 1.25 | 270.15 ± 6.92a | 11.00 ± 0.38a | 1.70 ± 0.011b | 1.65 ± 0.07b |
| quercetin | 238 ± 1.58 | 271.46 ± 6.68a | 10.78 ± 0.52b | 1.72 ± 0.10b | 1.44 ± 0.09b |
| SQ | 237 ± 1.27 | 270.33 ± 7.84a | 10.24 ± 1.16b | 1.69 ± 0.21b | 1.58 ± 0.04b |
| LDMSQ | 239 ± 1.49 | 270.66 ± 6.85a | 09.78 ± 1.65b | 1.73 ± 0.17b | 1.54 ± 0.09b |
| MDMSQ | 238 ± 1.32 | 271.06 ± 5.60a | 09.96 ± 1.23b | 1.72 ± 0.13b | 1.51 ± 0.05b |
| HDMSQ | 237 ± 1.07 | 278.25 ± 6.26a | 10.43 ± 0.54b | 1.73 ± 0.08b | 1.58 ± 0.14b |
Results are expressed as mean ± SEM of the measurements (n = 6). Different subscripts following mean values within a column indicate significantly different groups in Duncan’s multiple comparison test with p < 0.05.
2.6. Hematological Parameters
The hematological parameters of hepatotoxic rats treated with SQ are presented in Table 2. The results indicated that the rats treated with CCl4 showed decreased hemoglobin (Hb) concentration, red blood cell (RBC) count, mean corpuscular volume (MCV), mean corpuscular Hb (MCH) concentration, platelet count, and lymphocyte and lymphocyte number. The Hb concentration of rat group which received LDMSQ (14.00 ± 0.32 g/dL), MDMSQ (14.98 ± 0.10 g/dL), and HDMSQ (15.23 ± 1.30 g/dL) was significantly higher when compared with those treated with native silymarin (13.02 ± 0.30 g/dL) and quercetin (13.63 ± 0.50 g/dL). An increase in white blood cell (WBC) (16.32 ± 0.13 × 103/μL) was observed in rats induced with CCl4, and WBC was significantly higher (p < 0.05) in the CCl4 group when compared to all the treated groups. The lymphocyte number was higher in HDMSQ-treated rat groups (17.6 ± 1.24 × 103/μL) than compared to the control and rat groups treated with the native form of bioactives. The MCV in rat groups treated with MDMSQ (53.53 ± 1.15 fL) and HDMSQ (54.93 ± 1.03 fL) were higher when compared with those treated with the native form of silymarin (52.86 ± 0.69 fL), quercetin (52.16 ± 0.43 fL), and a combination of SQ (50.83 ± 0.41 fL). The results indicated that treatment with miniaturized SQ increased the Hb, MCV, and lymphocyte number, and the values are comparable to the experimental control group. Furthermore, the improved hematological factors in the rats treated with MSQ could be due to effective absorption of bioactive compounds.
Table 2. Effect of Miniaturized SQ Feed on Hematological Parameters at the End of 8 Week Studya.
| Particulars | WBC (x103/μL) | RBC (x106/μL) | Hb (g/dL) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (x103/μL) | LYM (%) | LYM(#) (x103/μL) |
|---|---|---|---|---|---|---|---|---|---|
| control | 15.10 ± 0.90b | 7.71 ± 0.85a | 14.43 ± 1.56a | 53.13 ± 1.70a | 18.43 ± 0.68a | 35.35 ± 1.22a | 506.33 ± 17.9a | 84.06 ± 2.06a | 14.7 ± 2.00a |
| CCl4 | 16.32 ± 0.13a | 6.42 ± 0.25b | 12.52 ± 1.15b | 49.28 ± 1.01b | 15.76 ± 2.10b | 29.53 ± 1.97b | 460.02 ± 19.89b | 72.73 ± 7.69b | 7.30 ± 0.96b |
| silymarin | 13.81 ± 2.34b | 7.92 ± 0.39a | 13.02 ± 0.30ab | 52.86 ± 0.69a | 17.66 ± 0.52a | 33.46 ± 0.15a | 591.66 ± 24.70a | 80.66 ± 5.32a | 14.5 ± 1.56a |
| quercetin | 13.63 ± 1.62b | 7.36 ± 0.28a | 13.63 ± 0.50ab | 52.16 ± 0.43a | 17.16 ± 0.66a | 33.85 ± 0.17a | 579.39 ± 31.43a | 75.28 ± 4.42a | 13.2 ± 0.25a |
| SQ | 13.96 ± 0.70b | 7.89 ± 0.39a | 13.33 ± 0.90ab | 50.83 ± 0.41b | 17.73 ± 0.52a | 31.53 ± 2.44a | 556.36 ± 14.37a | 78.82 ± 3.73a | 14.5 ± 0.95a |
| LDMSQ | 14.83 ± 0.32b | 7.91 ± 0.35a | 14.00 ± 0.32a | 52.67 ± 1.57a | 17.68 ± 0.52a | 33.46 ± 0.15a | 558.38 ± 32.86a | 80.67 ± 5.36a | 14.4 ± 2.13a |
| MDMSQ | 15.23 ± 0.50b | 7.77 ± 0.87a | 14.98 ± 0.10a | 53.53 ± 1.15a | 17.76 ± 0.65a | 33.66 ± 0.50a | 559.33 ± 12.50a | 81.33 ± 5.54a | 16.1 ± 1.87a |
| HDMSQ | 15.18 ± 2.08b | 7.84 ± 0.85a | 15.23 ± 1.30a | 54.93 ± 1.03a | 17.98 ± 1.38a | 33.86 ± 0.15a | 563.01 ± 13.52a | 83.62 ± 5.32a | 17.6 ± 1.24a |
Results are expressed as mean ± SEM of the measurements (n = 6 animal/group). Different subscripts following mean values within a column indicate significantly different groups in of Duncan’s multiple comparison test with p < 0.05. WBC—WBC count, RBC—RBC count, Hb—Hb concentration, MCV—mean corpuscular volume, MCH—mean corpuscular Hb, MCHC—mean corpuscular Hb concentration, PLT—platelet count, LYM—lymphocyte, and LYM#—lymphocyte number.
2.7. Liver Function Test and Lipid Profile of Rats Treated with MSQ
The enzymes, namely, alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT), are sensitive enzymes, whose assessment reflect the severity of liver damage.28 The effect of different treatments of bioactive compounds on the liver function test and lipid profile along with few essential biochemical parameters are presented in Table 3. In the serum, ALT and AST levels showed a significant increase (p < 0.05) for the rats treated with CCl4 compared to those treated with bioactives. Similarly, a significant increase in the activity of ALP was seen in the CCl4-treated group. The native SQ, low, medium, and high doses of miniaturized SQ had considerably reduced GGT compared to that of CCl4-treated groups. The elevated level of AST and ALT activities in the CCl4-treated group showed a sign of damage of the liver parenchymal cells, and increased enzyme activity was an apparent response toward the increase in ROS generation.29 Hence, there was no significant difference in the levels of enzymes such as AST, ALT, ALP, and GGT in MSQ-treated groups compared with the native form of silymarin, quercetin, and a combination of both (SQ).
Table 3. Effect of Miniaturized SQ in Liver Function and Lipid Profile among Different Rat Groups.
| particulars | control | CCl4 | silymarin | quercetin | SQ | LDMSQ | MDMSQ | HDMSQ |
|---|---|---|---|---|---|---|---|---|
| ALT (U/L) | 131.2 ± 2.51c | 170.9 ± 6.85a | 136.6 ± 2.17b | 137.5 ± 2.84b | 138.1 ± 3.21b | 129.9 ± 1.62c | 131.8 ± 2.45c | 130.7 ± 3.37c |
| AST (U/L) | 130.4 ± 2.26c | 167.5 ± 4.01a | 135.5 ± 2.91b | 136.1 ± 2.34b | 137.0 ± 3.05c | 130.8 ± 3.61c | 132.6 ± 3.47c | 129.1 ± 2.54c |
| ALP (U/L) | 188.2 ± 5.06b | 240.2 ± 7.12a | 198.2 ± 4.36b | 200.7 ± 3.29b | 198.0 ± 7.27b | 189.7 ± 5.12b | 184.2 ± 7.15b | 187.5 ± 6.84b |
| GGT (U/L) | 8.407 ± 0.68b | 11.430 ± 0.35a | 8.407 ± 0.34b | 8.522 ± 0.35b | 8.812 ± 0.26b | 8.627 ± 0.42b | 8.652 ± 0.68b | 8.603 ± 0.93b |
| HDL (mg/dL) | 40.95 ± 7.74a | 28.94 ± 4.47b | 38.94 ± 2.32a | 40.28 ± 3.73a | 39.61 ± 2.54a | 43.64 ± 3.70a | 44.31 ± 1.39a | 45.65 ± 2.47a |
| LDL (mg/dL) | 40.73 ± 4.40b | 78.33 ± 3.07a | 59.53 ± 3.90b | 50.13 ± 4.12b | 44.86 ± 2.36b | 43.86 ± 1.83b | 44.93 ± 3.13b | 46.42 ± 2.44b |
| total cholesterol (mg/dL) | 166.6 ± 14.6b | 183.3 ± 12.7a | 162.6 ± 10.3b | 165.0 ± 11.4b | 163.3 ± 7.69b | 157.6 ± 10.3b | 156.3 ± 13.0b | 158.0 ± 10.8b |
| triacylglycerols (mg/dL) | 124.8 ± 6.56b | 151.4 ± 4.52a | 131.5 ± 6.81b | 128.7 ± 5.42b | 128.7 ± 8.48b | 127.6 ± 6.01b | 124.6 ± 10.1b | 120.0 ± 8.96b |
| bilirubin (mg/dL) | 0.725 ± 0.27d | 2.634 ± 0.12a | 1.253 ± 0.06b | 1.356 ± 0.10b | 1.206 ± 0.08b | 1.122 ± 0.07c | 1.136 ± 0.06c | 1.142 ± 0.09c |
| total protein (g/dL) | 7.533 ± 0.21b | 4.992 ± 0.98c | 6.662 ± 0.50b | 6.321 ± 0.18b | 6.591 ± 0.38b | 7.871 ± 0.93a | 7.820 ± 0.64a | 7.863 ± 0.36a |
| glucose (mg/dL) | 85.82 ± 4.52b | 108.82 ± 4.75a | 89.70 ± 5.62b | 93.52 ± 5.33b | 95.21 ± 4.12b | 87.11 ± 5.10b | 85.88 ± 5.10b | 87.64 ± 4.52b |
| albumin (g/dL) | 3.853 ± 0.09a | 2.491 ± 0.10c | 3.091 ± 0.09b | 3.045 ± 0.06b | 3.089 ± 0.12b | 3.215 ± 0.18b | 3.442 ± 0.12b | 3.353 ± 0.16b |
| uric acid (mg/dL) | 4.404 ± 0.95b | 7.773 ± 0.73a | 6.073 ± 0.32b | 6.021 ± 0.69b | 6.146 ± 0.60b | 5.712 ± 0.92b | 5.621 ± 0.57b | 5.587 ± 0.65b |
| creatinine (mg/dL) | 0.612 ± 0.07b | 1.196 ± 0.05a | 0.912 ± 0.03b | 0.971 ± 0.06b | 0.975 ± 0.06b | 0.880 ± 0.03b | 0.892 ± 0.04b | 0.825 ± 0.05b |
The HDL levels in the serum of MSQ-treated groups, namely, HDMSQ (45.65 ± 2.47 mg/dL) and MDMSQ (44.31 ± 1.39 mg/dL), are significantly higher than those treated with native silymarin (38.94 ± 2.32 mg/dL), quercetin (40.28 ± 3.73 mg/dL), and SQ (39.61 ± 2.54 mg/dL). The LDL level in the HDMSQ (46.42 ± 2.44 mg/dL), MDMSQ (44.93 ± 3.13 mg/dL), LDMSQ (43.86 ± 1.83 mg/dL) was lower compared with the groups treated with native silymarin (59.53 ± 3.90 mg/dL) and quercetin (50.13 ± 4.12 mg/dL), respectively. Results are expressed as mean ± SEM of the measurements (n = 6 animal/group). Different subscripts following mean values within a row indicate significantly different groups in Duncan’s multiple comparison test with p < 0.05: ALT (alanine aminotransferase), AST (aspartate aminotransferase), ALP (alkaline phosphatase), GGT (gamma glutamyl transferase), HDL (high-density lipoprotein), and LDL (low-density lipoprotein).
A substantial increase in serum total protein was observed in a rat group treated with miniaturized bioactives LDMSQ (7.871 ± 0.93 g/dL), MDMSQ (7.820 ± 0.64 g/dL), and HDMSQ (7.863 ± 0.36 g/dL), when compared to rats treated with native form of silymarin (6.662 ± 0.50 g/dL), quercetin (6.321 ± 0.18 g/dL), and their combination of SQ (6.591 ± 0.38 g/dL). Lower protein evaluation indicates the diagnostic measurement of liver diseases.30 Serum albumin improved in rat groups treated with miniaturized bioactives; LDMSQ (3.215 ± 0.18 g/dL), MDMSQ (3.442 ± 0.12 g/dL), and HDMSQ (3.353 ± 0.16 g/dL) than the native form of silymarin, quercetin, and a combination of both SQs. Rat groups administrated with CCl4 showed lower albumin (2.491 ± 0.10 g/dL) when compared with the control (3.853 ± 0.49 g/dL) group.
The decrease in albumin levels had been related to biliary liver damages and active cirrhosis.31 A significant decrease in bilirubin was observed in the serum of rat groups treated with miniaturized bioactive LDMSQ (1.122 ± 0.07 mg/dL), MDMSQ (1.136 ± 0.06 mg/dL) and HDMSQ (1.142 ± 0.09 mg/dL), when compared to rats treated with native form of silymarin, quercetin and their combination SQ. The elevated level of bilirubin is an indication of biliary obstruction and hemolysis.32 The level of glucose showed significant decrease in serum of rats treated with miniaturized bioactives; LDMSQ mg/dL), MDMSQ (85.88 ± 5.10 mg/dL) and HDMSQ (87.64 ± 4.52 mg/dL) than the native form of silymarin, quercetin and a combination of both SQs. However, the rat group administrated with CCl4 showed a greater increase in the level of glucose when compared with the control group. Uric acid levels showed significant decrease in the rats treated with miniaturized bioactives such as LDMSQ (5.712 ± 0.92 mg/dL), MDMSQ (5.621 ± 0.57 mg/dL) and HDMSQ (5.587 ± 0.65 mg/dL) than compared with native form of silymarin, quercetin and SQ.
Similarly, creatinine levels also decreased in rat groups treated with miniaturized bioactive compound that is LDMSQ (0.880 ± 0.03 mg/dL) MDMSQ (0.892 ± 0.04 mg/dL) and HDMSQ (0.825 ± 0.05 mg/dL) than the native form of silymarin, quercetin and the combination. It was observed that CCl4 group had a significant elevation in the level of serum uric acid (7.773 ± 0.73 mg/dL) and creatinine (1.196 ± 0.05 mg/dL). The treatment with miniaturized bioactive compound demonstrated hepatoprotection by improvement on liver enzymes and hematological parameters of serum.
2.8. Antioxidant Enzymes and Molecules
ROS are formed during the process of fatty acid oxidation. ROS are minimized by the activity of antioxidant enzymes such as catalase (CAT), SOD and GR.33 The activity of antioxidant enzymes in rat liver was assayed to reveal the protective effects of the miniaturized scaffold of SQ. The activities of SOD, GR, and CAT in the liver were significantly (p < 0.05) decreased in CCl4 treated rats (95.22 ± 1.68, 08.36 ± 1.22 and 25.8 ± 0.88 U/mg of protein, respectively) than compared to the control rat group (101.71 ± 2.98, 12.93 ± 2.08 and 30.71 ± 0.99 U/mg of protein, respectively) as shown in Table 4.
Table 4. Effect of Antioxidant and Enzyme Activities of Miniaturized Bioactive Compounds in Different Rat Groupsa.
| particulars | control | CCl4 | silymarin | quercetin | SQ | LDMSQ | MDMSQ | HDMSQ |
|---|---|---|---|---|---|---|---|---|
| TBARS (nmol/MDA/mg) | 0.868 | 1.238 | 0.828 | 0.838 | 0.865 | 0.798 | 0.785 | 0.695 |
| SOD (U/mg of protein) | 101.71 ± 2.98a | 95.22 ± 1.68b | 99.91 ± 2.64a | 95.28 ± 4.59a | 96.99 ± 5.54a | 99.36 ± 2.87a | 99.66 ± 3.76a | 102.01 ± 4.82a |
| GR (U/mg of protein) | 12.93 ± 2.08a | 08.36 ± 1.22b | 10.43 ± 1.79a | 10.87 ± 0.99a | 10.39 ± 1.06a | 10.97 ± 1.44a | 10.78 ± 0.40a | 12.99 ± 0.96a |
| catalase (U/mg of protein) | 30.71 ± 0.99a | 25.89 ± 0.88b | 28.56 ± 0.76a | 29.22 ± 0.98a | 28.87 ± 0.69a | 28.59 ± 0.58a | 28.03 ± 0.36a | 30.46 ± 0.84a |
Results are expressed as mean ± SEM of the measurements (n = 6 animal/group). Different subscripts following mean values within a row indicate significantly different groups in Duncan’s multiple comparison test with p < 0.05.
The activity of SOD in rats treated with different doses of the miniaturized scaffold of SQ (LDMSQ, MDMSQ and HDMSQ) varied in the range of 99.36 ± 2.87 to 102.01 ± 4.82 U/mg of protein, which was higher in the rats with native form of SQ and a combination of SQ, further, it was changed in the range from 95.28 ± 4.59 to 99.91 ± 2.64 U/mg of protein. Similarly, the levels of GR were found higher in rat groups administrated with a high dose of miniaturized bioactives (HDMSQ; 12.99 ± 0.96 U/mg of protein), when compared with LDMSQ, MDMSQ, the native form of silymarin, quercetin and their combination (SQ), which ranged from 10.39 ± 1.06 to 10.97 ± 1.44 U/mg of protein. Similarly, the level of catalase enzyme showed a significant increase in the rat group treated with HDMSQ (30.46 ± 0.84 U/mg of protein) than the other treated groups. The HDMSQ group showed better free radical scavenging property compared to LDMSQ and MDMSQ groups. The obtained results suggested that administration of a high dosage of miniaturized bioactives protects the liver from oxidative damage. Furthermore, it could reduce ROS formation and can act as a better antioxidant. In addition, it was displayed that SQ complex in miniaturized scaffold increased in SOD and CAT activity more significantly (p < 0.05) than native SQ. Rat group treated with HDMSQ (0.695 nmol/MDA/mg) decreases significantly for lipid peroxidation, when compared to rat group of LDMSQ (0.798 nmol/MDA/mg), MDMSQ (0.785 nmol/MDA/mg), native form of silymarin (0.828 nmol/MDA/mg), quercetin (0.838 nmol/MDA/mg) and a combination of both SQs (0.865 nmol/MDA/mg), respectively. The level of thiobarbituric acid reactive substance (TBARS) in the livers of CCl4 treated rat group was found considerably increased (p < 0.05) compared to other rat groups.
2.9. Histological Examination
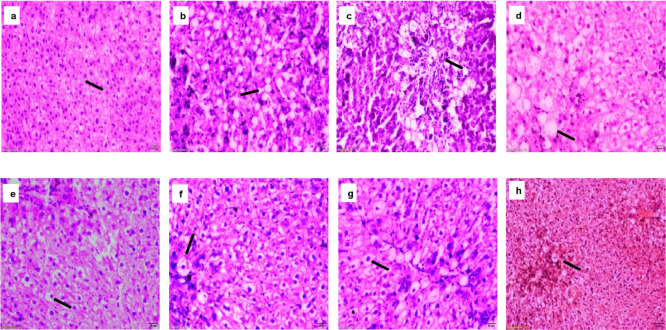
Histological study was performed using liver to determine the protective effect of the miniaturized scaffold of SQ. The observation of hepatic histology of rat liver cells is displayed in (Figure 5). The liver of control group rats showed a common appearance in portal areas of central veins and hepatic plates. In the CCl4 treated rats, fat accumulation in the liver and cytoplasm was observed. The hepatocytes were swollen with the presence of vacuoles, which occupied the cytoplasm and the nucleus was in the corner.34 The CCl4 has been reported to cause hepatotoxicity with apoptotic hepatocellular injury and necrotic, causing damage to liver function. The rat liver treated with low, medium and high dose MSQ showed improvement in the hepatocytes with less vacuolation, when compared to those administrated with native form of silymarin, quercetin and their combination. In the cases of LDMSQ, MDMSQ and HDMSQ, the hepatocytes appeared oval with homogenous cytoplasm and the nucleus was located in the center; with minimal hepatic damage, scattered and cytological ballooning. However, administration of miniaturized scaffold of SQ to CCl4-treated rats lowered the destruction in lobule structure compared to the native form of SQ. Furthermore, the histological recovery appeared more superior when applying a combined form of SQ. The administration of complex SQ resulted in a greater decrease in collagen deposition than SQ individually. Thus, the result indicates, the liver cells may regenerate in non-alcoholic induced rats when treated with miniaturized SQ.
Figure 5.
Morphological evaluation of liver histology in different groups (a) Control-shows normal hepatocytes (b) CCl4-shows hepatocytes ballooning with central nuclei with nuclear vacuolation of hepatocytes (c) silymarin, (d) quercetin & (e) SQ-shows less vacuolation, necrosis and ballooning of hepatocytes, (f) LDMSQ (g) MDMSQ & (h) HDMSQ-shows very few nuclear vacuolation of hepatocytes, hyperactive Kupffer cells and necrotic cells in hepatic parenchyma. (Scale bar = 20 μm with magnification 20×).
3. Conclusions
The present study investigated the miniaturized scaffold technique to increase the permeability of bioactive compounds, specifically SQ, as hepatoprotective agents. The properties of miniaturized scaffold of various biopolymers with highly active antioxidant molecules focus on the development of better therapeutic properties. High efficiency and slow release of SQ were observed. Oxidative damage induced by carbon tetrachloride in Wistar rats served as an appropriate in vivo model to study the hepatoprotective activity of miniaturized scaffold of SQ. The effect of MSQ was pronounced against oxidative stress as demonstrated by an increase in SOD, catalase and GR enzyme activities. Histological observations revealed less cell infiltration and morphological changes when treated with miniaturized scaffold bioactives. Dosage of the bioactive compound SQ (HDMSQ 200:50 mg of SQ/Kg BW of rat) with miniaturization technique showed enhanced hepatoprotective activity. The body weight of HDMSQ treated rats were high and comparable to control. Therefore, the overall MSQ has shown better hepatoprotection against NAFLD than a native form of SQ. The research findings revealed the efficacy of miniaturized bioactives on liver functions. Hence, the formulated SQ complex in a miniaturized scaffold system can be utilized as a novel delivery carrier against NAFLD.
4. Materials and Methods
4.1. Chemicals and Reagents
Silymarin seed powder, Quercetin hydrate (Q) with a purity of 95%, soy lecithin and β-cyclodextrin were procured from Himedia (Bangalore, India). Wheat germ oil was sourced from M/s. Global Merchant (Mumbai, India); groundnut oil from M/s. Shreeya Peanuts (Rajkot, India). CCl4 were procured from Ragu chemicals Mysore, India. Standard commercial pellet diet was procured from M/s. Sai Durga feeds and foods (Bangalore, India). All reagents used were of analytical grade.
4.2 HPLC Analysis of SQ
SQ standards were prepared in a concentration of 1 mg/mL in methanol (w/v). SQ was separated from miniaturized scaffold by centrifugation of 12,000 rpm for 30 min the concentration of free SQ in the supernatant was calculated by chromatographic analysis, using a stationary phase Shodex C18–250 × 4.6 mm (5 μm) column (Hitachi, Elite Lachrom 2000 series, Japan). A mixture of 1% acetic acid–methanol–water (1:49.5:49.5 v/v) was served as mobile phase, the injection volume were 10 μL and the elution has been made in gradient mode at a flow rate of 1 mL/min and the detection made at 288 and 366 nm respectively. Program for one analysis the time required was about 60 min.35
4.3. Preparation of Microspheres
Bioactive compounds, SQ were hosted in carrier wheat germ oil with surfactants to form microspheres. Microspheres were initially prepared with individual surfactants such as sorbitan monolaurate, sorbitan monooleate, polysorbate (40, 60) with wheat germ oil as a carrier. Considering the hydrophobic nature of SQ, about 200 mg of silymarin and 50 mg of quercetin were dissolved in 0.01% NaOH (w/v) at room temperature (28 ± 1 °C). A optimized mixture composed of soy lecithin (3.5%; w/v), dissolved SQ and 10 mL of wheat germ oil were homogenized to prepare emulsion using a homogenizer (Ultra turrax, T18 basic IKA-T18, Germany) at 16,000 rpm for 30 min and stored at 4 °C for further analysis.36
4.4. Size Distribution of Microspheres
The size of microspheres was determined by a trinocular microscope (Olympus BX-5, Japan) with software (Prog Res C-5 software) fixed with a digital camera to capture the images. Microspheres were observed under 100× magnification for analyzing the size distribution.
4.5. Particle Size Analysis
The particle size distribution of the microspheres was measured using a laser light diffraction particle size analyzer (S3500, Microtrac Inc., USA). About 100 μL microsphere sample was taken and was stirred to attain a proper mixture. The analysis was done in triplicates.
4.6. Preparation of Miniaturized Scaffold
Different biopolymers such as maltodextrin, cellulose, pectin, whey protein, beta-cyclodextrin and gum arabica were used for the preparation of miniaturization using a homogenizer at 10,000 rpm for 10 min and the prepared microspheres were added and mixed thoroughly. The mixture was freeze-dried at 0.4 torr at −20 °C using lyophilizer (Scanvac coolsafe—1104 pro, Denmark) to form a miniaturized scaffold. The miniaturized bioactive compound along with biopolymers was further standardized for their physio-chemical properties. After the preparation of miniaturization, the sample was kept in a screw-caped tube and stored at refrigeration (4 ± 0.1 °C) condition for studying the efficacy as hepatoprotectant using the in vitro and in vivo system.
4.7. FTIR Spectroscopy
Functional properties of bioactive compounds (SQ) were analyzed using an FTIR spectrophotometer (Bruker, Germany/Tensor II). The spectra were recorded in the wavelength region of 4000–400 cm–1. The standards of SQ were analyzed to observe the change in the functional group of miniaturized bioactive compounds.
4.8. Release of Bioactive Compound from a Miniaturized Scaffold
SQ release from the miniaturized scaffold, were studied by incubating the MSQ (30 mg) in phosphate-buffered solution (PBS), at pH 7.4, at 37 °C. 20 mg of MSQ was dispersed in 5 mL of release medium (PBS of pH 7.4 containing 0.1% w/v Tween 80) in a dialysis tube (Sigma dialysis tubes, molecular weight cutoff, 12 kDa), and the closed dialysis bag immersed in 20 mL release medium in a centrifuge tube. Tween 80 was used to increase the solubility of MSQ in the buffer solution to maintain sink condition. The tube was placed in a shaker bath at 37 °C and shaken horizontally at 100 cycles/min. About 15 mL of the sample were withdrawn and replaced with the same volume of fresh medium. The samples were filtered through a 0.22 μM filter and were analyzed for the content of SQ and their release was monitored by measuring the maximum absorption spectrophotometrically at 288 and 366 using a spectrophotometer (Shimadzu spectrophotometer Model UV-1800, Shimadzu, Japan).
4.9. Animals and Experimental Groups
Adult male Wistar rats (n = 48) weighing 250–300 g and aged eight weeks were sourced from institute’s animal house facility after approval of institutional animal ethics committee (CFT/IAEC/64/2016) as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Fisheries, Animal Husbandry and Dairying, Government of India (New Delhi, India).
The rats were randomly divided into eight groups with six animals each. The animals were housed at a room temperature of 21 ± 3 °C, a relative humidity of 50 ± 20%, and light and dark (L/D) cycle of 12 h each. The animals were acclimatized for 6 days before the start of the experiment, provided ad libitum feed and free access to drinking water. Table 5 summarizes the animal grouping and their treatment process with the dosage of bio-actives. Group 1 (control) was fed with normal diet and water. Group 2 (negative control) was given intraperitoneal injection of carbon tetrachloride 1 mL/kg body weight diluted in vegetable oil (1:1) twice a week for inducing NAFLD. Silymarin dose was designed based on previous investigations.37 The quercetin dose regimen was designed as per the earlier reported animal therapeutic study.38 Studies are also made on the hepatoprotective effect of quercetin with dose (2 × 250 mg/day) on human clinical trial.39 The CCl4-induced rat model described was used for scheduling the dose regimen.40 The animals were observed daily for mortality and appearance of changes if any. Once in a week, the body weights were recorded, and the dose administered was adjusted weekly according to animal weights to sustain the target dose. Further, routine clinical monitoring was carried out. Besides, food intake was recorded daily. The experiment was carried out for 8 weeks. After 24 h of the last dose, the rats were euthanized and efforts were made to minimize suffering and stress.
Table 5. Animal Grouping and Dose of Administration.
| s. no. | groups | abbreviation | dosage (per Kg body weight) |
|---|---|---|---|
| 1 | control group | - | - |
| 2 | carbon tetrachloride | CCl4 | 1 mL |
| 3 | native form of silymarin | S | 200 mg |
| 4 | native form of quercetin | Q | 50 mg |
| 5 | a combination of native form of SQ | SQ | 200:50 mg |
| 6 | low-dose miniaturized SQ | LDMSQ | 100:15 mg |
| 7 | medium dose miniaturized SQ | MDMSQ | 150:30 mg |
| 8 | high-dose miniaturized SQ | HDMSQ | 200:50 mg |
4.10. Blood and Organ Collection
The animals were euthanized with CO2 at the end of the experimental period (i.e., after 56 days of treatment). About 2 mL of blood was collected into a heparinized container to examine hematological parameters; an additional 5 mL of blood was collected in a non-heparinized container and centrifuged at 3000 rpm for 10 min; the resulting serum was used for bioassays. The animals were quickly dissected, and the liver was excised and weighed to calculate relative organ weight. The samples of the liver were placed in 10% formal saline for histological examination.
4.11. Hematology
The blood sample (approximately 20 μL) was collected with EDTA and used for the estimation of WBCs, RBCs, Hb, mean corpuscular volume (MCV), corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC), platelets (PLT), percentages of lymphocytes (LYM %), and lymphocytes number (LYM#) using a hematology analyzer (Sysmex XP-100).
4.12. Serum Biochemical Markers and Lipid Profile
The effect of miniaturized SQ was evaluated by assaying the serum biochemical parameters associated with liver function. The activities of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gamma glutamate transferase and concentrations of albumin, total proteins, total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, glucose, uric acid, bilirubin, and creatinine were measured in plasma samples obtained from all groups of rats. The analysis was done following the instructions in the diagnostic kits from Agappe Diagnostics (Ernakulam, Kerala, India).
4.13. Antioxidant Enzymes and Molecules in the Liver
4.13.1. Superoxide Dismutase
The SOD activity in the liver homogenate was analyzed spectrophotometrically.41 This method determines the ability of SOD to inhibit the oxidation of nitroblue tetrazolium (NBT). One unit of SOD signifies the amount of enzymes required to hinder the rate of NBT oxidation by 50% at 25 °C. The rate of activity is expressed as units/mg protein.
4.13.2. Catalase
The catalase (CAT) activity was measured as per the method reported by Aebi (1984). The decomposition of hydrogen peroxide (H2O2) was monitored kinetically by CAT enzymes at 240 nm. One unit of CAT activity is equal to the micromole of H2O2 degraded per minute per milligram of protein.42
4.13.3. Glutathione Reductase
About 150 μL of liver serum was prepared in 5% (w/v) trichloroacetic acid and centrifuged at 2000g for 10 min, and the glutathione (GSH) content in the deproteinized supernatant was estimated by Ellman’s reagent (5,5′-dithio-bis[2-nitrobenzoic acid]). GR catalyzes the reduction of GSSG (glutathione disulfide) to GSH (glutathione), and GSH is the sulfhydryl form of a molecule that helps against oxidative stress. The GR activity was expressed as mmol of GSH oxidized/min/mg of protein at 25 °C.43
4.13.4. Thiobarbituric Acid Analysis
Lipid peroxidation was measured through TBARSs to detect the level of MDA (malondialdehyde) using fluorescence absorption.44 Around 0.2 mL of plasma was mixed with 10% sodium dodecyl sulfate, 0.53% TBA, and 20% acetic acid and boiled for 1 h. Butanol: pyridine (15:1) was added, mixed, and centrifuged at 3000 rpm for 10 min. The aliquot was read at 535 nm and recorded.
4.13.5. Histological Analysis
Tissues were taken from the liver of each animal after dissection and fixed using 10% formalin saline. The fixed tissues were processed routinely for paraffin embedding and cut into 4–5 μM thick sections, and the sections of organs were stained by hematoxylin and eosin. Tissue slides were prepared and observed using an optical microscope (Olympus BX-5, Prog Res C-5 software fixed with a digital camera to capture the images).
4.14. Statistical Analysis
Statistical analysis was investigated by analysis of variance using SPSS statistical software version 16. Duncan’s multiple comparison test performed the comparison of means. The level of significance used was p < 0.05 for all the statistical tests.
Acknowledgments
The first author acknowledges the Indian Council of Medical Research (ICMR), New Delhi, India, for supporting this work through the award of research fellowship (3/1/2/31/2014-Nut). The authors thank the Director, CSIR-CFTRI, for continuous support and providing the facilities to carry out the research work.
Glossary
Abbreviations
- SQ
silymarin and quercetin
- MS
miniaturized scaffold
- MSQ
miniaturized silymarin and quercetin
- NAFLD
non-alcoholic fatty liver disease
- SQMD
silymarin and quercetin with maltodextrin
- SQP
silymarin and quercetin with pectin
- SQWP
silymarin and quercetin with whey protein
- SQGA
silymarin and quercetin with gum arabica
- SQBC
silymarin and quercetin with β-cyclodextrin
- SQC
silymarin and quercetin with cellulose
- LDMSQ
low dose miniaturized silymarin and quercetin
- MDMSQ
medium-dose miniaturized silymarin and quercetin
- HDMSQ
high-dose miniaturized silymarin and quercetin
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00555.
Miniaturized scaffold efficiency of quercetin and silymarin with different biopolymers and FTIR spectra of (a) silymarin (b) quercetin (PDF)
Author Contributions
J.M.S.R. and A.K.S. conceptualized and proposed the project. J.M.S.R. designed the study, established the protocols, executed the experiments, interpreted the results, and prepared the manuscript. M.S.P. designed and supervised the animal experiments. A.K.S. supervised the in vitro studies and revised and finalized the manuscript.
The work was funded and supported by the Council of Scientific and Industrial Research (CSIR).
The authors declare no competing financial interest.
This paper was published on the Web on August 3, 2021. Superscript a’s and b’s were inadvertently deleted from Tables 1 and 2 and were added back in. The corrected version was reposted on August 4, 2021.
Supplementary Material
References
- Dressman J. B.; Thelen K. Cytochrome P450-Mediated Metabolism in the Human Gut Wall. J. Pharm. Pharmacol. 2009, 61, 541–558. 10.1211/jpp/61.05.0002. [DOI] [PubMed] [Google Scholar]
- Younossi Z.; Tacke F.; Arrese M.; Chander Sharma B.; Mostafa I.; Bugianesi E.; Wai-Sun Wong V.; Yilmaz Y.; George J.; Fan J.; Vos M. B. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Cai J.; She Z.; Li H. Insights into the Epidemiology, Pathogenesis, and Therapeutics of Nonalcoholic Fatty Liver Diseases. Adv. Sci. 2019, 6, 1801585. 10.1002/advs.201801585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams L. A.; Angulo P. Treatment of Non-Alcoholic Fatty Liver Disease. Postgrad. Med. 2006, 82, 315–322. 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouri N.; Lopez R.; Berk M.; Feldstein A. E. Serum Retinol-Binding Protein 4 Levels in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Gastroenterol. 2009, 43, 985. 10.1097/mcg.0b013e3181a0998d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostovaneh M. R.; Ambale-Venkatesh B.; Fuji T.; Bakhshi H.; Shah R.; Murthy V. L.; Tracy R. P.; Guallar E.; Wu C. O.; Bluemke D. A.; Lima J. A. C. Association of Liver Fibrosis with Cardiovascular Diseases in the General Population: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ. Cardiovasc. Imaging. 2018, 11, e007241 10.1161/CIRCIMAGING.117.007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogaly H. A.; Eltablawy N. A.; Abd-Elsalam R. M. Antifibrogenic Influence of Mentha Piperita L. Essential Oil against CCl4-Induced Liver Fibrosis in Rats. Oxid. Med. Cell. Longevity 2018, 2018, 4039753. 10.1155/2018/4039753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuñón M. J.; Alvarez M.; Culebras J. M.; González-Gallego J. An Overview of Animal Models for Investigating the Pathogenesis and Therapeutic Strategies in Acute Hepatic Failure. World J. Gastroenterol. 2009, 15, 3086. 10.3748/wjg.15.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N.; Zou Y.; Zhu L.; Wang H.-F.; Dai M.-G. Antioxidant Properties of Proanthocyanidins Attenuate Carbon Tetrachloride (CCl4)-Induced Steatosis and Liver Injury in Rats via CYP2E1 Regulation. J. Med. Food 2014, 17, 663–669. 10.1089/jmf.2013.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C.; Barros L.; Ferreira I. C. Extraction, Identification, Fractionation and Isolation of Phenolic Compounds in Plants with Hepatoprotective Effects. J. Sci. Food Agric. 2016, 96, 1068–1084. 10.1002/jsfa.7446. [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F.; Duarte J.; Jimenez R.; Santos-Buelga C.; Osuna A. Antihypertensive Effects of the Flavonoid Quercetin. Pharmacol. Rep. 2009, 61, 67–75. 10.1016/s1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]
- Dixit N.; Baboota S.; Kohli K.; Ahmad S.; Ali J. Silymarin: A Review of Pharmacological Aspects and Bioavailability Enhancement Approaches. Indian J. Pharmacol. 2007, 39, 172. 10.4103/0253-7613.36534. [DOI] [Google Scholar]
- Kandemir F. M.; Kucukler S.; Caglayan C.; Gur C.; Batil A. A.; Gülçin İ. Therapeutic Effects of Silymarin and Naringin on Methotrexate-Induced Nephrotoxicity in Rats: Biochemical Evaluation of Anti-Inflammatory, Antiapoptotic, and Antiautophagic Properties. J. Food Biochem. 2017, 41, e12398 10.1111/jfbc.12398. [DOI] [Google Scholar]
- Miltonprabu S.; Tomczyk M.; Skalicka-Woźniak K.; Rastrelli L.; Daglia M.; Nabavi S. F.; Alavian S. M.; Nabavi S. M. Hepatoprotective Effect of Quercetin: From Chemistry to Medicine. Food Chem. Toxicol. 2017, 108, 365–374. 10.1016/j.fct.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Gao C.; Xing M.; Li Y.; Zhu L.; Wang D.; Yang X.; Liu L.; Yao P. Quercetin Prevents Ethanol-Induced Dyslipidemia and Mitochondrial Oxidative Damage. Food Chem. Toxicol. 2012, 50, 1194–1200. 10.1016/j.fct.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Ying H.-Z.; Liu Y.-H.; Yu B.; Wang Z.-Y.; Zang J.-N.; Yu C.-H. Dietary Quercetin Ameliorates Nonalcoholic Steatohepatitis Induced by a High-Fat Diet in Gerbils. Food Chem. Toxicol. 2013, 52, 53–60. 10.1016/j.fct.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Terao J.; Piskula M. K. Flavonoids and Membrane Lipid Peroxidation Inhibition. Nutrition 1999, 15, 790–791. 10.1016/s0899-9007(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Wu J.-W.; Lin L.-C.; Hung S.-C.; Chi C.-W.; Tsai T.-H. Analysis of Silibinin in Rat Plasma and Bile for Hepatobiliary Excretion and Oral Bioavailability Application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. 10.1016/j.jpba.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Bao C.; Jiang P.; Chai J.; Jiang Y.; Li D.; Bao W.; Liu B.; Liu B.; Norde W.; Li Y. The Delivery of Sensitive Food Bioactive Ingredients: Absorption Mechanisms, Influencing Factors, Encapsulation Techniques and Evaluation Models. Food Res. Int. 2019, 120, 130–140. 10.1016/j.foodres.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Zhang A.; Sun H.; Yuan Y.; Sun W.; Jiao G.; Wang X. An in Vivo Analysis of the Therapeutic and Synergistic Properties of Chinese Medicinal Formula Yin-Chen-Hao-Tang Based on Its Active Constituents. Fitoterapia 2011, 82, 1160–1168. 10.1016/j.fitote.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Anandam S.; Selvamuthukumar S. Fabrication of Cyclodextrin Nanosponges for Quercetin Delivery: Physicochemical Characterization, Photostability, and Antioxidant Effects. J. Mater. Sci. 2014, 49, 8140. 10.1007/s10853-014-8523-6. [DOI] [Google Scholar]
- Karathanos V. T.; Mourtzinos I.; Yannakopoulou K.; Andrikopoulos N. K. Study of the Solubility, Antioxidant Activity and Structure of Inclusion Complex of Vanillin with β-Cyclodextrin. Food Chem. 2007, 49, 8140–8153. 10.1016/j.foodchem.2006.01.053. [DOI] [Google Scholar]
- Kumari A.; Yadav S. K.; Pakade Y. B.; Singh B.; Yadav S. C. Development of Biodegradable Nanoparticles for Delivery of Quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. 10.1016/j.colsurfb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Sohrabi M. J.; Dehpour A.-R.; Attar F.; Hasan A.; Mohammad-Sadeghi N.; Meratan A. A.; Aziz F. M.; Salihi A.; Shekha M. S.; Akhtari K.; Shahpasand K.; Hojjati S. M. M.; Sharifi M.; Saboury A. A.; Rezayat S. M.; Mousavi S. E.; Falahati M. Silymarin-Albumin Nanoplex: Preparation and Its Potential Application as an Antioxidant in Nervous System in Vitro and in Vivo. Int. J. Pharm. 2019, 572, 118824. 10.1016/j.ijpharm.2019.118824. [DOI] [PubMed] [Google Scholar]
- Singireddy A.; Subramanian S. Cyclodextrin Nanosponges to Enhance the Dissolution Profile of Quercetin by Inclusion Complex Formation. Part. Sci. Technol. 2016, 34, 341–346. 10.1080/02726351.2015.1081658. [DOI] [Google Scholar]
- Smyth R.; Munday M. R.; York M. J.; Clarke C. J.; Dare T.; Turton J. A. Comprehensive Characterization of Serum Clinical Chemistry Parameters and the Identification of Urinary Superoxide Dismutase in a Carbon Tetrachloride-Induced Model of Hepatic Fibrosis in the Female Hanover Wistar Rat. Int. J. Exp. Pathol. 2007, 88, 361–376. 10.1111/j.1365-2613.2007.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M.-Y.; Fu T. Y.-C.; Shih P.-H.; Lee C.-P.; Yen G.-C. Du-Zhong (Eucommia Ulmoides Oliv.) Leaves Inhibits CCl4-Induced Hepatic Damage in Rats. Food Chem. Toxicol. 2006, 44, 1424–1431. 10.1016/j.fct.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Messner M.; Brissot P. Traditional Management of Liver Disorders. Drugs 1990, 40, 45–57. 10.2165/00003495-199000403-00005. [DOI] [PubMed] [Google Scholar]
- López-De León A.; Rojkind M. A Simple Micromethod for Collagen and Total Protein Determination in Formalin-Fixed Paraffin-Embedded Sections. J. Histochem. Cytochem. 1985, 33, 737–743. 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- Heemann U. Albumin Dialysis in Cirrhosis with Superimposed Acute Liver Injury: A Prospective, Controlled Study. Hepatology 2002, 36, 949–958. 10.1016/s0270-9139(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Thapa B. R.; Walia A. Liver Function Tests and Their Interpretation. Indian J. Pediatr. 2007, 74, 663–671. 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- Kirke D. A.; Smyth T. J.; Rai D. K.; Kenny O.; Stengel D. B. The Chemical and Antioxidant Stability of Isolated Low Molecular Weight Phlorotannins. Food Chem. 2017, 221, 1104–1112. 10.1016/j.foodchem.2016.11.050. [DOI] [PubMed] [Google Scholar]
- El-Lakkany N.; El-Din S.; Sabra A.-N.-A.; Hammam O.; Ebeid F.-L. Co-Administration of Metformin and N-Acetylcysteine with Dietary Control Improves the Biochemical and Histological Manifestations in Rats with Non-Alcoholic Fatty Liver. Results Pharma Sci. 2016, 11, 374. 10.4103/1735-5362.192487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovich K.; Li W.; DeAngelis R.; Greenbaum L. E.; Ciliberto G.; Taub R. Interleukin-6 Protects against Fas-Mediated Death by Establishing a Critical Level of Anti-Apoptotic Hepatic Proteins FLIP, Bcl-2, and Bcl-XL. J. Biol. Chem. 2001, 276, 26605–26613. 10.1074/jbc.m100740200. [DOI] [PubMed] [Google Scholar]
- Zu Y.; Li C.; Fu Y.; Zhao C. Simultaneous Determination of Catechin, Rutin, Quercetin Kaempferol and Isorhamnetin in the Extract of Sea Buckthorn (Hippophae Rhamnoides L.) Leaves by RP-HPLC with DAD. J. Pharm. Biomed. Anal. 2006, 41, 714–719. 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Hundre S. Y.; Karthik P.; Anandharamakrishnan C. Effect of Whey Protein Isolate and β-Cyclodextrin Wall Systems on Stability of Microencapsulated Vanillin by Spray-Freeze Drying Method. Food Chem. 2015, 174, 16–24. 10.1016/j.foodchem.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Hong R.; Tian T. Silymarin’s Protective Effects and Possible Mechanisms on Alcoholic Fatty Liver for Rats. Biomol. Ther. 2013, 21, 264. 10.4062/biomolther.2013.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias N.; Macarulla M. T.; Aguirre L.; Miranda J.; Portillo M. P. Liver Delipidating Effect of a Combination of Resveratrol and Quercetin in Rats Fed an Obesogenic Diet. J. Physiol. Biochem. 2015, 71, 569–576. 10.1007/s13105-015-0403-2. [DOI] [PubMed] [Google Scholar]
- Pasdar Y.; Oubari F.; Zarif M. N.; Abbasi M.; Pourmahmoudi A.; Hosseinikia M. Effects of Quercetin Supplementation on Hematological Parameters in Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Clin. Nutr. Res. 2020, 9, 11. 10.7762/cnr.2020.9.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obi F. O.; Usenu I. A.; Osayande J. O. Prevention of Carbon Tetrachloride-Induced Hepatotoxicity in the Rat by H. Rosasinensis Anthocyanin Extract Administered in Ethanol. Toxicology 1998, 131, 93. 10.1016/s0300-483x(98)00119-x. [DOI] [PubMed] [Google Scholar]
- Beyer W. F.; Fridovich I. Assaying for Superoxide Dismutase Activity: Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Aebi H. [13] Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Flohe L.; Günzler W. A.; Schock H. H. Glutathione Peroxidase: A Selfnoenzyme. FEBS Lett. 1973, 32, 132–134. 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- Yagi K. A Simple Fluorometric Assay for Lipoperoxide in Blood Plasma. Biochem. Med. 1976, 15, 212–216. 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.