Abstract
Inspired by the natural topological structure of skeletal muscle tissue, the topological surface construction of bionic scaffolds for skeletal muscle repair has attracted great interest. Many previous studies have focused on the effects of the topological structure on myoblasts. However, these studies used only specific repeating sizes and shapes to achieve the myoblast alignment and myotube formation; moreover, the regulatory effects of the size of a topological structure on myogenic differentiation are often neglected, leading to a lack of guidance for the design of scaffolds for skeletal muscle tissue engineering. In this study, we fabricated a series of microgroove topographies with various widths and depths via a combination of soft lithography and melt-casting and studied their effects on the behaviors of skeletal muscle cells, especially myogenic differentiation, in detail. Microgrooved poly(lactic-co-glycolic acid) substrates were found to effectively regulate the proliferation, myogenic differentiation, and myotube formation of C2C12 cells, and the degree of myogenic differentiation was significantly dependent on signals in response to the size of the microgroove structure. Compared with their depth, the width of the microgroove structures can more strongly affect the myogenic differentiation of C2C12 cells, and the degree of myoblast differentiation was enhanced with increasing groove width. Microgroove structures with relatively large groove widths and small groove depths promoted the myogenic differentiation of C2C12 cells. In addition, the integrin-mediated focal adhesion kinase signaling pathway and MAPK signaling pathway were activated in cells in response to the external topological structure, and the size of the topological structure of the material surface effectively regulated the degree of the cellular response to the external topological structure. These results can guide the design of scaffolds for skeletal muscle tissue engineering and the construction of effective bionic scaffold surfaces for skeletal muscle regeneration.
Introduction
Since 1912, through studies by Harrison,1 cell orientation and locomotion have been known to be directed by the geometric features of the underlying substratum, known as topography,2 in a phenomenon named contact guidance by Weiss. et al.3 Cells in native tissue are surrounded by neighboring cells and an extracellular matrix (ECM) secreted by the cells. Studies have repeatedly shown that a large number of topographies on the order of micro and nanometers exist in the ECM and contribute to the regulation of cell behaviors, which suggests the ubiquity and importance of contact guidance.4−6 The development of micro and nanofabrication technologies allows for in vitro explorations of cell responses to various artificial topographies, and topographies have shown an ability to influence cell features and behaviors, such as cell morphology,7,8 adhesion,9,10 migration,11 proliferation,12 and differentiation.13
Tissue engineering is a promising alternative to autograft or allograft transplantation and can ease the shortage of native donor tissues and organs. In addition, it allows the construction of in vitro models of some tissues or organs for use in biological studies, tissue repair, and drug research, among other fields.14 In tissue engineering, the adequate imitation of native tissue with respect to chemical, physical, and biological properties is required to avoid immunological rejection and help reconstruct tissue function. Because topographies on the ECM surface play a critical role in cell fate, micro or nanoscale topography cues on a tissue engineering scaffold can be powerful tools for mimicking the in vivo microenvironment of cells, achieving desired cell behavior, and ultimately forming tissues or organs that are both structurally and functionally similar to native tissues or organs. To date, many studies have incorporated topographical cues into bone,15,16 heart,17 neuronal,18,19 and blood vessel20 tissue engineering and studied their effects on the repair of different tissues. For example, Xu et al. developed a nanofibrous scaffold with aligned topography and found that smooth muscle cells aligned and migrated along the nanofibers and that cell adhesion and proliferation were also enhanced compared to those of a control. Their results indicated that the developed scaffold may be an ideal scaffold for blood vessel engineering.21
Skeletal muscle is an important part of the human body and accounts for 48% of its weight. The contraction and expansion of skeletal muscle provide a physical basis for body movement. Skeletal muscle diseases, such as sarcopenia and Duchenne muscular dystrophy, and severe sports injuries cause the loss and dysfunction of skeletal muscle.22−24 At present, the most conventional treatment for skeletal muscle diseases is autograft transplantation, which may cause morbidity at the donor site.25 The injection of skeletal muscle satellite cells is an alternative, but this strategy is accompanied by the poor survival of the delivered cells and the complications, such as immunological rejection. Skeletal muscle tissue engineering is gradually becoming a promising approach that lacks the above deficiencies. In the body, native skeletal muscle is mainly composed of a great number of aligned fibers, which are composed of multinucleated cells that are responsible for the formation and contraction of muscles.26,27 Therefore, the alignment of seed cells, such as skeletal myoblasts, in skeletal muscle tissue engineering simulates the structure of native skeletal muscle tissue and is essential to the physical function of regenerated skeletal muscle tissue. Many reports have confirmed that the surface micro/nanotopography of scaffolds can regulate the orientation of skeletal muscle cells and the formation of myotubes, which is conducive to skeletal muscle tissue repair.27−31 The disorganized arrangement of the skeletal muscle cells and the ECM leads to the formation of disorganized skeletal muscle tissue without the function of skeletal muscle and results in failed skeletal tissue repair. At present, the topological structures commonly used in skeletal muscle tissue engineering include nanofibers and microgrooves.32,33 These structures can regulate the orientation of muscle cells to mimic the natural morphology of skeletal muscle tissue. Therefore, the combination of topography and skeletal muscle tissue engineering is of great significance for skeletal muscle regeneration.
Inspired by the natural topological structure of skeletal muscle tissue, the bionic topological surface construction of scaffolds for skeletal muscle repair has attracted great interest. Although many studies have focused on the effects of the topological structure on myoblasts, these studies have used only specific repeating sizes and shapes to induce myoblast alignment and myotube formation. In addition, the regulatory effects of the size of the topological structure on myogenic differentiation are often neglected, which limits effective guidance in the design of skeletal muscle tissue engineering scaffolds. Therefore, in this work, we designed a series of microgroove topographies with various widths and depths via a combination of soft lithography and melt-casting and explored the regulatory effect of the microgroove structure on myogenic differentiation in detail. A schematic illustration of the fabrication procedure is shown in Figure 1. Herein, we chose C2C12 skeletal muscle cells as a model cell type for myogenic differentiation. Poly(lactic-co-glycolic acid) (PLGA) was adopted as a substrate material because of its biocompatibility and approval by the US Food and Drug Administration for clinical application.34 Our studies demonstrate that the microgroove structure plays an important role in the regulation of cell behaviors, especially myogenic differentiation and aligned myotube formation. In addition, the degree of myogenic differentiation was found to be significantly dependent on the size cues of the microgroove structure, and the degree of myoblast differentiation increased with the increase of groove width. These results can guide the design of scaffolds for skeletal muscle tissue engineering and the construction of effective bionic scaffold surfaces for skeletal muscle regeneration.
Figure 1.

Schematic illustration of the fabrication of microgrooved PLGA. First, polydimethylsiloxane (PDMS) was poured onto a silicon master to produce a PDMS master. Then PLGA was melted at 150 °C for 4 h on the PDMS master surface. Finally, the microgrooved PLGA substrate was peeled off from the PDMS template.
Results and Discussion
Fabrication and Characterization of Microgrooved PLGA Substrates
Microgrooved PLGA substrates were prepared by combining the two methods: soft lithography and melt-casting. In this study, a microgroove topography structure was chosen as the micropattern to mimic the structure of aligned fibers of native skeletal muscle. Herein, we designed a series of widths (100, 50, and 25 μm), and the depth was set to 50 μm. We also designed a series of depths (100, 50, and 25 μm), and the width was set to 50 μm. The prepared microgrooved PLGA substrates were first characterized via scanning electron microscopy (SEM) to observe their surface morphology. The results are shown in Figure 2. Each prepared microgroove in the PLGA displayed a well-ordered structure, and the size was consistent with the original design. In addition, the microgroove exhibited a clean surface without obvious defects or impurities. These results showed that the microgrooved PLGA surface was successfully fabricated by the combination of soft lithography and melt-casting.
Figure 2.

SEM photos of microgrooves of different widths and depths on the PLGA substrate: (a) W100D50 (width: 100 μm; depth: 50 μm); (b) W50D50 (width: 50 μm; depth: 50 μm); (c) W25D50 (width: 25 μm; depth: 50 μm); (d) W50D100 (width: 50 μm; depth: 100 μm); and (e) W50D25 (width: 50 μm; depth: 25 μm). (f) Schematic illustration of microgrooves. The ridge width was set to 200 μm.
Contact Angle Measurement of Patterned PLGA Materials
The surface topological structure has been reported to affect the hydrophilic–hydrophobic properties of a material’s surface.35 Therefore, the contact angle of each microgrooved PLGA material was measured using a contact angle instrument, and the results are shown in Figure 3. The contact angles of the smooth PLGA, W50D100, W50D50, W50D25, W100D50, and W25D50 were 81.8 ± 3.4, 93.4 ± 3.1, 91.4 ± 3.3, 91.3 ± 3.4, 89.5 ± 1.1, and 92.6 ± 2.9°, respectively, which showed that the preparation of microgroove structures on the surface of the PLGA substrate can slightly increase the contact angle by approximately 10.0° compared with that of smooth PLGA. There was no significant difference in contact angles among the patterned PLGA substrates with different groove widths and depths, which may have been due to the relatively large sizes of the microgrooves.
Figure 3.
Quantitative analysis of the contact angle of PLGA materials with grooves of different widths and depths.
Cell Proliferation
Previous reports have shown that the micro/nanotopological structure of a material surface can directly regulate cell proliferation.36−38 Therefore, the cell proliferation behaviors of C2C12 cells after culture for 1, 3, and 5 days on the microgrooved PLGA substrates were assessed via cell counting kit 8 (CCK-8) assay. The results (Figure 4a) demonstrated that the microgroove structure can effectively regulate the proliferation of C2C12 cells. The proliferation rate of the cells was significantly increased as the groove width increased. W100D50, W50D50, and W25D50 presented higher cell densities than the control samples (smooth) on the first day and maintained this trend at all time points. On the third and fifth days of culture, both W100D50 and W50D50 showed significant differences in cell density compared with W25D50 and the smooth samples. Although the C2C12 cells proliferated faster on W100D50 than on the other substrates, there was no significant difference in cell growth between W100D50 and W50D50. Nevertheless, our results clearly showed that the microgroove structure can promote cell proliferation, with a lower cell proliferation rate observed on smooth surfaces. This may be because the microgroove structure of the substrate surface increases the cell adhesion compared to that on the smooth PLGA surface (Figure 4b). In our previous work, we also investigated the effects of different microgroove depths on the proliferation of C2C12 cells.39 Our previous results demonstrated that C2C12 cells cultured on microgrooves of various depths exhibited a higher proliferation rate than cells cultured on the smooth samples, indicating that the groove depth affects cell proliferation.
Figure 4.
(a) Cell proliferation behavior measured via CCK-8 assay after the culture of C2C12 cells on microgrooved PLGA samples with different groove widths for 1, 3, and 5 days. (b) Cell adhesion rate measured via CCK-8 assay after the culture of C2C12 cells on microgrooved PLGA samples with different groove depths for 1 day. The results are presented as the means ± standard error for n = 6 (*p < 0.05).
Myogenic Differentiation
In addition to cell proliferation, the myogenic differentiation of C2C12 cells was investigated in detail. According to the literature, the microstructure of material surfaces can affect the direction and degree of cell differentiation.40−42 Regarding the mechanism, it is widely accepted that the surface microstructures of material surfaces can regulate cell differentiation behavior by influencing cell morphology. The current literature mostly focuses on the effects of nano and submicron-scale structures on cell differentiation; these structures are smaller than the size of individual cells and can greatly influence individual cells at the molecular level. However, topological structures of microns or even tens of microns often exist on the surface of tissue engineering scaffolds or biomaterials, which usually cooperate to regulate the cell behaviors. Therefore, it was necessary to determine the functions of a relatively large microscale structure. In this study, to investigate the effect of a large micron-scale groove structure on cell differentiation behavior, the C2C12 cells, as a model cell line, were seeded at a concentration of 1 × 104 cells/mL onto the surfaces of microgroove structures with different widths and depths. The cells were then cultured in growth medium for 3 days and myogenic differentiation medium for 5 days, after which TRIzol and protein lysis buffer were added to obtain samples for real-time-polymerase chain reaction (RT-PCR) and western blotting, respectively.
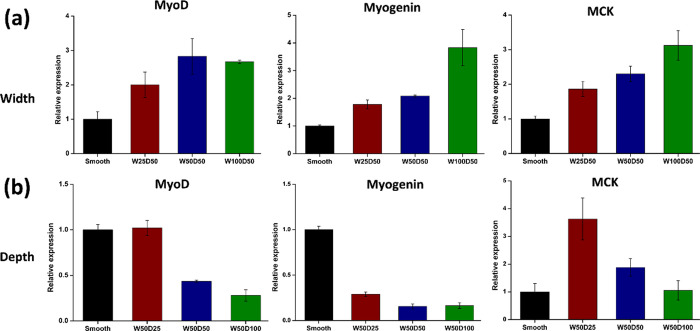
At the described times, the expression of the marker genes, MyoD, myogenin, and MCK was detected by RT-PCR. As shown in Figure 5, the microgrooved structure of the PLGA surface could significantly affect the differentiation of C2C12 cells into myotubes. The groove width and depth have contrasting effects on the myogenin differentiation of C2C12 cells. As the groove width increased, the degree of myogenic differentiation also increased (Figure 5a). Marker genes of myogenic differentiation (MyoD, myogenin, and MCK) were significantly upregulated as the groove width increased. In contrast, as the groove depth increased, the degree of myogenic differentiation was reduced (Figure 5b). The marker genes of myogenic differentiation (MyoD, myogenin, and MCK) were significantly downregulated as the groove depth increased. To further verify the RT-PCR results, the protein expression levels of MYH and myogenin were measured via western blotting. The primary outcomes confirmed the previous patterns observed for the groove width and depth. The expression levels of both proteins were upregulated as the groove width increased (Figure 6) and the groove depth decreased (Figure 7), which was consistent with the RT-PCR results. Therefore, it can be concluded that the microgroove structures can effectively regulate the myogenic differentiation. Furthermore, the results demonstrated that the dimensions of the microgroove are important parameters for cell differentiation. For myogenic differentiation, structures with a large groove width and a shallow groove depth are beneficial. Unlike smooth materials, the microgroove structures can partially suppress the differentiation of C2C12 cells into myotubes to a certain extent, which can be explained as follows: as the depth increases, it is increasingly difficult for cells to cross the deeper groove; thus, the groove hinders the migration of C2C12 cells on the microgrooved material surface, which hinders the fusion of C2C12 cells and their formation into myotubes on the surface of the material.43
Figure 5.
Characterization of myogenic differentiation of C2C12 cells using RT-PCR to measure the gene expression level of markers. (a) MyoD, myogenin, and muscle creatine kinase (MCK) mRNA levels measured in C2C12 cells cultured on microgrooves of different widths. (b) MyoD, myogenin, and MCK mRNA levels measured in C2C12 cells cultured on microgrooves of different depths.
Figure 6.
Photograph of a western blot showing (a) MYH and (b) myogenin expression of C2C12 cells cultured on microgrooves with different depths and quantitative densitometric analysis of the western blot image using Image J software.
Figure 7.
Photograph of a western blot showing (a) MYH and (b) myogenin expression of C2C12 cells cultured on microgrooves with different widths and quantitative densitometric analysis of the western blot image using Image J software.
In mammals, the orientation of muscle fibers plays an important role in maintaining the normal function of skeletal muscle tissue.28−30 Therefore, determining how to regulate the orientation of muscle fibers in the design of skeletal muscle tissue engineering is the key to successful skeletal muscle tissue repair. In this study, we attempted to regulate the alignment of myotubes using a patterned microgroove structure on a PLGA substrate surface. Herein, W100D50, W50D50, and W25D50 were chosen as substrates because of their optimal differentiation effects on C2C12 cells. C2C12 cells were seeded onto the surfaces of microgroove structures with different groove widths at a concentration of 1 × 104 cells/mL and cultured in growth medium for 3 days and myogenic differentiation medium for 5 days. Then, immunohistochemical staining was carried out to observe the morphology of the myotubes. The results are shown in Figure 8. We found that the myotubes formed during C2C12 cell differentiation displayed significant alignment along the direction of the groove, while the myotubes on the smooth substrate were randomly arranged. These results demonstrated that the designed microgroove structures with various groove widths could not only prompt the myogenic differentiation of C2C12 cells but also control the alignment of myotubes, which is important for skeletal muscle tissue engineering.
Figure 8.
Morphological observation of myotubes on microgrooved PLGA substrates with different groove widths. (a) W100D50, (b) W50D50, (c) W25D50, and (d) smooth substrate. The depth of the microgrooves was 50 μm for all samples, and their locations are indicated in the images by blue triangles. The locations of the myotubes in the images are indicated with red arrows. (e) Percentage of aligned myotubes out of the total number of myotubes (alignment index). (*p < 0.05 and **p < 0.01).
To intuitively show the alignment degree of myotube alignment, the percentage of aligned myotubes, defined as the alignment index, was calculated. On a microgrooved surface, a myotube was considered aligned with the grooves when the angle between the longest myotube axis and the groove axis was less than 10°; otherwise, the myotube was considered randomly arranged. As shown in Figure 8e, the alignment index was significantly increased from 12% in the control group to approximately 70∼91% on the grooved substrates. The alignment indices were 91.37% for W100D50, 78.29% for W50D50, 70.50% for W25D50, and 12.06% for the smooth material, respectively. Although no significant difference in the alignment index among the grooved substrates with different groove widths was observed, the alignment index gradually increased as the groove width increased. These results demonstrate that grooves of the appropriate size can regulate the orientations of the myotubes during myoblast differentiation, which has great significance for skeletal muscle repair.
Signaling Pathway Analysis
Many previous studies have reported that the activation of the integrin-mediated focal adhesion kinase (FAK) signaling pathway and MAPK signaling pathway plays an important role in the regulation of cell behavior by the topological structure of the material surface.44−46 The integrin-mediated FAK signaling pathway can mediate the adhesion of cells to the ECM and is involved in many important biological processes, such as cell movement, proliferation, differentiation, survival, and the regulation of gene expression. FAK, as a key signaling pathway molecule, often acts as a stimulus sensor that can be activated by stimulation from the external environment and plays a role in controlling cell movement through its own phosphorylation. Cells can sense stimulation of the microgroove topological microenvironment through transmembrane integrin proteins.47,48 The integrin-mediated FAK signaling pathway is first activated; then, external environmental signals are transmitted from the membrane to the nucleus through cascade reactions involving the MAPK signaling pathway. Then, the expression of genes and proteins is regulated.49,50 In the MAPK signaling pathway, the protein kinase ERK1/2 plays a key role; it is an extracellular signal-regulated kinase that can respond to external stimuli through its own protein phosphorylation.51−53 Therefore, in this study, the responses of the integrin-mediated FAK signaling pathway and MAPK signaling pathway to microenvironments with different groove sizes were studied by detecting the key protein kinases FAK and ERK1/2 and their phosphorylation expression levels. As shown in Figure 9, the results demonstrated that the expression and phosphorylation levels of these protein kinases FAK and ERK1/2 were significantly dependent on the groove width of the microgroove structure. With increasing microgroove width, the expression and phosphorylation level of the protein kinases gradually increased. These results indicated that the integrin-mediated FAK and MAPK signaling pathways were activated in response to the external topological structure of the cells and that the dimensions of the topological structure of the material surface could effectively regulate the cellular response to the structure.
Figure 9.
(a) Photograph of a western blot showing the expression and phosphorylation levels of key protein kinases (FAK and ERK1/2). (b) Quantitative analysis of the western blot image using Image J software.
Conclusions
In summary, we fabricated a series of microgroove topographies with different groove widths and depths via a combination of soft lithography and melt-casting and studied their ability to regulate the behaviors of skeletal muscle cells, especially myogenic differentiation, in detail. It was found that the microgroove structure of the PLGA substrate surface could affect the proliferation, myogenic differentiation, and aligned myotube formation of the C2C12 cells. In addition, the size of the microgroove structure affected the degree of myogenic differentiation and myotube formation. Compared with the depth, the width of the microgroove structure more effectively promoted the myogenic differentiation of skeletal muscle cells. Microgroove structures with relatively large groove widths and shallow groove depths can promote the myogenic differentiation of C2C12 cells. In addition, the groove structure effectively regulated the orientations of the myotubes during myoblast differentiation. The alignment index was significantly increased from 12% in the control group to approximately 70∼91% on the grooved substrates, which is important for the biomimetic repair of skeletal muscle tissue function. In addition, the integrin-mediated FAK signaling pathway and MAPK signaling pathway were activated in response to the external topological structure of the cells, and the size cues of the topological structure of the material surface regulated the degree of the cellular response to the external topological structure. These results can effectively guide the design of skeletal muscle tissue engineering scaffolds and the construction of effective scaffold surfaces for skeletal muscle regeneration.
Materials and Methods
Fabrication and Characterization of Microgrooved PLGA Substrates
The patterned PLGA material was fabricated in the same way as reported previously.39 A PDMS template was obtained by soft photolithography. In brief, a silicon wafer was first spin-coated with a negative photoresist (NR21-20000P, Futurrex, USA). After baking at 80 °C for 10 min and then 150 °C for 5 min, the resist was exposed to UV light through a photomask (3 in × 3 in) and developed in an RD6 developer solution. Then, we poured a mixture of PDMS base (Sylgard 184, Dow Corning, USA) and curing agent (10:1 w/w) onto a silicon wafer. After heating at 60 °C for 4 h, a PDMS mask was prepared. A PLGA (50:50, MW = 50,000 g/mol) replica was prepared via a melt-casting method (150 °C for 4 h) using the PDMS master as a template. Then, the patterned PLGA material was cut using a nonmetallic laser cutter to prepare a substrate with the same size as the 24-well cell culture plate used for subsequent cell experiments.
The surface morphology of the grooved PLGA substrates was first characterized using SEM (Quanta 200 SEM, FEI, the Netherlands). Prior to microscopy, the samples were coated with gold (120 s). The static contact angles of the patterned PLGA and smooth PLGA (as a control) were investigated via the seat-drop method using an OCA15 contact angle goniometer (DATAPHYSICS, Germany). The droplet volume was 2 μL, and the velocity was medium. Five points on each sample were randomly selected for assessment.
In this study, a series of grooved PLGA substrates with various pattern depths (width: 50 μm; depth: 25, 50, or 100 μm) and widths (depth: 50 μm; width: 25, 50, or 100 μm) was prepared through the methods described above. For ease of reference, we named these different microgrooved substrates W50D100, W50D50, W50D25, W100D50, and W25D50 according to their width (W) and depth(D).
Cell Culture
Mouse myoblasts (C2C12; Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; PAA, Germany) with high glucose supplemented with 10% fetal bovine serum (Gibco, USA). The PLGA samples were placed in 24-well plates, sanitized in 75% (v/v) ethanol for 2 h, rinsed with sterilized phosphate-buffered saline (PBS) three times, and pretreated with culture medium for 12 h. Finally, each sample was seeded with 1 mL of a C2C12 cell suspension (1 × 104 cells/well) and incubated at 37 °C in a humidified incubator with 5% CO2.
Cell Proliferation
A CCK-8 (Dojindo Laboratories, Japan) was used to determine the effects of microgrooves with different widths (W100D50, W50D25, and W25D50) and depths (W50D100, W50D50, and W50D25) on the proliferation of C2C12 cells at different time intervals. In brief, samples from both the experimental and control groups were transferred to 24-well plates, seeded with 1 mL of a C2C12 cell suspension (1 × 104 cells/well), and incubated at 37 °C in a humidified incubator with 5% CO2. After culturing for 1, 3, and 5 days, 300 μL of DMEM and 30 μL of CCK-8 solution were added and incubated with the cells at 37 °C for 1 h. Subsequently, the medium supernatant was extracted for absorbance detection using a Thermo 3001 microplate reader (Thermo, USA) (n = 6) at 450 nm. In addition, the adhesion rate of cells on the surface of the groove substrates with different widths was also evaluated by CCK-8 assay after culture for 1 day.
Myogenic Differentiation
The expression of marker genes related to myogenic differentiation was detected via RT-PCR to investigate the effects of grooves of different widths and depths on the differentiation of C2C12 cells. In brief, C2C12 cells were seeded on the surfaces of W100D50, W50D50, W25D50, W50D100, W50D25, and the smooth materials at a concentration of 1 × 104 cells/mL and cultured in growth medium for 3 days. Then, the growth medium was exchanged for myogenic differentiation medium, and incubation was continued for another 5 days followed by the addition of TRizol reagent to homogenize the cells. RNA was then extracted from the homogenized samples following the manufacturer’s protocol. The following primer sequences were used: MyoD gene, (forward) 5’-GGACCCAGGAACTGGGATATG-3′ and (reverse) 3’-TCATAGAAGTCGTCTGCTGTCTCAA-5′; myogenin gene, (forward) 5’-CAGTGAATGCAACTCCCACAG-3′ and (reverse) 3’-TGGACGTAAGGGAGTGCAGA-5′; MCK gene, (forward) 5’-ACCTCTACAATAAGCTTCGCGATAA-3′ and (reverse) 3′- ATGGCGGTCCTGGATGATG-5′; MLC2 gene, (forward) 5’-TTCCATCTGGAGCTACTGCCTTG-3′ and (reverse) 3′- CCTTGAACTCCTGGATCTGAGTCTG-5′; and GAPDH gene, (forward) 5′- AAATGGTGAAGGTCGGTGTGAAC-3′ and (reverse) 3′- CAACAATCTCCACTTTGCCACTG-5′. The relative quantification of each target gene was normalized to GAPDH expression and calculated using the 2–ΔΔCt method.
In addition, the expression of proteins related to myogenic differentiation was also measured via western blotting to investigate the effect of the groove depth on the differentiation of C2C12 cells. In brief, the C2C12 cells were seeded on the surfaces of W100D50, W50D50, W25D50, W50D100, W50D25, and the smooth materials at a concentration of 1 × 104 cells/mL. The cells were cultured in growth medium for 3 days and in myogenic differentiation induction medium for 5 days; then, protein lysis buffer was added to extract the total protein from the samples for western blot analysis.
Immunohistochemical Staining
C2C12 cells were seeded onto the surfaces of microgrooved structures with different widths at a concentration of 1 × 104 cells/mL and cultured in growth medium for 3 days and myogenic differentiation medium for 5 days. Then, the cells were washed with PBS and fixed using 4% formaldehyde for 15 min at room temperature. Next, the cells were immersed in 0.1% Triton X-100 for 10 min and 1% bovine serum albumin (BSA) in 1x PBS for 30 min at room temperature. The samples were then incubated with 1:200 mouse antisarcomeric α-actinin antibody (Sigma) in 1% BSA at 4 °C overnight. Cells were washed 3 times with 1× PBS before incubation with Alexa 488-conjugated goat antimouse antibody at 1:400 in 1% BSA at room temperature in the dark for 60 min (Invitrogen). Cell nuclei were stained with DAPI (Invitrogen) for 10 min at room temperature in the dark. Finally, the cells were observed by confocal laser scanning microscopy (Leica, Germany).
Signaling Pathway Analysis
The mechanism by which microgroove topography regulates cell behavior was further explored via western blotting. Briefly, C2C12 cells were seeded onto PLGA samples (W100D50, W50D50, W25D50, and smooth material) at a density of 1 × 104 cells/mL and cultured for 5 days. The cells were then washed with PBS, and the protein lysis buffer was added to extract the total protein for western blot analysis. The phosphorylation levels of the key protein kinases (ERK1/2 and P38) in the MAPK signaling pathway and FAK proteins were detected.
Image and Statistical Analysis
Using SPSS 22.0 statistical analysis software, one-way analysis of variance followed by Tukey’s test for means comparison was used to assess the level of significance. Quantitative experimental results are expressed as the mean ± standard error, and differences for which *p < 0.05 were designated statistically significant.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2017YFC1105000), the National Nature Science Foundation of China (31971261, 51603074, and U1801252), the Fundamental Research Funds for the Central Universities (D2192170), the Guangdong Basic and Applied Basic Research Foundation (2020A1515010668), the Science and Technology Planning Project of Guangzhou (202102021048), and Medical Scientific Research Foundation of Guangdong Province (A2018169).
Author Contributions
H.G. and J.X. contributed equally to this work. H.G. designed the study, analyzed the data, and wrote the manuscript. J.X. participated in the analysis of the data and a certain degree of grammar editing of the article. Y.W. and H.W. participated in the material characterization experiment. H.W. assisted in the design and operation of cell experiments. S.L. provided the experimental materials and guided the experimental design plan.
The authors declare no competing financial interest.
References
- Harrison R. G. The cultivation of tissues in extraneous media as a method of morpho-genetic study. Anat. Rec. 1912, 6, 181–193. 10.1002/ar.1090060404. [DOI] [Google Scholar]
- Nikkhah M.; Edalat F.; Manoucheri S.; Khademhosseini A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials 2012, 33, 5230–5246. 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P. The problem of specificity in growth and development. Yale J. Biol. Med. 1947, 19, 235–278. [PMC free article] [PubMed] [Google Scholar]
- Abrams G. A.; Goodman S. L.; Nealey P. F.; Franco M.; Murphy C. J. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000, 299, 39–46. 10.1007/s004410050004. [DOI] [PubMed] [Google Scholar]
- Brody S.; Anilkumar T.; Liliensiek S.; Last J. A.; Murphy C. J.; Pandit A. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Eng. 2006, 12, 413–421. 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D.; Marcum B. A. Nematocyte migration in hydra: evidence for contact guidance in vivo. J. Cell Sci. 1980, 41, 33–51. 10.1242/jcs.41.1.33. [DOI] [PubMed] [Google Scholar]
- Loesberg W.; Teriet J.; Vandelft F.; Schon P.; Figdor C.; Speller S.; Vanloon J.; Walboomers X.; Jansen J. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 2007, 28, 3944–3951. 10.1016/j.biomaterials.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Yang S.; Min J. H.; Cho K.; Seo I. H.; Ryu W.; Koh W.-G. Fabrication of microgrooved scaffolds using near-field electrospinning-assisted lithography (NFEAL). J. Ind. Eng. Chem. 2019, 80, 471–478. 10.1016/j.jiec.2019.08.025. [DOI] [Google Scholar]
- Lu J.; Rao M. P.; MacDonald N. C.; Khang D.; Webster T. J. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008, 4, 192–201. 10.1016/j.actbio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Karuri N. W.; Liliensiek S.; Teixeira A. I.; Abrams G.; Campbell S.; Nealey P. F.; Murphy C. J. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J. Cell Sci. 2004, 117, 3153–3164. 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim E. K. F.; Reano R. M.; Pang S. W.; Yee A. F.; Chen C. S.; Leong K. W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005, 26, 5405–5413. 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinger C. J.; Zhang Z. T.; Gerecht S.; Borenstein J. T.; Langer R. Enhancement of in vitro capillary tube formation by substrate nanotopography. Adv. Mater. 2008, 20, 99–103. 10.1002/adma.200702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby M. J.; Gadegaard N.; Tare R.; Andar A.; Riehle M. O.; Herzyk P.; Wilkinson C. D. W.; Oreffo R. O. C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- Hosseini V.; Ahadian S.; Ostrovidov S.; Camci-Unal G.; Chen S.; Kaji H.; Ramalingam M.; Khademhosseini A. Engineered Contractile Skeletal Muscle Tissue on a Microgrooved Methacrylated Gelatin Substrate. Tissue Eng. Part A 2012, 18, 2453–2465. 10.1089/ten.tea.2012.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger O.; Zhao G.; Schwartz Z.; Simpson J.; Wieland M.; Landolt D.; Boyan B. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials 2005, 26, 1837–1847. 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Dalby M. J.; McCloy D.; Robertson M.; Agheli H.; Sutherland D.; Affrossman S.; Oreffo R. O. C. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials 2006, 27, 2980–2987. 10.1016/j.biomaterials.2006.01.010. [DOI] [PubMed] [Google Scholar]
- McDevitt T. C.; Angello J. C.; Whitney M. L.; Reinecke H.; Hauschka S. D.; Murry C. E.; Stayton P. S. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J. Biomed. Mater. Res. 2002, 60, 472–479. 10.1002/jbm.1292. [DOI] [PubMed] [Google Scholar]
- Yim E. K. F.; Pang S. W.; Leong K. W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res. 2007, 313, 1820–1829. 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Wu Y.; Hu T.; Ma P. X.; Guo B. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019, 96, 175–187. 10.1016/j.actbio.2019.06.035. [DOI] [PubMed] [Google Scholar]
- Wise S. G.; Byrom M. J.; Waterhouse A.; Bannon P. G.; Ng M. K. C.; Weiss A. S. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011, 7, 295–303. 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Xu C. Y.; Inai R.; Kotaki M.; Ramakrishna S. Aligned biodegradable nanotibrous structure: a potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. 10.1016/S0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- Järvinen T. A. H.; Järvinen T. L. N.; Kääriäinen M.; Kalimo H.; Järvinen M. Muscle injuries: biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- Porter M. M.; Vandervoort A. A.; Lexell J. Aging of human muscle: structure, function and adaptability. Scand. J. Med. Sci. Sports 1995, 5, 129–142. 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Emery A. E. H. The muscular dystrophies. Lancet 2002, 359, 687–695. 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Bach A. D.; Beier J. P.; Stern-Staeter J.; Horch R. E. Skeletal muscle tissue engineering. J. Cell. Mol. Med. 2004, 8, 413–422. 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé S. B. P.; Rudnicki M. A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Jiao A.; Moerk C. T.; Penland N.; Perla M.; Kim J.; Smith A. S. T.; Murry C. E.; Kim D.-H. Regulation of skeletal myotube formation and alignment by nanotopographically controlled cell-secreted extracellular matrix. J. Biomed. Mater. Res. A 2018, 106, 1543–1551. 10.1002/jbm.a.36351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zhang Z.; Wang Y.; Su Y.; Chen M. 3D myotube guidance on hierarchically organized anisotropic and conductive fibers for skeletal muscle tissue engineering. Mater. Sci. Eng.: C 2020, 116, 111070 10.1016/j.msec.2020.111070. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-W.; Shiwarski D. J.; Ball R. L.; Whitehead K. A.; Feinberg A. W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. 10.1021/acsbiomaterials.0c00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almonacid Suarez A. M.; Brinker M. G. L.; Brouwer L. A.; van der Ham I.; Harmsen M. C.; van Rijn P. Topography-Mediated Myotube and Endothelial Alignment, Differentiation, and Extracellular Matrix Organization for Skeletal Muscle Engineering. Polymer 2020, 12, 1948. 10.3390/polym12091948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.; Kim W.; Kim G. Topologically Micropatterned Collagen and Poly(epsilon-caprolactone) Struts Fabricated Using the Poly(vinyl alcohol) Fibrillation/Leaching Process To Develop Efficiently Engineered Skeletal Muscle Tissue. ACS Appl. Mater. Interfaces 2017, 9, 43459–43469. 10.1021/acsami.7b14192. [DOI] [PubMed] [Google Scholar]
- Charest J. L.; García A. J.; King W. P. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials 2007, 28, 2202–2210. 10.1016/j.biomaterials.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Chen M. C.; Sun Y. C.; Chen Y. H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Park G. E.; Pattison M. A.; Park K.; Webster T. J. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials 2005, 26, 3075–3082. 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Zhu C.; Zuhlke C.; Alexander D.; Francisco J. S.; Zeng X. C. Turning a Superhydrophilic Surface Weakly Hydrophilic: Topological Wetting States. J. Am. Chem. Soc. 2020, 142, 18491–18502. 10.1021/jacs.0c07224. [DOI] [PubMed] [Google Scholar]
- Cun X.; Hosta-Rigau L. Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications. Nanomaterials 2020, 10, 2070. 10.3390/nano10102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer M. H.; Alvarez-Paino M.; McLaren J.; Pappalardo F.; Trujillo S.; Wong J. Q.; Shrestha S.; Abdelrazig S.; Stevens L. A.; Lee J. B.; Kim D.-H.; Gonzalez-Garcia C.; Needham D.; Salmeron-Sanchez M.; Shakesheff K. M.; Alexander M. R.; Alexander C.; Rose F. R. A. J. Designing topographically textured microparticles for induction and modulation of osteogenesis in mesenchymal stem cell engineering. Biomaterials 2021, 266, 120450 10.1016/j.biomaterials.2020.120450. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhang T.; Jiang M.; Yin X.; Luo X.; Sun H. Effect of the immune responses induced by implants in a integrated three-dimensional micro-nano topography on osseointegration. J. Biomed. Mater. Res. A 2021, 109, 1429–1440. 10.1002/jbm.a.37134. [DOI] [PubMed] [Google Scholar]
- Gao H.; Cao X.; Dong H.; Fu X.; Wang Y. Influence of 3D Microgrooves on C2C12 Cell Proliferation, Migration, Alignment, F-actin Protein Expression and Gene Expression. J. Mater. Sci. Technol. 2016, 32, 901–908. 10.1016/j.jmst.2016.01.011. [DOI] [Google Scholar]
- Gong H. Y.; Park J.; Kim W.; Kim J.; Lee J. Y.; Koh W.-G. A Novel Conductive and Micropatterned PEG-Based Hydrogel Enabling the Topographical and Electrical Stimulation of Myoblasts. ACS Appl. Mater. Interfaces 2019, 11, 47695–47706. 10.1021/acsami.9b16005. [DOI] [PubMed] [Google Scholar]
- Li M.; Fu X.; Gao H.; Ji Y.; Li J.; Wang Y. Regulation of an osteon-like concentric microgrooved surface on osteogenesis and osteoclastogenesis. Biomaterials 2019, 216, 119269 10.1016/j.biomaterials.2019.119269. [DOI] [PubMed] [Google Scholar]
- Patel M.; Min J. H.; Hong M.-H.; Lee H.-J.; Kang S.; Yi S.; Koh W.-G. Culture of neural stem cells on conductive and microgrooved polymeric scaffolds fabricated via electrospun fiber-template lithography. Biomed. Mater. 2020, 15, 045007 10.1088/1748-605X/ab763b. [DOI] [PubMed] [Google Scholar]
- Gao H.; Dong H.; Cao X.; Fu X.; Zhu Y.; Mao C.; Wang Y. Effective Spatial Separation of PC12 and NIH3T3 Cells by the Microgrooved Surface of Biocompatible Polymer Substrates. Langmuir 2015, 31, 6797–6806. 10.1021/acs.langmuir.5b01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K.; Ingber D. Micromechanical control of cell and tissue development: Implications for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 1306–1318. 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Mechanosensation through integrins: Cells act locally but think globally. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 1472–1474. 10.1073/pnas.0530201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N.; Tytell J. D.; Ingber D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D.; Hauck C. R.; Sieg D. J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999, 71, 435–478. 10.1016/S0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Hanks S. K.; Polte T. R. Signaling through focal adhesion kinase. BioEssays 1997, 19, 137–145. 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- Cox E. A.; Sastry S. K.; Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol. Biol. Cell 2001, 12, 265–277. 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez E.; Engel E.; Planell J. A.; Samitier J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. 2009, 191, 126–135. 10.1016/j.aanat.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ma Y.; Zhang J.; Xie Q.; Wang Z.; Yu S.; Yuan Y.; Liu C. MBG-Modified beta-TCP Scaffold Promotes Mesenchymal Stem Cells Adhesion and Osteogenic Differentiation via a FAK/MAPK Signaling Pathway. ACS Appl. Mater. Interfaces 2017, 9, 30283–30296. 10.1021/acsami.7b02466. [DOI] [PubMed] [Google Scholar]
- Xia L.; Lin K.; Jiang X.; Xu Y.; Zhang M.; Chang J.; Zhang Z. Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. J. Mater. Chem. B 2013, 1, 5403–5416. 10.1039/c3tb20945h. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Li H.; Lin C.; Ning C.; Lin K. Synergetic topography and chemistry cues guiding osteogenic differentiation in bone marrow stromal cells through ERK1/2 and p38 MAPK signaling pathway. Biomater. Sci. 2018, 6, 418–430. 10.1039/C7BM01044C. [DOI] [PubMed] [Google Scholar]