Abstract
More than 50% of individuals who are HIV positive report insomnia, which can reduce HIV treatment adherence, impair quality of life, and contribute to metabolic dysfunction. Major depressive disorder is also highly comorbid in this population, leading to further impairment. There is evidence that treating insomnia may improve not only sleep, but depression and metabolic function, as well. The present study aimed to examine the effects of pharmacotherapeutic treatment of insomnia on sleep, depression, and metabolic functioning in individuals with HIV. 20 individuals with asymptomatic seropositive HIV and comorbid insomnia and depression were administered zaleplon for 6 weeks. Insomnia severity was assessed using the Insomnia Severity Index and Epworth Sleepiness Scale, and depression severity was assessed using the Quick Inventory of Depression, both prior to treatment and 6 weeks post treatment. Metabolomic changes were assessed using a comprehensive platform measuring ~2000 lipid features and polar metabolites. Linear mixed effects models demonstrated that 6 weeks of treatment with zaleplon significantly improved symptoms of both insomnia and depression. Metabolomic analyses also demonstrated that changes in insomnia severity were associated with significant changes in key branched chain amino acid metabolites. Our results show that improvement in insomnia is associated with reduced depressive symptoms and beneficial metabolomic changes. Additionally, changes in key branched chain amino acid metabolites following treatment may serve as useful biomarkers of treatment response.
Keywords: Insomnia, HIV, depression, metabolomics, zaleplon
Introduction
In people living with human immunodeficiency virus (HIV), approximately 50% will present with a psychiatric disorder during the course of the illness.1 Of these psychiatric disorders, major depressive disorder (MDD) is the most prevalent, occurring in approximately 42% of individuals.2 In individuals with HIV, MDD does not only affect mood, but is also associated with increased morbidity and mortality related to impaired immune function. For example, Ickovics and colleagues3 demonstrated a two-fold increase in mortality risk for women with HIV and chronic MDD compared to those with limited or no depressive symptoms, even when controlling for other contributing factors. Additionally, chronic MDD is associated with a reduction in NK cells and CD8+ T lymphocytes.4 Moreover, while MDD in HIV seropositive patients reduces adherence to antiretroviral treatment,1 thereby impacting mortality, pharmacological treatment of MDD not only improves adherence to treatment5 but improves immune function, as well.6
In addition to MDD, sleep disturbance is also highly prevalent in individuals with HIV, with an estimated 70% of individuals reporting symptoms of insomnia.7 Insomnia is a sleep disorder that has serious ramifications for health and well-being, and this is especially the case for those with HIV as it has the potential to impact multiple physiological processes. Similar to MDD, insomnia negatively impacts outcomes in the HIV+ population by reducing quality of life, impairing psychosocial functioning, and decreasing treatment adherence.8,9 Disease trajectory might also be impacted directly by way of abnormal immune function secondary to insomnia.10 Indeed, sleep disturbance has broad physiological effects that likely exacerbate many of the medical comorbidities commonly found in persons with HIV. A particular emerging area of interest is at the intersection of sleep and metabolic function. Individuals with HIV are at higher risk for metabolic dysfunction due to metabolic and lipidomic changes.11–13 We recently demonstrated that individuals with insomnia exhibit increased energy metabolites and decreased branched-chain amino acid catabolic products, which we interpreted as evidence of metabolic dysregulation associated with physiological hyperarousal.14 Evidence also suggests that sleep loss, more broadly, directly impacts metabolic pathway function. For example, sleep restriction has been found to disrupt insulin sensitivity15 and is associated with reduced plasma glucose, sugars, and sugar alcohols.16 Furthermore, other studies have established that sleep deprivation or chronic sleep restriction impacts lipid levels.17–18 Given the high prevalence of insomnia and metabolic dysfunction in persons with HIV, the association between insomnia and metabolomics in individuals with HIV is increasingly important to understand.
In patients with MDD, persistent sleep disturbance and insomnia is associated with worse outcomes,19 including increased risk for relapse and decreased treatment response to antidepressant therapies.20,21 Because the prevalence of MDD and insomnia are so high in HIV, the interaction of these two disorders may be particularly detrimental to this population. Fortunately, evidence suggests that managing insomnia in patients with MDD improves treatment response and reduces risk of relapse. In fact, several large, randomized studies have demonstrated that, in conjunction with appropriate treatment for co-existing mood or anxiety disorders, non-benzodiazepine hypnotics benefit sleep, mood, and next-day functioning in those with insomnia and MDD.22–24 Because these agents have been associated with fewer side effects and reduced abuse potential as compared to classical benzodiazepines,22 non-benzodiazepine hypnotics represent an attractive pharmacotherapy for treatment of insomnia in those with MDD. Given that pharmacologic management is currently the most commonly used intervention for insomnia,25 utilizing pharmacotherapy to target symptoms of insomnia in individuals with HIV and comorbid MDD could improve clinical outcomes.
Non-benzodiazepine hypnotics acting through GABA-A receptors including zolpidem, zaleplon, and eszopiclone do show promise in the treatment of insomnia in the HIV+ population. While zolpidem is perhaps the best characterized non-benzodiazepine hypnotic, there is evidence to suggest that it might interact with protease inhibitors used to treat HIV.26 Zaleplon, on the other hand, has demonstrated efficacy in treating insomnia27 and by virtue of its pharmacodynamics and metabolic profile, it has a low risk of interaction with HIV medication. Therefore, zaleplon is an attractive candidate for treating sleep onset difficulties in this population.
While targeted behavioral interventions for insomnia, such as acupuncture or caffeine reduction, have been shown to improve sleep quality in patients with HIV,8 to date, there have been no pharmacotherapeutic studies on treating insomnia in the HIV+ population, emphasizing the need to determine whether known hypnotic medications are effective. This is a particularly relevant question because treating insomnia with pharmacotherapy may not only improve sleep but also depressive and metabolic symptoms. Moreover, improvement in depressive symptoms has alone been shown to lead to better outcomes in patients with HIV, and concomitantly improve metabolic function. This study aims to examine the impact of targeted pharmacotherapy of insomnia in HIV seropositive patients with comorbid depressive disorders on measures of sleep and mood in addition to serum metabolite profiles. Given that zaleplon has shown to be efficacious in the treatment of insomnia in both healthy and depressed populations, we hypothesize that treatment of sleep onset insomnia with zaleplon will result in improved sleep quality and mood, which will also be reflected in the metabolite profile.
Methods
Participants
20 adults, between the ages of 18 and 65, with a diagnosis of asymptomatic seropositive HIV, were recruited by fliers and posters, and through referrals from patients receiving care at HIV clinics at the Hospital of the University of Pennsylvania (HUP) and Penn Presbyterian. All participants had a SCID-confirmed DSM-IV diagnosis of Major Depressive Disorder, Dysthymic Disorder, or Bipolar Depression, and were currently receiving pharmacological treatment with benefit. Participants also agreed to continue this medication for the course of the study (6 weeks). All participants were screened to have difficulties with insomnia, as determined by a total score of at least 2 on insomnia items of the 17-item HAM-D, and specific complaint of sleep onset insomnia (sleep onset latency of at least 1 hour, 3 or more times per week). Exclusionary criteria included significant chronic, systemic illness or significant neurologic disorder, including traumatic brain injury, clinically significant history of liver disease, psychiatric instability as indicated by current acute suicidality, current homicidal thoughts, or current psychosis as judged by study investigators, lifetime history of schizophrenia, schizoaffective disorder, or any psychotic illness, history of substance abuse or dependence over the past 6 months, current (past 2 weeks) use of medication to assist with sleep (e.g., Ambien/zolpidem, Dalmane/flurazepam, Doral/quazepam, Halcion/triazolam, Lunesta/eszopiclone, Prosom/estazolam, Restoril/temazepam, Rozerem/ramelteon, Sonata/zaleplon, melatonin, Unisom, Benadryl), or pregnancy. All participants understood the study requirements and procedures and provided written informed consent.
Procedures
Following initial screening to verify study inclusion and exclusion criteria, participants were enrolled in the study. All participants were administered zaleplon as described below for six weeks. Subjective self-report questionnaires, including the Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS), Quick Inventory of Depressive Symptomatology (QIDS), and laboratory measures were administered at baseline, and again at the 6-week follow-up to determine how improvement in insomnia impacted mood and metabolic function. All study visits and procedures occurred within the University of Pennsylvania Mood and Anxiety Disorders Treatment and Research Program and were approved by the Institutional Review Board of the University of Pennsylvania School of Medicine. The study was registered at ClinicalTrials.gov (identifier: NCT03489304).
Zaleplon
Zaleplon is a non-benzodiazepine sedative-hypnotic agent, available in 5 and 10 mg capsules. All participants were started with zaleplon 5 mg capsules and were seen every other week by a study doctor during treatment. Dosage was increased to 10mg if the participant failed to respond to the lower dose within the first two weeks of treatment, as determined by the study psychiatrist at visit 3 by assessing response on the Clinical Global Impression Improvement scale (CGI-I). Dose was increased to 10mg unless the patient was “much” or “very much” improved with regard to insomnia. Regular study drug reconciliation was performed (every other week during the 6-week treatment phase) to document drug dose prescribed, drug consumed, and drug remaining.
Clinical Measures
Structured Clinical Interview for DSM-IV (SCID).28 The SCID is a semi-structured clinician-administered interview designed to assess the presence or absence of Axis I psychiatric disorders based on the DSM-IV criteria.
Hamilton Depression Rating Scale (HAM-D).29 The HAM-D is a 17-item, clinician-administered, scale for assessing current depressive symptom severity. Scores range from 0 to 52, with higher scores indicating higher depression severity. Scores from 8–13 are indicative of “mild depression,” while scores from 14–18 are indicative of “moderate depression,” scores from 19–22 are indicative of “severe depression,” and scores over 23 are indicative of “very severe depression.” The HAM-D includes one item pertaining to suicidal ideation.
The Clinical Global Impression Improvement scale (CGI-I).30 The CGI is a single-item, clinician-assessed rating of treatment response. This rating, on a scale from 1–7, is assessed relative to a baseline prior to the initiation of an intervention. Lower scores indicate higher treatment response.
Self-Report Measures
Insomnia Severity Index (ISI).31 The ISI is a 7-item questionnaire designed to assess subjective symptoms of insomnia including daytime consequences. Scores range from 0 to 28, with scores from 0–7 indicating “no clinically significant insomnia,” scores from 8–14 indicating “subthreshold insomnia,” scores from 15–21 indicating “moderately severe clinical insomnia,” and scores from 22–28 indicating “severe clinical insomnia.”
Epworth Sleepiness Scale (ESS).32 The ESS is an 8-item questionnaire designed to assess an individual’s level of daytime sleepiness via the likelihood of falling asleep in common situations. Scores range from 0 to 24, with higher scores indicating more daytime sleepiness.
Quick Inventory of Depressive Symptomatology (QIDS).33 The QIDS is a 16-item questionnaire designed to assess DSM-IV diagnostic domains of major depressive disorder over the past 7 days. Scores range from 0 to 48, with higher scores indicating more severe depression.
Laboratory Measures
Labs were conducted at baseline and again at 6 weeks, at PMHARC’s Laboratory Core. 50 mL of blood were drawn (10 mL each in 3 EDTA and 2 SST tubes) from each individual, between approximately 9–10 am to control for circadian effects.
For metabolomics analysis, blood plasma samples were extracted using a biphasic method of extraction34 and the polar and non-polar fractions were obtained. The polar fraction was divided into two parts and dried using vacuum concentrator. The two parts were further evaluated by targeted quantitative NMR profiling and semi-quantitative UPLC-TQD-ESI-MS profiling. NMR spectroscopy was performed as described previously.14,35 Briefly, the dried fraction was reconstituted in 200 ml phosphate buffer (pH ~ 7.4) containing DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid) as internal standard. The samples were taken in a 3mm NMR tube and the spectra were acquired using a 700 MHz AVANCE III HD NMR spectrometer equipped with a 3mm TXI probe and samplejet (Bruker Biospin, Billerica, MA). NOESYGPPR1D pulseprogram was used for acquisition of the spectra. Acquired data was exported to Chenomx NMR suite 8.0 (Chenomx, Edmonton, Alberta) and processed and analyzed using targeted profiling.36 38 metabolites were identified and quantified using this approach.
The remaining part from the polar fraction was reconstituted in 1:1 acetonitrile/water and used for UPLC-MS based profiling as described previously.37 Briefly, the samples were injected in analytical triplicate to a XBridge BEH amide column (2.5 mm × 100mm × 2.1mm) using an Acquity H-class UPLC system (Waters Corp. Milford, MA). The column eluent was analyzed using a Waters Xevo TQS-micro ESI-MS operating with polarity switching mode. The injections were randomized and pooled quality control samples were run in regular interval to correct for any instrumental drift. Using this approach, 144 metabolites were identified.
The non-polar fraction was dried under the hood and was used for untargeted lipidomics analysis using UPLC-qTOF-ESI-MS. Dried fractions were reconstituted in 60% A (2:3 water/acetonitrile, 100 mm ammonium formate) and 40% B (9:1 isopropanol/acetonitrile, 10 mm ammonium formate). Samples were injected into a reverse phase XSelect column (2.1mm × 100mm × 2.5 mm) using an Acquity H-class UPLC system (Waters Corp, Milford, MA). The UPLC gradient was as follows - 75% A and 25% B for 0.5 min, a quick ramp of 50% A and 50% B for 0.5 min, 25% A and 75% B for 4 min, followed by a ramp of 10% A and 90% B for 2 min, and finally a ramp to 1% A and 99% B for 2 min. The eluents were introduced into a Waters G2S qTOF ESI-MS in positive and negative modes separately using capillary voltage of 3,000 V and a sampling cone voltage of 40 °C. The desolvation gas flow was set to 800 l/h, and the temperature was set to 450 °C. The source temperature was set to 80 °C. A lock-spray mass (Leucine-Enkephalin) was used for assessment of accurate mass. Data was collected in MSE mode using both low (without collision energy) and high (with collision energy) modes. Analytical triplicates were run with regular injections of pooled quality control samples. The data was exported into Progenesis QI software (Nonlinear dynamics) for further analysis. Overall, 4709 lipid features were relatively quantified using this untargeted lipidomics analysis. Most significantly associated lipids were putatively identified using lipidmaps database search.
Data Analysis
First, patient characteristics, including demographic and baseline clinical variables were summarized.
Next, in order to determine the effects of zaleplon administration on sleep (ISI, ESS) and clinical outcomes (QIDS,), a linear mixed effects model with an unstructured variance-covariance structure was used in order to account for the repeated-measures design and within-person correlation of measurements over time. Age, gender, and race were included in the model as potential confounders. The mixed effects model uses all available patients and data regardless of dropout, with the assumption that missingness due to dropout is completely at random conditional on covariates. For each outcome, model estimated means at baseline and 6 weeks, along with the change in outcomes, were reported with 95% confidence intervals.
Lastly, Pearson’s correlation coefficients were used to assess the relationship between percent change of the metabolites and the percent change of the primary sleep measures (i.e. ISI) between baseline and 6 weeks. Metabolite assays from all three data analytic platforms (Nuclear Magnetic Resonance -NMR, TQD-mass spectrometry, TOF-mass spectrometry) were used for this purpose.
Results
Participant Characteristics and Baseline Variables
Demographic and clinical variables were summarized for the sample (Table 1). The sample of 20 individuals was comprised of mostly African American (80%) males with a mean age of 50.2 (range 28 to 60). The participants were characterized, on average, as having a moderate level of insomnia as assessed by the ISI, a mild level of depression as measured by the HAM-D, and a mild level of excessive daytime sleepiness on the ESS. We observed a 25% attrition rate, with 16 individuals participating for 6 weeks. Of the 4 individuals who were lost to follow-up, all (100%) were male, half were African American (50%), with a mean age of 51.7 (range 41 to 58). At the 6-week follow-up, one individual had missing HAM-D and another individual had missing ISI and ESS. No significant adverse events were reported during the course of the study.
Table 1. Demographic and Baseline Clinical Characteristics (N = 20). Mean (Standard Deviation) Unless Otherwise Specified.
| Patient Characteristics | ||
| Age, mean years (range) | 50.2 (28, 60) | |
| Female, n (%) | 6 (30) | |
| Race, n (%) | ||
| Black/African American | 16 (80) | |
| White | 4 (20) | |
| Hamilton Depression Rating Scale, 17-item (HAM-D) | 13.0 (6.8) | |
| Insomnia Severity Index (ISI) | 20.8 (4.3) | |
| Epworth Sleepiness Scale (ESS) | 11.1 (5.4) | |
| Quick Inventory of Depressive Symptoms (QIDS) | 11.9 (4.9) |
Effects of Zaleplon on Sleep
All individuals measured at 6 weeks experienced some improvement in insomnia severity. A mixed effects linear model revealed that treatment with zaleplon significantly decreased symptoms of insomnia by 6 weeks as measured by the ISI [−9.3, 95% CI (−12.2, −6.4)]. Daytime sleepiness measured on the ESS was likewise reduced [−4.1 (−6.4, −1.8)].
Effects of Zaleplon on Mood
A mixed effects linear model also demonstrated that treatment with zaleplon significantly decreased depressive symptoms as measured on the QIDS [−3.4 (−5.6, −1.1)]. With regard to the QIDS, zaleplon administration equally affected both sleep and non-sleep items, with both domains decreasing by approximately 25% (see Table 2).
Table 2. Change in Sleep and Clinical Outcome Variables Following Zaleplon Administration. Model Estimated Means (95% Confidence Interval) at Baseline, 6 Weeksa.
| Outcome | Baseline predicted mean [95%CI] |
Week 6 predicted mean [95%CI] |
Change in predicted means at 6 weeks from baseline [95% CI] |
p | ||||
| Insomnia Severity Index (ISI) | 21.9 [19.2, 24.6] | 12.6 [9.4, 15.8] | −9.3 [−12.2, −6.4] | <.001 | ||||
| Epworth Sleepiness Scale (ESS) | 12.4 [9.0, 15.9] | 8.3 [4.7, 12.0] | −4.1 [−6.4, −1.8] | 0.002 | ||||
| Secondary Outcomes | ||||||||
| Quick Inventory of Depressive Symptoms (QIDS) | 12.3 [8.5, 16.0] | 8.9 [5.0, 12.7] | −3.4 [−5.6, −1.1] | 0.006 | ||||
| QIDS Sleep Items | 2.7 [2.1, 3.2] | 1.9 [1.4, 2.5] | −0.7 [−1.3, −0.2] | 0.016 | ||||
| QIDS Non-Sleep Items | 9.6 [6.2, 13.2] | 6.9 [3.3, 10.6] | −2.7 [−4.6, −0.8] | 0.010 |
aModel adjusted for age, gender and race.
Effects of Zaleplon on Metabolic Profiles
In order to identify the metabolites associated with improvement in insomnia post-zaleplon treatment, Pearson’s correlation coefficients were calculated between changes in metabolite level and changes in ISI from pre- and post-treatment. Table 3 shows the small molecular weight metabolites significantly associated with changes in ISI. Supplementary Table 1 includes the Pearson statistic for all low molecular weight metabolites. Four branched chain amino acid (BCAA) (Leucine, Isoleucine, 3-Methyl-2-oxovaleric acid and 2-hydroxyisocaproic acid, Figure 1) and aromatic amino acid metabolites (tyrosine, phenylpyruvate, Figure 1) were among the most strongly associated metabolites. Energy metabolites such as glucose, acetone and hydroxydodecanoylcarnitine were also associated with the improvement in insomnia (p = 0.06 for all three metabolites; Figure 1). Further, changes in ISI were associated with changes in g-aminobutyrate, an inhibitory neurotransmitter and target of zaleplon (Figure 1).
Table 3. Association between Changea in Low Molecular Weight Metabolite and Change in ISI.
| Metabolite | Pearson’s r | P value | ||
| Metabolites profiled by NMR | ||||
| Leucine | 0.72 | 0.03 | ||
| 3-Methyl-2-oxovalerate | 0.62 | 0.05 | ||
| Acetone | 0.62 | 0.06 | ||
| Glucose | 0.61 | 0.06 | ||
| Tyrosine | 0.61 | 0.06 | ||
| Isoleucine | 0.60 | 0.06 | ||
| Metabolites profiled by UPLC-TQD-ESI-MS | ||||
| 2-Hydroxyisocapoic acid | 0.79 | 0.006 | ||
| γ-Aminobutyric acid | 0.87 | 0.02 | ||
| Phenylpyruvic acid | −0.65 | 0.05 | ||
| Hydroxydodecanoylcarnitine | −0.62 | 0.06 | ||
aChange is defined as percent change: ((6wk – baseline)baseline)*100.
Figure 1.
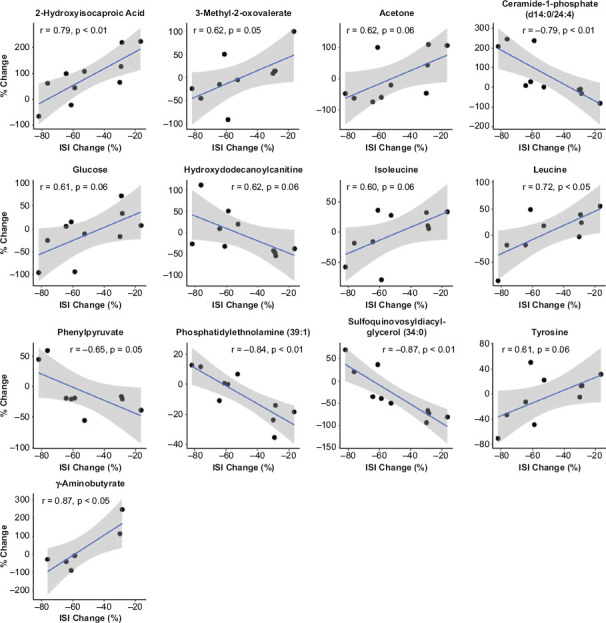
Association of Percent Change in ISI with Percent Change in Metabolites Following 6 Weeks of Treatment with Zaleplon
r = Pearson’s correlation coefficient.
Among the lipid features, 84 significant associations (p < 0.05, Supplementary Table 2) were identified. Putative identifications were carried out for the top hits. Sulfoquinovosyldiacylglycerol (34:0) (r = −0.87, p < 0.01), phosphatidylethanolamine (39:1) (r = −0.84, p < 0.01) and ceramide-1-phosphate d14:0/24:4 (r = −0.79, p < 0.01) were the top three identified lipids (Figure 1).
Discussion
The results of the present study demonstrated that a 6-week course of treatment with zaleplon in individuals who are HIV positive significantly improved symptoms of insomnia, which can include perceived time to fall asleep, amount of time spent awake in the middle of the night, or waking too early. Additionally, subjective reports of sleepiness decreased following zaleplon administration, indicating that treating nighttime sleep disturbance can significantly improve daytime functioning. Although cognitive-behavioral therapy for insomnia (CBT-i) is now recommended as the first line treatment for insomnia,38 hypnotic medications have previously been shown to be equally effective for the short-term treatment of insomnia. Hypnotic medications also impact symptoms acutely, with improvements shown in 1–2 nights,39 which can be a critical benefit when treating patients with pre-existing conditions such as HIV. Importantly, zaleplon was well-tolerated in participants of this study.
Our results also show that improvements in insomnia were accompanied by improvements in depressive symptoms, as measured by the QIDS. This is crucially important, as comorbid insomnia and MDD are associated with worse outcomes than having one condition alone.19 The ability to improve symptoms of both disorders is significant in the treatment of individuals with HIV. Importantly, these data also showed that the reductions in scores on the QIDS were not only due to changes in sleep-related items, indicating that mood may improve through mechanisms that may not be solely sleep-mediated. The idea that treating insomnia can improve symptoms of depression has motivated the study of sleep as a modifiable risk factor in MDD.40,41 Our findings raise the possibility that treating insomnia might likewise benefit depression in individuals with HIV, though future work in a larger, randomized controlled trial is required.
With regard to metabolic functioning, reduction in insomnia severity was significantly associated with several metabolite and lipid changes. Among the polar metabolites, association of branched chain amino acid (BCAA) metabolites with changes in insomnia severity was striking. Specifically, changes in BCAAs (leucine, isoleucine) and their catabolites (3-methyl-2-oxovalerate, 2-hydroxyisocaproic acid) were decreased with improvement in insomnia. Previously, our group showed that BCAA metabolism is specifically perturbed during bedtime in individuals with insomnia, indicating that catabolism may be the primary metabolic activity in this population at night.14 In addition to increased nighttime BCAA catabolism, there was also evidence of elevated glucose concentrations in those with insomnia, providing metabolic evidence that insomnia may result in glucose intolerance. In fact, type 2 diabetes and insulin resistance have been shown to be long term effects of chronic insomnia.42
We now show that improvement in insomnia is associated with changes in BCAA metabolism, suggesting a functional association. In the present study, treatment of insomnia improved blood glucose and increased ceramide-1-phosphate. Mitsutake and colleagues43 have reported that increased activity of ceramide kinase induces glucose intolerance possibly via inflammatory signaling of immune cells. Ceramide-1-phosphate, on the other hand, produces the opposite effect of ceramide,44 and thus may be associated with improvements in glucose metabolism. Therefore, our results suggest that pharmacologic treatment of insomnia may reverse the harmful metabolic dysregulation associated with sleep disturbance. These results, however, require replication; a recent study investigating the relationship between the treatment of insomnia using zopiclone and glucose metabolism failed to show significant improvements in insulin sensitivity or glucose tolerance.45 It should be noted, however, that this study failed to demonstrate an improvement in ISI-measured insomnia severity, which we did find in the present study. Taken together, our data suggest that in individuals with HIV, insomnia is functionally related to disturbances in pathways that are linked to insulin resistance, and that the treatment of insomnia has potential to normalize this disturbance.
The present data should be interpreted in light of study limitations. First, insomnia in this study was only measured using the ISI. Although the ISI has been shown to be valid and reliable measure, utilizing only one subjective measure may limit our ability to fully characterize the effects of zaleplon in individuals with HIV. Indeed, studies have shown that subjective reports of insomnia symptoms are not often reflected in objective measures of sleep, such as polysomnography.46 In order to more comprehensively understand the relationship between hypnotics, insomnia severity, mood, and metabolic function, future studies should assess insomnia using additional subjective measures in addition to objective measures. Second, this study investigated the drug zaleplon, a nonbenzodiazepine sedative hypnotic. Although zaleplon is in the same class as other ‘Z’ drugs such as zopiclone and zolpidem, the present data cannot be extrapolated to these other drugs, or other sedative hypnotics. Future studies should therefore investigate the safety and efficacy of other nonbenzodiazepine hypnotics for treatment of insomnia in individuals with HIV. Lastly, the pre-post study design does not control for other changes during the study period and thus effects cannot be fully attributed to the medication.
In summary, the present study is the first pharmacotherapeutic intervention for insomnia in patients with HIV and depression. We found that 6 weeks of treatment with zaleplon was associated with a significant reduction of symptoms of insomnia and depression and with alterations in systemic metabolic profiles. These results have important implications for the HIV+ population given that both depression and insomnia are common and known risk factors for poor outcomes. In addition, changes in key branched chain amino acid metabolites following treatment may serve as useful biomarkers of treatment response.
Acknowledgments
Funding/support: This research was supported by pilot funds to M.S.K. and A.W. from the Penn Mental Health AIDS Research Center Grants, an NIH-funded program (P30 MH 097488), a Burroughs Wellcome Career Award for Medical Scientists and Doris Duke Charitable Foundation Clinical Scientist Development Award to M.S.K., and an NIH Career Development (K23 MH 118580) award to J.R.G.
Conflicts of interest: The authors report no financial or other relationship relevant to the subject of this article.
Previous presentation: Poster presented at the ACNP annual meeting, Palm Springs, California, December 3–7, 2017.
Role of the sponsors: The supporters had no role in the design, analysis, interpretation, or publication of this study.
References
- 1.Del Guerra FB, Fonseca J, Figueiredo VM, Ziff EB, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J Neurovirol. 2013;19(4):314–327. doi: 10.1007/s13365-013-0177-7. [DOI] [PubMed] [Google Scholar]
- 2.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep. 2015;17(1):530. doi: 10.1007/s11920-014-0530-4. [DOI] [PubMed] [Google Scholar]
- 3.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 4.Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus—infected men: a 2-year follow-up study. Arch Gen Psychiatry. 1997;54(3):279–285. doi: 10.1001/archpsyc.1997.01830150105015. [DOI] [PubMed] [Google Scholar]
- 5.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. JAIDS. 2005;38(4):432–438. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 6.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25(2):221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinstein ML, Selwyn PA. High prevalence of insomnia in an outpatient population with HIV infection. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;19:260–265. doi: 10.1097/00042560-199811010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Omonuwa TS, Goforth HW, Preud’homme X, Krystal AD. The pharmacologic management of insomnia in patients with HIV. JCSM. 2009;5(3):251. [PMC free article] [PubMed] [Google Scholar]
- 9.Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De PL et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28(5):445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cruess DG, Evans DL, Repetto MJ, Gettes D, Douglas SD, Petitto JM. Prevalence, diagnosis, and pharmacological treatment of mood disorders in HIV disease. Biol Psychiatry. 2003;54(3):307–316. doi: 10.1016/s0006-3223(03)00318-4. [DOI] [PubMed] [Google Scholar]
- 11.Bandaru VVR, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S et al. A lipid storage–like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81(17):1492–1499. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickens AM, Anthony DC, Deutsch R, Mielke MM, Claridge TD, Grant I et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS. 2015;29(5):559. doi: 10.1097/QAD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khuder SS, Chen S, Letendre S, Marcotte T, Grant I, Franklin D et al. Impaired insulin sensitivity is associated with worsening cognition in HIV-infected patients. Neurology. 2019;92(12):e1344–e1353. doi: 10.1212/WNL.0000000000007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehrman P, Sengupta A, Harders E, Ubeydullah E, Pack AI, Weljie A. Altered diurnal states in insomnia reflect peripheral hyperarousal and metabolic desynchrony: a preliminary study. Sleep. 2018;41(5):zsy043. doi: 10.1093/sleep/zsy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157(8):549–557. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell LN, Kilkus JM, Booth JN, III, Bromley LE, Imperial JG, Penev PD. Effects of sleep restriction on the human plasma metabolome. Physiol Behav. 2013;122:25–31. doi: 10.1016/j.physbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP et al. Effect of sleep deprivation on the human metabolome. PNAS. 2014;111(29):10761–10766. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weljie AM, Meerlo P, Goel N, Sengupta A, Kayser MS, Abel T et al. Oxalic acid and diacylglycerol 36: 3 are cross-species markers of sleep debt. PNAS. 2015;112(8):2569–2574. doi: 10.1073/pnas.1417432112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pigeon WR, Hegel M, Unützer J, Fan M, Sateia MJ, Lyness JM et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481–488. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997 doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 21.Dew MA, Reynolds CF, Buysse DJ, Houck PR, Hoch CC, Monk TH et al. Electroencephalographic sleep profiles during depression: effects of episode duration and other clinical and psychosocial factors in older adults. Arch Gen Psychiatry. 1996;53(2):148–156. doi: 10.1001/archpsyc.1996.01830020066008. [DOI] [PubMed] [Google Scholar]
- 22.Jindal RD, Thase ME. Treatment of insomnia associated with clinical depression. Sleep Medicine reviews. 2004;8(1):19–30. doi: 10.1016/S1087-0792(03)00025-X. [DOI] [PubMed] [Google Scholar]
- 23.Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59(11):1052–1060. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Pollack M, Kinrys G, Krystal A, McCall WV, Roth T, Schaefer K et al. Eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):551–562. doi: 10.1001/archpsyc.65.5.551. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Family Practice. 2012;13(1):40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenblatt DJ, Harmatz JS, Durol AL, Daily JP, Graf JA, Mertzanis P et al. Differential impairment of triazolam and zolpidem clearance by ritonavir. J Acquir Immune Defic Syndr. 2000;24(2):129–136. doi: 10.1097/00126334-200006010-00007. [DOI] [PubMed] [Google Scholar]
- 27.Walsh JK, Fry J, Erwin CW, Scharf M, Roth T, Vogel GW. Efficacy and tolerability of 14-day administration of zaleplon 5mg and 10mg for the treatment of primary insomnia. Clinical Drug Investigation. 1998;16(5):347–354. [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams J. New York, NY: Biometrics Research, New York State Psychiatric Institute 1996; Structured clinical interview for DSM-IV axis I disorders research version (SCID-I) [Google Scholar]
- 29.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23(1):56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guy W. Clinical global impression. Assessment manual for psychopharmacology. 1976:217–222. [Google Scholar]
- 31.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 32.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 33.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 34.Malik DM, Rhoades S, Weljie A. Extraction and Analysis of Pan-Metabolome Polar Metabolites by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS) Bio-protocol. 2018;8(3) doi: 10.21769/BioProtoc.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sengupta A, Weljie AM. NMR Spectroscopy–Based Metabolic Profiling of Biospecimens. Current protocols in protein science. 2019;98(1):e98. doi: 10.1002/cpps.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM. Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem. 2006;78(13):4430–4442. doi: 10.1021/ac060209g. [DOI] [PubMed] [Google Scholar]
- 37.Rhoades SD, Weljie AM. Comprehensive optimization of LC-MS metabolomics methods using design of experiments (COLMeD) Metabolomics. 2016;12(12):183. doi: 10.1007/s11306-016-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 39.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep medicine reviews. 2009;13(3):205–214. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Manber R, Edinger JD, Gress JL, Pedro-Salcedo MGS, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR et al. Efficacy of Cognitive-Behavioral Therapy for Insomnia Combined With Antidepressant Pharmacotherapy in Patients With Comorbid Depression and Insomnia: A Randomized Controlled Trial. J Clin Psychiatry. 2016;77(10):e1316–e1323. doi: 10.4088/JCP.15m10244. [DOI] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitsutake S, Date T, Yokota H, Sugiura M, Kohama T, Igarashi Y. Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett. 2012;586(9):1300–1305. doi: 10.1016/j.febslet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 44.Arana L, Gangoiti P, Ouro A, Trueba M, Gómez-Muñoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids in health and disease. 2010;9(1):15. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buxton OM, Pavlova MK, O’Connor SP, Wang W, Winkelman JW. Lack of change in glucose metabolism in eszopiclone-treated primary insomnia patients. Nat Sci Sleep. 2017;9:187. doi: 10.2147/NSS.S130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edinger JD, Fins AI, Sullivan RJ, Jr, Marsh GR, Dailey DS, Hope TV et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20(12):1119–1126. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]


