Abstract
We report a click-chemistry based modular strategy for antibody labeling with 64Cu (t1/2 = 12.7 h; β+ 0.656 MeV, 17.4%; β− 0.573 MeV, 39%; EC 43%) under ambient condition utilizing a cross-bridged tetraazamacrocyclic (CB-TE2A) analogue, which otherwise requires harsh conditions that make the CB-TE2A analogues underutilized for protein labeling despite the fact that they form kinetically inert copper complexes with high in vivo stability. Our strategy involves prelabeling a CB-TE2A based scaffold (CB-TE2A-1C) with 64Cu and its subsequent reaction with an antibody via the tetrazine-norbornene mediated click chemistry. The effectiveness of this strategy was demonstrated by labeling two monoclonal antibodies, an anti-PSMA antibody (YPSMA-1) and a chimeric anti-phosphatidylserine antibody (Bavituximab). The immunoreactivity of the antibodies remained unchanged after the tetrazine modification and click-chemistry 64Cu labeling. To further demonstrate the practicality of the modular 64Cu labeling strategy, we tested positron emission tomography (PET) imaging of tumor with the 64Cu-labeled bavituximab in a mouse xenograft model. The tumor visualization and uptake of the labeled antibody exhibited the versatility of the click-chemistry strategy.
Graphical Abstract
INTRODUCTION
Copper-64 (t1/2 = 12.7 h; β+ 0.656 MeV, 17.4%; β− 0.573 MeV, 39%; EC 43%) has been investigated for use in monoclonal antibodies (mAb) based radiopharmaceuticals1–4 for both positron emission tomography (PET) imaging and radiotherapy.2,5–7 The half-life of 64Cu (t1/2 = 12.7 h) enables an imaging procedure up to 48 h after administration, which accommodates the in vivo distribution and target localization of mAbs. Recently, the concept of using the same radiopharmaceutical constructs for both imaging and therapy has made 64Cu an attractive radioisotope for the development of theranostic agents.3,8
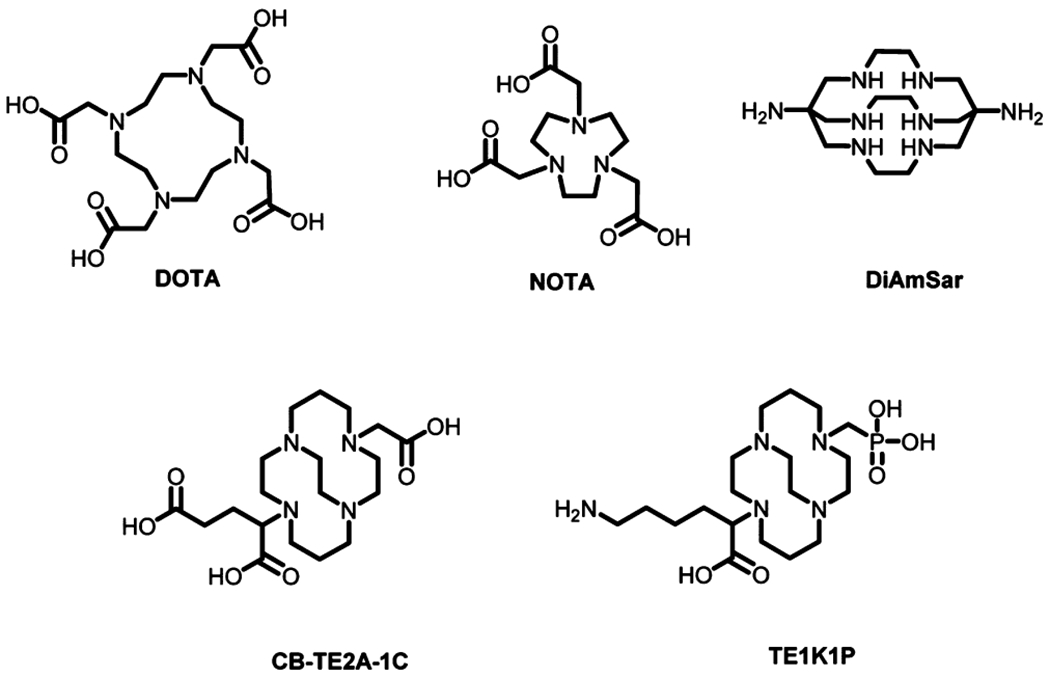
A major step toward the development of 64Cu based radiopharmaceuticals is the identification of bifunctional chelator (BFC) ligands that can stably complex 64Cu2+ under physiological conditions.5,9,10 Most of the currently available BFCs used for this purpose are not ideal because of their poor in vivo stability or the harsh conditions required for efficient incorporation of 64Cu. One of the most widely used bifunctional chelators in radiopharmaceuticals, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), has been extensively exploited for 64Cu labeling of biomolecules including antibodies and peptides (Figure 1). However, 64Cu labeled DOTA bioconjugates are only moderately stable under in vivo conditions, undergoing demetalation subsequently causing high liver accumulation.1,11–19 Copper complexes with improved stability have been reported. Hexaazamacrobicyclic cage-type ligands, based upon the sepulchrate or sarcophagine cage motifs, have been efficiently radiolabeled under mild conditions and have been shown to possess high in vivo stability (Figure 1).4,20,21 However, the yield of antibody conjugation with these chelators is often low because of side reactions including protein cross-linking caused by the use of 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC).22 Moreover, copper complexes of these ligands carry a net positive charge, which leads to high hepatic uptake and slow kidney clearance.23–25 Derivatives of 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA)26,27 and cross-bridged 4,11-bis-(carboxymethyl)-1,4,8,11-tetraaza-bicyclo[6.6.2]hexadecane (CB-TE2A)19,20 form negative or neutral complexes with Cu(II) and demonstrate high in vivo stability (Figure 1). In fact, the latter forms one of the most stable complexes with 64Cu(II)28 and the Cu(II)-CB-TE2A complex is more resistant to the reductive metal loss than any other known tetramacrocyclic complexes.29 However, CB-TE2A and its analogues require harsh reaction conditions such as elevated temperature for 64Cu labeling, which makes them unsuitable for use in antibody labeling (Figure 2). Recently, attempts have been made to develop CB-TE2A based chelators for 64Cu labeling at room temperature. For instance, a CB-TE2A variant with two phosphonate arms, which could be radiolabeled with 64Cu at room temperature, was communicated.30 However, conjugation of the phosphonate group to primary amine side chains of peptides and antibodies proved challenging. Recently, a new cross-bridged cyclam chelator, CB-TE1K1P (Figure 1), which could be labeled with 64Cu under mild conditions, was reported.31 In vivo imaging of epidermal growth-factor receptor (EGFR) using the same chelator has also been reported.32 Though TE1K1P is an excellent chelator for antibody labeling, it carries an extra negative charge under the physiological pH, which may alter the pharmacokinetics of the antibody. More recently, click chemistry via Cu(I)-catalyzed azide–alkyne cycloaddition has been used to conjugate peptides with the cross-bridged tetraazamacrocyclic chelating core for 64Cu labeling.33,34 However, the chelating core forms a positively charged Cu(II) complex, which is suboptimal for the preservation of in vivo behavior of the targeting molecules.
Figure 1.
Bifunctional chelators for copper radiopharmaceuticals.
Figure 2.
Click chemistry strategy for 64Cu-labeling of antibodies using a cross-bridged tetraazamacrocyclic analogue (CB-TE2A-1C).
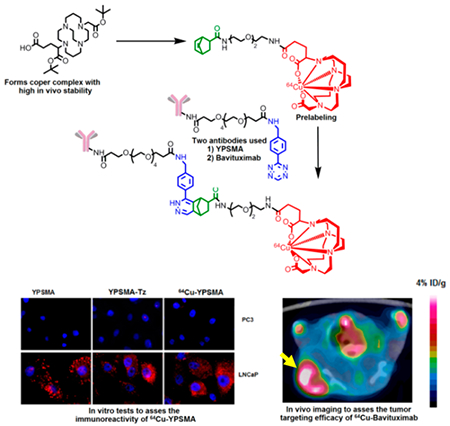
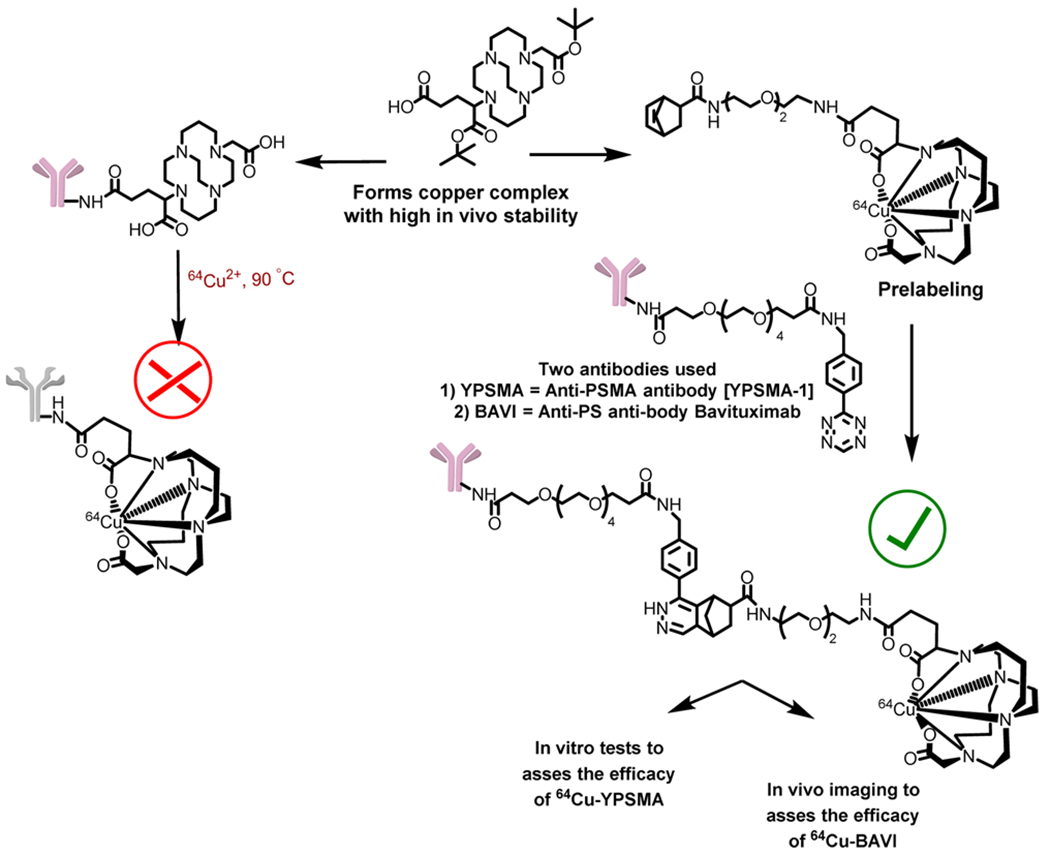
In this work, we describe a modular strategy for the construction of 64Cu labeled antibodies utilizing the chemical inertness of Cu(II)-CB-TE2A-1C, a neutral moiety, without exposing antibodies to elevated temperature, thereby better preserving the immunoreactivity of the antibody (Figure 2).
RESULTS AND DISCUSSION
This work was to develop a modular synthetic strategy to readily label antibody conjugates with 64Cu through CB-TE2A-1C under mild conditions. To achieve the goal, we employed a “copperless click reaction” variant, which involves the reaction between a tetrazine moiety and a strained alkene dienophile.35–37 The labeling is accomplished in a two-step procedure in which a tetrazine-modified antibody is reacted with a 64Cu-labeled chelator-modified strained alkene. Depicted in Scheme 1 and Figure 3, the synthetic route to the construction of radiolabeled antibodies involves four steps: (1) preparation of a stock of tetrazine-modified antibody via carbodiimide coupling followed by checking the immunoreactivity of the tetrazine modified antibody by immunohistochemistry; (2) creation of a stock of strained alkene-modified chelator via carbodiimide coupling; (3) radiolabeling of the strained alkene-modified chelator; and (4) construction of the desired radiolabeled antibody by the click reaction between the alkene and tetrazine moieties.
Scheme 1.
Derivatization of Tetrazine and Norbornene with Functionalized Spacers
Figure 3.
Tetrazine conjugation to antibody and subsequent labeling through click reaction.
Similar prelabeling strategies involving ligations of dienophiles with tetrazines have already been employed with success for radiochemistry with 18F, 111In, and 64Cu.38–41 It should be noted that copper catalyzed click reaction, though synthetically less challenging, was not employed due to the concern on the possible interference of cold copper (catalyst) with 64Cu labeling.
To test our modular strategy, we chose two antibodies. The first one was anti-PSMA (prostate-specific membrane antigen) antibody YPSMA-1 (YPSMA), which has high binding specificity to PSMA in prostate cancer but little or no cross-reactivity to benign prostate hyperplasia or to normal prostatic tissue. In vitro assays were performed on this antibody conjugate to validate our method. The second one was Bavituximab (BAVI), a phosphatidylserine (PS) targeting antibody. It is currently under clinical trials in cancer patients as an adjuvant to chemotherapy. In vivo PET imaging with 64Cu-labeled BAVI (64Cu-BAVI) was conducted in a tumor xenograft mouse model to further validate the potential use of our click-chemistry startegy.
Chemical Synthesis.
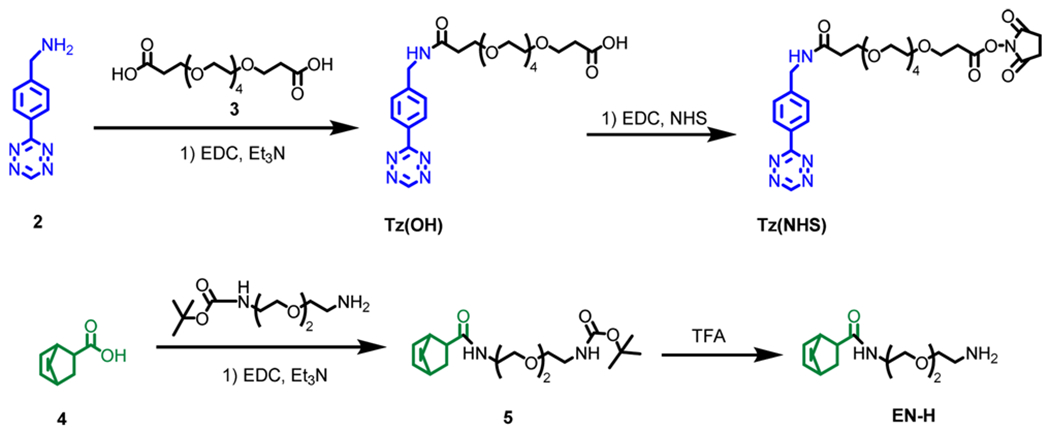
The synthetic route was designed considering the stability of tetrazine (2) and norbornene (4). Since tetrazine is sensitive to high temperature, it was used to modify the antibody. Norbornene being stable at high temperature was conjugated to CB-TE2A(tBu)-COOH as the cross bridged scaffold requires harsh labeling conditions. Compound 2 was successfully synthesized through the reaction of commercially available reactants, 4-(aminomethyl)-benzonitrile hydrochloride, formamidine acetate, and elemental sulfur. The resultant product, a dihydrotetrazine intermediate ((4-(1,2-dihydro-1,2,4,5-tetrazin-3-yl)phenyl)methanamine) was oxidized with sodium nitrite forming the aromatic tetrazine product. To enable the tethering of the tetrazine moiety to an antibody and to make 2 water-soluble, a tetraethylene glycol (PEG-4) based linker was introduced (Scheme 1). The amino group of 2 was converted to a carboxyl group via a PEG-4 prosthetic arm by conjugating 2 with a dicarboxylate spacer, 3, via carbodiimide chemistry. To ensure a 1:1 stoichiometric reaction, an excess amount of 3 (5 equiv) was used. The carboxylate group of the resultant compound, Tz(OH), was then activated via the formation of an NHS ester, Tz(NHS). The carboxylic group of 5-norbornene-2-carboxylic acid (4) was converted to an amino functionality by tethering it with N-Boc-2,2′-(ethylene-dioxy)diethylamine prosthetic arm. The Boc protection of the resultant compound was removed under the treatment of TFA. The resultant product, EN-H, was conjugated to CB-TE2A(tBu)-COOH, which was synthesized per our published procedure42 via the carbodiimde coupling reaction using EDC.
Antibody Modification and Radiolabeling.
Prior to modifications, the antibodies, YPSMA and BAVI, were subjected to a buffer exchange through Micro Bio-Spin chromatography columns with a 6000 Da molecular weight cutoff and reconstituted in phosphate-buffered saline (PBS, pH 8.0). This step was necessary, as the antibodies were stored at pH 7.4 mixed with additives such as sodium azide. At pH 7.4, the rate of reaction between the antibody and Tz(NHS) is slow as compared to the hydrolysis of activated acid. Reaction at the slightly basic pH (pH = 8.0) ensures a fast and efficient reaction of Tz(NHS) with the lysine moieties of the antibody. The reaction was incubated at room temperature for 6 h, followed by centrifugal filtration with a 10 000 Da molecular weight cutoff to purify the resultant antibody conjugate. This step removes the unreacted Tz(NHS) and hydrolyzed small molecule reactants. For prelabeling, CB-EN conjugate was used, as norbornene derivatives are stable at high temperatures. Prelabeling was realized by heating a solution of CB-EN and 64Cu(II) in 0.4 M (pH = 6.5) NH4OAc buffer at 85 °C for 30 min. After labeling, the reaction mixture was purified via Sep-Pak C-18 light cartridge. Since the eluted conjugate, 64Cu-CB-EN, was in 80% ethanol, it was reconstituted in PBS buffer to avoid denaturing the antibody. After reconstitution, the resultant Tz-modified antibody (YPSMA-Tz or BAVI-Tz) was mixed with 44.4 MBq of 64Cu-CB-EN. Since the rate and efficiency of the above reaction heavily relies on the concentration, attention was paid to keep the volume of the reaction to minimal (80–150 μL). The resultant mixture was then incubated for 1–4 h at 37 °C. Of note, the volumes for 64Cu-CB-EN to react with YPSMA-Tz and BAVI-Tz were kept the same but the reaction time was different as the respective radiochemical yields were different. This discrepancy may be attributed to the use of different antibodies, which may have different numbers of functional points for conjugation. As the reaction mixture of labeled antibody contained unreacted 64Cu-CB-EN, it was purified using centrifugal filter units with a 10 000 Da molecular weight cutoff. The radiochemical purity of the final radiolabeled antibody was assayed by size exclusion radio-HPLC (high-performance liquid chromatography) and found to be >99%.
Immunoreactivity Assay of YPSMA-Tz and 64Cu-YPSMA.
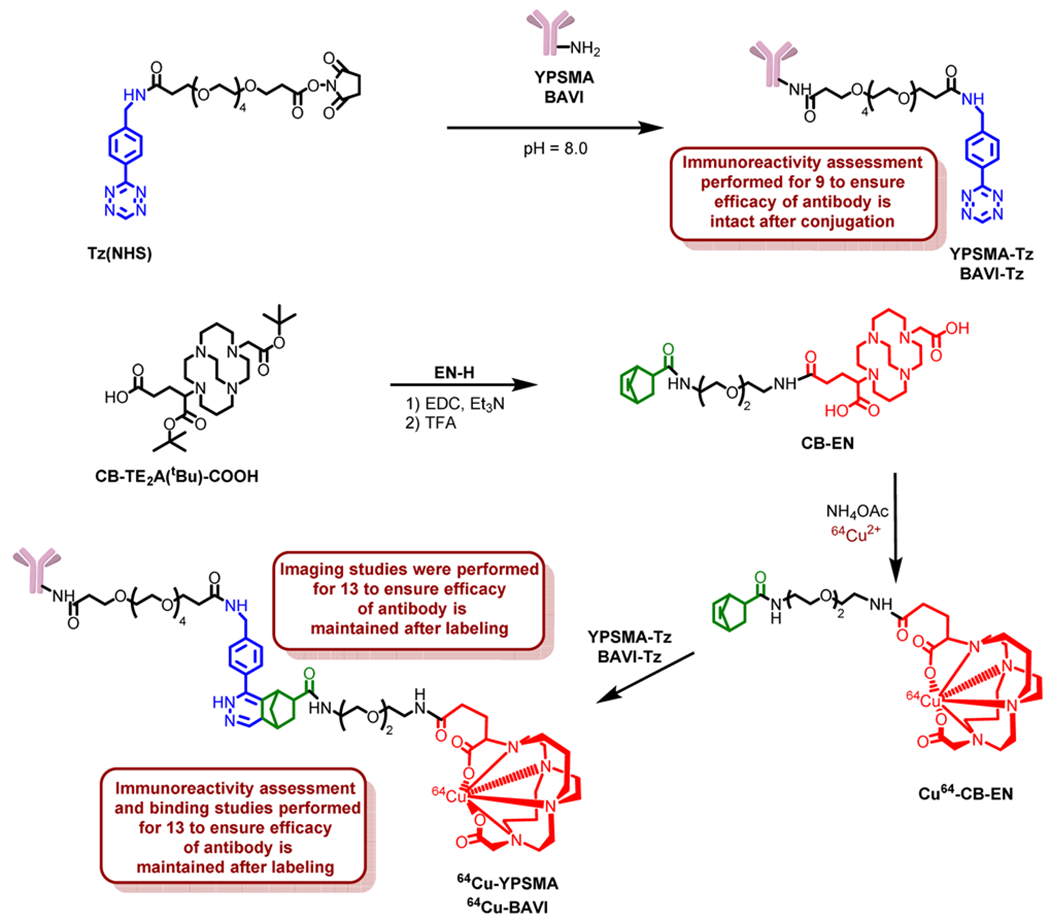
Our modular click-chemistry strategy of antibody labeling encompasses chemical modification (tethering of antibody with tetrazine) and radiolabeling (reaction of tetrazine tethered antibody with CB-EN). To evaluate the effect of chemical modification and radiolabeling on the immunoreativity of the antibody, several tests were carried out (Figure 4). Western blot was performed on membrane proteins obtained from LNCaP and PC3 cell line and tumor tissues to see whether the specific binding affinity of YPSMA had been compromised after the tetrazine modification. As shown in Figure 4a, no detectable signal was observed on PC3 lanes confirming the fact that PC3 cells did not have PSMA expression. The LNCaP lanes showed that the immunoreactivity of the Tz-modified antibody was maintained through the chemical modification.
Figure 4.
Western blot results of PC3 and LNCaP cell and tumor tissue extracts treated with unmodified YPSMA and tetrazine modified YPSMA-Tz (a). Radioactivity measurement of 64Cu-YPSMA uptaken by PC3 and LNCaP cells (b). Immunochemistry staining of PC3 and LNCaP cell lines using YPSMA, YPSMA-Tz, and 64Cu- YPSMA (c).
To further assess the effects of chemical modification and radiolabeling on the immunoreactivity of YPSMA, 64Cu-YPSMA was incubated with PC3 and LNCaP cells. The incubated cells were then thoroughly washed with buffer to remove any nonspecific interaction before the cell bound radioactivity was measured using a gamma counter. Shown in Figure 4b, 64Cu-YPSMA displayed significantly higher uptake in the PSMA+ LNCaP cells than in the PSMA− PC3 cells, further confirming that the immunoreactivity was maintained through the chemical modification and labeling conditions. In addition, we performed immunochemistry staining of PC3 and LNCaP cell lines with unmodified YPSMA, tetrazine modified YPSMA-Tz, and 64Cu-labeled YPSMA. Depicted in Figure 4c, the PSMA expression stained with YPSMA, YPSMA-Tz, and 64Cu-YPSMA is displayed in red and the cell nuclei stained in blue. Both YPSMA-Tz and 64Cu-YPSMA showed the same level of immune-staining of PSMA as YPSMA, while no meaningful red staining occurred with PC3 cells. Taken together, these results demonstrate that our modular click-chemistry strategy of antibody modification and radiolabeling had minimal or negligible effects on the immunoreactivity of the antibody.
It should be noted that YPSMA binds to the intracellular domains of PSMA.43 Like ProstaScint, YPSMA may not be able to be developed as an optimal imaging agent for noninvasive assessment of PSMA in vivo.44 In addition, despite the fact that our modular click-chemistry strategy was successfully applied to YPSMA without compromising its immunoreactivity, the overall 64Cu-labeling yield of YPSMA was low. Therefore, we did not pursue further in vivo imaging with 64Cu-YPSMA after we had proven the feasibility of our modular click-chemistry strategy. Instead, we extended the application of our validated strategy to a chimeric antiphosphatidylserine antibody, BAVI, which was developed at our institution and is currently undergoing clinical trials.45
Tumor Imaging with 64Cu-BAVI.
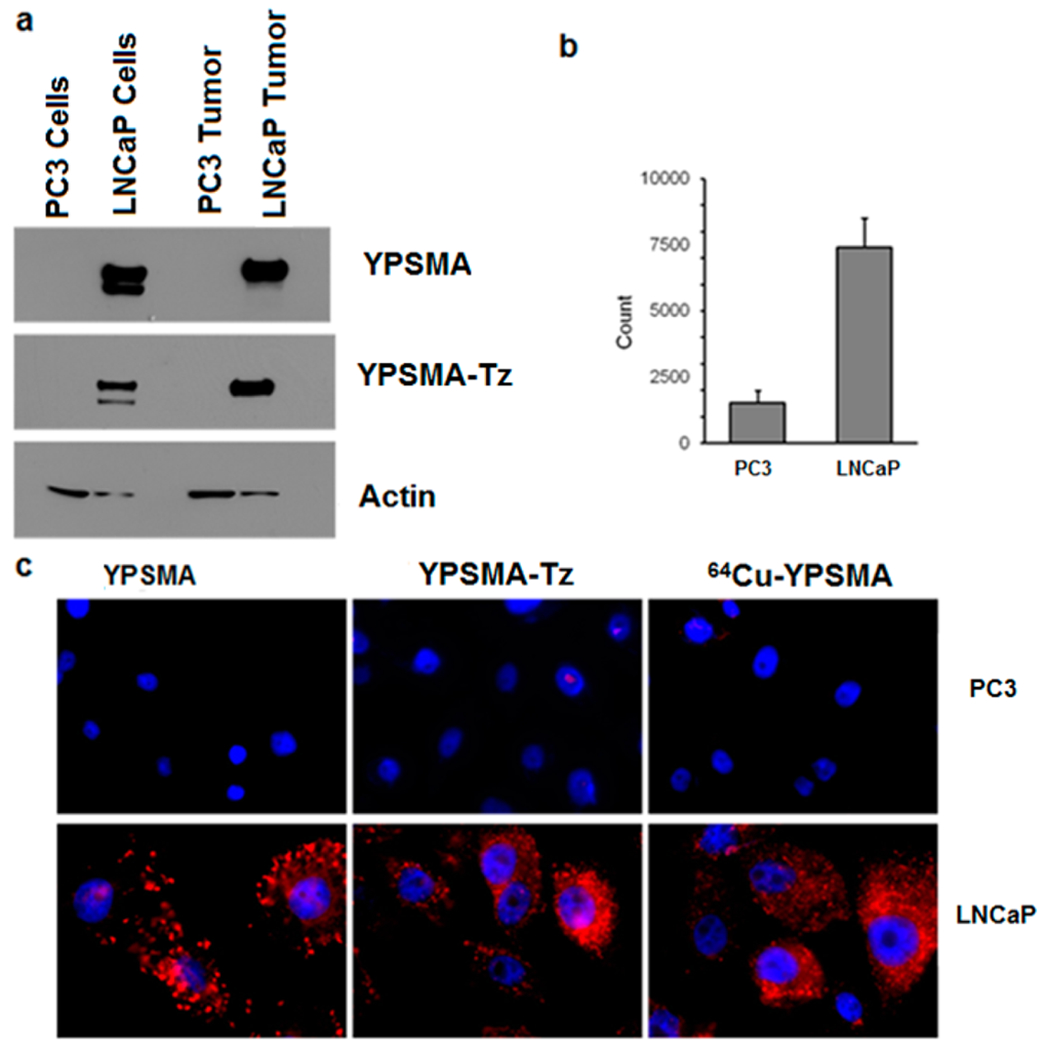
In order to assess the in vivo imaging profile of 64Cu-BAVI prepared by our click-chemistry strategy, a PET/CT imaging study was performed in a mouse model bearing LNCaP xenografts. As shown in Figure 5, the LNCaP tumor were clearly visualized with 64Cu-BAVI after 2 h p.i. and a steady tumor retention of the radioactivity was observed up to 46 h p.i. The quantitative analysis performed on the images further revealed that the tumor uptake levels were 3.4%, 3.2%, 3.1%, and 3.2% ID/g at 2, 16, 34, and 46 h, respectively. Due to the intrinsic nature of radiolabeled antibodies, a substantial level of liver uptake was also observed. However, the hepatic uptake showed a decreasing trend from 38.4% ID/g at 2 h to 20.6% ID/g at 46 h.
Figure 5.
Representative PET/CT images of LNCaP tumor xenografts with 64Cu-BAVI in a mouse model at 2, 16, 34, and 46 h p.i. Images are shown by maximum intensity projection (MIP). The tumors are indicated by white arrow.
Immunohistochemical Staining of PS.
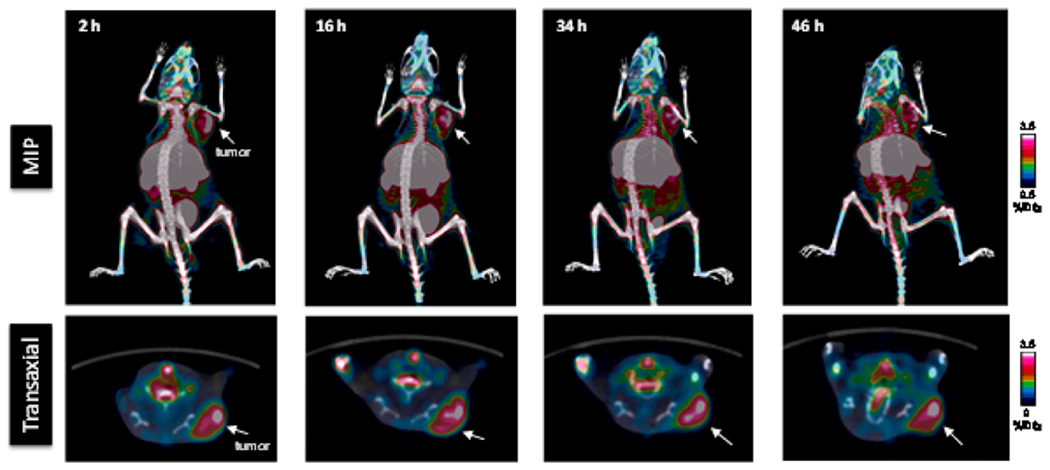
To validate the PS-targeted tumor imaging of 64Cu-BAVI, the LNCaP tumor xenografts were sectioned and doubly stained with anti-CD31 (red) and BAVI (green) as shown in Figure 6. LNCaP tumor sections were stained by H&E (left), The merged images (second column from the left) of tumor vasculatures stained by anti-CD31 (red; third column from the left) and PS positive tissues stained by BAVI (green; right) indicates a 29% ± 10% (yellow) of prevalence in the whole tumor.
Figure 6.
Immunohistochemical staining of phosphatidylserine (PS) expression in LNCaP tumor xenografts.
Although more comprehensive evaluations on the practicality of using 64Cu-BAVI for phosphatidylserine imaging are warranted (e.g., imaging under the condition of chemo-drug or radiation induced apoptosis46), our proof of concept PET/CT imaging study with 64Cu-BAVI demonstrated the feasibility of our modular click-chemistry strategy for 64Cu-labeling of antibodies using cross-bridged tetraazamacrocyclic chelators.
MATERIALS AND METHODS
All chemicals, unless otherwise noted, were acquired from Sigma-Aldrich (St. Louis, MO) and used as received without further purification. All water used was ultrapure (Milli-Q, Millipore, Billerica, MA) and passed through a 10 cm column of Chelex resin (Bio-Rad Laboratories, Hercules, CA) before use. DMSO was of molecular biology grade (>99.9%), 5-norbornene-2-carboxylic acid, N-Boc-2,2′-(ethylenedioxy)-diethylamine, 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide hydrochloride, triethylamine, and all other solvents were of the highest grade commercially available. NMR (nuclear magnetic resonance) spectroscopy was performed on a Bruker 400 MHz NMR. HPLC was performed using a Waters HPLC equipped with a Waters Xterra Shield RP18 semiprep column (250 × 10 mm, 10 μm) and read by a Waters 2996 photodiode array detector and an in-line Shell Jr. 2000 radio-detector, using a gradient of 0:100 MeCN/H2O (both with 0.1% TFA) to 100:0 MeCN/H2O within 50 min. The anti-PSMA antibody, YPSMA, was purchased from Abcam (Cambridge, MA). BAVI was obtained from Dr. Philip E. Thorpe’s laboratory at the University of Texas Southwestern Medical Center. Copper-64 was purchased from Washington University in St. Louis, MO. For accurate quantification of radioactivity, experimental samples were counted for 1 min on a calibrated PerkinElmer (Waltham, MA) Automatic Wizard2 Gamma Counter. Matrix-assisted laser desorption/ionization (MALDI) mass spectra were acquired on an Applied Biosystems Voyager-6115 mass spectrometer.
Compound 2.
The synthesis was performed according to a published procedure with modifications.36 In brief, 4-(aminomethyl)-benzonitrile hydrochloride (0.84 g, 0.005 mol), formamidine acetate (2.08 g, 0.02 mol), and elemental sulfur (0.16 g, 0.005 mol) were added to a dry, 50 mL round-bottom flask. Anhydrous hydrazine (2.0 mL) was then added to the flask, and the resultant orange reaction mixture was stirred for 20 h. After the allotted time, 1% HCl (aq) (50 mL) was slowly added to the reaction mixture, and the resultant solution was stirred for 10 min and subsequently filtered through a medium glass frit. The remaining orange solution was cooled in an ice bath to 0 °C, and a solution of 1.7 g of NaNO2 in 15 mL of water was then added dropwise to the reaction mixture. While still cooling in an ice bath, acetic acid (50 mL) was added slowly, and the reaction mixture immediately turned bright pink. After allowing this solution to warm up to room temperature over the course of 3 h, the solvent was evaporated at 50 °C and 20 Torr on a rotary evaporator. The resultant red crude solids were purified by flash chromatography using a gradient of MeOH (0.01% TFA)/CHCl3 (0.01% TFA) 30:70 after the removal of solvent, the pure product was obtained in 35% yield (0.33 g, 0. 1.80 mmol). 1H NMR (400 MHz, D2O): δ = 10.46 (s, 1H), 8.54 (d, 2H), 7.77 (d, 2H), 4.41 (s, 2H). MS (MALDI) m/z calcd. for C9H9N5: 187.1; found: 188.4 ([M + H]+).
Compound Tz(OH).
To a solution of amino terminated tetrazine derivative 2 (0.10 g, 0.53 mmol) in DMF (2.0 mL) was added the dicarboxylic acid derived from tetraethylene glycol (3, 0.72 g, 2.13 mmol), 1-ethyl-3-(3-(dimethylamino)-propyl) carbodiimide hydrochloride (101 mg, 0.53 mmol), and triethylamine (0.05 g, 0.53 mmol). The resultant solution was stirred for 12 h, filtered, and the solvent evaporated. The crude product was purified by flash chromatography (ethyl acetate) to give carboxylate carrying PEG-4 tetrazine derivative Tz(OH) (0.08 g, 0.16 mmol, 30%) as a red liquid. 1H NMR (400 MHz, CDCl3): δ = 10.21 (s, 1H), 10.08 (s, 1H), 8.35 (m, 1H), 7.71 (m, 1H), 7.36 (m, 1H), 4.97–4.08 (m, 4H), 4.08–3.00 (m, 16H), 2.84–2.59 (m, 2H), 2.31–2.56 (m, 2H). 13C NMR (100 MHz, CDCl3): δ = 174.1, 173.2, 166.7, 165.9, 157.7, 143.9, 130.33, 128.2, 70.4, 70.2, 70.0, 69.9, 66.9, 66.4, 65.4, 42.9, 36.3, 34.6, 31.9. MS (MALDI) m/z calcd. for C23H33N5O8: 507.2; found: 530.7 ([M + Na]+).
Compound Tz(NHS).
To a solution of PEG-4 tetrazine derivative Tz(OH) (0.08 g, 0.16 mmol) in DMF (1.0 mL) was added N-hydroxysuccinimide (0.15 g, 0.21 mmol) and 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide hydrochloride (0.04 g, 0.21 mmol). The resultant solution was stirred for 12 h, filtered, and the solvent evaporated. The crude product was purified by flash chromatography (ethyl acetate) to give NHS activated ester of PEG-4 tetrazine derivative Tz(NHS) (0.04 g, 0.08 mmol, 50%) as a red solid. 1H NMR (400 MHz, CDCl3): δ = 10.17 (s, 1H), 8.50 (m, 2H), 7.50 (m, 2H), 4.54 (m, 2H), 4.98–3.37 (m, 20H), 2.88–2.73 (m, 2H), 2.62 (s, 4H), 2.60–2.43 (m, 2H). 13C NMR (100 MHz, CDCl3): δ = 172.6, 172.2, 169.1, 166.2, 157.7, 144.3, 130.33, 128.4, 128.2, 70.4, 70.4, 70.1, 67.2, 66.5, 65.6, 51.7, 42.9, 36.7, 34.7, 32.06, 25.4, MS (MALDI) m/z calcd. for C27H36N6O10: 604.2; found: 627.4 ([M + Na]+).
Compound 5.
To a solution of 5-norbornene-2-carboxylic acid (4, 0.10 g, 0.72 mmol) in DMF (2.0 mL) was added the N-Boc-2,2′-(ethylenedioxy)diethylamine (0.23 g, 0.92 mmol), 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide hydrochloride (0.14 g, 0.72 mmol), and triethylamine (0.07 g, 0.72 mmol). The resultant solution was stirred for 12 h, and the solvent evaporated. The crude product was purified by flash chromatography (ethyl acetate) to give a triethylene glycol (PEG-3) modified norbornene derivative 5 (0.19 g, 0.50 mmol, 70%) as a colorless oil. 1H NMR (400 MHz, CDCl3): δ = 6.37 (s, 1H), 5.93 (m, 2H), 5.13 (s, 1H), 3.43 (m, 2H), 3.36 (m, 2H), 3.27 (m, 2H), 3.11 (m, 2H), 2.72 (m, 2H), 1.85 (m, 1H), 1.73 (m, 1H), 1.56 (m, 1H), 1.25 (s, 9H), 1.11 (m, 2H). 13C NMR (100 MHz, CDCl3): δ = 175.7, 155.9, 137.9, 135.9, 78.9, 70.1, 70.0, 69.8, 47.1, 46.1, 41.4, 40.1, 39.1, 28.26. MS (MALDI) m/z calcd. for C19H32N2O5: 368.2; found: 369.3 ([M + H]+).
Compound EN-H.
The solution of PEG-3 norbornene derivative (5, 0.19 g, 0.50 mmol) was stirred with TFA (2.0 mL) for 4 h. After deprotection, the solvent was evaporated and the product was purified by flash chromatography (ethyl acetate) to give EN-H (0.12 g, 0.44 mmol, 88%) as a colorless vicious liquid. 1H NMR (400 MHz, CDCl3): δ = 6.95 (s, 1H), 6.08 (m, 1H), 6.04 (m, 1H), 3.82–3.48 (m, 7H), 3.41 (m, 2H), 3.20 (m, 2H), 2.86 (m, 2H), 2.43 (m, 1H), 2.11 (m, 1H), 1.73 (m, 1H), 1.50 (m, 1H), 1.32 (m, 1H). 13C NMR (100 MHz, CDCl3): δ = 178.8, 137.9, 135.6, 69.9, 69.8, 66.1, 46.7, 44.4, 41.4, 39.9, 39.6, 30.5. MS (MALDI) m/z calcd. for C14H24N2O3: 268.2; found: 269.3 ([M + H]+).
Compound CB-EN.
To a solution of PEG-3 norbornene derivative (EN-H, 0.12 g, 0.43 mmol) in DMF (2.0 mL) was added protected cross-bridged chelator (CB-TE2A(tBu)-COOH, 0.19 g, 0.36 mmol), 1-ethyl-3-(3-(dimethylamino)-propyl) carbodiimide hydrochloride (0.07 g, 0.36 mmol), and triethylamine (0.04 g, 0.36 mmol). The resultant solution was stirred for 12 h, filtered, and the solvent evaporated. The crude protected product was purified by HPLC (0.11 g, 0.14 mmol, 40%) as a white solid. 1H NMR (400 MHz, CDCl3): δ = 9.09 (m, 2H), 6.08 (m, 2H), 3.95–3.45 (m, 10H), 3.48–3.00 (m, 12H), 2.97–2.54 (m, 10H), 2.52–2.19 (m, 4H), 2.15–1.65 (m, 10H), 1.58–1.33 (m, 15H), 1.33–1.05 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 176.1, 175.9, 138.2, 136.1, 82.5, 81.9, 70.3, 70.1, 70.0, 68.7, 62.4, 56.2, 55.9, 51.1, 47.9, 47.2, 46.3, 44.6, 44.4, 41.5, 39.6, 39.2, 32.9, 30.4, 28.1, 25.2. MS (MALDI) m/z calcd. for C41H72N6O8: 776.5; found: 799.5 ([M + Na]+). The protected product was mixed with TFA (2.0 mL) and the solution was stirred for 12 h. After the reaction, the solvent was evaporated and the product was purified by HPLC to give CB-EN (0.08 g, 0.13 mmol, 90%) as a red viscous liquid. 1H NMR (400 MHz, CDCl3): δ = 6.13 (m, 2H), 3.96–3.49 (m, 12H), 3.49–2.97 (m, 13H), 2.97–2.70 (m, 5H), 2.70–2.31 (m, 3H), 2.31–1.91 (m, 5H), 1.91–1.61 (m, 3H), 1.61–1.43 (m, 2H), 1.43–1.25 (m, 2H),1.03 (s 1H). 13C NMR (100 MHz, CDCl3): δ 179.9, 175.4, 141.9, 139.9, 73.9, 73.6, 72.7, 61.3, 54.1, 51.0, 50.0, 48.1, 46.6, 45.4, 43.4, 42.9, 36.3, 34.2, 31.7, 31.2, 26.9. MS (MALDI) m/z calcd. for C33H56N6O8: 664.4; found: 665.8 ([M + H]+).
Modification of YPSMA with Tetrazine (YPSMA-Tz).
A 100 μL solution of the antibody (1.0 mg/mL) was subjected to a buffer exchange by using Micro Bio-Spin chromatography columns (Bio-Rad Laboratories) with a 6000 Da molecular weight cutoff. After centrifugation, the antibody was reconstituted with (PBS, 1×, pH 8.0). To the resulting solution was added 50 equiv of the NHS activated tetrazine derivative, Tz(NHS), in 10 μL of PBS, pH 7.4. The reaction was incubated at room temperature for 6 h, followed by centrifugal filtration with a 10 000 Da molecular weight cutoff to purify the resultant antibody conjugate, YPSMA-Tz. The purified antibody conjugate was reconstituted with 100 μL PBS, pH 7.4, and used directly for further reaction.
Modification of BAVI with Tetrazine (BAVI-Tz).
A 90 μL solution of the antibody (18.5 mg/mL) was subjected to a buffer exchange by using Micro Bio-Spin chromatography columns (Bio-Rad Laboratories) with a 6000 Da molecular weight cutoff. After centrifugation, the antibody was reconstituted in PBS, pH 8.0. To the resultant antibody was added 50 equiv of the NHS activated tetrazine derivative, Tz(NHS), in 10 μL of PBS, pH 7.4. The reaction was incubated at room temperature for 6 h, followed by centrifugal filtration with a 10 000 Da molecular weight cutoff to purify the resultant antibody conjugate, BAVI-Tz.
Prelabeling of CB-EN with 64Cu (64Cu-CB-EN).
To a 0.5 mL eppendorf tube containing 2.2 μg of conjugate CB-EN in 40 μL of 0.6 M NH4OAc buffer was added 70.3 MBq of 64CuCl2 solution. The reaction mixture was shaken and incubated at 85 °C for 30 min. To remove the nonspecifically bound and free 64CuCl2 from the 64Cu-labeled conjugate, 2 μL of 5 mM diethylene triamine pentaacetic acid (DTPA) was added to the reaction mixture. The mixture was allowed to incubate for 5 min followed by passing the mixture through a preconditioned Sep-Pak C-18 light cartridge. The cartridge was rinsed thoroughly with water (5 mL) followed by elution of 64Cu-labeled norbornene conjugate (64Cu-CB-EN) by 0.5 mL of ethanol/10 mM PBS mixture (80:20). The elute yielded 48.1 MBq of 64Cu-labeled product. The product was analyzed by radio-HPLC to determine the radiochemical yield and purity. The eluted product was dried under stream of nitrogen at 50 °C and then reconstituted in 10 mM PBS solution (50 μL).
64Cu Labeling of YPSMA (64Cu-YPSMA).
To the previously purified antibody tethered with tetrazine (YPSMA-Tz, 100 μL) was added 64Cu-labeled norbornene conjugate (CB-EN, 44.4 MBq). The mixture was incubated at 37 °C for 4 h. The resulting mixture was then purified by centrifugal filtration with a 10 000 molecular weight cutoff to give 2220 KBq of labeled antibody, 64Cu-YPSMA. The radiochemical purity of the final radiolabeled bioconjugate was assayed by radio-HPLC fitted with size exclusion column (BioSuite SEC Column, 125 Å, 10 ,μm, 7.5 mm × 300 mm) and was found to be >99%.
64Cu Labeling of BAVI (64Cu-BAVI).
To the purified antibody tethered with tetrazine (BAVI-Tz, 30 μL) was added 64Cu-labeled norbornene conjugate (CB-EN, 118.4 MBq). The mixture was incubated at 37 °C for 1 h. The resulting mixture was then purified by centrifugal filtration with a 10 000 molecular weight cutoff to give 22.9 MBq of labeled antibody, 64Cu-BAVI. The radiochemical purity of the final radiolabeled bioconjugate was assayed by radio-FPLC fitted with Superdex 200 Increase GL and was found to be >99%.
Small Animal PET/CT Imaging.
Small animal PET/CT imaging was performed on a Siemens Inveon PET/CT Multimodality System in LNCaP tumor-bearing SCID mice that had been intravenously injected with 17.2 MBq of 64Cu-BAVI via the tail vein. The mouse was sedated on the imaging bed under 2% isoflurane for the duration of imaging. Immediately after the CT data acquisition that was performed at 80 kV and 500 μA with a focal spot of 58 μm, static PET scans were conducted at the given time points post injection (p.i.) (2, 16, 23, 34, and 46 h) for 15 min. Both CT and PET images were reconstructed with manufacturer’s software. Reconstructed CT and PET images were fused for quantitative data analysis; regions of interest (ROIs) were drawn as guided by CT and quantitatively expressed as percent injected dose per gram of tissue (% ID/g).
Immunohistochemistry for YPSMA, YPSMA-Tz, and 64Cu-YPSMA.
A volume of 500 μL RPIM 1640 culture media containing approximately 20 000 cells, either PSMA+ LNCaP or PSMA− PC3, was added to a 24 well plate containing gelatin-coated coverslips. When the cells reached the desired density, the culture media were removed from each well and washed twice with PBS. A volume of 200 μL of 4% formaldehyde fixing solution was added to each well, and incubated for 20 min at room temperature. Each well was washed twice with PBS. The solution of 200 μL of the primary antibody YPSMA, diluted in a ratio of 1:500, 1:250 YPSMA-Tz, or 1:125 64Cu-YPSMA (64Cu-YPSMA, the labeled antibody was stored for 64 h at 4 °C and tests were performed when the radioactivity was decayed) at 4 °C for 4 h. The goat anti-mouse tagged with Texas red (abcam) was then added to the plate stored at room temperature for 1 h. Each well was then rinsed twice with 400 μL of wash buffer. A diluted solution of (1:200; 200 μL) Alexa Fluor 488 Phalloidin (Invitrogen) was then added to each well, and incubated for 20 min at room temperature. After two rinsings (once with PBS and once with water), the coverslips were removed from the wells and excess water was carefully blotted out. One drop of ProLong Gold antifade reagent (Life Technologies) was then added along with DAPI (4′,6-diamidino-2-phenylindole, Invitrogen) onto the microscope slide per coverslip. The glass coverslip was placed on the slide and the edges sealed with nail polish. The slides were visualized using Zeiss AxioObserver Epifluorescence Microscope using filter sets appropriate for the label used under 63× objective oil lens.
Western Blot Analysis.
Protein from PC3 and LNCaP cells and tumor tissue were extracted by standard methods. Western blots were performed with 30 μg of total protein and transferred to PVDF membrane, blocked by 5% skim milk and probed using YPSMA primary antibody (ab19071, Abcam) (1:500) and modified YPSMA-Tz (1:250) and anti-Actin antibody (1:5000, Sigma) 4 °C overnight. Following incubation with HRP-labeled secondary antibody (GE Life Sciences, 1:1000) 1 h, immunoblots were visualized by ECL Plus (Millipore) and the target bands were recorded on X-ray film. Actin protein levels were used as a control to verify equal protein loading.
Cell Uptake Assay.
The cell uptake of 64Cu-YPSMA was measured using the PSMA+ LNCaP and PSMA− PC3 cells. The cells were washed with PBS buffer following by RPMI 1640 medium without FBS, and then resuspended in RPMI 1640 medium without FBS at the concentration of 2 × 106 cells/mL. The cell suspension was then dispensed into 1.5 mL eppendorf tubes (1 × 106 cells per tube), to each of which was added approximately 12 000 cpm of 64Cu-YPSMA in 0.02 mL of PBS buffer. After the cells were incubated at room temperature for 60 min (n = 5), the cell suspension was centrifuged and the supernatant from each vial was stored separately. Each pellet was resuspended in 0.5 mL of ice-cold PBS buffer. To remove the unbound 64Cu-YPSMA, the above procedure was repeated five times. The combined supernatant solutions and the remaining cell pellet from each tube were counted with a γ-counter to determine the cell bound 64Cu-YPSMA level.
Phosphatidylserine (PS) Expression in LNCaP Tumors Measured by Immunohistochemical Staining.
SCID/Nude male mice bearing LNCaP tumors were injected intravenously (i.v.) with 100 μg of BAVI and allowed to circulate for 2 h. Anesthetized mice were then perfused by transcardial perfusion with heparinized normal saline followed immediately by 4% paraformaldehyde (PFA) in PBS (pH 7.4) at a rate of 90 mL/h using a syringe pump. The tumors were then excised and dissected. All tumor pieces were placed in 4% PFA at 4 °C overnight with continuous gentle agitation. All tumor tissues were then transferred to PBS and equilibrated overnight at 4 °C. Tissues for cryoembedding were transferred to a 10% sucrose/PBS solution overnight at 4 °C and then transferred to an 18% sucrose/PBS solution. After removed from solution, tissues were blotted and submerged in optimal cutting temperature (OCT) compound. Tissues in OCT were then frozen rapidly by partial immersion in liquid N2 cooled isopentane and stored at −80 °C until sectioning. Vascular endothelium was stained using a rat anti-mouse CD31 antibody (BD Biosciences, San Jose, CA) followed by Cy3-labeled goat anti-rat IgG; BAVI-positive vessels were identified with biotinylated goat anti-human IgG conjugated to Cy2-labeled streptavidin. Fluorescent Images were captured using CarlZeiss Axioscan (Carl Zeiss, Jena, Germany) (whole tumor and low power) and Zeiss confocal microscope (Carl Zeiss, Jena, Germany, high power, 40× oil lens) mounted on Elipse E600 fluorescent microscope (Nikon, Melville, NY) and analyzed with Zen software. Doubly labeled endothelial cells (CD31 positive/PS positive) were identified by yellow fluorescence on merged images. The percentage of doubly positive vessels was calculated as follows: (mean number of yellow vessels per field/mean number of total vessels) × 100.
ACKNOWLEDGMENTS
This work was partially supported by the Prostate Cancer Research Program of the United States Army Medical Research and Materiel Command (W81XWH-12–1–0336), the Dr. Jack Krohmer Professorship Funds, and the National Institutes of Health (CA159144).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Niu G, Li Z, Cao Q, and Chen X (2009) Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with 64Cu-DOTA-trastuzumab. Eur. J. Nucl. Med. Mol. Imaging 36, 1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Anderson CJ, Connett JM, Schwarz SW, Rocque PA, Guo LW, Philpott GW, Zinn KR, Meares CF, and Welch MJ (1992) Copper-64-labeled antibodies for PET imaging. J. Nucl. Med 33, 1685–1691. [PubMed] [Google Scholar]
- (3).Bryan JN, Jia F, Mohsin H, Sivaguru G, Miller WH, Anderson CJ, Henry CJ, and Lewis MR (2005) Comparative uptakes and biodistributions of internalizing vs. noninternalizing copper-64 radioimmunoconjugates in cell and animal models of colon cancer. Nucl. Med. Biol 32, 851–858. [DOI] [PubMed] [Google Scholar]
- (4).Voss SD, Smith SV, DiBartolo N, McIntosh LJ, Cyr EM, Bonab AA, Dearling JLJ, Carter EA, Fischman AJ, Treves ST, et al. (2007) Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates. Proc. Natl. Acad. Sci. U.S.A 104, 17489–17493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Smith SV (2004) Molecular imaging with copper-64. J. Inorg. Biochem 98, 1874–1901. [DOI] [PubMed] [Google Scholar]
- (6).Connett JM, Buettner TL, and Anderson CJ (1999) Maximum tolerated dose and large tumor radioimmunotherapy studies of Cu-64-labeled monoclonal antibody 1A3 in a colon cancer model. Clin. Cancer Res 5, 3207S–3212S. [PubMed] [Google Scholar]
- (7).Wu AM, Yazaki PJ, Tsai S.-w., Nguyen K, Anderson A-L, McCarthy DW, Welch MJ, Shively JE, Williams LE, Raubitschek AA, et al. (2000) High-resolution microPET imaging of carcinoembryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. Proc. Natl. Acad. Sci. U.S.A 97, 8495–8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Bryan JN, Lewis MR, Henry CJ, Owen NK, Zhang J, Mohsin H, Jia F, Sivaguru G, and Anderson CJ (2004) Development of a two-antibody model for the evaluation of copper-64 radioimmunotherapy. Vet. Comp. Oncol 2, 82–90. [DOI] [PubMed] [Google Scholar]
- (9).Bass LA, Wang M, Welch MJ, and Anderson CJ (2000) In vivo transchelation of copper-64 from TETA-octreotide to superoxide dismutase in rat liver. Bioconjugate Chem. 11, 527–532. [DOI] [PubMed] [Google Scholar]
- (10).Jones-Wilson TM, Deal KA, Anderson CJ, McCarthy DW, Kovacs Z, Motekaitis RJ, Sherry AD, Martell AE, and Welch MJ (1998) The in vivo behavior of copper-64-labeled azamacrocyclic complexes. Nucl. Med. Biol 25, 523–530. [DOI] [PubMed] [Google Scholar]
- (11).Shokeen M, and Anderson CJ (2009) Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET). Acc. Chem. Res 42, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, and Conti PS (2004) MicroPET imaging of breast cancer αv-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol. Imaging Biol 6, 350–359. [DOI] [PubMed] [Google Scholar]
- (13).Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, and Chen X (2005) MicroPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J. Nucl. Med 46, 1707–1718. [PubMed] [Google Scholar]
- (14).Anderson CJ, Pajeau TS, Edwards WB, Sherman ELC, Rogers BE, and Welch MJ (1995) In vitro and in vivo evaluation of copper-64-octreotide conjugates. J. Nucl. Med 36, 2315–2325. [PubMed] [Google Scholar]
- (15).Anderson CJ, Jones LA, Bass LA, Sherman ELC, McCarthy DW, Cutler PD, Lanahan MV, Cristel ME, Lewis JS, and Schwarz SW (1998) Radiotherapy, toxicity and dosimetry of copper-64-TETA-octreotide in tumor-bearing rats. J. Nucl. Med 39, 1944–1951. [PubMed] [Google Scholar]
- (16).McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, and Lewis JS (2005) Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of α-MSH. J. Med. Chem 48, 2985–2992. [DOI] [PubMed] [Google Scholar]
- (17).Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I, Bading JR, Laug WE, and Conti PS (2004) Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor αvβ3-integrin expression. J. Nucl. Med 45, 1776–1783. [PubMed] [Google Scholar]
- (18).Elsässer-Beile U, Reischl G, Wiehr S, Bühler P, Wolf P, Alt K, Shively J, Judenhofer MS, Machulla H-J, and Pichler BJ (2009) PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J. Nucl. Med 50, 606–611. [DOI] [PubMed] [Google Scholar]
- (19).Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, and Anderson CJ (2004) Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem 47, 1465–1474. [DOI] [PubMed] [Google Scholar]
- (20).Sprague JE, Peng Y, Fiamengo AL, Woodin KS, Southwick EA, Weisman GR, Wong EH, Golen JA, Rheingold AL, and Anderson CJ (2007) Synthesis, characterization and in vivo studies of Cu(II)-64-labeled cross-bridged tetraazamacrocycle-amide complexes as models of peptide conjugate imaging agents. J. Med. Chem 50, 2527–2535. [DOI] [PubMed] [Google Scholar]
- (21).Di Bartolo NM, Sargeson AM, Donlevy TM, and Smith SV (2001) Synthesis of a new cage ligand, SarAr, and its complexation with selected transition metal ions for potential use in radioimaging. Dalton Trans., 2303–2309. [Google Scholar]
- (22).Di Bartolo N, Sargeson AM, and Smith SV (2006) New 64Cu PET imaging agents for personalised medicine and drug development using the hexa-aza cage, SarAr. Org. Biomol. Chem 4, 3350–3357. [DOI] [PubMed] [Google Scholar]
- (23).Smith SV, Waters DJ, and DiBartolo N (1996) Separation of Cu-64 from Ga-67 waste products using anion exchange and low acid aqueous/organic mixtures. Radiochim. Acta 75, 65–68. [Google Scholar]
- (24).Anderson CJ, and Ferdani R (2009) Copper-64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother. Radiopharm 24, 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dearling JLJ, Voss SD, Dunning P, Snay E, Fahey F, Smith SV, Huston JS, Meares CF, Treves ST, and Packard AB (2011) Imaging cancer using PET — the effect of the bifunctional chelator on the biodistribution of a 64Cu-labeled antibody. Nucl. Med. Biol 38, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chong H-S, Mhaske S, Lin M, Bhuniya S, Song HA, Brechbiel MW, and Sun X (2007) Novel synthetic ligands for targeted PET imaging and radiotherapy of copper. Bioorg. Med. Chem. Lett 17, 6107–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, and Smith CJ (2007) [64Cu-NOTA-8-Aoc-BBN(7–14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc. Natl. Acad. Sci. U.S.A 104, 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, and Anderson CJ (2001) Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J. Med. Chem 45, 469–477. [DOI] [PubMed] [Google Scholar]
- (29).Woodin KS, Heroux KJ, Boswell CA, Wong EH, Weisman GR, Niu WJ, Tomellini SA, Anderson CJ, Zakharov LN, and Rheingold AL (2005) Kinetic inertness and electrochemical behavior of copper(II) tetraazamacrocyclic complexes: Possible implications for in vivo stability. Eur. J. Inorg. Chem, 4829–4833. [Google Scholar]
- (30).Stigers DJ, Ferdani R, Weisman GR, Wong EH, Anderson CJ, Golen JA, Moore C, and Rheingold AL (2010) A new phosphonate pendant-armed cross-bridged tetraamine chelator accelerates copper(II) binding for radiopharmaceutical applications. Dalton Trans. 39, 1699–1701. [DOI] [PubMed] [Google Scholar]
- (31).Zeng D, Ouyang Q, Cai Z, Xie X-Q and Anderson CJ (2014) New cross-bridged cyclam derivative CB-TE1K1P, an improved bifunctional chelator for copper radionuclides. Chem. Commun 50, 43–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zeng D, Guo Y, White AG, Cai Z, Modi J, Ferdani R, and Anderson CJ (2014) Comparison of conjugation strategies of cross-bridged macrocyclic chelators with cetuximab for copper-64 radiolabeling and PET imaging of EGFR in colorectal tumor-bearing mice. Mol. Pharm 11, 3980–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lebedev AY, Holland JP, and Lewis JS (2010) Clickable bifunctional radiometal chelates for peptide labeling. Chem. Commun 46, 1706–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Cai Z, Li BTY, Wong EH, Weisman GR, and Anderson CJ (2015) Cu(i)-assisted click chemistry strategy for conjugation of non-protected cross-bridged macrocyclic chelators to tumour-targeting peptides. Dalton Trans. 44, 3945–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, and Weissleder R (2009) Fast and sensitive pretargeted labeling of cancer cells through a tetrazine/trans-cyclooctene cycloaddition. Angew. Chem., Int. Ed 48, 7013–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Devaraj NK, Weissleder R, and Hilderbrand SA (2008) Tetrazine-based cycloadditions: application to pretargeted live cell imaging. Bioconjugate Chem. 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Blackman ML, Royzen M, and Fox JM (2008) Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels–Alder reactivity. J. Am. Chem. Soc 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Rossin R, Renart Verkerk P, van den Bosch SM, Vulders RCM, Verel I, Lub J, and Robillard MS (2010) In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem., Int. Ed 49, 3375–3378. [DOI] [PubMed] [Google Scholar]
- (39).Reiner T, Keliher EJ, Earley S, Marinelli B, and Weissleder R (2011) Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew. Chem. Int. Ed 50, 1922–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zeglis BM, Mohindra P, Weissmann GI, Divilov V, Hilderbrand SA, Weissleder R, and Lewis JS (2011) Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels–Alder click chemistry. Bioconjugate Chem. 22, 2048–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, Weissleder R, and Lewis JS (2013) A pretargeted PET imaging strategy based on bioorthogonal diels–alder click chemistry. J. Nucl. Med 54, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Liu W, Hao GY, Long MA, Anthony T, Hsieh JT, and Sun XK (2009) Imparting multivalency to a bifunctional chelator: A scaffold design for targeted PET imaging probes. Angew. Chem., Int. Ed 48, 7346–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Rybalov M, Ananias HJK, Hoving HD, van der Poel HG, Rosati S, and de Jong IJ (2014) PSMA, EpCAM, VEGF and GRPR as imaging targets in locally recurrent Prostate cancer after radiotherapy. Int. J. Mol. Sci 15, 6046–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Taneja SS (2004) ProstaScint® scan: Contemporary use in clinical practice. Rev. Urol 6, S19–S28. [PMC free article] [PubMed] [Google Scholar]
- (45).Stafford JH; Hao G; Best AM; Sun X; Thorpe PE (2013) Highly specific PET imaging of prostate tumors in mice with an Iodine-124-labeled antibody fragment that targets phosphatidylserine. PLoS One 8; DOI: 10.1371/journal.pone.0084864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ogasawara A, Tinianow JN, Vanderbilt AN, Gill HS, Yee S, Flores JE, Williams SP, Ashkenazi A, and Marik J (2013) ImmunoPET imaging of phosphatidylserine in pro-apoptotic therapy treated tumor models. Nucl. Med. Biol 40, 15–22. [DOI] [PubMed] [Google Scholar]


