Abstract
Background
Right-sided tricuspid valve (TV) endocarditis can be difficult to identify and may be under-recognized in the absence of traditional risk factors. While generally identified with aortic valve pathology, infective endocarditis that extends beyond the leaflets of the TV have been reported to cause conduction disease.
Case summary
We present the case of a 63-year-old patient who presented with haemodynamically unstable complete heart block requiring temporary venous pacemaker support. Despite the absence of traditional risk factors or significant valvular disease on transthoracic echocardiogram, she was found to be persistently bacteraemic and subsequent transoesophageal echocardiogram identified large vegetation on the septal leaflet of the TV. Conduction disease was noted to reverse with antibiotic therapy and resolution of bacteraemia.
Discussion
Although rare, right-sided endocarditis involving the triangle of Koch may present with conduction disease due to local inflammation and mechanical compression. Conduction disease associated with right-sided disease appears to be readily reversible with medical therapy and temporary device support may be appropriate in the acute setting.
Keywords: Infective endocarditis, Complete heart block, Case report, Pacemaker
Learning points
Right-sided infective endocarditis (IE) can be difficult to identify given its atypical presentation that may mimic upper respiratory pathology.
The presence of acute conduction deficits and sepsis should prompt thorough evaluation of all cardiac valves, even in the absence of traditional right-sided valvular risk factors.
Complete heart block due to native tricuspid valve IE can occur with septal leaflet involvement. It is often transient and responds well to medical therapy.
Introduction
Right-sided infective endocarditis (IE) accounts for only 5–10% of IE cases, with the tricuspid valve (TV) being most commonly affected.1 Case reports assessing native TV IE in the absence of traditional risk factors [intravenous (IV) drug use or catheter placement] have generally been associated with Staphylococcus aureus infection, as was the case in our patient.2 Despite the virulent nature of the associated organism, right-sided IE may be underdiagnosed due to its atypical presentation, which can mimic respiratory infections (fever, dyspnoea, pulmonary infiltrates). Additionally, a majority of the surgical criteria for IE apply primarily to left-sided disease, and in cases of right-sided disease the decision regarding surgical intervention is often delayed until significant TV disease has developed or patients have developed multiple, recurrent pulmonary emboli as a result of the large, mobile vegetations fostered by the low-pressure right-sided system.2,3 Given that TV IE tends to affect younger patients, this may result in significant long-term morbidity if not promptly identified.
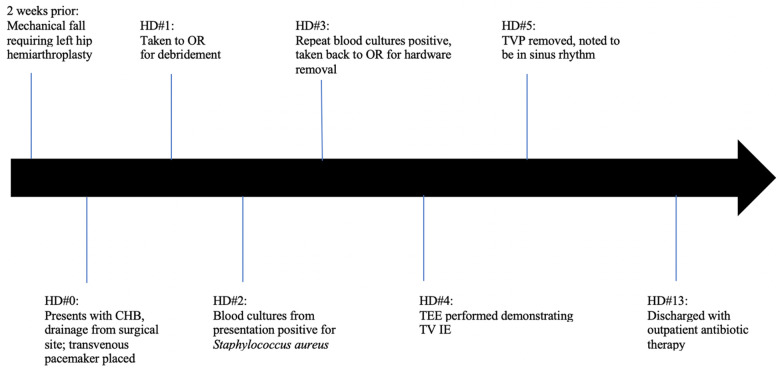
Timeline
CHB, complete heart block; IE, infective endocarditis; OR, operating room; TOE, transoesophageal echocardiogram; TV, tricuspid valve; TVP, transvenous pacemaker.
Case presentation
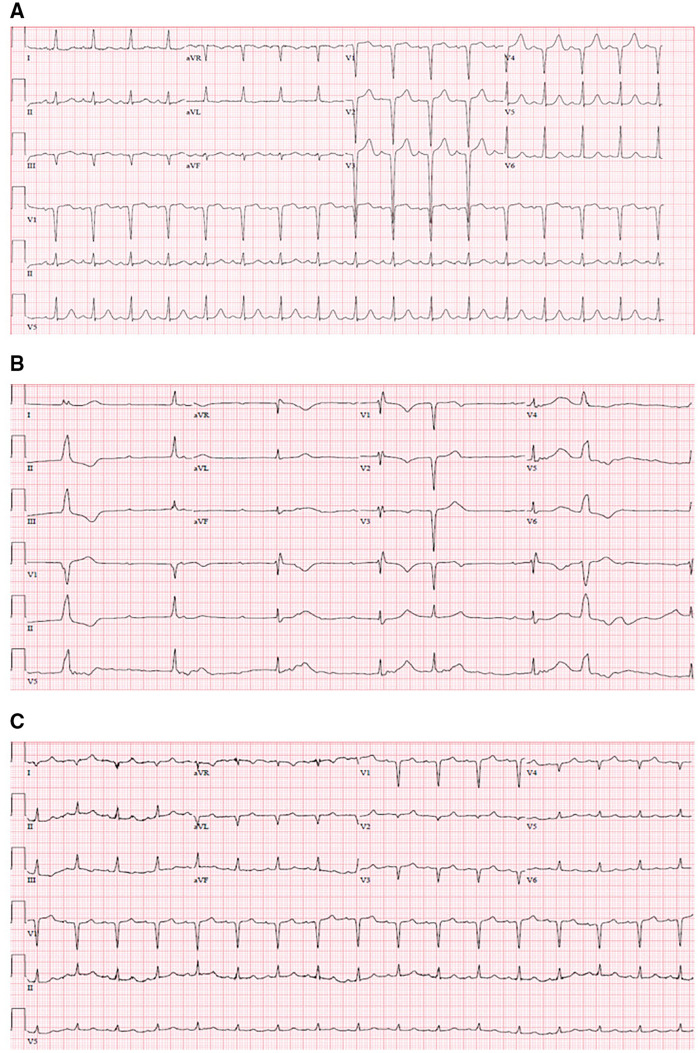
We discuss the case of a 63-year-old African-American female with recent mechanical fall complicated by left femoral neck fracture requiring left hip hemiarthroplasty ∼2 weeks prior, who presented for evaluation of purulent drainage from her surgical site. She was altered and haemodynamically unstable on presentation, with heart rate ∼30 b.p.m. and blood pressure 80/60. She was cool to the touch, with crackles at bilateral lung bases and jugular venous distention to the angle of the mandible. An electrocardiogram (EKG) demonstrated complete heart block (CHB) with junctional escape rhythm (Figure 1), and she was started on a continuous dopamine infusion and urgent temporary pacemaker was placed with improvement in her haemodynamics and mental status. She denied any lightheadedness or recurrent falls, but reported worsening fatigue over the week prior to presentation.
Figure 1.
Electrocardiogram at (A) baseline, (B) at presentation, and (C) following antibiotic therapy.
She had no known history of coronary artery disease or structural heart defects, and EKG done prior to recent surgery demonstrated no evidence of conduction disease (Figure 1).
The differential diagnosis of CHB includes age-related degenerative disease, metabolic derangements (hypothyroidism, hypoglycaemia, hyperkalaemia), medication toxicity (beta-blockers, calcium channel blockers, digoxin), mechanical complications (following valvular interventions, endocarditis), or coronary ischaemia. In our patient, an acute insult from either ischaemia, metabolic derangements, or medication effects appeared more likely given her recently normal EKG.
Initial laboratory data were significant for leukocytosis of 29.7 × 103/μL with neutrophilic predominance (85%), elevated lactic acid (6.2 mmol/L), and acute kidney injury (Cr 1.7 mg/dL from 0.7 mg/dL 3 weeks prior). High sensitivity troponin was mildly elevated at presentation (41 ng/L) but trended downwards thereafter. There were no other significant metabolic derangements, and thyroid-stimulating hormone was within normal limits. The patient denied taking additional doses of metoprolol prior to presentation. Blood cultures were drawn prior to initiation of empiric antibiotic therapy.
EKG at presentation is shown in Figure 1, which demonstrated CHB with junctional escape and intermittent premature ventricular complexes. Transthoracic echocardiogram (TTE) demonstrated normal left ventricular function with no significant valvular abnormalities or evidence of abscesses.
Management
Blood and wound cultures were drawn, and empiric antibiotic therapy was started with vancomycin and cefepime. A temporary transvenous pacemaker was placed from the right internal jugular approach prior to going to the operating room (OR) for wound debridement on Day 1 of her hospitalization. Initial blood cultures from presentation, prior to transvenous pacemaker insertion, returned positive for S. aureus and rifampin and gentamicin were added until sensitivities returned methicillin-resistant organisms. Further cultures remained positive, and she returned to the OR for complete removal of all hardware with antibiotic spacer placement. Given lack of clear reversible aetiology and normal TTE, she was planned for a leadless pacemaker placement to minimize device-related infective risk.
Prior to this, a transoesophageal echocardiogram (TOE) was completed due to persistent bacteraemia despite apparent source control, which demonstrated a mobile echo-density measuring 1.6 × 0.9 cm on the atrial side of the septal leaflet of the TV. This appeared to be attached to the annulus and highly mobile with only mild tricuspid regurgitation (Videos 1 and 2). There was no clear evidence of abscess formation or other valvular pathology. With this finding, pacemaker implantation was deferred and she was continued on antibiotic therapy. She was noted to intermittently demonstrate sinus rhythm with first-degree atrio-ventricular block after 72 h of therapy, but the predominant rhythm remained CHB necessitating backup pacing. On hospital Day 5, she was able to sustain normal sinus rhythm with normal conduction pattern on EKG, and transvenous pacemaker was removed.
She was evaluated by cardiothoracic surgery given persistent bacteraemia and underwent coronary angiogram as part of her pre-operative evaluation which demonstrated no obstructive coronary artery disease. Given her clinical improvement, resolution of sinus rhythm, and hesitancy about cardiac surgery, she was continued on 6 weeks of IV antibiotics with plan for repeat TOE and close cardiology and cardiothoracic surgery follow-up in the outpatient setting. Per documentation, she has been doing well, although she did not make her scheduled cardiology appointment or repeat TOE, which are now in the process of being rescheduled.
Discussion
Approximately 10% of native valve IE cases are complicated by conduction deficits due to inflammatory oedema, myocarditis, and abscess formation.4 This primarily occurs with aortic valve involvement, and to the best of our knowledge, there have only been three previously reported cases of conduction disease due to native TV IE.5–7 As was the case in our patient, these patients improved with medical therapy, and permanent pacemaker implantation was not required.
Right-sided IE presents predominantly with lethargy and respiratory symptoms, and diagnosis can often be delayed in favour of respiratory illnesses. Improved diagnostic techniques and awareness have resulted in higher rates of right-sided IE noted in recent years. Congenital heart disease, indwelling lines or catheters, and IV drug use are the dominant risk factors, and native valve disease remains extremely rare. As such, the index of suspicion should be high in patients who present with a constellation of symptoms, persistent respiratory symptoms, or evidence of conduction disease.8
The presence of conduction disease does not affect mortality, but has been associated with increased risk of embolization and should be treated aggressively.9 Resolution of conduction disease with acute IE is rare and seems to be a unique characteristic of right-sided disease based on the few available reports. Of all potential factors, location of infection appears most relevant to the development of conduction disease, and may explain why right-sided IE tends to resolve with medical therapy.4 The triangle of Koch, located on the septal wall of the right atrium, contains the AV node and penetrating AV bundle at its apex, which is bound by the septal leaflet of the TV.10 The AV node and conduction tissue sit at the intersection of the anterior and septal leaflets of the TV, with the aortic valve on the other side. Inflammation, oedema, and extension of TV IE involving the septal leaflet may induce variations in nearby conduction tissue, and serve as a potential mechanism of TV IE-related conduction disease. Despite right-sided IE forming larger vegetations, leaflet positioning results in relatively small surface area of direct contact with conduction tissue. This may account for the low rate of noted conduction disease, and the relatively high rates of improvement with resolution of initial oedema and inflammation.
CHB related to IE is currently a Class I indication for surgical intervention, regardless of completion of antibiotic therapy with no specific recommendations based on location of disease (left- vs right-sided).3 With regards to TV-related pathology, most cases appear to resolve with medical therapy alone, and the role for surgical intervention or pacemaker implantation is less clear. In these cases, patients may benefit from a delay in surgery to assess for clinical response with antibiotics and source control. In those who require permanent pacemaker implantation and have demonstrated high infective risk despite the absence of traditional risk factors, leadless pacemakers such as the Micra Transcatheter Pacemaker System (Medtronic, Minneapolis, MN, USA) may be a suitable alternative to traditional pacemakers given their low risk of developing infection.11
Conclusions
Although typically associated with aortic valve IE, conduction deficits have been reported with right-sided valvular disease and TV IE should be considered in the differential of acute conduction disease in the appropriate clinical context. Definitive pacemaker implantation should be delayed, as case reports have suggested high rates of resolution with medical therapy alone.
Lead author biography

Dr Nikhil Singh is a second-year cardiology fellow at the University of Chicago Medical Center in Chicago, IL. He completed his Internal Medicine training at the University of Southern California, where he stayed for an additional year as Chief Resident. He has an interest in quality improvement and outcomes research, and plans on pursuing a career in academic non-invasive cardiology.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Hussain S, Witten J, Shrestha N, Blackstone E, Pettersson G.. Tricuspid valve endocarditis. Ann Cardiothorac Surg 2017;6:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revilla A, Lopez J, Villacorta E, Gomez I, Sevilla T, del Pozo M. et al. Isolated right-sided valvular endocarditis in non-intravenous drug users. Rev Esp Cardiol 2008;61:1253–1259. [DOI] [PubMed] [Google Scholar]
- 3.Pettersson G, Hussain S.. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg 2019;8:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiNubile M, Calderwood S, Steinhaus D, Karchmer A.. Cardiac conduction abnormalities complicating native valve active infective endocarditis. Am J Cardiol 1986;58:1213–1217. [DOI] [PubMed] [Google Scholar]
- 5.Agu C, Salhan D, Bakhit A, Basheer H, Basunia M, Bhattarai B. et al. Tricuspid valve endocarditis complicated by Mobitz type II heart block—a case report and literature review. J Community Hosp Intern Med Perspect 2015;5:29689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Uruena N, Hernandez C, Duro I, Sandin M, Zatarain E, San Roman A.. Transient trifascicular block secondary to tricuspid valve endocarditis. Rev Esp Cardiol 2012;65:767–768. [DOI] [PubMed] [Google Scholar]
- 7.Fordyce C, Leather R, Partlow E, Swiggum E.. Complete heart block associated with tricuspid valve endocarditis due to extended spectrum β-lactamase-producing Escherichia coli. Can J Cardiol 2011;27:263.e17–263.e20. [DOI] [PubMed] [Google Scholar]
- 8.Yuan S.Right-sided infective endocarditis: recent epidemiologic changes. Int J Clin Exp Med 2014;7:199–218. [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu H, Bae M, Lee S, Lee J, Lee J, Kwon Y. et al. Presence of conduction abnormalities as a predictor of clinical outcomes in patients with infective endocarditis. Heart Vessels 2011;26:298–305. [DOI] [PubMed] [Google Scholar]
- 10.Klimek-Piotrowska W, Holda M, Koziej M, Salapa K, Piatek K, Holda J.. Geometry of Koch’s triangle. EP Europace 2017;19:452–457. [DOI] [PubMed] [Google Scholar]
- 11.El-Chami MF, Bonner M, Holbrook R, Stromberg K, Mayotte J, Molan A. et al. Leadless pacemakers reduce risk of device-related infection: review of the potential mechanisms. Heart Rhythm 2020;17:1393–1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.