Abstract
Background
Capnocytophaga canimorsus, a bacterium found in the oral cavities of healthy cats and dogs, is rarely reported as a cause of infective endocarditis. In this report we describe such a case in a young, male dog owner who presented acutely unwell in heart failure.
Case summary
A 47-year-old male presented with a subacute onset of fever, night sweats, weight loss, dyspnoea, and peripheral oedema. On clinical examination typical features of infective endocarditis, heart failure, and aortic regurgitation were found. The patient had no conventional risk factors for infective endocarditis but was a dog owner. Transthoracic echocardiography revealed vegetations on the right coronary and non-coronary cusps of the aortic valve causing severe eccentric aortic regurgitation and left ventricular dilatation. Initial blood cultures taken prior to the initiation of antimicrobial therapy showed no growth. The patient underwent aortic valve and root replacement and a 16S ribosomal RNA polymerase chain reaction (16S rRNA PCR) of the resected aortic valve tissue, using the additional primer set 785F/1175R targeting the V5–7 region of 16S rRNA, identified C. canimorsus. The patient was treated post-operatively with a 6-week course of meropenem and made a good recovery.
Discussion
Suspicion of C. canimorsus causing infective endocarditis should be considered in culture-negative infective endocarditis in individuals who have close contact with dogs or cats. Those who are immunocompetent can be susceptible to this infection and so this diagnosis should not be disregarded in healthy individuals. A 16S rRNA PCR can help identify this bacterium and should be used early in cases of culture-negative infective endocarditis.
Keywords: Capnocytophaga canimorsus, Infective endocarditis, 16s ribosomal RNA polymerase
Learning points
This case highlights a need for an increased awareness of Capnocytophaga canimorsus as a potential cause of culture-negative infective endocarditis
A 16S rRNA sequence polymerase chain reaction with the primer pair (785F/1175R) targeting the V5–7 region of 16S rRNA genes should be used when looking for a culture-negative infective endocarditis.
Immunocompetant individuals can be susceptible to infections caused by C. canimorsus, including infective endocarditis.
Introduction
Capnocytophaga canimorsus is emerging as an atypical cause of infective endocarditis in humans. These Gram negative rod-shaped bacteria are found in the oral cavities of healthy cats and dogs and can be transmitted via animal bites, scratches, or tactile exposure(1).1 It mostly affects individuals who are immunocompromised and those who are functionally or physically asplenic(1).1 There have been 19 cases of C. canimorsus infective endocarditis reported in the literature and these are associated with a high case fatality rate(2–7).2–7 We report a young immunocompetent gentleman who developed C.canimorsus infective endocarditis requiring urgent surgical intervention.
Timeline
| Day 1 | Admission to hospital witd 4 weeks history of progressively worsening dyspnoea, unintentional weight loss, and pyrexia. Fluid overloaded witd systolic and diastolic murmurs on examination. Elevated N-terminal prohormone of brain natriuretic peptide, high-sensitivity troponin I, and C-reactive protein. Started on intravenous antibiotics. |
| Day 2 |
Transthoracic echocardiogram confirms infective endocarditis of aortic valve with free-flowing AR. Left ventricular end-diastolic volume 316 mL. Magnetic resonance imaging of the brain demonstrates a small embolic lesion and mycotic aneurysm of the left internal carotid artery. Computed tomography scan of chest, abdomen, and pelvis showed imaging features of cardiac failure, splenomegaly, and absence of embolic lesions in other organs. |
| Day 3 | Inpatient transfer to a tertiary cardiothoracic centre. Given multiple broad-spectrum antibiotics. |
| Day 4 | Underwent emergency cardiothoracic surgery for aortic valve endocarditis. |
| Day 7 | Microbiology report shows no growth from aortic tissue sample, after 48 h incubation in standard culture. |
| Day 25 | No growth of bacterium after 12 days in enrichment culture. |
| Day 30 | 16S ribosomal RNA polymerase chain reaction identifies Capnocytophaga canimorsus. |
| Day 32 | Good operative outcome and continued good response to antibiotic regime. |
| Week 10 | Patient discharged after completing 6 weeks of intravenous meropenem. |
Case presentation
A 47-year-old male was admitted with dyspnoea at rest, reduced exercise tolerance, low-grade fever, night sweats, and unintentional weight loss of 7 kg over the two weeks leading up to admission. He was previously fit and well, had no past medical history, and was not taking regular medications. He was a labourer by trade, an ex-smoker, denied intravenous drug use, drank 2–3 units of alcohol per week, and owned a pet dog. Observations on admission were a temperature of 38.9 degrees Celsius, blood pressure 131/61 mmHg, and pulse rate 114 beats per minute. A Grade 4 early diastolic murmur and mid-systolic flow murmur were audible over the left sternal edge and loudest at the apex. A collapsing pulse was palpable in the upper limbs and Corrigan pulse sign at the carotids was positive. Conjunctival haemorrhages and fingernail splinter haemorrhages were seen. There were signs of heart failure with a raised jugular venous pressure and bilateral basal crackles in the chest. An electrocardiogram revealed a sinus tachycardia with a normal PR interval.
Urinalysis revealed a microscopic haematuria. Initial blood tests revealed a raised C-reactive protein of 72 mg/L and an elevated white cell count of 14.9 x 10 9 /L with a neutrophilia and monocytosis. There was a normocytic normochromic anaemia (haemoglobin of 91 g/L) and thrombocytopenia (platelet count of 80 x10 9 /L). HIV and hepatitis B and C screens were negative. Serum N-terminal pro-brain natriuretic peptide was markedly elevated at 27,931ng/L (assay cut-off suggestive of heart failure > 2,000 ng/L) and high sensitivity cardiac troponin I level was measured at 1,506 ng/L trending down to 1,209 ng/L (range 0-34.2ng/L).
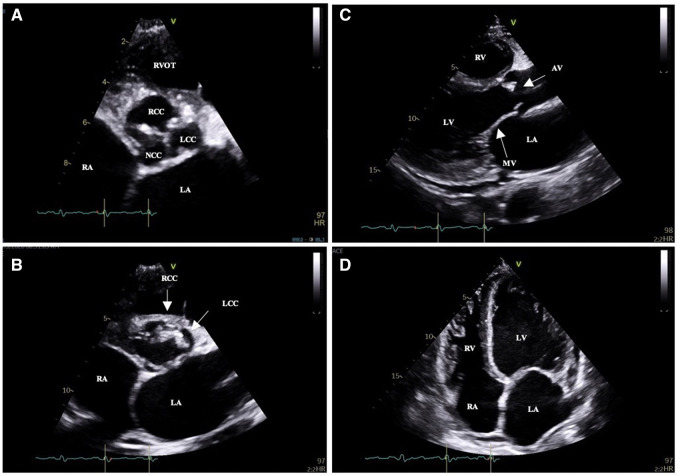
Transthoracic echocardiography revealed mobile vegetations attached to a tricuspid aortic valve that oscillated into the aorta during systole (Figure 1A–D; Video 1). This was associated with severe eccentric aortic regurgitation (AR) with a pressure half time of 72 ms and significant diastolic flow reversal in the descending aorta (Figure 2; Supplementary material online, Video S1). The aortic root dimensions were normal. The left ventricle was markedly dilated with a left ventricular end-diastolic volume (LVEDV) of 316 mL. Left ventricular systolic function was preserved (left ventricular ejection fraction of 0.61 by Simpson’s biplane method of discs). The estimated systolic pulmonary artery pressure by echocardiography was 75 mmHg and this was associated with mild dilatation of the right ventricle and severe tricuspid regurgitation (Video 2). There was a posteriorly directed jet of mitral regurgitation secondary to chordal tethering of the anterior leaflet of the mitral valve (Video 3).
Figure 1.
Transthoracic echocardiogram images. (A,B) Parasternal short-axis views of the tricuspid aortic valve showing vegetations of the right and left coronary cusp. (C) Parasternal long-axis view showing aortic valve vegetation of the right coronary cusp. (D) Apical four-chamber view showing left ventricular dilatation in response to severe aortic regurgitation.
Figure 2.
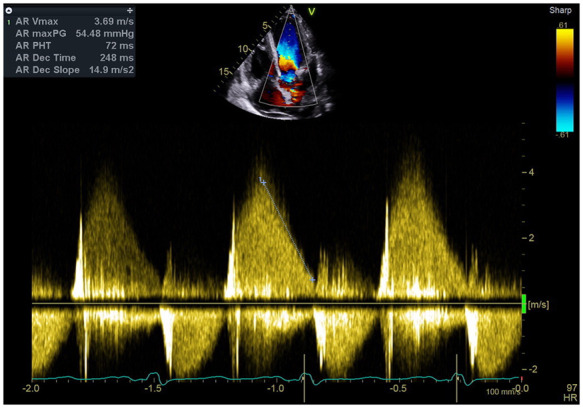
Continuous wave Doppler in the apical five chamber window showing a high density envelope with rapid deceleration. Pressure half time measured at 72 ms.
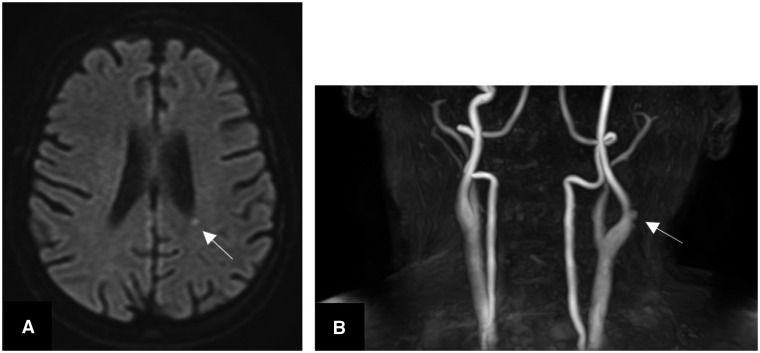
A diagnosis of definite infective endocarditis of the native aortic valve by the modified Duke’s criteria was made. A magnetic resonance imaging scan of the brain showed a small area of hyperintensity adjacent to the posterior body of the left lateral ventricle (Figure 3A,B). Time-of-flight angiography of the carotid arteries showed small mycotic aneurysms in the left internal carotid artery. A computed tomography scan of the chest, abdomen, and pelvis revealed cardiomegaly, moderate bilateral pleural effusions, pulmonary oedema, and splenomegaly. Three sets of blood cultures were taken which were negative after extended culture. Intravenous amoxicillin was given followed by intravenous vancomycin and gentamicin.
Figure 3.
(A) Magnetic resonance imaging of the brain showing an acute punctate infarct next to the left lateral ventricle caused by an embolus. (B) Time of flight angiography of the carotids showing a couple of small mycotic aneurysms arising from the lateral wall of the left internal carotid artery.
The patient was transferred to a cardiothoracic centre for definitive surgical management following a Heart team discussion. Intraoperative transoesophageal echocardiography confirmed severe and torrential aortic regurgitation (Figure 4). Multiple vegetations were attached to the left ventricular outflow tract muscular septum and between the junction of the right and left coronary cusps with destruction of the aortic annulus. Purulent material was seeping out of an abscess cavity adjacent to the right coronary cusp. No vegetations were present on the mitral or tricuspid valves. A tissue aortic valve replacement plus aortic root replacement with re-implantation of the coronary arteries was performed. The anterior mitral valve leaflet was found to be mildly tethered and so freed with a tricuspid valve annuloplasty ring sited due to the presence of severe functional regurgitation.
Figure 4.
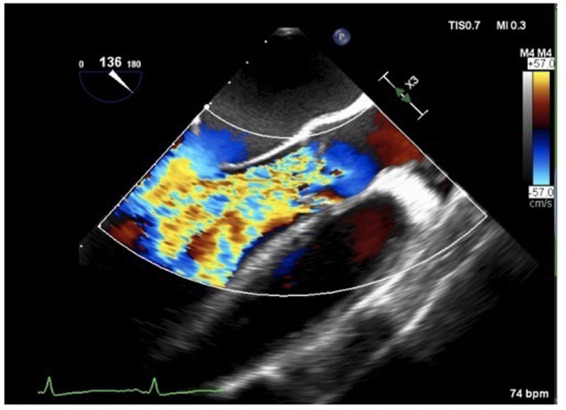
(A) Colour Doppler transoesophageal echocardiogram image. Long-axis view demonstrating severe aortic regurgitation.
No organisms were seen by Gram’s stain of aortic valve tissue collected intraoperatively and no growth occurred after 48 h of standard culture or 12 days of enrichment culture. As a causal organism had not been identified a sample was submitted for 16S ribosomal RNA (16S rRNA) gene sequencing which identified C. canimorsus. An additional primer pair (785F/1175R) targeting the V5–7 region of 16S rRNA gene8–10 (Figure 5) had been recently added to routine diagnostic 16S rRNA, and this helped identify C. canimorsus, which has been shown to be amplified much less efficiently by the other primer sets targeting the V1–2 regions of 16s rRNA gene.8
Figure 5.
The 16S sequences obtained from the sample of the patient’s aortic tissue valve produced by the clinical scientists at The Great Ormand Street Hospital.
None of the three sets of blood cultures taken at admission grew any organisms after 14 days of routine culture. As the patient had demonstrated a good initial response to intravenous meropenem this antibiotic was continued with daily monitoring of his inflammatory markers to good effect. Capnocytophaga canimorsus is shown to be sensitive to imipenem, clindamycin, linezolid, and tetracylines.11
Four weeks post-surgery an echocardiogram showed a well-seated aortic valve replacement with no transvalvular or paravalvular regurgitation (Supplementary material online, Video S2). Left ventricular size had returned to normal (LVEDV 137 mL) and left ventricular ejection fraction was maintained at 0.55–0.60. There was no residual tricuspid regurgitation after siting of the tricuspid annuloplasty ring (Supplementary material online, Video S3) and minimal residual mitral regurgitation with improvement of mitral valve leaflet coaptation (Supplementary material online, Video S4). The estimated pulmonary artery pressure had normalized to 16 mmHg. The patient’s post-discharge has been uneventful with regular outpatient follow-up.
Discussion
This Gram-negative facultative bacillus triggers a host immune response due to the lack of interaction between its lipopolysaccharide and the human toll-like receptor 4, the receptor which triggers an inflammatory response.12 Capnocytophaga canimorsus grows slowly in blood culture bottles and on agar plates with the requirement of meticulous growth conditions, including an anaerobic atmosphere of 5–10% carbon dioxide.1 Longer incubation periods, for up to 14 days, and/or terminal subculture should be considered when the causal organism proves difficult to identify, and the addition of 16S rRNA gene sequencing, including the primer 785F/1175R targeting the V5–7 region of 16S rRNA gene, should also be considered.8–10,13,14
The case highlights that an occupational history and history of pets are important in the context of a recurrent fever at presentation. Previous case reports have described C. canimorsus transmitted by dog bites and scratches.2–7 It is assumed that with small open wounds from our patient’s job as a labourer, transmission of the organism leading to his acute cardiac admission was from the saliva of his dog. Finally, although previous descriptions have involved those who are immunocompromised, this case illustrates the destructive nature of the infection in young, immunocompetent patients with no co-morbidities. Therefore, a high clinical index of suspicion along with early use of advanced microbiological culture techniques and early intervention is required in order to mitigate the poor prognosis associated with this zoonosis.
Lead author biography

Dr Anita Sri is a junior doctor in the Thames Valley Deanery NHS England and worked in the cardiology department for six months in Buckinghamshire. She attended medical school at Imperial College London and did her BSc project with the paediatric cardiology team in The Royal Brompton Hospital. Her main interests are inherited heart conditions and sudden cardiac deaths.
Supplementary Material
Acknowledgements
Dr Kathryn Harris, Principal Clinical Scientist, Great Ormond Street Hospital NHS Foundation Trust. Professor Andrew Nicholson, Consultant Histopathologist, Harefield Histopathology Department.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1.Janda JM, Graves MH, Lindquist D, Probert WS.. Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis 2006;12:340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coutance G, Labombarda F, Pellissier A, Legallois D, Hamon M, Bachelet C. et al. Capnocytophaga canimorsus endocarditis with root abscess in a patient with a bicuspid aortic valve. Heart Int 2009;4:hi.2009.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso RM, Rodrigues J, Garcia D, Campos ID.. Tricuspid valve endocarditis due to Capnocytophaga canimorsus. BMJ Case Rep 2019;12:e233721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayani O, Higginson LA, Toye B, Burwash IG.. Man’s best friend? Infective endocarditis due to Capnocytophaga canimorsus. Can J Cardiol 2009;25:2008–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry M.Double native valve infective endocarditis due to Capnocytophaga canimorsus: first reported case caused by a lion bite. Case Rep Infect Dis 2018;2018:4821939–4821933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai J, Imanaka K, Kodana M, Ohgane K, Sekine S, Yamamoto K. et al. Infective endocarditis caused by Capnocytophaga canimorsus; a case report. BMC Infect Dis 2019;19:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngaage DL, Kotidis KN, Sandoe JA, Unnikrishnan Nair R.. Do not snog the dog: Infective endocarditis due to Capnocytophaga canimorsus. Eur J Cardiothorac Surg 1999;16:362–363. [DOI] [PubMed] [Google Scholar]
- 8.Doyle RM, Harris K, Kamiza S, Harjunmaa U, Ashorn U, Nkhoma M. et al. Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLoS One 2017;12:e0180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris KA, Hartley JC.. Development of broad-range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service. J Med Microbiol 2003;52:685–691. [DOI] [PubMed] [Google Scholar]
- 10.Harris KA, Yam T, Jalili S, Williams OM, Alshafi K, Gouliouris T. et al. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. Eur J Clin Microbiol Infect Dis 2014;33:2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolivet-Gougeon A, Sixou JL, Tamanai-Shacoori Z, Bonnaure-Mallet M.. Antimicrobial treatment of Capnocytophaga infections. Int J Antimicrob Agents 2007;29:367–373. [DOI] [PubMed] [Google Scholar]
- 12.Shin H, Mally M, Kuhn M, Paroz C, Cornelis GR.. Escape from immune surveillance by Capnocytophaga canimorsus. J Infect Dis 2007;195:375–386. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell D, Yuen M, Lasaitis R.. Detection of capnocytophaga species in simulated blood cultures by bactec-NR. Pathology 1992;24:35. [Google Scholar]
- 14.Fida M, Dylla BL, Sohail MR, Pritt BS, Schuetz AN, Patel R.. 2019. Role of prolonged blood culture incubation in infective endocarditis diagnosis.Eur J Cin Microbiol Infect Dis 2019;38:197–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.