Abstract
Background
Immune checkpoint inhibitors (ICI) have revolutionized the management of many cancer types by drastically improving the median survival rate of patients. However, this efficiency comes at the cost of a high rate of immune-related adverse events, including lethal cardiac manifestations. Rapidly fatal cases of ICI-induced myocarditis have been reported and drawn considerable attention over the past years. However, it is essential to bear in mind that not all cardiac events occurring under ICI therapy are necessarily myocarditis.
Case summary
A 61-year-old female treated with pembrolizumab for a stage IV melanoma was admitted for chest pain leading to the diagnosis of ICI-related myocarditis based on the description of a discrete left ventricular subepicardial late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) imaging. ICI were suspended and intravenous methylprednisolone initiated. A second line anti-MEK therapy was initiated. After a month of treatment, similar chest pain occurred. CMR revealed a midventricular stress cardiomyopathy and no LGE was detected. A posteriori interrogation revealed emotional stressors preceding both episodes. Review of the first CMR, performed 2 weeks after symptom onset, indicated a pattern compatible with the recovery phase of a stress cardiomyopathy and the presence of LGE was questioned. ICI were reintroduced without recurrence of cardiac events.
Discussion
Not all cardiac manifestations occurring under ICI therapy are drug-related adverse events, therefore differential diagnoses must systematically be considered as the contraindication of ICI may have a major impact on patient prognosis. Cardiac imaging should be performed early and plays a key role in the management strategy.
Keywords: Cardio-oncology, Stress cardiomyopathy, Immunotherapy, Immune checkpoint inhibitors, Myocarditis, Case report
Introduction
Immune checkpoint inhibitors (ICI) have revolutionized the management of many cancer types by drastically improving the median survival rate of patients. However, this efficiency in fighting cancer comes at the cost of a high rate of immune-related adverse events, including potentially lethal cardiac manifestations.1 Rapidly fatal cases of ICI-induced myocarditis have been reported and drawn considerable attention over the past years.1–3 However, it is of utmost importance to bear in mind that not all cardiac events occurring under ICI therapy are myocarditis or ICI related as illustrated by the present case report.
Learning points
Not all cardiac manifestations occurring under immune checkpoint inhibitors (ICI) therapy are drug-related adverse events and differential diagnoses should systematically be considered.
The contraindication of ICI may have a major impact on patient prognosis.
Cardiac imaging plays an important role and should be perfomed early.
Timeline
The timeline of the case report is presented in Figure 1.
Figure 1.
Timeline of the case report.
Case presentation
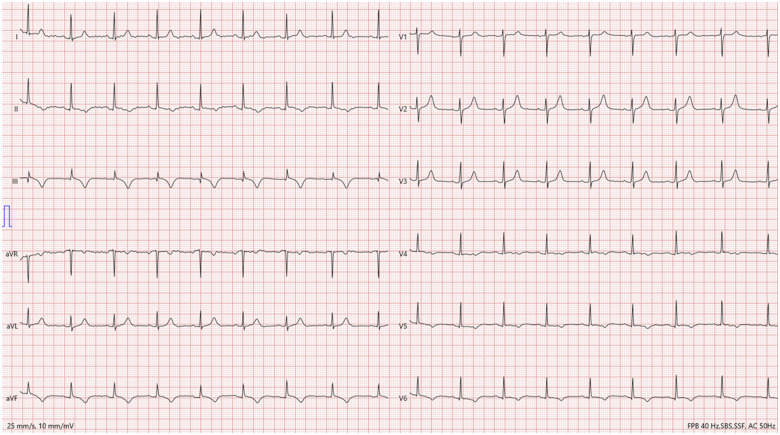
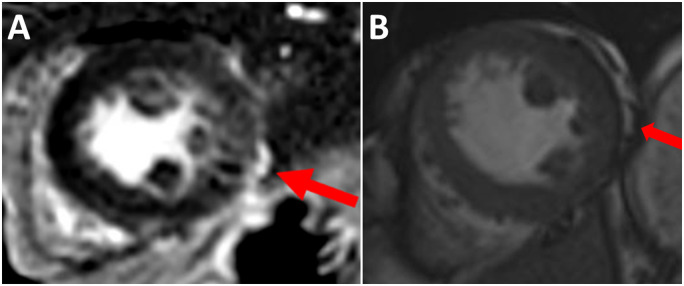
A 61-year-old Caucasian female patient with history of smoking, hypertension, and depression, was admitted to our institution for acute, intense, substernal chest pain, characterized as burning, without radiation. At the time of admission, she was treated for a stage IV melanoma of the nasal cavity with the ICI pembrolizumab because of tumour progression despite local debulking surgery and adjuvant radiotherapy. The current episode occurred after five cycles of pembrolizumab administered over a period of 3 months. On admission, blood pressure was 142/72 mmHg, pulse rate 90/min, respirations 12/min, temperature 37.2°C, and the physical examination, including the cardiovascular examination, was unremarkable. Electrocardiography (ECG) revealed sinus tachycardia with negative T waves in the infero-lateral leads (Figure 2). Troponin T hs levels were 266 ng/L (normal 14<ng/L), and total creatine kinase levels were 158 U/L (normal 25–140 U/L). Computed tomography (CT) excluded acute pulmonary embolism. The coronary angiography was unremarkable. The transthoracic echocardiography performed one week after symptom onset revealed a left ventricular ejection fraction (LVEF) of 50% and no regional wall motion abnormalities (Video 1). At this stage, our team retained the diagnosis of ICI-induced myocarditis based on the presence of a discrete subepicardial late gadolinium enhancement (LGE) of the infero-lateral segment, despite the absence of myocardial oedema on T2 mapping images (Figures 3 and 4). ICI were suspended and intravenous methylprednisolone 1 g/day initiated for 3 days followed by oral corticosteroid therapy. The 1-month follow-up revealed complete recovery of the cardiac function. ICI were contraindicated and anti-MEK therapy (trametinib 2 mg/day) was introduced as a second line treatment.
Figure 2.
Initial electrocardiogram of the patient showing sinus tachycardia with negative T waves in the infero-lateral leads.

Figure 3.
(A) Suspicion of the presence of a discrete subepicardial late gadolinium enhancement of the infero-lateral segment of the left ventricle on cardiac magnetic resonance. (B) Cine steady-state free precession imaging showing that the hyperintensity seen on the first late gadolinium sequences is consistent with fat rather than with true late gadolinium enhancement of the left ventricle.
Figure 4.
T2 mapping sequences at the (A) basal (B) mid ventricular and (C) apical levels of the left ventricle showing normal range T2 values. Corresponding late gadolinium sequences at the (D) basal (E) mid ventricular and (F) apical levels of the left ventricle.
One month after anti-MEK therapy initiation, the patient was again admitted for chest pain, mild troponin T hs elevation to 717 ng/mL and similar ECG modifications. The transthoracic echocardiography (Video 2) as well as the cardiac magnetic resonance (CMR) revealed a reduced LVEF to 46% with severe hypokinesia of all mid-ventricular segments (Video 3). T2 mapping values were markedly increased in all midventricular segments (up to 77 ms) and normal in all other segments (Figure 5). All these parameters were in favour of a midventricular stress cardiomyopathy and anti-MEK therapy-induced left ventricular dysfunction was excluded. Beta-blockers and angiotensin-converting enzyme inhibitors were introduced.
Figure 5.
T2 mapping sequence on cardiac magnetic resonance showing increased values in midventricular segments (up to 77 ms).
The follow-up at 1 month revealed complete recovery of the cardiac function. In the light of this diagnosis, both episodes of cardiac events were re-examined by our cardio-oncology team. A posteriori patient interrogation revealed clear emotional stressors preceding both episodes of chest pain. During the first episode of care, cardiac symptoms occurred immediately after the patient was informed that the follow-up positron emission tomography (PET) CT did not show cancer regression under ICI. The second time, the chest pain occurred after she discovered on a report a discrete elevation of the CK level, which is a relatively common side effect associated with anti-MEK therapy. Regarding the first episode, although no clear regional wall motion abnormalities were noted on echocardiography and CMR, it is worth emphasizing that they were performed late at respectively 1 and 2 weeks after the first symptoms. Review of the first CMR, performed 2 weeks after symptom onset, led us to question the presence of significant LGE. No LGE was indeed detected on the second CMR (Figure 6) and the hyperintensity seen on the first late gadolinium sequences was attributed to fat rather than to a true LGE of the left ventricle (Figure 3).
Figure 6.
Late gadolinium sequences of the second cardiac magnetic resonance showing no enhancement in the (A and B) long-axis and (C and D) short-axis views.
Two months after the second cardiac event, the follow-up PET-CT revealed tumour progression. After complete review of the case, the first cardiac event was also attributed to a midventricular stress cardiomyopathy and not to a case of ICI-induced myocarditis, based on the clinical history, typical ECG tracings, and the absence of any detectable subepicardial LGE on the second CMR scan. Anti-MEK therapy was suspended and combination immunotherapy, including ipilimumab and nivolumab, was successfully introduced. Neither recurrence of stress cardiomyopathy, nor occurrence of myocarditis was diagnosed during the follow-up.
Discussion
Myocarditis is the most feared cardiac adverse event occurring under ICI therapy.4,5 Indeed the diagnosis should systematically be considered in the presence of cardiac manifestations in patients treated with ICI therapy because of the broad range of possible clinical presentations and the risk of ventricular arrhythmia, atrioventricular block, fulminant myocarditis with cardiogenic shock and death.6,7 However, clinicians must keep in mind that not all cardiac events occurring under ICI therapy are myocarditis or ICI related. Retaining the diagnosis of myocarditis has significant implications on patients’ overall prognosis as it leads to definitive ICI therapy interruption, which is often the only treatment that may allow durable response, particularly in case of advanced stage cancer.4 Therefore, it is essential to exclude differential diagnoses beforehand as illustrated in the present article. Moreover, an integrated approach including clinical, biological, and imaging parameters is the best strategy to characterize cardiac adverse events occurring during ICI therapy.
CMR imaging plays an important role to discriminate the many differential diagnoses that should be considered in the presence of acute myocardial injury. Although non-contributive in up to 50% of cases in the literature,8 CMR may provide incremental diagnostic value if performed as early as possible after symptom onset, particularly with the use of tissue characterization techniques including LGE sequences for myocardial necrosis and T2 mapping (or equivalent) sequences for myocardial oedema detection and to better capture typical signs of myocardial involvement.9–11 A few cases of stress cardiomyopathy have been reported but none described the midventricular pattern, which can be difficult to diagnose.12,13 In the present case, T2 values were significantly increased (>60 ms) in midventricular segments of the left ventricle suggesting the presence of myocardial oedema (Figure 4) and cine images clearly showed midventricular severe hypokinesia (Video 3) pleading in favour of the diagnosis of stress cardiomyopathy. In addition, T2 values were normal in the other segments of the left ventricle. Furthermore, a strong argument supporting the diagnosis is the complete resolution of CMR abnormalities at the 1-month follow-up including normalization of T2 values and the absence of recurrence of myocarditis following reintroduction of ICI therapy. It is worth noting that the pattern of the first episode of stress cardiomyopathy (apical, midventricular, basal, or focal) cannot be confirmed. It is also important to mention that distinguishing ICI-induced myocarditis and stress cardiomyopathy can be an arduous task, particularly in case of focal type stress cardiomyopathy. Both diagnoses are most often based on a body of evidence, and the pros and cons of the reintroduction of ICI should be carefully weighed up, as the risk of fatal recurrence in case of ICI-induced myocarditis remains probably high.
In conclusion, not all cardiac manifestations occurring under ICI therapy are drug-related adverse events, therefore differential diagnoses must systematically be considered as the contraindication of ICI may have a major impact on overall patient prognosis. Cardiac imaging should be performed early and plays a key role in the management strategy, which must be elaborated in constant collaboration with oncologists.
Lead author biography
Dr Dimitri Arangalage is Associate Professor in the Department of Cardiology of Bichat Hospital, Paris, France. His main areas of interest are multimodality cardiovascular imaging, valvular heart disease, and cardio-oncology. He wrote the present article while he was completing a 1-year fellowship in cardiovascular imaging at the Centre Hospitalier Universitaire Vaudois, in Lausanne, Switzerland, under the supervision of Dr Pierre Monney. He was awarded the best clinical case of the ESC congress 2020.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: J.S. receives an unrestricted grant from Bayer Healthcare, Switzerland. The other authors have no conflict of interest related to the present paper.
Funding: None declared.
Supplementary Material
References
- 1.Arangalage D, Delyon J, Lermuzeaux M, Ekpe K, Ederhy S, Pages C. et al. Survival after fulminant myocarditis induced by immune-checkpoint inhibitors. Ann Intern Med 2017;167:683–684. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y. et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi JJ, Salem J-E, Sosman JA, Lebrun-Vignes B, Johnson DB.. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball S, Ghosh RK, Wongsaengsak S, Bandyopadhyay D, Ghosh GC, Aronow WS. et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol 2019;74:1714–1727. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM. et al. ; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J-R, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019;115:854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Awadalla M, Mahmood SS, Groarke JD, Nohria A, Liu S. et al. Late gadolinium enhancement in patients with myocarditis from immune checkpoint inhibitors. J Am Coll Cardiol 2019;73:675. [Google Scholar]
- 9.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT. et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A. et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart 2011;97:1312–1318. [DOI] [PubMed] [Google Scholar]
- 11.Dastidar AG, Rodrigues JCL, Johnson TW, De Garate E, Singhal P, Baritussio A. et al. Myocardial infarction with nonobstructed coronary arteries: impact of CMR early after presentation. JACC Cardiovasc Imaging 2017;10:1204–1206. [DOI] [PubMed] [Google Scholar]
- 12.Ederhy S, Cautela J, Ancedy Y, Escudier M, Thuny F, Cohen A.. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging 2018;11:1187–1190. [DOI] [PubMed] [Google Scholar]
- 13.Geisler BP, Raad RA, Esaian D, Schwartz SE, Apical DR.. ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer 2015;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.