Abstract
In-transit metastases (ITMs) in patients with malignant melanoma (MM) are associated with poor prognosis and a worse disease burden compared with MM without ITMs. A substantial population of patients with ITMs show no or only poor responses to newly developed therapies, such as immune checkpoint inhibitors or molecular-targeted agents. It is difficult to control the exudate and bleeding from ITMs when these medications are ineffective. In Japan, local injection of interferon-β (IFN-β) has been licensed for years as adjuvant therapy for MM. However, the evidence for IFN-β effectiveness for ITMs remains low. The present report describes a case of MM with multiple ITMs that did not respond to a programmed cell death-1 inhibitor and local injections of IFN-β at 3 million IU/day for 5 days/4 weeks but remitted upon increasing the amount of IFN-β injections to 10 consecutive days/4 weeks. Local IFN-β therapy could be an option for improving the quality of life of patients.
Keywords: malignant melanoma, in-transit metastasis, interferon-β, local injection, quality of life
Introduction
In-transit metastasis (ITM) is a cutaneous or subcutaneous metastasis that develops between the primary lesion and regional lymph nodes, and is seen in approximately 4-10% of patients with malignant melanoma (MM) (1). ITMs are associated with poor prognosis. ITM negatively impacts patients' quality of life, and its treatment is essentially palliative (2). When patients develop multiple ITMs, especially after lymph node dissection, it is difficult to control the exudate and bleeding from ITMs. Newly-developed therapies [i.e., BRAF/MEK inhibitors and immune checkpoint inhibitors (ICIs)] have improved the prognosis of many patients with MM (3-5). However, a substantial population of patients do not respond to even these medications. In Japan, local injection of interferon-β (IFN-β) has been used for years as adjuvant therapy licensed for MM. Nevertheless, the evidence of IFN-β effectiveness for ITM remains low, and the use of IFN has decreased following launches of ICIs and molecular-targeted agents. Herein, we report a case of MM with multiple ITMs that did not respond to a PD-1 inhibitor, but went into remission upon local injection of IFN-β.
Case report
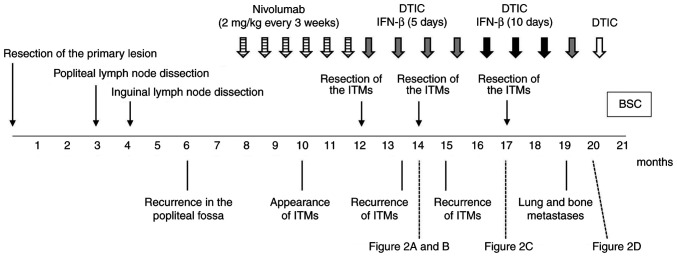
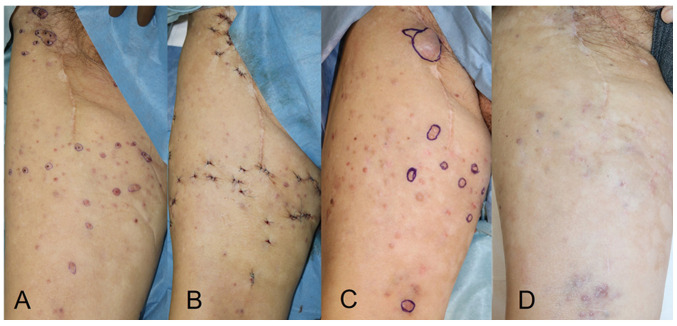
An 81-year-old man presented with acral lentiginous melanoma on his right sole. The clinical course of the patient is shown in Fig. 1. The primary lesion was resected, but the tumour spread to the popliteal and inguinal lymph nodes without any distant metastasis. No BRAF mutation was detected in the tumour, and PD-L1 expression was not examined. He received 2 mg/kg nivolumab every 3 weeks for the recurrence following popliteal and inguinal lymph node dissections. However, ITMs appeared and increased. Moreover, colitis as an immune-related adverse event associated with nivolumab was observed. The treatment was switched to dacarbazine (DTIC) (100 mg/day for 5 days every 4 weeks) and local injections of IFN-β (3 million IU/day for 5 days every 4 weeks) after the treatment with nivolumab for 4 months. The ITMs were resected twice (Fig. 2A and B) during the treatment, but recurred within a month. Because no distant metastasis was detected, local control of tumours was important to improve the patient's quality of life. Other ICIs were not considered advisable due to the side effects developed with nivolumab. In contrast, no serious adverse events were observed with local injections of IFN-β. The local injections of IFN-β were increased from 5 to 10 consecutive days and administered every 4 weeks, followed by a third resection of ITMs (Fig. 2C). Thereafter, ITMs were markedly suppressed and no longer needed to be resected (Fig. 2D). However, lung and bone metastases appeared 4 months after the increase in IFN-β, and then the treatment was switched to the best supportive care.
Figure 1.
Clinical course of the patient. ITM, in-transit metastasis; DTIC, dacarbazine; IFN-β, interferon-β; BSC, best supportive care.
Figure 2.
Treatment course of ITMs. (A) Just before the second resection of ITMs 2 months after starting treatment with dacarbazine + IFN-β (5 consecutive days injections). (B) Just after the second resection. (C) Just before the third resection 1 month after the increase of IFN-β (10 consecutive days injections). (D) At 4 months after the third resection of ITMs (5 months after the increase of IFN-β). The ITMs were markedly suppressed. ITM, in-transit metastasis; IFN-β, interferon-β.
Discussion
It has been reported that IFN-α contributed to recurrence-free survival in patients with MM (6). A case of MM with ITMs treated with intravenous IFN-β has also been reported (7). However, there is little evidence for the efficacy of local IFN injection for ITMs. An in-vitro study revealed that human IFN-β inhibited proliferation of melanoma cells and induced apoptosis in a dose-dependent fashion (8). In our patient, increasing the amount of IFN-β led to good control of ITMs without visible skin impairment, bleeding, or exudation, suggesting a remarkable anti-tumour effect of the increase in dose of IFN-β.
ICIs potently reinvigorate the usual anti-tumour response that the immune system is capable of, but tumour cells have managed to evade. However, the overall response rate of ICIs to ITMs for itself was reported to be 54% in Australia (9), and presumably even lower in Japanese melanoma which is less commonly accompanied by chromic sun-induced damage. IFN has multiple immunomodulatory effect, such as increasing tumour-infiltrating cells, downregulating T-regulatory cells, modulating the STAT1/3 balance in tumour cells and host lymphocytes, direct inhibition of cell proliferation, induction of apoptosis of tumour cells, and inhibition of tumour angiogenesis (8,10). The synergistic effect of PD-1 inhibitor and IFN-β has been reported in a small size study of advance melanoma (11). The effect of IFN-β and PD-1 inhibitor on ITMs in our case appears to be additive rather than synergistic, because it was observed only after the increase in dose of IFN-β.
Nevertheless, local IFN-β therapy could be an option for improving quality of life of patients with ITMs refractory to ICIs. Moreover, when the ITMs are also refractory to IFN-β, increasing the dose of IFN-β may lead to better control of the skin lesions.
Acknowledgements
The authors would like to thank Dr Faiz Kermani (World Medical Fund, Alsace, France) for his language editing, writing assistance and support.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YTa, ST, TK and MH made contributions to the conception and design of the work, and YTa, TK and MH were major contributors in writing the manuscript. YTe, DM and AT evaluated the patient data and the therapeutic effects of IFN-β. ST, AT and MH revised the manuscript. AT and MH supervised treatment of the patient and confirmed the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This is a case report retrospectively reviewed and analyzed without any new interventions. The report does not include information which may identify the patients, and did not require ethical committee approval.
Patient consent for publication
Written informed consent was obtained from the patient's next of kin for publication of this case report.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pawlik TM, Ross MI, Thompson JF, Eggermont AM, Gershenwald JE. The risk of in-transit melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol. 2005;23:4588–4590. doi: 10.1200/JCO.2005.12.245. [DOI] [PubMed] [Google Scholar]
- 2.Hayes AJ, Clark MA, Harries M, Thomas JM. Management of in-transit metastases from cutaneous malignant melanoma. Br J Surg. 2004;91:673–682. doi: 10.1002/bjs.4610. [DOI] [PubMed] [Google Scholar]
- 3.Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, Larkin J, Nyakas M, Dutriaux C, Haydon A, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 4.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J, Punt CJ, Salès F, Dummer R, Robert C, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 7.Arima M, Iwata Y, Morita Y, Kobayashi T, Sasaki R, Suzuki K, Matsunaga K. A case of malignant melanoma with in-transit metastasis that responded to intravenous infusion of interferon-β. Case Rep Dermatol. 2014;6:74–79. doi: 10.1159/000360729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubo H, Ashida A, Matsumoto K, Kageshita T, Yamamoto A, Saida T. Interferon-beta therapy for malignant melanoma: The dose is crucial for inhibition of proliferation and induction of apoptosis of melanoma cells. Arch Dermatol Res. 2008;300:297–301. doi: 10.1007/s00403-008-0841-6. [DOI] [PubMed] [Google Scholar]
- 9.Nan Tie E, Lai-Kwon J, Rtshiladze MA, Na L, Bozzi J, Read T, Atkinson V, Au-Yeung G, Long GV, McArthur GA, et al. Efficacy of immune checkpoint inhibitors for in-transit melanoma. J Immunother Cancer. 2020;8(e000440) doi: 10.1136/jitc-2019-000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Trolio R, Simeone E, Di Lorenzo G, Buonerba C, Ascierto PA. The use of interferon in melanoma patients: A systemic review. Cytokine Growth Factor Rev. 2015;26:203–212. doi: 10.1016/j.cytogfr.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura T, Hidaka T, Kambayashi Y, Furudate S, Kakizaki A, Tono H, Tsukada A, Haga T, Hashimoto A, Morimoto R, et al. Phase I study of nivolumab combined with IFN-β for patients with advanced melanoma. Oncotarget. 2017;8:71181–71187. doi: 10.18632/oncotarget.17090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.