Abstract
Agonists of peroxisome proliferator-activated receptor (PPAR)-γ have been suggested as potential adjuvant therapy in bacterial pneumonia because of their capacity to inhibit inflammation and enhance bacterial clearance. Previous studies only assessed the effects of pretreatment with these compounds, thereby bearing less relevance for the clinical scenario. Moreover, PPAR-γ agonists have not been studied in pneumonia caused by Klebsiella pneumoniae, a common human respiratory pathogen of which antibiotic treatment is hampered by increasing antimicrobial resistance. Here we show that administration of the PPAR-γ agonist pioglitazone 6 or 8 h after infection of mice with a highly virulent strain of Klebsiella pneumoniae via the airways results in reduced cytokine and myeloperoxidase levels in the lungs at 24 h after infection, as well as reduced bacterial growth in the lungs and decreased dissemination to distant organs at 42 h post-infection. These results suggest that pioglitazone may be an interesting agent in the treatment of Klebsiella pneumonia.
Keywords: PPAR-γ, Pioglitazone, Pneumonia, Klebsiella
To the editor,
Peroxisome proliferator-activated receptor (PPAR)-γ is a transcription factor belonging to the PPAR subfamily of nuclear hormone receptors [1]. While originally studied for its role in lipid and glucose metabolism, by now the role of PPAR-γ in the regulation of immune responses has been widely recognized [1, 2]. Endogenous ligands that can stimulate PPAR-γ activity include fatty acids and eicosanoids. Considering the broad effects resulting from PPAR-γ activation several synthetic agonists have been developed, in particular thiazolidinediones such as pioglitazone, rosiglitazone, troglitazone, and ciglitazone [1]. Amongst others, these PPAR-γ agonists have been studied as a potential adjunctive therapeutic strategy in bacterial infections [2].
Pneumonia is a major cause of morbidity and mortality worldwide [3]. Treatment of pneumonia by antimicrobial agents has become more difficult due to the emergence of multidrug resistant pathogens. Antimicrobial resistance is especially problematic in infections caused by gram-negative bacteria such as Pseudomonas (P.) aeruginosa and microorganisms belonging to the Enterobacteriaceae family, such as Klebsiella (K.) pneumoniae [4, 5]. Previous research on the potential of PPAR-γ agonists in gram-negative pneumonia has focused on P. aeruginosa [6]. PPARγ activation enhanced phagocytosis and killing of Pseudomonas by macrophages, and pretreatment with pioglitazone resulted in lower bacterial counts in lung homogenates of mice infected with this bacterium via the airways [7]. With regard to gram-positive pneumonia, our group showed that pretreatment with ciglitazone lessens Streptococcus (S.) pneumoniae-induced lung inflammation in mice by reducing bacterial outgrowth and proinflammatory cytokine production [8]. Of note, however, rosiglitazone treatment compromised bacterial clearance during post-influenza super-infection by Staphylococcus aureus [9]. To the best of our knowledge the effect of PPAR-γ stimulation during Klebsiella pneumonia in vivo has not been studied. In vitro, troglitazone and rosiglitazone were shown to increase the ability of rat alveolar macrophages to phagocytose opsonized K. pneumoniae [10]. In the present study we aimed to study the effect of pioglitazone posttreatment, (i.e., administered during an ongoing infection) in pneumonia caused by a highly virulent K. pneumoniae strain.
Pneumonia was induced in female C57BL/6 mice (Charles River, Maastricht, the Netherlands; 8–10 weeks of age) by intranasal inoculation of K. pneumoniae serotype 2 (43816; ATCC, Rockville, MD; 104 colony‐forming units, CFU) as previously described [11–13]. Bacterial counts were determined in organ homogenates and blood as described [11–13]. Tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, IL-10, C-X-C motif ligand (CXCL)2 and myeloperoxidase (MPO) were measured in lung homogenates by ELISA according to manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Statistical analysis was done using GraphPad Prism 7.03. The number of mice and the statistical tests used for each data set are described in the figure legends. A P value < 0.05 was considered statistically significant.
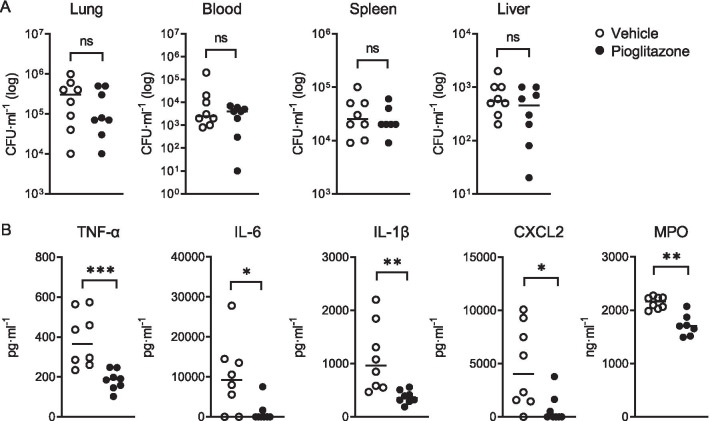
Two treatment strategies with pioglitazone (administered by intraperitoneal injection) were evaluated. In a first experiment mice were infected with K. pneumoniae via the airways and treated with a single dose of pioglitazone (20 mg/g body weight; Cayman Chemical, Ann Arbor, MI, USA) or vehicle (10% dimethylsulfoxide in PBS) intraperitoneally 8 h later. At 24 h after infection all mice showed high bacterial loads in the lungs, which were not different between treatment groups; likewise, bacterial burdens in distant body sites (blood, spleen and liver) were similar in pioglitazone and vehicle treated mice (Fig. 1A). Pioglitazone strongly reduced TNF, IL-6, IL-1β, CXCL2 and MPO concentrations in whole lung homogenates (Fig. 1B). We next studied the longer-term consequences of pioglitazone treatment in an experiment in which pioglitazone (10 mg/g) or vehicle was given at 6 and 30 h after infection and effects were assessed 42 h post-infection. In this setting, pioglitazone reduced bacterial loads in lungs and distant organs (Fig. 2A) and inflammatory mediators and MPO levels in lungs, although the difference between groups did not reach statistical significance for IL-6 and IL-1β (Fig. 2B).
Fig. 1.
Short-term effect of pioglitazone in Klebsiella-induced pneumonia. Mice were infected with K. pneumoniae via the airways and treated with a single dose of pioglitazone (20 mg/g body weight) or vehicle intraperitoneally (n = 8 per group) 8 h later; measurements were done 24 h after infection. A Bacterial loads in lungs, blood, spleen and liver. B TNF, IL-6, IL-1β, CXCL2 and MPO levels in lung homogenates. Graphs show median and every dot represents one individual mouse. P values were calculated using Mann–Whitney U tests. *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant
Fig. 2.
Influence of pioglitazone administration 42 h after induction of pneumonia. Mice were infected with K. pneumoniae via the airways and treated with pioglitazone (10 mg/g) or vehicle at 6 and 30 h after infection (n = 8 per group); effects were assessed 42 h post-infection. A Bacterial loads in lungs, blood, spleen and liver. B TNF, IL-6, IL-1β, CXCL2 and MPO levels in lung homogenates. Graphs show median and every dot represents one individual mouse. P values were calculated using Mann–Whitney U tests. *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant
The present results of pioglitazone mediated inhibition of inflammatory responses in the lungs during Klebsiella pneumonia is in agreement with the previously described effects of ciglitazone during pneumonia caused by S. pneumoniae-induced pneumonia [8]. PPAR-γ agonists can exert anti-inflammatory effects on macrophages, including inhibition of proinflammatory cytokine production, via multiple mechanisms, encompassing inhibition of nuclear factor-ĸB and mitogen-activated protein kinases [2, 6]. The role of PPAR-γ in the anti-inflammatory potential of macrophages is further supported by the finding that disruption of endogenous PPAR-γ in myeloid cells impairs alternative macrophage activation [14], and is associated with a spontaneous low-grade inflammatory response in lungs of mice [15]. In pneumonia caused by S. pneumoniae mice with macrophage-specific PPAR-γ deficiency displayed impaired antibacterial defense [15]. Together these data suggest that the inhibitory effect of both endogenous PPAR-γ and synthetic PPAR-γ agonists like thiazolidinediones on inflammation in the lungs drives its bacterial growth limiting effect during pneumonia.
PPAR-γ agonists have been extensively studied in the context of lung inflammation [6, 16]. Previous studies have reported on the effects of pre-treatment with a thiazolidinedione in mouse models of Pseudomonas and pneumococcal pneumonia [6–8], and bacterial superinfection following influenza [9]. The present study is the first to describe the effect of a PPAR-γ agonist during pneumonia caused by K. pneumoniae, a common human respiratory pathogen for which adjunctive therapies are urgently needed [5]. Moreover, this investigation is the first to investigate the effect of administration of a PPAR-γ agonist in mice with an ongoing lung infection, thereby more closely mimicking a clinical scenario. Our results argue for clinical evaluation of PPAR-γ agonists as immune modulating agents in the treatment of bacterial pneumonia.
Acknowledgements
The authors thank Marieke ten Brink and Joost Daalhuisen (Center for Experimental and Molecular Medicine, Amsterdam-UMC) for their technical support carrying the animal experiments.
Authors’ contributions
Conceptualization: IR-M and TvdP. Methodology: IR-M and BLF, Validation: IR-M. Formal analysis: IR-M. Investigation: IR-M, BLF and AFdV. Resources: TvdP. Writing—original draft: TvdP. Writing—review and editing: IR-M, BLF, AFdV and TvdP. Visualization: IR-M. Funding acquisition: TvdP. All authors read and approved the final manuscript.
Funding
Ivan Ramirez-Moral was funded by the Era-Net JPIAMR/ZonMW (Grant 50-52900-98-201); and Bianca Lima Ferreira was supported by FAPESP (Grant 2019/02224-0).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee of the Academic Medical Center approved all experiments.
Consent of publication
Not applicable.
Competing interests
The authors declare no commercial of financial conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. Pparγ signaling and metabolism: the good, the bad and the future. Nat Med Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy AT, Lakshmi SP, Reddy RC. PPAR γ in bacterial infections: a friend or foe? PPAR Res. 2016;2016:7963540. doi: 10.1155/2016/7963540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres A, Cilloniz C, Niederman MS, Menéndez R, Chalmers JD, Wunderink RG, et al. Pneumonia. Nat Rev Dis Prim. 2021;7:25. doi: 10.1038/s41572-021-00259-0. [DOI] [PubMed] [Google Scholar]
- 4.Enne VI, Personne Y, Grgic L, Gant V, Zumla A. Aetiology of hospital-acquired pneumonia and trends in antimicrobial resistance. Curr Opin Pulm Med. 2014;20:252–258. doi: 10.1097/MCP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 5.Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Carvalho MV, Gonçalves-De-albuquerque CF, Silva AR. PPAR gamma: from definition to molecular targets and therapy of lung diseases. Int J Mol Sci. 2021;22:805. doi: 10.3390/ijms22020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedi B, Yuan Z, Joo M, Zughaier SM, Goldberg JB, Arbiser JL, et al. Enhanced clearance of Pseudomonasaeruginosa by peroxisome proliferator-activated receptor gamma. Infect Immun. 2016;84:1975–1985. doi: 10.1128/IAI.00164-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stegenga ME, Florquin S, De Vos AF, Van Der Poll T. The thiazolidinedione ciglitazone reduces bacterial outgrowth and early inflammation during Streptococcuspneumoniae pneumonia in mice. Crit Care Med. 2009;37:614–618. doi: 10.1097/CCM.0b013e31819599b6. [DOI] [PubMed] [Google Scholar]
- 9.Gopal R, Mendy A, Marinelli MA, Richwalls LJ, Seger PJ, Patel S, et al. Peroxisome proliferator-activated receptor gamma (PPARγ) suppresses inflammation and bacterial clearance during influenza-bacterial super-infection. Viruses. 2019;11:505. doi: 10.3390/v11060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronoff DM, Serezani CH, Carstens JK, Marshall T, Gangireddy SR, Peters-Golden M, et al. Stimulatory effects of peroxisome proliferator-activated receptor-γ on Fcγ receptor-mediated phagocytosis by alveolar macrophages. PPAR Res. 2007;2017:52546. doi: 10.1155/2007/52546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claushuis TAM, de Vos AF, Nieswandt B, Boon L, Roelofs JJTH, de Boer OJ, et al. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood. 2018;131:864–876. doi: 10.1182/blood-2017-06-788067. [DOI] [PubMed] [Google Scholar]
- 12.Ding C, Scicluna BP, Stroo I, Yang J, Roelofs JJ, de Boer OJ, et al. Prekallikrein inhibits innate immune signaling in the lung and impairs host defense during pneumosepsis in mice. J Pathol. 2020;250:95–106. doi: 10.1002/path.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez-Moral I, Blok DC, Bernink JH, Garcia-Laorden MI, Florquin S, Boon L, et al. Interleukin-33 improves local immunity during gram-negative pneumonia by a combined effect on neutrophils and inflammatory monocytes. J Pathol. 2020;253:374–383. doi: 10.1002/path.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautier EL, Chow A, Spanbroek R, Marcelin G, Greter M, Jakubzick C, et al. Systemic analysis of PPARγ in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J Immunol. 2012;189:2614–2624. doi: 10.4049/jimmunol.1200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobs SP, Kopf M. PPAR-γ in innate and adaptive lung immunity. J Leukoc Biol. 2018;104:737–741. doi: 10.1002/JLB.3MR0118-034R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.