Abstract
There is limited data on the in-hospital outcomes of cardiogenic shock (CS) secondary to takotsubo syndrome (TS). We aimed to assess the incidence, predictors, and outcomes of CS in hospitalized patients with TS. All patients with TS were identified from the National Inpatient Sample database from September 2006 to December 2017. The cohort was divided into those with versus without CS and logistic regression analysis was used to identify predictors of CS and mortality in patients admitted with TS. A total of 260,144 patients with TS were included in our study, of whom 14,703 (6%) were diagnosed with CS. In-hospital mortality in patients with CS was approximately six-fold higher compared with those without CS (23% vs 4%, p <0.01). TS patients with CS had a higher incidence of malignant arrhythmias like ventricular tachycardia or ventricular fibrillation (15.0% vs 4%, p <0.01) and non-shockable cardiac arrests (12% vs 2%, p <0.01). Independent predictors of CS were male gender, Asian and Hispanic ethnicity, increased burden of co-morbidities including congestive heart failure, chronic pulmonary disease, and chronic diabetes. Independent predictors of mortality were male gender, advanced age, history of congestive heart failure, chronic renal failure, and chronic liver disease. In conclusion, CS occurs in approximately 6% of patients admitted with TS, in-hospital mortality in TS patients with CS was approximately six-fold higher compared with those without CS (23% vs 4%, p <0.01), male gender and increased burden of co-morbidities at baseline were independent predictors of CS and mortality.
Takotsubo syndrome (TS) is a neurologically mediated acute heart failure syndrome characterized by transient reversible left ventricular dysfunction affecting more than 1 coronary artery territory in a circumferential distribution.1,2 The syndrome is classically associated with a lack of culprit lesion on coronary angiography and is often triggered by physical or emotional stress.2 TS is variably referred to as stress cardiomyopathy, apical ballooning syndrome, or broken heart syndrome and is increasingly recognized in approximately 2% of patients initially presenting with acute coronary syndromes.2–4 It was initially thought to be a benign disease characterized by complete recovery and favorable overall prognosis, however, contemporary studies have shown that TS can be associated with several acute complications including heart failure, cardiogenic shock (CS), malignant arrhythmias, left ventricular thrombus with thromboembolism, and stroke.5 Moreover, acute and long-term mortality associated with TS is comparable to acute coronary syndromes.6–9 There is paucity of real-world data on the trends, predictors, and outcomes of CS in patients with TS. Our objective is to assess these parameters from a large nationally representative sample of hospitalized patients in the United States.
Methods
The National inpatient sample (NIS) is the largest publicly available all-payer administrative claims-based database and is a part of Healthcare Cost and Utilization Project databases. These data are stratified to represent 20% of US inpatient hospitalizations across different hospitals and geographic regions (random sample). National estimates of the entire US hospitalized population were calculated using discharge weights provided. NIS has data on demographics (age, gender, race, and ethnicity), hospital characteristics, income, and insurance status in addition to diagnostic and procedural fields.10 Standard Elixhauser co-morbidities were used in basleine tables and for regression anaylsis.11 Institutional review board approval and informed consent were not required for this study given the de-identified nature of the NIS database and public availability.
We analyzed NIS data from September 2006 to December 2017 using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes and International Classification of Diseases, 10th Revision, Clinical Modification ICD-10-CM codes (ICD-10-CM). All patients with TS who are 18 years and older were identified using ICD-9-CM code of 429.83 & ICD-10-CM code of I51.81. The study cohort was then divided into 2 groups (TS with CS vs without CS). CS was identified using ICD-9-CM of 785.51 and ICD-10-CM of R57.0 (Figure 1). Patients without CS but with concomitant codes suggesting possibility of shock were removed from analysis (concomitant codes for vasopressors [0017, 3E033XZ, 3E043XZ], Intra-aortic balloon pump [P3761, 5A02110, 5A02210], and percutaneous ventricular assist devices [3768, 02HA3RZ, 5A0221D]).The primary end point of the study was the incidence of CS and mortality in patients admitted with TS and to study the temporal trends in these complications over time. Secondary end points were surrogates of severe disability, cost of hospitalization, and length of stay in TS with CS compared with those without CS. Descriptive statistics were presented as frequencies with percentages for categorical variables and as medians with interquartile range (IQR) for continuous variables. Baseline characteristics were compared using a Pearson chi-squared test and independent samples t test for continuous variables. Mann Whitney U test was used for comparing medians.
Figure 1.
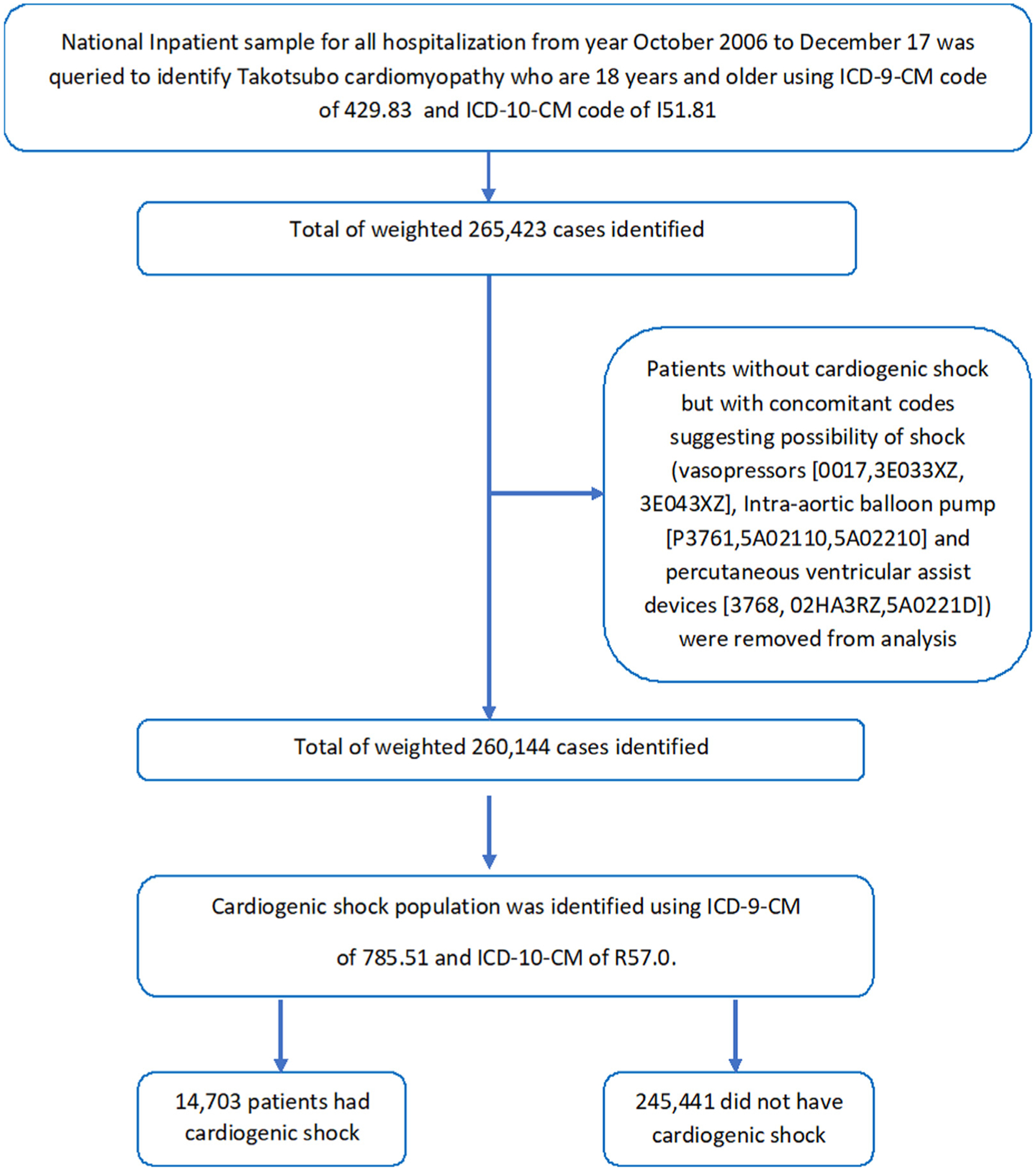
Flow chart of our paper.
Initially, binomial logistic regression model was used to identify variables from demographic data (Table 1) that were significantly associated with patient mortality (p < 0.05). These variables were then subsequently utilized in a multiple forward entry stepwise logistic regression model (with removal for p > 0.1) to identify predictors of CS in TS cohort. Forward entry stepwise logistic regression model (with removal for p > 0.1) was also used to identify predictors of mortality in study cohort. For this study, a p < 0.05 was considered statistically significant. All statistical analyses were performed using statistical package for social science (SPSS) version 26 (IBM Corp) and R studio 3.5.
Table 1.
Baseline characteristics of study cohort
| Variable | Cardiogenic Shock | ||
|---|---|---|---|
| No (n = 245,441) | Yes (n = 14,703) | p Value | |
| Median age, (years) | 68 (58–78) | 67 (57–75) | p<0.01 |
| Women | 212,191 (87%) | 11,621 (79%) | p<0.01 |
| White | 184,284 (82%) | 10,469 (78%) | p<0.01 |
| Black | 16,627 (7%) | 1,025 (7%) | |
| Hispanic | 12,935 (5%) | 857 (6%) | |
| Asians | 4,266 (2%) | 431 (3%) | |
| Others | 13,16 (0.6%) | 531 (4.0%) | |
| Atrial fibrillation | 45,075 (18%) | 3,695 (25%) | p<0.01 |
| Non-shockable Arrests | 5,140 (2%) | 1,790 (12%) | p<0.01 |
| Ventricular flutter, ventricular fibrillation, and Ventricular Tachycardia | 10,786 (4%) | 2,209 (15%) | p<0.01 |
| Acquired immune deficiency syndrome | 233 (0.1%) | 20 (0.1%) | 0.16 |
| Alcohol abuse | 10,656 (5%) | 827 (6%) | p<0.01 |
| Anemias | 43,981 (19%) | 3,410 (24%) | p<0.01 |
| collagen vascular diseases | 12,441 (5%) | 544 (4%) | p<0.01 |
| Congestive heart failure | 99,765 (41%) | 9,658 (66%) | p<0.01 |
| Chronic pulmonary disease | 69,237 (29%) | 4,403 (31%) | p<0.01 |
| Coagulopathy | 13,611 (6%) | 2,896 (20%) | p<0.01 |
| Depression | 41,528 (18%) | 1,837 (13.0%) | p<0.01 |
| Diabetes, uncomplicated | 39,843 (17%) | 2,144 (15%) | p<0.01 |
| Diabetes with chronic complications | 13,092 (5%) | 1,055 (7%) | p<0.01 |
| Drug abuse | 9,839 (4%) | 843 (6%) | p<0.01 |
| Hypertension | 149,642 (63%) | 7,023 (50%) | p<0.01 |
| Hypothyroidism | 43,197 (18%) | 2,032 (14%) | p<0.01 |
| Liver disease | 7,107 (3.0%) | 749 (5%) | p<0.01 |
| Lymphoma | 2,205 (1%) | 168 (1.5%) | p<0.01 |
| Metastatic cancer | 5,456 (2%) | 456 (3%) | p<0.01 |
| Cerebrovascular disease | 17,680 (7%) | 1,634 (11%) | p<0.01 |
| Coronary artery disease | 98,297 (40.0%) | 5,082 (35%) | p<0.01 |
| Smokers | 40,231 (16%) | 2,471 (17%) | 0.19 |
| Obesity | 23,917 (10%) | 1,394 (10%) | 0.32 |
| Paralysis | 6,984 (3%) | 591 (4%) | p<0.01 |
| Peripheral vascular disorders | 19,379 (8%) | 1,402 (10%) | p<0.01 |
| Psychoses | 11,515 (5%) | 589 (4%) | p<0.01 |
| Pulmonary circulation disorders | 6,817 (3%) | 655 (5%) | p<0.01 |
| Renal failure | 26,328 (11%) | 1,874 (13%) | p<0.01 |
| Solid tumor without metastasis | 5,325 (2%) | 439 (3%) | p<0.01 |
| Weight loss | 20,535 (9%) | 2,689 (19.0%) | p<0.01 |
| Insurance/payer | |||
| Medicare | 154,569 (63%) | 8,549 (58%) | p<0.01 |
| Medicaid | 20,731 (9%) | 1,600 (11%) | |
| Private insurance | 56,476 (23%) | 3,716 (25%) | |
| Self-pay | 7,703 (3%) | 449 (3%) | |
| No charge/Others | 5,696 (2%) | 366 (3%) | |
| Household income (percentile) | |||
| 0–25th | 59,981 (25%) | 3,499 (24%) | 0.04 |
| 26–50th | 62,511 (26%) | 3,762 (26%) | |
| 51–75th | 61,519 (26%) | 3,619 (25%) | |
| 76–100th | 57,246 (24%) | 3,571 (2%) | |
| Urban versus rural hospital | |||
| Rural | 16,861 (7%) | 515 (4%) | p<0.01 |
| Urban non-teaching | 71,229 (29%) | 3,411 (23%) | |
| Urban teaching | 157,352 (64%) | 10,776 (73%) | |
| Hospital size | |||
| Small | 30,832 (13%) | 1,260 (9%) | p<0.01 |
| Home | 64,156 (26%) | 3,261 (22%) | |
| Large | 150,453 (61%) | 10,182 (69%) | |
Results
Our total cohort included 260,144 patients with TS of whom 14,703 (6%) were diagnosed with CS. The baseline characteristics are shown in (Table 1). In our total study cohort of 260,144 patients with TS, mortality was approximately 5% (12,367). In-hospital mortality in patients with TS-CS was almost six-fold higher compared with those without CS (23% vs 4%, p <0.01). CS patients also had a much higher burden of resource utilization, including use of mechanical ventilation (65.1% vs 13.5%, p <0.01), tracheostomy (4.1% vs 0.8%, p <0.01). Patients with CS also had a longer median length of stay (9 [IQR, 5 to 15] days vs 4 [IQR, 2 to 7] days, p <0.01) and a higher median cost of stay ($116,860 [IQR, 63,732 to 219,886], vs ($39,560 [IQR, 23,371 to 72,442], p <0.01). Patients with CS were less likely to be discharged home (28.5% vs 58.9%, p <0.01) and more likely to be discharged to short term rehabilitation facility (5.1% vs 2.6%, p <0.01) or skilled nursing facility (28.6% vs 19.4%, p <0.01) (Table 2).
Table 2.
Outcomes of study cohort
| Variable | Cardiogenic Shock | p Value | |
|---|---|---|---|
| No (n = 245,441) | Yes (n = 14,703) | ||
| Died | 9,004 (4%) | 3,363 (23%) | p<0.01 |
| Home | 144,456 (59%) | 4,186 (29%) | |
| Short term Rehab | 6,300 (3%) | 743 (5%) | |
| Skilled nursing facility | 47,543(19%) | 4,195 (29%) | |
| Home with home health | 36,538 (15%) | 2,137 (14%) | |
| Against medical advice | 1,448 (0.6%) | 69 (0.5%) | |
| Resource utilization and procedures | |||
| Median (IQR) cost $ | 39,560 (23,371–72,442) | 116,860 (63,732–219,886) | p<0.01 |
| Median (IQR) length of stay, days | 4 (2–7) | 9(5–15) | p<0.01 |
| Ventilator use | 33,048 (14%) | 9,577 (65%) | p<0.01 |
| PEG | 3,891 (2%) | 707 (5%) | p<0.01 |
| Tracheostomy | 1,966 (1%) | 604 (4%) | p<0.01 |
| IABP | - | 3,167 (22%) | p<0.01 |
| pVADS | - | 411 (3%) | p<0.01 |
| Vasopressors | - | 1,842 (13%) | p<0.01 |
Abbreviations: IABP = Intra-aortic balloon pump; PEG = Percutaneous gastrostomy tube; pVADS = Percutaneous ventricular assist devices.
Our analysis showed a significant uptrend in CS in patients with TS (Figure 2). There has been uptrend in mortality in patients admitted with TS and CS during our study period from 16% in 2007 to 25 % in 2017 (p <0.01) (Figure 3). Mean length of stay and mean cost of stay have also continued to increase in TS patients with CS during study period (2007 to 2017; from 8 days to 11 days and 103,034$ to 207,270$ respectively) (Figure 4). Also, the use of Intra-Aortic Balloon Pump (IABP) in CS patients has shown a significant decrease from 2007 to 2017 (38.3% to 13.3%, p <0.01) with a simultaneous increase in the use of percutaneous ventricular assist devices (pVAD) (0.7% to 4.9%, p <0.01) (Figure 4).
Figure 2.
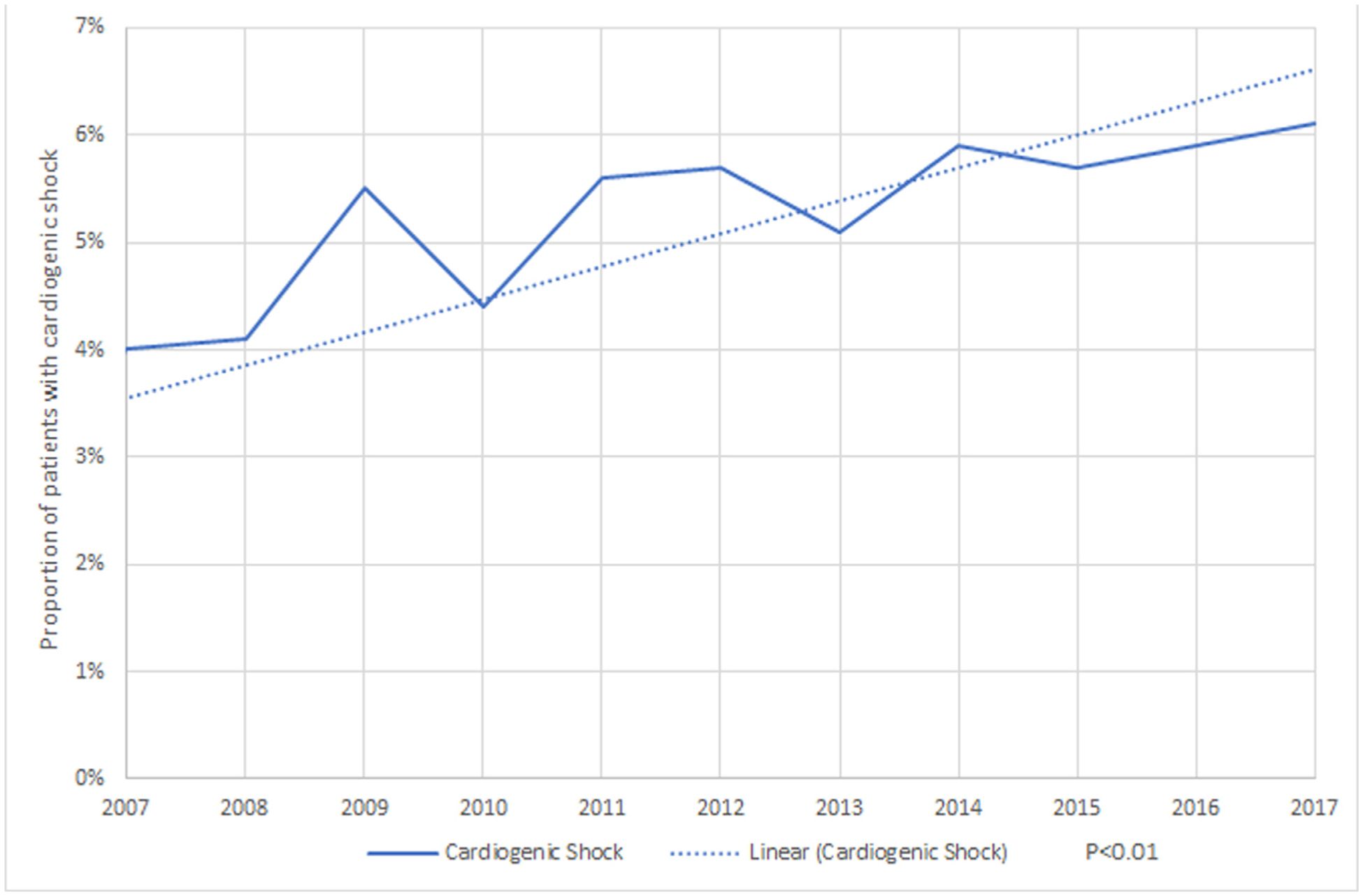
Trends in Proportion of cardiogenic shock in Takotsubo Syndrome.
Figure 3.
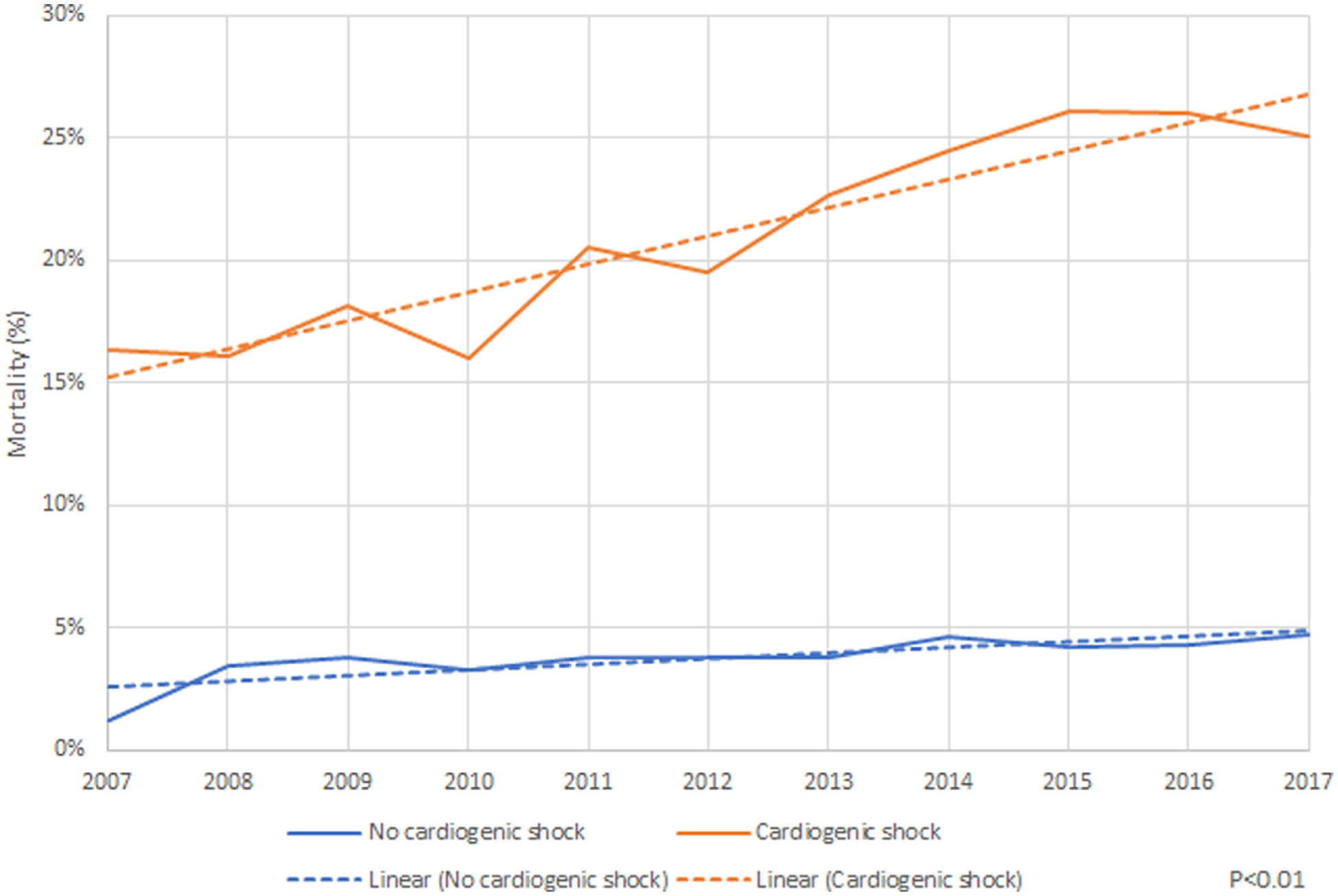
Trends in mortality in Takotsubo Syndrome.
Figure 4.
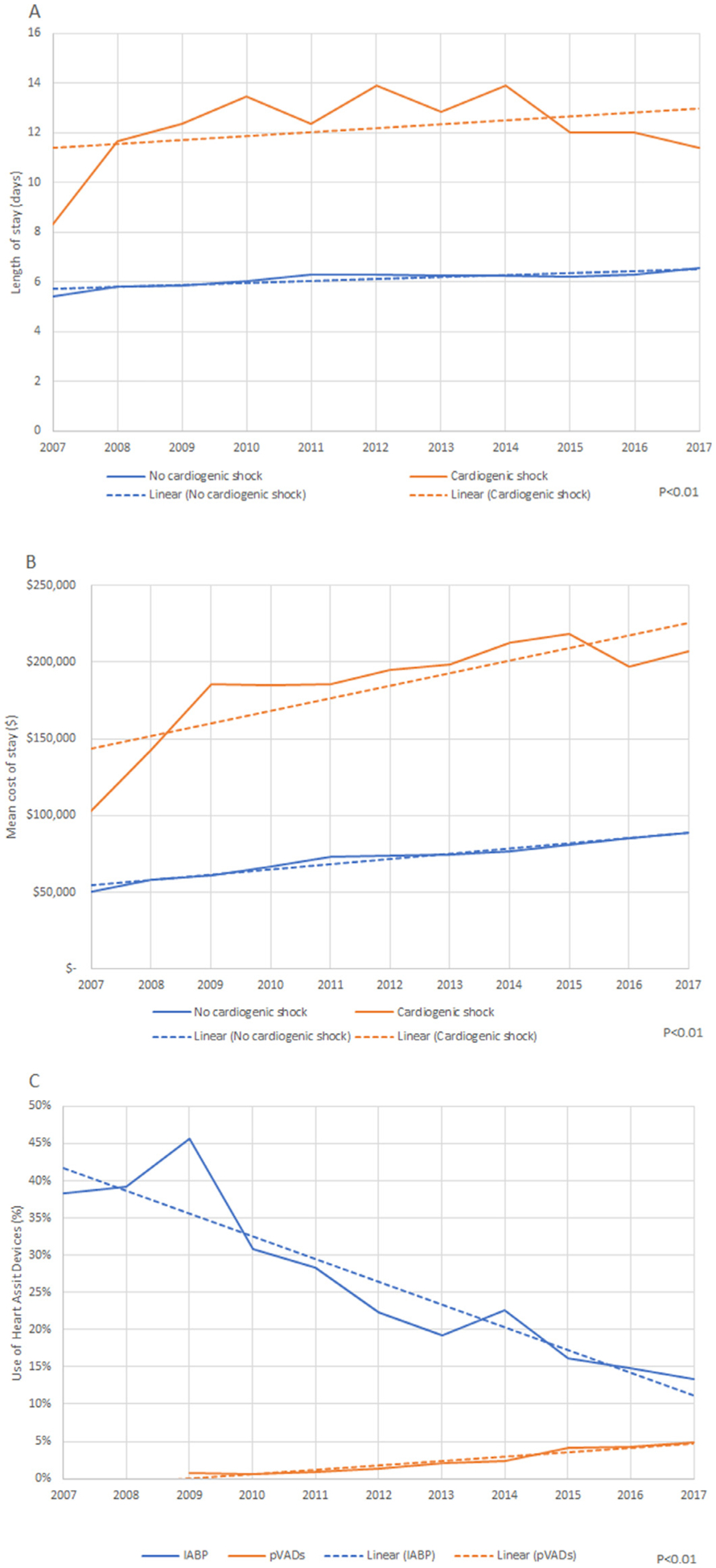
(A) Trends in mean length of stay in Takotsubo syndrome. (B) Trends in mean cost of stay in Takotsubo syndrome. (C) Trends in use of heart assist devices in Takotsubo syndrome.
In regression analysis, independent predictors of CS were male gender (odds ratio [OR], 1.2, 95% confidence interval [CI] [1.15 to 1.23], p <0.01), Asian (OR, 1.37, 95% CI [1.23 to 1.53], p <0.01), Hispanic ethnicity (OR 1.08, 95% CI [1.00 to 1.169], p <0.01), history of congestive heart failure (OR, 1.52, 95% CI [1.45 to 1.58], p <0.01), chronic pulmonary disease (OR 1.10, 95% CI [1.07 to 1.16], p <0.01), cerebrovascular disease (OR, 1.25, 95% CI [1.17 to 1.33], p <0.01), chronic diabetes (OR, 1.13, 95% CI [1.06 to 1.22], p <0.01),obesity (OR, 1.07, 95% CI [1.01 to 1.15], p <0.01) and peripheral vascular disease (OR, 1.30, 95%CI [1.31 to 1.28], p <0.01) (Figure 5). Independent predictors of mortality in our analysis were male gender (OR, 1.78, 95% CI [1.70 to 1.85], p <0.01), advanced age (OR, 1.02, 95% CI [1.01 to 1.02], p <0.01), congestive heart failure (OR, 1.36, 95% CI [1.30 to 1.42], p <0.01), coagulopathy (OR, 2.35, 95%CI [2.23 to 2.48], p <0.01), chronic liver disease (OR, 1.53, 95% CI [1.41 to 1.67], p <0.01), chronic renal failure (OR, 1.2, 95% CI [1.16 to 1.30], p <0.01), cerebrovascular disease (OR, 2.6, 95% CI [2.48 to 2.75], p <0.01), and peripheral vascular disease (OR, 1.34, 95% CI [1.26 to 1.43], p <0.01). On the contrary, factors associated with lower risk of in-hospital mortality in TS include female gender (OR, 0.56, 95% CI [0.54 to 0.59], p <0.01),and psychiatric co-morbidities like depression (OR, 0.63, 95% CI [0.60 to 0.69], p <0.01) and psychoses (OR, 0.60, 95% CI [0.53 to 0.67], p <0.01) (Figure 5).
Figure 5.
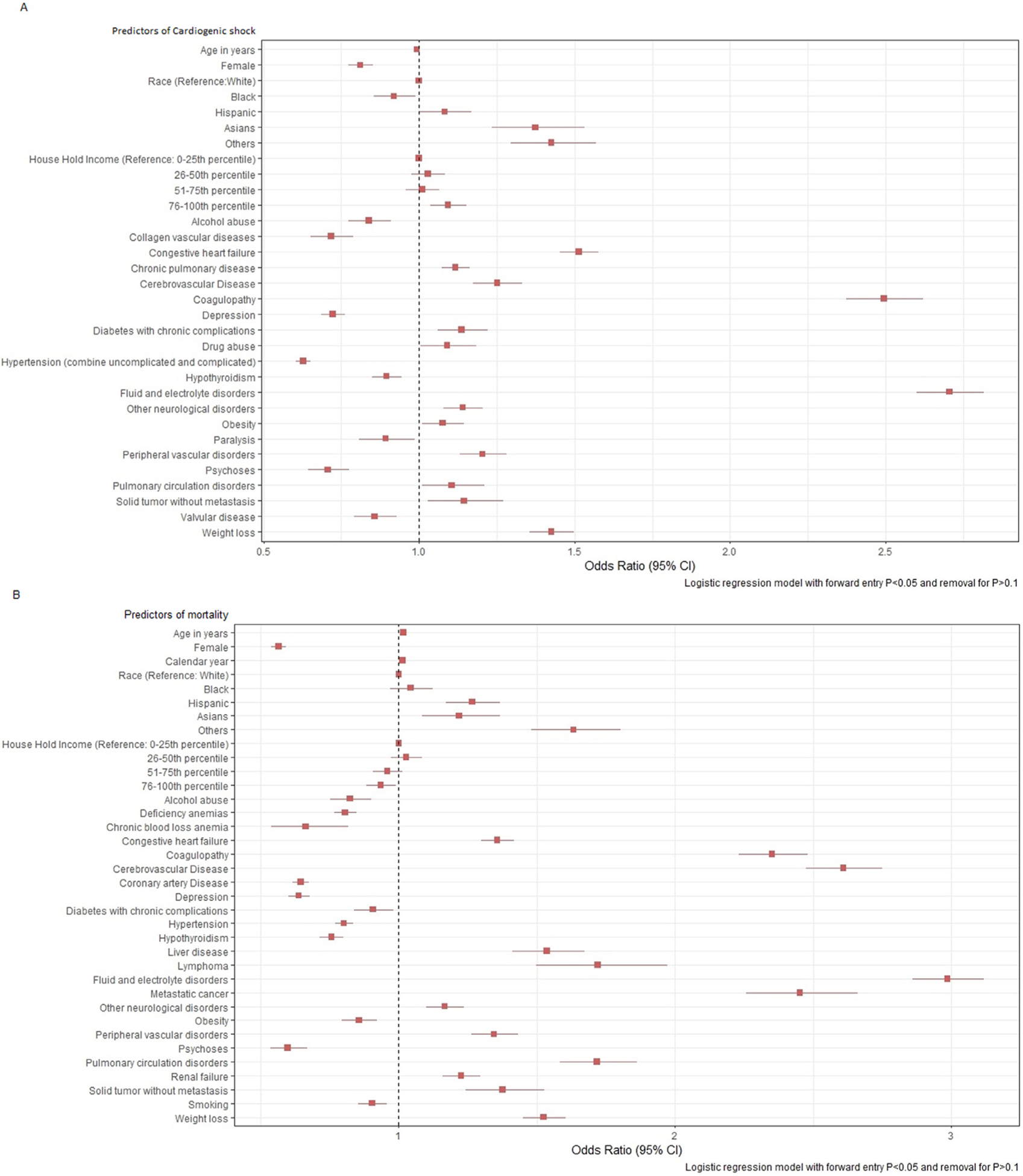
(A) Predictors of cardiogenic shock in Takotsubo syndrome. (B) Predictors of mortality in Takotsubo syndrome.
Discussion
The major findings of our study are: (1) TS is not a benign syndrome and is associated with significant risk of in-hospital mortality even in patients without CS. (2) CS occurs in approximately 6% of patients admitted with TS and is associated with a six-fold increase in mortality (23% vs 4.0%) compared with TS patients without CS. (3) TS patients with CS are more likely to be males and have underlying medical co-morbidities such as congestive heart failure and diabetes. (4) There has been a significant uptrend in the incidence of CS and in hospital mortality in patients admitted with TS during our study period.
Since the first description of TS in 1990, the incidence of TS has been increasing, likely secondary to the increase in awareness and recognition of the disease.12 The classical patient with TS is a post-menopausal woman who presents with sudden onset of chest pain or dyspnea in setting of an emotional stress. The disease is characterized by transient reversible LV systolic dysfunction typically in the form of apical ballooning and return of normal LV contractile function within a few days to weeks.4,9,13 Although initially thought to be a benign and reversible cardiac dysfunction associated with favorable long term prognosis, contemporary data from several studies have revealed the vicious nature of the disease with significant in-hospital complications including malignant arrhythmias, CS and mortality.5,13–17
In our study, TS was associated with a risk of in-hospital mortality of approximately 4.0% in patients without CS and much higher mortality rate of 23% in patients with CS. The mortality rate we report is similar to mortality rates reported in previous studies and a meta-analysis of TS studies with mortality ranging from 2.3% to 5%.18 Ventricular arrhythmias and nonshockable cardiac arrests from underlying physical illness could be the events leading to a malignant outcome even in the absence of hemodynamic deterioration secondary to CS. The arrhythmias are likely attributable to high levels of circulating catecholamines.19
In our study, CS occurred in approximately 6% of patients with TS and was associated with a six-fold increase in-hospital mortality. The incidence of CS in our study is lower than reported in smaller registries where the reported incidence of CS was around 10%.18 The mortality in the CS subgroup is similar to in-hospital mortality rate in the international takotsubo registry of 23.5%. The increased mortality in CS could be from multiple factors––multi organ dysfunction that complicates CS and also fatal arrhythmias that are more common in CS patients. Moreover, acute respiratory failure secondary to pulmonary edema, frequently complicates CS in TS.20
Our analysis is consistent with previous literature that shows that CS is more likely to occur in males and younger patients18,21–24 with medical co-morbidities including congestive heart failure, cerebrovascular disease, malignancy, chronic lung disease, and chronic liver disease as opposed to the prototypical TS patient who is an older post-menopausal woman with psychiatric co-morbidities like depression, higher mortality, and worse outcomes in men has also been shown in the data from national Registry on Takotsubo Syndrome registry and other single center studies.25,26 A “secondary” phenotype of TS has been described that occurs in the setting of physical or surgical illness and predominantly in hospitalized patients. Secondary TS has much worse prognosis and higher mortality compared with “primary” TS.27 Our analysis showing worse outcomes of TS in men is likely because they are more likely to develop a secondary “In hospital TS” which is known from to be associated with poorer outcomes and a higher mortality27,28 compared with “primary” TS which is more likely to occur in setting of emotional stress in women.
Increasing trend in CS and mortality in TS patients is concerning. Possible explanations for the increasing trends in CS and mortality in TS is due to the aging population with multiple baseline co-morbidities predisposing to a secondary form of TS which is associated with higher incidence of CS and mortality, moreover the uptrend in the use of pVAD at the cost of IABP is also concerning especially in light of recent evidence from large retrospective studies showing higher mortality with use of pVADs compared with IABP.29 Understanding the physiology of CS––pump failure versus left ventricular outflow obstruction is key to guide the management of these patients to improve outcomes.16 Decision pathways to treat CS due to above etiologies need further study to improve outcomes. The utility of mechanical circulatory support, the type of mechanical support (IABP vs pVAD) and extracorporeal life support in this patient population also needs evaluation.
Our study has a few limitations. First, the current study is retrospective in nature and hence it is vulnerable to potential selection bias and residual confounding, therefore, findings from the current analysis should be considered hypothesis generating, rather than hypothesis testing. Second, the NIS is an administrative claim-based database that uses ICD-9-CM and ICD-10-CM codes, which are prone to coding errors, however, the hard clinical end points used in this study such as CS, hospital mortality, and discharge disposition are less prone to diagnostic and coding errors. Third, patients are not followed longitudinally in NIS so long-term outcomes could not be assessed from present dataset. Finally, echocardiographic, laboratory, and medication data were not available due to inherent limitation of the dataset. Nonetheless our study is the largest to date to analyze the frequency of adverse outcomes including CS and in hospital morality in patients with TS and gives important insights into the predictors of CS and in hospital mortality in patients admitted with TS.
In conclusion, CS complicates approximately 6% of patients admitted with TS and is associated with almost six-fold increase in mortality (23% vs 4%, p <0.01). Independent predictors of CS and mortality are male gender and increased burden of co-morbidities at baseline like congestive heart failure, chronic lung disease, complicated diabetes mellitus, chronic kidney disease, and malignancy.
Supplementary Material
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relations that could have appeared to influence the work reported in this study.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2020.09.014.
References
- 1.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008;118:2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi YJ, Nef HM, Lyon AR. Epidemiology and pathophysiology of takotsubo syndrome. Nat Rev Cardiol 2015;12:387–397. [DOI] [PubMed] [Google Scholar]
- 3.Kurowski V, Kaiser A, von Hof K, Killermann DP, Mayer B, Hart-mann F, Schunkert H, Radke PW. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest 2007;132:809–816. [DOI] [PubMed] [Google Scholar]
- 4.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 5.Schneider B, Athanasiadis A, Schwab J, Pistner W, Gottwald U, Schoeller R, Toepel W, Winter KD, Stellbrink C, Muller-Honold T, Wegner C, Sechtem U. Complications in the clinical course of takotsubo cardiomyopathy. Int J Cardiol 2014;176:199–205. [DOI] [PubMed] [Google Scholar]
- 6.Citro R, Rigo F, Previtali M, Ciampi Q, Canterin FA, Provenza G, Giudice R, Patella MM, Vriz O, Mehta R, Baldi C, Mehta RH, Bossone E. Differences in clinical features and in-hospital outcomes of older adults with tako-tsubo cardiomyopathy. J Am Geriatrics Soc 2012;60:93–98. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey SW. Takotsubo cardiomyopathy: natural history. Heart Fail Clin 2013;9:123–136. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey SW. Cardiogenic shock complicating takotsubo events. JACC: Heart Failure 2018;6:937–939. [DOI] [PubMed] [Google Scholar]
- 9.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. New Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and, Quality R MD. HCUP Databases. Healthcare Cost and Utilization Project (HCUP) 2019. [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12.Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J 2016;172:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Pina IL. Stress-induced cardiomyopathy. Heart failure clinics 2019;15:41–53. [DOI] [PubMed] [Google Scholar]
- 14.Hurst RT, Prasad A, Askew JW 3rd, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging 2010;3:641–649. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Senecal C, Lewis B, Prasad A, Rajiv G, Lerman LO, Lerman A. Natural history and predictors of mortality of patients with Takotsubo syndrome. Int J Cardiol 2018;267:22–27. [DOI] [PubMed] [Google Scholar]
- 16.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1955–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, Vonthein R, Schuler G, Thiele H, Eitel I. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail 2016;18:650–656. [DOI] [PubMed] [Google Scholar]
- 18.Singh K, Carson K, Shah R, Sawhney G, Singh B, Parsaik A, Gilutz H, Usmani Z, Horowitz J. Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am J Cardiol 2014;113:1420–1428. [DOI] [PubMed] [Google Scholar]
- 19.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on takotsubo syndrome: a position statement from the taskforce on takotsubo syndrome of the heart failure association of the European society of cardiology. Eur J Heart Fail 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
- 20.Vallabhajosyula S, Dunlay SM, Murphree DH Jr., Barsness GW, Sandhu GS, Lerman A, Prasad A. Cardiogenic shock in Takotsubo cardiomyopathy versus acute myocardial infarction: an 8-year national perspective on clinical characteristics, management, and outcomes. JACC Heart failure 2019;7:469–476. [DOI] [PubMed] [Google Scholar]
- 21.Almendro-Delia M, Núñez-Gil IJ, Lobo M, Andrés M, Vedia O, Sionis A, Martin-García A, Cruz Aguilera M, Pereyra E, Martín de Miguel I, Linares Vicente JA, Corbí-Pascual M, Bosch X, Fabregat Andrés O, Sánchez Grande Flecha A, Pérez-Castellanos A, Pais JL, De Mora Martín M, Escudier Villa JM, Martín Asenjo R, Guillen Marzo M, Rueda Sobella F, Aceña Á, García Acuña JM, García-Rubira JC, Figueras J, Barrabes JA, Andrés M, Núñez Gil IJ, Mejía HD, Vedia O, Feltes G, Worner F, Bascompte Claret R, Pereyra E, Jiménez Candil J, García Sánchez MJ, Martín García AC, Martín García A, Bodi V, Bonanad C, Bastante T, Cruz Aguilera M, Palazuelos J, Sancho Car-mona D, López Pais J, Alonso JJ, Almendro Delia M, Lobo M, Rodríguez de Leiras S, García Rubira JC, Corbí-Pascual M, Córdoba Soriano JG, De Mora Martín M, Pérez B, Martín Asensio R, Rueda Sobella F, Santos Pardo I, Manzano Nieto MC, Escudier Villa JM, Fabregat Andrés O, Ridocci-Soriano F, Parias Ángel MN, Gaebelt HP, Aceña A, Martin Reyes R, Bergua C, Sanz Puértolas P, Echeverria Lucotti I, Vidal Pérez R, Sionis A, Duran Cambra A, Tómas Ortiz J, Bosch Genover X, Guillen Marzo M, Bardají RA, García Acuña JM, Sánchez Grande Flecha A, García González MJ, García de la Villa Redondo G, Pérez Castellanos A, Piqueras-Flores J, Ruíz Valdepeas Herrero L, Linares Vicente JA, Ruiz Arroyo JR, García J, Giner Caro JA, Martínez Selles M, Martín de Miguel I. Short- and long-term prognostic relevance of cardiogenic shock in takotsubo syndrome: results from the RETAKO registry. JACC: Heart Failure 2018;6:928–936. [DOI] [PubMed] [Google Scholar]
- 22.Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the national inpatient sample 2008 to 2009. Am Heart J 2012;164:215–221. [DOI] [PubMed] [Google Scholar]
- 23.Di Vece D, Citro R, Cammann VL, Kato K, Gili S, Szawan KA, Micek J, Jurisic S, Ding KJ, Bacchi B, Schwyzer M, Candreva A, Bossone E, D’Ascenzo F, Sarcon A, Franke J, Napp LC, Jaguszewski M, Noutsias M, Münzel T, Knorr M, Heiner S, Katus HA, Burgdorf C, Schunkert H, Thiele H, Bauersachs J, Tschöpe C, Pieske BM, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Hasenfuβ G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Empen K, Felix SB, Delmas C, Lairez O, El-Battrawy I, Akin I, Borggrefe M, Gilyarova E, Shilova A, Gilyarov M, Horowitz J, Kozel M, Tousek P, Widimský P, Winchester DE, Ukena C, Di Mario C, Prasad A, Böhm M, Bax JJ, Lüscher TF, Ruschitzka F, Ghadri JR, Templin C. Outcomes associated with cardiogenic shock in takotsubo syndrome. Circulation 2019;139:413–415. [DOI] [PubMed] [Google Scholar]
- 24.Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Sakata K, Nagao K, Yamamoto T, Takayama M. Gender differences in patients with takotsubo cardiomyopathy: multi-center registry from Tokyo CCU network. PloS one 2015;10:e0136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Castellanos A, Martínez-Sellés M, Mejía-Rentería H, Andrés M, Sionis A, Almendro-Delia M, Martín-García A, Aguilera MC, Pereyra E, Linares Vicente JA, García de la Villa B, Núñez-Gil IJ. Tako-tsubo syndrome in men: rare, but with poor prognosis. Rev Esp Cardiol (Engl Ed). 2018;71:703–708. [DOI] [PubMed] [Google Scholar]
- 26.Weidner KJ, El-Battrawy I, Behnes M, Schramm K, Fastner C, Kuschyk J, Hoffmann U, Ansari U, Borggrefe M, Akin I. Sex differences of in-hospital outcome and long-term mortality in patients with Takotsubo cardiomyopathy. Ther Clin Risk Manag 2017;13:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isogai T, Yasunaga H, Matsui H, Tanaka H, Ueda T, Horiguchi H, Fushimi K. Out-of-hospital versus in-hospital Takotsubo cardiomyopathy: analysis of 3719 patients in the diagnosis procedure combination database in Japan. Int J Cardiol 2014;176:413–417. [DOI] [PubMed] [Google Scholar]
- 28.Guo S, Xie B, Tse G, Roever L, Xia Y, Li G, Wang Y, Liu T. Malignancy predicts outcome of Takotsubo syndrome: a systematic review and meta-analysis. Heart Fail Rev 2020;25:513–522. [DOI] [PubMed] [Google Scholar]
- 29.Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, Berkowitz A, Masoudi FA, Messenger JC, Parzynski CS, Ngufor C, Girotra S, Amin AP, Shah ND, Desai NR. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. Jama 2020;323:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


