Abstract
Background
Randomized controlled trials showed that sodium/glucose cotransporter-2 inhibitors (SGLT2i) protect the heart and kidney in an array of populations with type 2 diabetes (T2D) and increased cardiorenal risk. However, the extent of these benefits also in lower kidney-risk T2D populations needs further investigation.
Methods
Members of Maccabi Healthcare Systems listed in their T2D registry who initiated new glucose lowering agents (GLA), were divided into SGLT2i initiators and other GLAs (oGLAs). Groups were propensity score-matched by baseline demographic and medical characteristics. Two composite cardiovascular outcomes were defined: all-cause mortality (ACM) or hospitalization for heart failure (hHF); and ACM, myocardial infraction (MI) or stroke. The cardiorenal outcome was: ACM, new end-stage kidney disease (ESKD) or ≥ 40% reduction from baseline estimated glomerular filtration rate (eGFR). Renal-specific outcome was new ESKD or ≥ 40% eGFR reduction. Single components of cardiovascular and kidney outcomes were also assessed. Three subgroup definitions of low baseline kidney-risk were used: eGFR > 90 ml/min/1.73 m2; urinary albumin below detectable levels; and low risk according to Kidney Disease: Improving Global Outcomes (KDIGO) classification. Analyses were performed utilizing an unadjusted model, and a model adjusted to baseline eGFR and urinary albumin-to-creatinine ratio.
Results
Between April 1, 2015 and June 30, 2018; 68,187 patients initiated new GLAs — 11,321 SGLT2i initiators and 42,077 oGLAs initiators were eligible. Propensity score-matching yielded two comparable cohorts; each included 9219 participants. Median follow-up was 1.7 years. Compared to oGLAs, SGLT2i initiators had lower incidence of ACM or hHF [HR95%CI = 0.62(0.51–0.75)]; ACM, MI or stroke [0.67(0.57–0.80)]; the cardiorenal outcome [0.65(0.56–0.76)]; and the renal-specific outcome [0.70(0.57–0.85)]. SGLT2i initiators also had lower risk for ACM, hHF and ≥ 30%, ≥ 40%, ≥ 50%, ≥ 57% eGFR reduction. No difference between groups was observed for MI or stroke. In the low baseline kidney-risk subgroups, SGLT2i initiation was generally associated with lower risk of the cardiovascular and cardiorenal outcomes, driven mainly by lower ACM incidence.
Conclusions
Our findings in the general population of patients with T2D demonstrates lower risk of cardiorenal outcomes associated with initiation of SGLT2i compared with oGLAs, including specifically in patients with low baseline kidney-risk.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-021-01362-y.
Keywords: SGLT2i, Cardiorenal outcomes, Real world evidence, Type 2 diabetes
Introduction
Cardiovascular (CV) and kidney outcomes trials (CVOTs and KOTs) have shown that sodium/glucose cotransporter-2 inhibitors (SGLT2i) protect the heart and kidney in a variety of high-risk populations. In the context of type 2 diabetes (T2D) these benefits were shown in populations with established cardiovascular disease (CVD; EMPA-REG OUTCOME and to a lesser degree at VERTIS CV) [1–3] and/or with multiple CVD risk factors (CANVAS program, DECLARE-TIMI 58) [4–7]; chronic kidney disease (CKD; CREDENCE, SCORED, DAPA-CKD) [8–11], or with heart failure and reduced ejection fraction (HFrEF; DAPA-HF, EMPEROR-Reduced, SOLOIST-WHF) [12–15].
Based on these findings, recent position statements such as the 2020 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [16] and the 2021 American Diabetes Association (ADA) Standards of Care [17] recommended SGLT2i use in patients with T2D and increased risk for CKD, HFrEF and/or atherosclerotic CVD (AsCVD). An open question persists however, whether SGLT2i also exerts these protective effects in lower risk populations of patients with T2D, such as those with normal kidney markers, comprising most of the patients with T2D in the primary-care setting. This debate has practical clinical consequences—should SGLT2i be recommended to the general population of patients with T2D for the purpose of cardiorenal protection, independently of glycemic control?
In this observational study, we used the registry of Maccabi Healthcare Services (MHS), Israel’s second largest Health Maintenance Organization (HMO) insuring approximately 2.2 million subjects. Patients with T2D, who initiated a new glucose lowering agent (GLA) therapy between April 2015 and June 2018 were identified. SGLT2i initiators were propensity-scored matched with patients starting other GLAs (oGLAs), according to patients’ demographics, medical history, background medications and socioeconomic status. CV and kidney outcomes were analyzed in the entire population and in specific populations with low baseline kidney risk.
Methods
Study population
The study population was composed of patients registered in MHS Diabetes Registry [18] who initiated a new GLA treatment between April 1, 2015 and June 30, 2018. Index date was defined as the date of first filled prescription. Individuals with a previous prescription of that GLA class during the 365 days prior index date were not regarded as new users. Patients had to be ≥ 18-year-old with at least 1 year of data history in the MHS prior to index date. Only those with at least one eGFR measurement during the 180 days prior to the index date were included. Excluded were patients defined as type 1 diabetes in the MHS Diabetes Registry [18] or treated with insulin alone with no other GLA in the year prior to the index date. Also omitted were those with end-stage kidney disease (ESKD), on dialysis, or after kidney transplantation.
The sample population was also part of the main CVD-REAL 3 main report that compared kidney outcomes amongst SGLT2i initiators and oGLAs in five countries [19]. However, the protocol of this analysis has been adjusted for the data in the MHS database that has baseline urinary albumin-to-creatinine ratio (UACR) values for a remarkable portion of the participants, enabling specific focus on lower kidney-risk populations. Specifically, baseline UACR (and eGFR) values were used to develop the propensity-score model, and were adjusted to in the cox model. Specific subgroups of low-kidney risk patients were defined based on baseline UACR (and eGFR) values. To avoid immortal time bias [20, 21], the CVD-REAL 3 study included all episodes of GLA initiation during follow up. In the current analysis, to avoid this bias, each patient was included once, and only the first GLA initiation during the study period was defined as index date. The index medication was defined accordingly. In the main analysis patients who initiated a second GLA were kept in their original cohort. In a sensitivity analysis [‘strict on treatment’ (sOT) follow up definition], initiation of a second GLA resulted in follow-up termination (see below ‘follow up definition’).
The study received approval from MHS Institutional Review Board (IRB) committee at Bait Balev Hospital. Due to de-identified data extracting, informed consent was not requested by the IRB.
Definitions of main variables
Laboratory measurements were performed in certified laboratories run by the MHS. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation [22], and the lower limit of detection of urinary albumin was 1.1 mg/dL. Baseline eGFR slope (per year) was calculated during the 4 years prior to the index date. Slope was calculated only for the participants with at-least 180 days interval between their first and their last (i.e., before index date) eGFR measurement during this period. All measurements were taken in a community setting, rather than during hospitalization, to reduce variations due to acute states, and defined as last evaluation within a year prior to the index date [except for baseline eGFR (180 days) and eGFR-slope calculation (4 years), as above]. Additional file 1: Table S1 presents the relevant ICD-9 (diagnosis) and ATC (medications) codes used in this study. History of established CVD, myocardial infarction (MI), heart failure (HF), stroke, transient ischemic attack (TIA), atrial fibrillation, hypertension and cancer were defined by inclusion in specific validated MHS registries [23–25] until the index date. Other co-morbidities were defined as having that diagnosis within a year prior to the index date. Baseline medications were defined as having at least one medication purchased during the year prior to index-date (not including index-date). Residential socioeconomic status (SES) was coded on a 1–10 scale developed by the Israeli Central Bureau of Statistics. This parameter was categorized into 4 groups (low [1–3], low-medium [4, 5], medium [6, 7] and high [8–10]).
Follow up definitions
Patients were followed until migration, leaving the MHS, last date of data collection (set on June 30, 2018) or death date. The end of the intention to treat (ITT) period was defined by the last date of data collection or date of leaving the MHS (due to death or other reason), whichever came first. In a first sensitivity analysis, on treatment (OT) follow-up period was defined as the exposure time until last date of data collection, date of leaving the MHS or until treatment discontinuation—whichever came first. For this purpose, treatment discontinuation was defined as having a gap of more than 90 days, plus the treatment-period specified in the last prescription before treatment-cessation. In those that discontinued treatment, the follow-up ended once the number of days specified in the last prescription had passed, with the addition of 30-day grace period. In a second sensitivity analysis, a strict on treatment (sOT) follow-up period was defined. Criteria for sOT follow-up termination were those used for the OT definition, added by occasions of new GLA initiation. Analysis by the sOT definition was added to correct for possible biases associated with additional GLA(s) initiations (including SGLT2i’ in the oGLAs arm) during follow-up.
Outcomes and subgroup definitions
Two composite CV outcomes were defined: (1) all-cause mortality (ACM) or hHF; and (2) ACM, stroke or MI. The cardiorenal outcome was defined as ACM, new ESKD or ≥ 40% reduction in eGFR from the last measurement prior the index date. The renal specific outcome included new ESKD or ≥ 40% reduction in eGFR. Single event outcomes were components of the composite outcome, as well as ≥ 30%, ≥ 50%, or ≥ 57% (i.e., doubling of serum creatinine) reduction in eGFR.
Incidence analyses of the study outcome in the matched cohort were conducted by treatment groups of the entire cohort and by baseline low kidney risk subgroups, defined in three different ways: (1) low KDIGO risk (UACR < 30 mg/g and eGFR > 60 ml/min/1.73 m2) [26]; (2) eGFR > 90 ml/min/1.73 m2; (3) or urinary albumin below detectable levels (BDL). All patients had baseline eGFR values (i.e., 180 days prior the index date). Patients lacking baseline UACR values were included in the low KDIGO risk cohort, as long as they had eGFR > 60 ml/min/1.73 m2.
Statistical analysis
Propensity score was developed using a multivariate logistic regression model where the dependent binary variable indicated if the indexed medication was SGLT2i (= 1) or indexed medication was oGLA (= 0). Matching was generated on each eGFR layer separately (eGFR > 90, 60–90 and < 60 ml/min/1.73 m2). More than 40 demographics and medical covariates prior to treatment initiation were used for the propensity score. Continuous laboratory measurements were categorized, and a missing value was defined as “missing” category to allow all patients to be matched. All variable definitions are described in the supplementary methods. Briefly, amongst the included covariates were age, sex, index year, HbA1c, BMI, eGFR, UACR (below detectable levels (BDL), < 30, 30–300 and > 300 mg/g), diabetes and cardiovascular complications (based on diagnosis or MHS’s CVD registries [23–25]; Additional file 1: Table S1), glucose lowering agents, and cardiovascular or other medications.
Propensity score estimates the probability of initiating SGLT2i or other GLA given the covariates in the model. Patients were matched in 1:1 ratio by using greedy matching, which select SGLT2i treated patients and match the nearest oGLA-initiating subject. Caliper matching was defined as caliper = 0.25 multiplied by the standard deviation of the propensity score distribution [27].
Patients’ demographic and medical characteristics were described at baseline period (prior to treatment initiation). Mean and standard deviation were used to describe continuous variables, while numbers of patients and percentages were used to describe categorical variables. Differences between patients who initiated SGLT2i or oGLA were assessed using standardized difference (STD). Significant difference in STD between groups was considered to be higher than 10%.
Only the first episode of the pre-defined cardiovascular or kidney event was included in the incidence analysis. Person-time at risk for each patient was the length of the index exposure episode, defined as the number of days from the day after the index prescription start date to the last day of follow-up. For each outcome of interest, the crude incidence rate in each index exposure group was the number of incident events divided by the total number of person-years at risk and was expressed per 100 person-years. The incidence rates for the SGLT2i group and the control group were then compared using a hazard ratio and corresponding 95% confidence interval. This analysis was performed using Cox proportional hazards regression and repeated in an unadjusted model and a model adjusted for baseline eGFR as a continuous variable and to baseline UACR as categorical variable (urine albumin BDL; UACR > 0– < 30; 30– < 300; ≥ 300 mg/g).
In addition, these models were repeated within each of the low baseline kidney risk categories. Specifically for the analysis of the urinary albumin BDL category, the model was adjusted only by baseline eGFR.
Role of the funding sources
No funding was received for this analysis.
Results
Study structure, baseline characteristics, and initiated medications
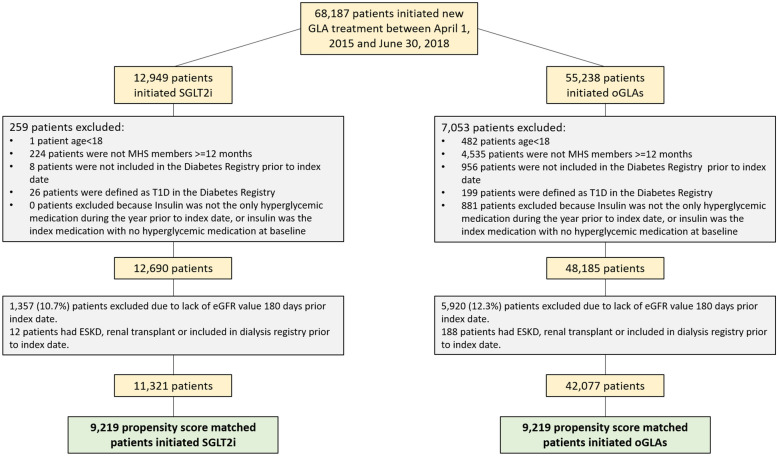
Between April 1st 2015 and June 30th 2018; 12,949 patients initiated treatment with SGLT2i and 55,238 patients initiated other GLAs (oGLAs). 11,321 SGLT2i initiators and 42,077 oGLAs initiators met the criteria to be included in this analysis (Fig. 1). Both groups were propensity score-matched according to their baseline demographic and clinical characteristics resulting in two comparable cohorts each included 9219 patients (Table 1, Additional file 1: Table S2). The population in this analysis included 39.7% women, mean (SD) age of 62.4 (10.3) years, most of them overweight (BMI ≥ 25 kg/m2) and 57.0% with diabetes duration longer than 10 years. Approximately 29.3% had established CVD history [24] and 19.5% had history of MI, coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) with stent. Mean (SD) baseline eGFR was 88.3 (18.5) ml/min/1.73 m2, mean annual eGFR slope was − 1.1 (2.7) mL/min/1.73 m2/year and median [IQR] baseline UACR was 13 (urine albumin BDL-49) mg/g. 11,768 (63.8%) had low KDIGO risk at baseline.
Fig. 1.
Participants flow chart. GLA glucose lowering agent; SGLT2i Sodium/glucose cotransporter-2 inhibitors; ESKD end stage kidney disease; MHS Maccabi Health Systems; T1D type 1 diabetes; eGFR estimated glomerular filtration rate
Table 1.
Patients’ baseline characteristics post propensity-matching
| Characteristic | Level | Study group | ||
|---|---|---|---|---|
| SGLT2-I (N = 9219) | oGLAs (N = 9219) | STD | ||
| Demographic characteristics | ||||
| Women | n (%) | 3683 (40.0%) | 3635 (39.4%) | 0.01 |
| Age (years) | Mean (SD) | 62.3 (9.6) | 62.5 (11.0) | − 0.01 |
| Years in diabetes registry, n (%) | ≤ 2 | 369 (4.0%) | 362 (3.9%) | 0.4 |
| 2–5 | 1124 (12.2%) | 1112 (12.1%) | ||
| 5–10 | 2504 (27.2%) | 2457 (26.7%) | ||
| > 10 | 5222 (56.6%) | 5288 (57.4%) | ||
| Socioeconomic status, n (%) | 1–3 (low) | 962 (10.4%) | 881 (9.6%) | 0.00 |
| 4–5 (low-medium) | 2825 (30.6%) | 2930 (31.8%) | ||
| 6–7 (medium) | 3530 (38.3%) | 3539 (38.4%) | ||
| 8–10 (high) | 1891 (20.5%) | 1858 (20.2%) | ||
| Missing | 11 (0.1%) | 11 (0.1%) | ||
| Baseline measures | ||||
| BMI | Mean (SD) in kg/m2 | 31.7 (5.4) | 31.6 (5.4) | 0.02 |
| HbA1c (%)/mmol/mol | Mean (SD) | 8.3 (1.5)/67.2 (2.3) | 8.3 (1.6)/67.2(2.3) | − 0.04 |
| eGFR (mL/min/1.73 m2), n (%)a | > 90 ml/min/1.73 m2 | 5026 (54.5%) | 5026 (54.5%) | |
| 60–90 ml/min/1.73 m2 | 3407 (37.0%) | 3407 (37.0%) | ||
| < 60 ml/min/1.73 m2 | 786 (8.5%) | 786 (8.5%) | ||
| UACR, n (%) | Urinary albumin BDL | 3510 (38.1%) | 3566 (38.7%) | 0.06 |
| < 30 mg/g | 2370 (25.7%) | 2372 (25.7%) | ||
| 30– < 300 mg/g | 2312 (25.1%) | 2295 (24.9%) | ||
| > 300 mg/g | 664 (7.2%) | 655 (7.1%) | ||
| Missing | 363 (3.9%) | 331 (3.6%) | ||
| KDIGO risk [26], n (%) | Low-risk | 5832 (63.3%) | 5936 (64.4%) | 0.05 |
| Moderate-risk | 2439 (26.5%) | 2250 (24.4%) | ||
| High and very high-risk | 948 (10.3%) | 1033 (11.2%) | ||
| Change in eGFR, n (%)b | < 3 mL/min/1.73 m2/year | 8757 (95.0%) | 8645 (93.8%) | − 0.04 |
| ≥ 3 mL/min/1.73 m2/year | 358 (3.9%) | 430 (4.7%) | ||
| ≥ 5 mL/min/1.73 m2/year | 110 (1.2%) | 124 (1.3%) | − 0.01 | |
| Missing | 104 (1.1%) | 144 (1.6%) | ||
| Baseline medications | ||||
| Metformin | n (%) | 8571 (93.0%) | 8544 (92.7%) | 0.01 |
| Sulfonylureas | n (%) | 2502 (27.1%) | 2549 (27.6%) | − 0.01 |
| DPP4i | n (%) | 4402 (47.7%) | 4373 (47.4%) | 0.01 |
| GLP-1 RA | n (%) | 1806 (19.6%) | 1880 (20.4%) | − 0.02 |
| Metiglinides | n (%) | 1093 (11.9%) | 1074 (11.6%) | 0.01 |
| TZDs | n (%) | 620 (6.7%) | 667 (7.2%) | − 0.02 |
| Acarbose | n (%) | 210 (2.3%) | 214 (2.3%) | − 0.00 |
| Insulin | n (%) | 2474 (26.8%) | 2295 (24.9%) | 0.04 |
| Short-acting | n (%) | 582 (6.3%) | 557 (6.0%) | 0.01 |
| Long-acting | n (%) | 2171 (23.5%) | 2105 (22.8%) | 0.02 |
| ACEi/ARBs | n (%) | 6595 (71.5%) | 6436 (69.8%) | 0.04 |
| Beta blockers | n (%) | 3664 (39.7%) | 3634 (39.4%) | 0.01 |
| Aldosterone antagonists | n (%) | 413 (4.5%) | 415 (4.5%) | − 0.00 |
| Medical history | ||||
| Established CVD history [24] | n (%) | 2705 (29.3%) | 2697 (29.3%) | 0.00 |
| Myocardial infarction/CABG/PCI with stent | n (%) | 1795 (19.5%) | 1796 (19.5%) | − 0.00 |
| Microvascular complicationsc | n (%) | 5624 (61.0%) | 5495 (59.6%) | 0.03 |
| Heart failure (24) | n (%) | 344 (3.7%) | 307 (3.3%) | 0.02 |
ACEi angiotensin-converting enzyme inhibitors; ARBs Angiotensin II receptor blocker; BDL below detectable levels; CABG coronary artery bypass grafting; DPP4i Dipeptidyl peptidase-4 inhibitor; eGFR estimated glomerular filtration rate; GLP-1 RAs glucagon-like peptide-1 receptor agonists; KDIGO Kidney Disease: Improving Global Outcome; oGLA other glucose lowering agent; PCI percutaneous coronary intervention; STD standardized difference; TZD Thiazolidinediones; UACR urinary albumin to creatinine ratio
aThe propensity score matching was generated on each eGFR layer separately, therefore the number of participants in each arm's eGFR layer is equal by definition
bBaseline eGFR slope (per year) was calculated during the 4 years prior to the index date. Slope was calculated only for the participants with at-least 180 days interval between their first and their last (i.e., before index date) eGFR measurement during this period
cMicrovascular complications was defined as: diabetic eye complication, neuropathy, diabetic foot/ peripheral angiopathy or diabetic kidney disease (i.e., nephropathy, eGFR < 60 or UACR > 100)
In the SGLT2i group, 34.0% of treatment initiations were with dapagliflozin and 66.0% were with empagliflozin. In the oGLAs group most patients initiated DPP4i (31.1%), metformin (18.2%), glucagon-like peptide-1 receptor agonists (GLP-1 RAs; 16.8%), insulin (11.1%), sulfonylurea (9.7%) or meglitinides (6.6%). Others initiated a regimen which included thiazolidinediones or acarbose. For the ITT follow up definition the mean exposure time was 1.7 (0.9) years. The distribution of each GLA within the cohorts, including the duration of follow up per each definition is presented in Additional file 1: Table S3.
CV and kidney outcomes in the total cohort
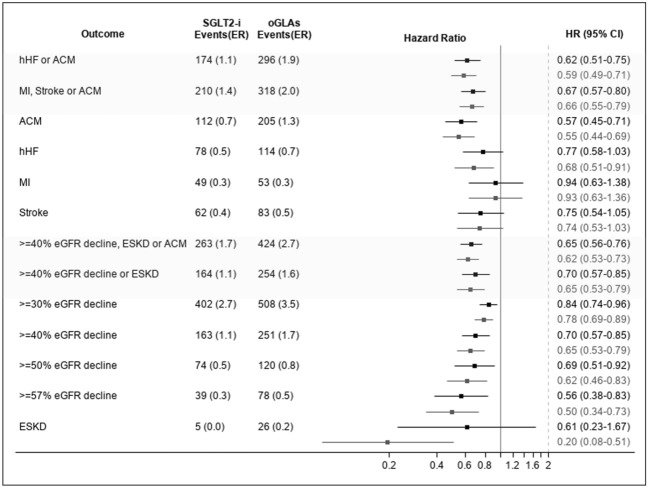
Figure 2 presents the hazard ratios for the different CV and kidney outcomes in those initiating SGLT2i compared with oGLAs in the ITT follow-up definition. Generally consistent results were obtained in an unadjusted model and in a model adjusted to baseline UACR and eGFR. Those that initiated SGLT2i experienced lower event-rates of both CV composite outcomes: hHF or ACM [HR (95% CI) = 0.62 (0.51–0.75)adjusted] as well as ACM, MI or stroke [HR (95% CI) = 0.67 (0.57–0.80)adjusted]. These differences were mostly driven from lower risk of ACM in the SGLT2i cohort [HR (95% CI) = 0.57 (0.45–0.71)adjusted], and for hHF [HR (95% CI) 0.77 (0.58–1.03)adjusted]. The event rate for stroke and MI were not significantly different between initiators of SGLT2i and oGLAs.
Fig. 2.
Risk for cardiovascular and kidney outcome in SGLT2i initiators compared to oGLAs in the entire cohort, during the ITT follow up definition. Event rates are presented as number of events per 100 person years of follow up. In black—the unadjusted model; and in grey—the model adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). SGLT2i sodium/glucose cotransporter-2 inhibitors; oGLAs other glucose lowering agents; ITT intention to treat; hHF hospitalization for heart failure; ACM all-cause mortality; MI myocardial infract; eGFR estimated glomerular filtration rate; ESKD end stage kidney disease; ER event rate
SGLT2i initiators had lower risk for adverse kidney events, including the composite cardiorenal [ACM, ESKD or ≥ 40% reduction in eGFR; HR (95% CI) = 0.65 (0.56–0.76)adjusted] and renal specific outcomes [ESKD or ≥ 40% reduction in eGFR; HR (95% CI) = and 0.70 (0.57–0.85) adjusted]. The SGLT2i cohort had lower risk for most of the tested single kidney outcomes, except for the risk of new ESKD outcome, which was limited by small number of events, and in which adjustment to baseline kidney markers attenuated the between group differences [n(SGLT2i) = 5 and n(oGLAs) = 26; HR (95% CI) = 0.20 (0.08–0.51)unadjusted and 0.61 (0.23–1.67)adjusted].
CV and kidney outcomes in populations of low kidney risk at baseline
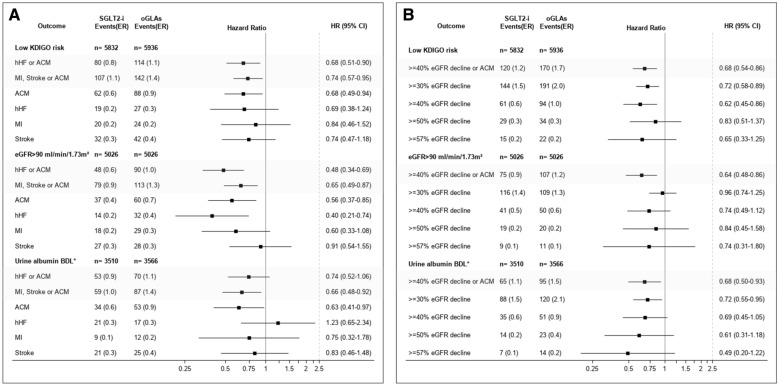
The risk for adverse CV and kidney outcomes was also tested in the three different definitions of low baseline kidney risk subgroups: low KDIGO risk [UACR < 30 mg/g and eGFR > 60 ml/min/1.73 m2; 11,768 (63.8%) patients]; baseline eGFR > 90 ml/min/1.73 m2 [10,052 (54.5%) patients]; urinary albumin BDL [7076 (38.4%) patients]. Due to lack of ESKD events in these low-risk subgroups, kidney outcomes were analyzed without new ESKD events. The presented results were obtained with the model adjusted to baseline kidney function; the unadjusted model yielded highly similar results.
Compared with oGLAs, initiation of SGLT2i was generally associated with lower event rates of the two composite cardiovascular outcomes as well as the cardiorenal outcome, across the different low kidney risk subgroups (Fig. 3). Specifically, the hazard ratio of the risk for the composite CV outcome of ACM, stroke or MI in SGLT2i initiators ranged between 0.67 and 0.76 in the low kidney risk subgroups. For the hHF or ACM composite outcome, SGLT2i initiation was associated with lower event rates in those with baseline low KDIGO risk and those with eGFR > 90 ml/min/1.73 m2, but no statistical significance was observed in the group of baseline urinary albumin below detectable levels [HR (95% CI) = 0.74 (0.52–1.06)] (Fig. 3A). The risk of composite cardiorenal outcome was consistently lower in SGLT2i initiators in all the tested low baseline kidney risk subgroups, with HR ranging between 0.69 and 0.72 (Fig. 3B).
Fig. 3.
Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the ITT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR > 60 ml/min/1.73 m2 and UACR < 30 mg/g. For the low KDIGO risk and eGFR > 90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. *BDL = Below detectable levels. SGLT2i sodium/glucose cotransporter-2 inhibitors; oGLAs other glucose lowering agents; ITT intention to treat; hHF hospitalization for heart failure; ACM all-cause mortality; MI myocardial infract; eGFR estimated glomerular filtration rate; UACR urinary albumin to creatinine ratio; KDIGO kidney disease: improving global outcomes; ER event rate
Single component CV and kidney outcomes were also analyzed. While the risk for MI or stroke was not significantly different between groups, the incidence of ACM was lower in SGLT2i initiators across all low kidney risk subgroups. The risk of hHF was lower in SGLT2i initiators within the eGFR > 90 ml/min/1.73 m2 subgroup, while no significant changes were observed in the other tested baseline kidney functions subgroups (Fig. 3A). The single kidney outcomes are presented in Fig. 3B.
Analysis of the CV and kidney outcomes by OT and sOT follow up definitions showed generally consistent results. The outcomes for the entire cohort are presented in Additional file 2: Figure S1, Additional file 5: FigureS3 and for the low baseline kidney risk subgroups in Additional files 3, 4: Figure S2, Additional files 6, 7: Figure S4, for the OT and sOT definitions, respectively.
Discussion
In this observational study, patients with T2D who initiated SGLT2i were propensity-score matched with those who initiated oGLAs. SGLT2i initiation was associated with improvement in two tested composite CV outcomes; [1] ACM, MI or stroke; [2] ACM or hHF, as well as in the cardiorenal and the renal specific composite outcomes. SGLT2i initiators also experienced lower event rates of most of the single outcomes: ACM, hHF, eGFR reduction from baseline of ≥ 30%, ≥ 40%, ≥ 50% or ≥ 57%. The incidence of stroke or MI was not significantly different between the groups across all follow-up definitions. Importantly, similar trends were observed in subgroups defined by their low kidney risk at baseline: low KDIGO risk; eGFR > 90 ml/min/1.73 m2; and urinary albumin BDL. To the best of our knowledge, this is the first time that SGLT2i associated reduction in cardiorenal risk is shown in populations with urinary albumin BDL. Together these findings consolidate the CV and kidney protective role of SGLT2i in a real-world setting in comparison to other GLAs, and in populations with healthy kidney status.
Robust observational studies from well-phenotyped patient populations are a good data source for evaluations of low-risk patients, that are often excluded from RCTs. Several CVOTs (EMPA-REG OUTCOME [1, 2], CANVAS program [4], DECLARE-TIMI 58 [6] and VERTIS CV [3]) tested the CV safety and efficacy of SGLT2i in patients with T2D and increased CV risk. Other studies specifically focused on populations with T2D and CKD or HFrEF [8, 9, 14]. They generally found that compared with placebo SGLT2i protect the kidney and reduce the incidence of hHF [2–4, 6, 8–10, 12–14, 28, 29]. However, due to the relatively high baseline risk of the participants in these trials, external validity to the general T2D population has been questioned [30]. For example, the prevalence of established CVD in these CVOTs varied between 40.6% in the DECLARE-TIMI 58 trial [31], 65.6% in the CANVAS program [4], and the entire sample populations of EMPA-REG OUTCOME and VERTIS CV [1, 3]. In comparison, our propensity-matched cohort had only 29.3% baseline prevalence of established CVD (Table 1), similar to other cross-sectional reports of the general T2D population in different countries [30, 32, 33]. Kidney-wise, baseline mean eGFR of the participants in our analysis was 88.3 ml/min/1.73 m2 compared with 74.2–77.2 ml/min/1.73 m2 in the EMPA-REG OUTCOME, CANVAS program and VERTIS CV [2–4], and 85.3 ml/min/1.73 m2 in the DECLARE-TIMI 58 [6]. Baseline eGFR slope of this trial's subjects was − 1.1 ml/min/1.73 m2/year; only 4.3% had a baseline annual eGFR slope of ≥ 3 ml/min/1.73 m2/year (fast-decliners) and 1.2% of ≥ 5 ml/min/1.73 m2/year (severe-decliners). All in all, our sample population seems to have lower baseline kidney and CV risk than the participants in these CVOTs. Thus, the observed reduction in cardiorenal risk associated with SGLT2i initiation suggests that these benefits may apply to broader populations with T2D.
While associated risks for ACM or hHF were generally lower following treatment with SGLT2i, we did not find a significant reduction in the risk for MI and stroke (especially in the OT and sOT follow-up definitions). Such trends were observed in other CVOTs and other real-world evidence (RWE) [2–4, 6, 34]. Of note, in these reports SGLT2i reduce hHF episodes more overtly relative to ACM [6, 28]. Here, however, in the low baseline kidney risk categories ACM incidence was consistently lower in SGLT2i initiators, while the risk for hHF was not stable, i.e., significant between group differences were observed only in the eGFR > 90 ml/min/1.73 m2 category. Unlike ACM, hHF is a clinical diagnosis that relies on physicians’ reporting, introducing a limitation to the interpretation of this outcome in RWE settings. Importantly, hHF event, as well as ESKD, rarely occur in patients with healthy kidney markers, precluding the capture of outcome differences in low kidney risk populations [35].
The cardiorenal improvement seen here with SGLT2i treatment has been similarly documented in other RWE studies [19, 34, 36–40]. The sample used for this study has been part of the CVD-REAL 3 study [19] that found better kidney outcomes in SGLT2i initiators compared to oGLAs across five countries. Here we used slightly different follow up definitions, further consolidating the results of the CVD-REAL 3, using a complementary approach (see “Methods” Section). While CVD-REAL 3 mainly focused on kidney outcomes, here we also tested CV outcomes. Importantly, the cardiorenal risk associated with SGLT2i initiation compared to oGLAs was specifically tested in different populations defined by their low baseline kidney risk. The current analysis was designed to emphasize the strengths of the MHS database. Additional variables were introduced into the propensity score matching, such as patients’ SES information and baseline UACR. Presence of UACR values for most of the participants (96.5%), provided a unique opportunity to analyze populations defined as low-kidney risk by both baseline eGFR and UACR. Specifically, we found SGLT2i-associated improvement in the cardiorenal outcome even in those with urinary albumin BDL, a relatively novel kidney definition based on cumulative results that any urinary albumin excretion, even within the normoalbuminuric range, is associated with worse outcomes [41–45]. Relevantly, SGLT2i were reported to improve albuminuria status—serving as a surrogate for kidney decline and a possible mediator– even in patients with T2D and normal kidney markers [5, 45, 46]. These results suggest a beneficial role for SGLT2i early in the disease process.
According to the 2021 ADA Standards of Care, SGLT2i are indicated for cardiorenal protection purposes in patients with T2D and CKD, HF or atherosclerotic cardiovascular disease (AsCVD)—independent of glycemic control or previous metformin use [17]. Similarly, the KDIGO guidelines recommend to treat most patients with T2D and CKD with SGLT2i [16]. A controversy persists whether SGLT2i have a cardiorenal preventive role in patients with T2D and normal kidney markers. In this analysis, the lower incidence of the composite CV and cardiorenal outcomes was generally conserved in the different low baseline kidney risk categories, with some variations. Importantly, lower risk for ACM was observed in the defined low baseline kidney risk categories. However, variation of the HR values for the other single component outcomes in this population precludes a specific conclusion. Thus, although our findings support a cardiorenal protective role of SGLT2i in patients with T2D and low baseline kidney risk, longer follow up on more participants may be required for more definitive answers.
Recent years have brought a renaissance in the treatment of diabetes kidney disease (DKD). The gold standard of risk factor modifications and angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEi/ARBs) [47–49] is now joined by SGLT2i therapy. Finerenone, a non-steroidal mineralocorticoid receptor antagonist (MRA), improved kidney outcomes in patients with DKD (the FIDELIO-DKD trial [50], and an ongoing study tests CV outcome (The FIGARO-DKD trial, NCT02545049). Cumulative evidence indicate that GLP-1 RAs may also have a kidney protective role [38, 51, 52], and the FLOW KOT [NCT03819153 (53)] is expected to provide a more definite answer. The SONAR trial indicated that specific patients with DKD could benefit from endothelin receptor antagonists (ERA) [54]. However, to date no medications have been approved for DKD prevention purposes in patients with T2D and healthy kidney markers. Our findings of lower cardiorenal risk following SGLT2i initiation, as well as recent post-hoc analyses from RCTs and other RWE, suggest that SGLT2i may exert such a role [7, 40, 52].
This study enjoys several strengths. In the final matched-cohort 3.5% lacked UACR measurement and 1.1% did not have a calculated eGFR slope at baseline. The completeness of the data, including medical history, background medication, and socioeconomic status, has allowed the formation of comparable cohorts. Baseline eGFR slope was not included in the propensity-score and yet was comparable in both arms, testifying for the relatively balanced nature of the cohorts. The relatively large proportion of patients with low baseline kidney risk enabled meaningful results in these specific subgroups of interest.
The study also has several limitations. First and foremost, treatment allocation was not assigned in a randomized controlled manner. It is possible that unknown confounders, that were not part of the propensity-matching, influenced the study outcome. Initiation of other GLAs or other cardiovascular or kidney medications (e.g., GLP-1 RAs or ACEi/ARBs amongst others) following the index date was also not accounted for. Baseline allocation of UACR and eGFR values were based on a single measurement, although these markers have some day-to-day variability. Another limitation is the lack of data regarding treatment adherence and specific changes in GLAs regimen during follow up. The risk for each outcome was therefore calculated for ITT, OT and sOT follow-up definitions; each has its own advantages and limitations, with generally similar findings. Lack of prospective data collection also affected the outcome definitions. Our registry does not specify the cause of death, precluding us from testing the association between SGLT2i initiations and CV death, as a component of the classical major adverse cardiovascular event (MACE) composite outcome, amongst other possible causes of death. Relevantly, several SGLT2i randomized controlled trials (RCTs) and RWEs have found lower incidence of ACM, suggesting an effect that may extend beyond MACE (EMPA-REG OUTCOME, DAPA-CKD) [1, 10, 28, 34]. Other outcomes (e.g., hHF) may be affected by variations between physicians’ reporting. Finally, we focused on populations with low baseline kidney risk, however we did not test stricter low baseline risk definitions e.g., those lacking CVD—this question should be analyzed in larger cohorts with longer follow up.
Conclusion
In conclusion, this observational, propensity-score matched analysis demonstrates that initiation of SGLT2i treatment in patients with T2D is associated with lower risk for adverse cardiorenal outcomes. These findings were generally consistent in populations of patients defined by their lower baseline kidney risk.
Supplementary Information
Additional file 1: Table S1. Variable’s definitions, including medications and diagnosis. ACE – Angiotensin-converting enzyme; ARB – Angiotensin II receptor blocker; CABG – coronary artery bypass grafting; CVD – cardiovascular disease; DPP4 – Dipeptidyl-peptidase 4; eGFR – estimated glomerular filtration rate; GLP-1 RA – glucagon-like peptide-1 receptor agonist; ICD-9 – International Classification of Diseases 9; MHS – Maccabi Healthcare Services; PCI – percutaneous coronary intervention. Table S2. Additional patients’ baseline characteristics post propensity-matching. Table S3. Distribution of index medications post-match and by follow up definitions. PP4i – Dipeptidyl peptidase-4 inhibitor; GLP1-RA – Glucagon-like peptide-1 receptor agonists; ITT – intention to treat; oGLAs – other glucose lowering agents; OT – on treatment; SGLT2i – Sodium-glucose cotransporter 2 inhibitors; sOT – strict on treatment; TZDs – Thiazolidinediones.
Additional file 2:Figure S1. Risk for cardiovascular and kidney outcome in SGLT2i initiators compared to oGLAs in the entire cohort, during the OT follow up definition. Event rates are presented as number of events per 100 person years of follow up. In black—the unadjusted model; and in grey—the model adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; OT = on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; ESKD = end stage kidney disease; ER = event rate.
Additional file 3: Figure S2. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the OT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. * BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; OT = on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Additional file 4: Figure S2. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the OT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. * BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; OT = on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Additional file 5: Figure S3. Risk for cardiovascular and kidney outcome in SGLT2i initiators compared to oGLAs in the entire cohort, during the sOT follow up definition. Event rates are presented as number of events per 100 person years of follow up. In black—the unadjusted model; and in grey—the model adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; sOT = strict on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; ESKD = end stage kidney disease; ER = event rate.
Additional file 6: Figure S4. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the sOT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. *BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; sOT = strict on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Additional file 7: Figure S4. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the sOT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. *BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; sOT = strict on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Acknowledgements
The authors would like to express their gratitude to Prof. Hiddo Heerspink from the University of Groningen, the Netherlands for reading the manuscript and providing critical comments as well as Ms. Rebecca Sprung for editing the manuscript.
Abbreviations
- ACEi
Angiotensin-converting enzyme inhibitors
- ACM
All-cause mortality
- ARBs
Angiotensin II receptor blocker
- AsCVD
Atherosclerotic cardiovascular disease
- BDL
Below detectable levels
- CABG
Coronary artery bypass grafting
- CKD
Chronic kidney disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CVOT
Cardiovascular outcomes trial
- DKD
Diabetes kidney disease
- eGFR
Estimated glomerular filtration rate
- ERA
Endothelin receptor antagonist
- ESKD
End-stage kidney disease
- GLA
Glucose lowering agent
- GLP-1 RAs
Glucagon-like peptide-1 receptor agonists
- HF
Heart failure
- hHF
Hospitalization for heart failure
- HMO
Health Maintenance Organization
- IRB
Institutional Review Board
- ITT
Intention to treat
- KDIGO
Kidney Disease Improving Global Outcome
- KOT
Kidney outcomes trial
- MACE
Major adverse cardiovascular event
- MHS
Maccabi Healthcare Services
- MI
Myocardial infraction
- MRA
Mineralocorticoid receptor antagonist
- oGLA
Other glucose lowering agent
- PCI
Percutaneous coronary intervention
- OT
On treatment
- RCT
Randomized controlled trial
- RWE
Real world evidence
- SES
Socioeconomic status
- SGLT2i
Sodium/glucose cotransporter-2 inhibitors
- sOT
Strict on treatment
- STD
Standardized difference
- T2D
Type 2 diabetes
- UACR
Urinary albumin to creatinine ratio
Authors’ contributions
MS, CMC, AK and OM conceived the idea. CMC, MK, GC and AK collected the data. MS, CMC, AR, IY and OM planned the analyses. CMC performed the analyses. MS and CMC designed the figures. MS and OM wrote the first draft. All authors read and approved the final manuscript.
Funding
No funding was received for this analysis.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study received approval from MHS Institutional Review Board (IRB) committee at Bait Balev Hospital. Due to de-identified data extracting, informed consent was not requested by the IRB.
Consent for publication
Not applicable.
Competing interests
MS, CMC and GC declare no conflict of interests. AR and IY declare consulting fees from Novo Nordisk and CuraLife. AK has received Grants and consulting fees from Astra Zeneca, Novonordisk and Boeheringer Ingelheim. MK has served on the advisory board/consultant for Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novo Nordisk, Sanofi, and Vifor Pharma; has received research grants from Astra Zeneca and Boehringer Ingelheim; has received other research support from Astra Zeneca. OM declares advisory board membership from AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, BOL Pharma; Speaker’s bureau honorarium from AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme and Boehringer Ingelheim; and research grants from Novo Nordisk and AstraZeneca.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meir Schechter and Cheli Melzer-Cohen contributed equally to this work
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 3.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 6.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 7.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22–31. doi: 10.1016/S2213-8587(20)30369-7. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano D, Longo M, Scappaticcio L, Caruso P, Esposito K. Sodium-glucose transporter-2 inhibitors for prevention and treatment of cardiorenal complications of type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):17. doi: 10.1186/s12933-021-01213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer IH, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839–848. doi: 10.1016/j.kint.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 18.Chodick G, Heymann AD, Shalev V, Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol. 2003;18(12):1143–1146. doi: 10.1023/B:EJEP.0000006635.36802.c8. [DOI] [PubMed] [Google Scholar]
- 19.Heerspink HJL, Karasik A, Thuresson M, Melzer-Cohen C, Chodick G, Khunti K, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35. doi: 10.1016/S2213-8587(19)30384-5. [DOI] [PubMed] [Google Scholar]
- 20.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 21.Suissa S. Reduced mortality with sodium-glucose cotransporter-2 inhibitors in observational studies: avoiding immortal time bias. Circulation. 2018;137(14):1432–1434. doi: 10.1161/CIRCULATIONAHA.117.032799. [DOI] [PubMed] [Google Scholar]
- 22.Schwandt A, Denkinger M, Fasching P, Pfeifer M, Wagner C, Weiland J, et al. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J Diabetes Complicat. 2017;31(9):1376–1383. doi: 10.1016/j.jdiacomp.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Weitzman D, Chodick G, Shalev V, Grossman C, Grossman E. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension. 2014;64(3):501–507. doi: 10.1161/HYPERTENSIONAHA.114.03718. [DOI] [PubMed] [Google Scholar]
- 24.Shalev V, Chodick G, Goren I, Silber H, Kokia E, Heymann AD. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol. 2011;152(3):345–349. doi: 10.1016/j.ijcard.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Israel National Cancer Registry, Israel Center for Disease Control, Ministry of Health. 2018. https://www.health.gov.il/English/MinistryUnits/HealthDivision/Icdc/Icr/Pages/default.aspx. Accessed 6 May 2021.
- 26.KDIGO CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [PubMed] [Google Scholar]
- 27.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Lin M, Chen Y, Xu H, Yan L, Dai H. Effects of sodium-glucose cotransporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. Cardiovasc Diabetol. 2021;20(1):83. doi: 10.1186/s12933-021-01272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherney DZI, Charbonnel B, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley R, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64(6):1256–1267. doi: 10.1007/s00125-021-05407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birkeland KI, Bodegard J, Norhammar A, Kuiper JG, Georgiado E, Beekman-Hendriks WL, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21(4):968–974. doi: 10.1111/dom.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raz I, Mosenzon O, Bonaca MP, Cahn A, Kato ET, Silverman MG, et al. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab. 2018;20(5):1102–1110. doi: 10.1111/dom.13217. [DOI] [PubMed] [Google Scholar]
- 32.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20(1):154. doi: 10.1186/s12933-021-01344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birkeland KI, Bodegard J, Banerjee A, Kim DJ, Norhammar A, Eriksson JW, et al. Lower cardiorenal risk with sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes without cardiovascular and renal diseases: a large multinational observational study. Diabetes Obes Metab. 2021;23(1):75–85. doi: 10.1111/dom.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg DD, Wiviott SD, Scirica BM, Gurmu Y, Mosenzon O, Murphy SA, et al. Heart failure risk stratification and efficacy of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus. Circulation. 2019;140(19):1569–1577. doi: 10.1161/CIRCULATIONAHA.119.042685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birkeland KI, Jørgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709–717. doi: 10.1016/S2213-8587(17)30258-9. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Bowe B, Gibson AK, McGill JB, Yan Y, Maddukuri G, et al. Comparative effectiveness of the sodium-glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care. 2020 doi: 10.2337/dc20-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Yan Y, et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care. 2020 doi: 10.2337/dc20-1890. [DOI] [PubMed] [Google Scholar]
- 39.Kohsaka S, Lam CSP, Kim DJ, Cavender MA, Norhammar A, Jørgensen ME, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8(7):606–615. doi: 10.1016/S2213-8587(20)30130-3. [DOI] [PubMed] [Google Scholar]
- 40.Qiu M, Ding L-L, Zhang M, Lin J-H, Gu J-S, Zhou X, et al. SGLT2 inhibitors for prevention of cardiorenal events in people with type 2 diabetes without cardiorenal disease: a meta-analysis of large randomized trials and cohort studies. Pharmacol Res. 2020;161:105175. doi: 10.1016/j.phrs.2020.105175. [DOI] [PubMed] [Google Scholar]
- 41.Scirica BM, Mosenzon O, Bhatt DL, Udell JA, Steg PG, McGuire DK, et al. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 Trial. JAMA Cardiol. 2018;3(2):155–163. doi: 10.1001/jamacardio.2017.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosenzon O. Albumin creatinine ratio and cardiovascular outcomes in the SAVOR-TIMI 53 trial. European Association for the Study of Diabetes (EASD) annual meeting. European Association for the Study of Diabetes: Stockholm; 2015. [Google Scholar]
- 43.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blecker S, Matsushita K, Köttgen A, Loehr LR, Bertoni AG, Boulware LE, et al. High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis. 2011;58(1):47–55. doi: 10.1053/j.ajkd.2011.02.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosenzon O, Wiviott SD, Heerspink HJL, Dwyer JP, Cahn A, Goodrich EL, et al. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diabetes Care. 2021 doi: 10.2337/dc21-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 47.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 48.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 49.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 50.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 51.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 52.Mosenzon O, Del Prato S, Schechter M, Leiter LA, Ceriello A, DeFronzo RA, et al. From glucose lowering agents to disease/diabetes modifying drugs: a “SIMPLE” approach for the treatment of type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):92. doi: 10.1186/s12933-021-01281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease. https://clinicaltrials.gov/ct2/show/NCT03819153. Accessed 26 Mar 2020
- 54.Heerspink HJL, Parving H-H, Andress DL, Bakris G, Correa-Rotter R, Hou F-F, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393(10184):1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Variable’s definitions, including medications and diagnosis. ACE – Angiotensin-converting enzyme; ARB – Angiotensin II receptor blocker; CABG – coronary artery bypass grafting; CVD – cardiovascular disease; DPP4 – Dipeptidyl-peptidase 4; eGFR – estimated glomerular filtration rate; GLP-1 RA – glucagon-like peptide-1 receptor agonist; ICD-9 – International Classification of Diseases 9; MHS – Maccabi Healthcare Services; PCI – percutaneous coronary intervention. Table S2. Additional patients’ baseline characteristics post propensity-matching. Table S3. Distribution of index medications post-match and by follow up definitions. PP4i – Dipeptidyl peptidase-4 inhibitor; GLP1-RA – Glucagon-like peptide-1 receptor agonists; ITT – intention to treat; oGLAs – other glucose lowering agents; OT – on treatment; SGLT2i – Sodium-glucose cotransporter 2 inhibitors; sOT – strict on treatment; TZDs – Thiazolidinediones.
Additional file 2:Figure S1. Risk for cardiovascular and kidney outcome in SGLT2i initiators compared to oGLAs in the entire cohort, during the OT follow up definition. Event rates are presented as number of events per 100 person years of follow up. In black—the unadjusted model; and in grey—the model adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; OT = on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; ESKD = end stage kidney disease; ER = event rate.
Additional file 3: Figure S2. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the OT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. * BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; OT = on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Additional file 4: Figure S2. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the OT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. * BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; OT = on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Additional file 5: Figure S3. Risk for cardiovascular and kidney outcome in SGLT2i initiators compared to oGLAs in the entire cohort, during the sOT follow up definition. Event rates are presented as number of events per 100 person years of follow up. In black—the unadjusted model; and in grey—the model adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; sOT = strict on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; ESKD = end stage kidney disease; ER = event rate.
Additional file 6: Figure S4. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the sOT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. *BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; sOT = strict on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Additional file 7: Figure S4. Risk for cardiovascular and kidney outcomes in SGLT2i initiators compared to oGLAs in low kidney risk populations, during the sOT follow up definition. A Cardiovascular outcomes. B Kidney outcomes. Event rates are presented as number of events per 100 person-years of follow up. Low KDIGO risk is defined as eGFR>60 ml/min/1.73 m2 and UACR<30 mg/g. For the low KDIGO risk and eGFR>90 ml/min/1.73 m2, the model was adjusted to baseline eGFR (as continuous variable) and UACR (as categorical variable). Outcome analysis of the urine albumin BDL category was only adjusted to baseline eGFR as continuous variable. *BDL= Below detectable levels. SGLT2i = sodium/glucose cotransporter-2 inhibitors; oGLAs = other glucose lowering agents; sOT = strict on treatment; hHF = hospitalization for heart failure; ACM =all-cause mortality; MI = myocardial infract; eGFR = estimated glomerular filtration rate; UACR = urinary albumin to creatinine ratio; KDIGO = kidney disease: improving global outcomes; ER =event rate.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.