Abstract
New classes of unexplored benzo[b]thiolanes are synthesized from trisubstituted thioamides through copper-catalyzed intramolecular S-arylation of thioamides for the first time. This method provides good to excellent yields with fully controlled chemoselectivity. Unusually, iminobenzo[b]thiolanes are very stable under mild acidic conditions. A plausible mechanism is proposed for the chemoselective S-arylation process.
Introduction
Benzothiophene is a predominant structural core compound in drugs and device materials.1−4 In particular, 2-aminobenzothiophenes show various biological activities, such as acetyl-CoA carboxylase inhibitor, tubulin polymerization, and mycobacterium inhibition.5 They are also precursors for the synthesis of the well-known drug raloxifene and its analogues.6 Therefore, several elegant synthetic methods have been developed for the construction of 2-aminobenzothiophene derivatives,7 whereas those for the 2-iminobenzothiophene core remain elusive in the literature.8,9 Recently, a 2-iminothiophene-fused polyaromatic compound A were synthesized from thioamides (Figure 1).10 The photophysical studies show emission in the range of 500–606 nm with quantum yields up to 0.64.10 Hence, compound A has been considered a potential core for developing optical device materials. Furthermore, the 2-aminobenzothiolane core is found in marine alkaloid makaluvamine F, which has potent cytotoxicity.11 Hydrolysis of 2-iminobenzothiophenes 1 provides unknown classes of 2-oxobenzothiolanes 2 (Figure 1).12 They are S-analogues of 2-oxindole derivatives. The indole derivatives are found in numerous natural products such as horsfiline, a 3,3′-spiro-2-oxindole alkaloid, alstonisine, gelsemine, and spirotryprostatin B.12 Until now, the synthesis of 2-oxobenzothiolanes has not been reported, whereas synthesis of benzothiolane and its 3-oxo derivatives is well developed.11a,13,14 Hence, developing a new protocol to synthesize 2-iminobenzothiolanes and their 2-oxo derivatives is highly desirable for therapeutic and materials science applications.
Figure 1.
Selected 2-imino-fused thiophenes and 2-aminobenzo[b]thiolane.
Thioamides are versatile synthons and are extensively used in the synthesis of heterocyclic building blocks.15 Notably, a wide range of thiazoles and their benzo derivatives is synthesized by inter/intramolecular S-alkylation and arylation of appropriate thioamides with the respective electrophilic partners.16,17 They proceed through either metal or metal-free reaction conditions. Similarly, thiophenes are prepared from thioamides and biselectrophiles.18 However, thioamides are rarely employed as reactive partners to synthesize 2-aminobenzothiophenes compared to benzothiazole synthesis.19 The chemoselective S-arylation of thioanilides has significant limitations. Recently, Olofsson and co-workers reported intermolecular chemoselective S-arylation of thioamides with diaryliodonium salts affording arylthioimidates.20 We recently reported copper-catalyzed intramolecular S-arylation of thioamides at room temperature providing 2-aminobenzothiophenes in good to excellent yields.21 Due to our ongoing interest in chemoselective heterocycle synthesis,22 herein we wish to report a chemoselective Ullmann reaction of α-trisubstituted thioamides under ligand-free conditions affording new classes of 2-iminobenzothiolanes for the first time.
Results and Discussion
The required trisubstituted thioamides 3 were prepared from α-(2-bromoaryl)-propanenitriles 4 or α-(2-bromophenyl)-propanoate 6 and aromatic/alkyl isothiocyanates 5 in the presence of NaHMDS affording the corresponding thioamides 3a–y in moderate to good yields (Table S1, Supporting Information). Spectral and analytical data characterized all newly synthesized 2-(2-bromoaryl)-2-cyano/carbmethoxy-N-substituted propanethioamide derivatives 3a–y. Having all the thioamides 3a–y, we started to investigate the suitable conditions for a chemoselective intramolecular S-arylation process. Hence, thioamide 3a was chosen as the model substrate for this process. The optimization began with CuI, KOtBu, and 1,10-phenanthroline at room temperature. The reaction was sluggish and afforded only 20% of the benzo[b]thiolane derivative 1a (Table 1, entry 1). Keeping the rest of the conditions the same, the reaction at different temperatures was studied. When the reaction was carried out at 60 °C, the yield of benzo[b]thiolane 1a increased to 42% (Table 1, entry 2). Subsequently, the reaction temperature was further raised to 90 °C, and the yield enhanced to 68% (Table 1, entry 3).
Table 1. Optimization of S-Arylation of α-Trisubstituted Thioamide 3.
| entry | 3 | Cu salt | base | solvent | temp (°C) | time (h) | yield (%)a |
|---|---|---|---|---|---|---|---|
| 1. | X = Br | CuI | KOtBu | DMF | Rt | 4 | 20b |
| 2. | X = Br | CuI | KOtBu | DMF | 60 | 3 | 42b |
| 3. | X = Br | CuI | KOtBu | DMF | 90 | 3 | 68b |
| 4. | X = Br | CuI | KOtBu | DMF | 90 | 3 | 77 |
| 5. | X = Br | CuI | Cs2CO3 | DMF | 90 | 3 | 77 |
| 6. | X = Br | CuI | K3PO4 | DMF | 90 | 3 | 85 |
| 7. | X = Br | CuI | Et3N | DMF | 90 | 3 | 91 |
| 8. | X = Br | CuI | K2CO3 | DMF | 90 | 3 | 69 |
| 9. | X = Br | CuI | Et3N | toluene | 90 | 3 | 91 |
| 10. | X = Br | CuI | Et3N | dioxane | 90 | 3 | 86 |
| 11. | X = Br | CuI | Et3N | DMSO | 90 | 3 | 92 |
| 12. | X = Br | CuBr | Et3N | DMF | 90 | 1.5 | 98 |
| 13. | X = Br | CuCl | Et3N | DMF | 90 | 1.5 | 92 |
| 14. | X = Br | Cu(OAc)2 | Et3N | DMF | 90 | 1.5 | 95 |
| 15. | X = I | CuBr | Et3N | DMF | 90 | 4 | 82 |
| 16. | X = Cl | CuBr | Et3N | DMF | 90 | 10 | 20 |
| 17. | X = Br | CuBr | Et3N | DMF | 90 | 2 | 83c |
Isolated yields.
With 1,10-phenanthroline ligand (10 mol %).
TEMPO (1 equiv) was used, TEMPO-(2,2,6,6-Tetramethylpiperidin-1-yl)ox.
When the reaction was carried out in the absence of a ligand, to our surprise, thioamide 3a yielded 77% of product 1a (Table 1, entry 4). This indicates that the ligand did not affect the S-arylation process. The structure of 1a was also confirmed by single-crystal X-ray analysis (Table 1). To improve the reaction’s efficiency, we screened different bases such as Cs2CO3, K3PO4, Et3N, and K2CO3, out of which Et3N gave 91% of benzo[b]thiolane 1a (Table 1, entries 5–8). Furthermore, we screened solvents like toluene, dioxane, and dimethyl sulfoxide (DMSO) (Table 1, entries 9–11). Only DMSO gave a comparable yield to that of DMF. Next, copper salts were tested for optimization, such as CuCl, CuBr, and Cu(OAc)2. We found that CuBr yielded 98% of 1a (Table 1, entries 12–14). Instead of aryl bromide, iodo and chloro derivatives were also examined. Aryl iodide gave 82% of benzo[b]thiolane 1a (Table 1, entry 15), whereas only 20% of 1a was observed after 10 h in the case of a chloro derivative (Table 1, entry 16). We did not find any trace amount of the N-arylated product during the optimization studies. In the presence of the radical scavenger TEMPO, we obtained 83% of benzo[b]thiolane 1a (Table 1, entry 17). This indicates that the S-arylation process does not proceed through a radical path.
The optimized reaction conditions were tested on the various trisubstituted thioamides 3b–y. Initially, N-aryl-substituted thioamides 3b–g were studied. The electron-donating groups on N-phenyl derivatives 3b,c gave the corresponding benzo[b]thiolanes 1b,c in 85–91% yields (Table 2). Similarly, the para-fluoro- and nitro-substituted N-phenyl thioamides 3d,e were smoothly transformed to benzo[b]thiolanes 1d,e in good to excellent yields (Table 2). The reaction was continued with ortho-substituted thioanilides 3f,g. The benzo[b]thiolanes 1f,g were obtained from the respective thioanilides 3f,g in 75–90% yields (Table 2). Next, we examined alkyl-substituted thioamides 3h–k under the optimized reaction conditions. The S-arylation of thioamides 3h–k took a bit longer reaction time of about 6–12 h to afford the corresponding benzo[b]thiolanes 1h–k in good to excellent yields (Table 2). Furthermore, we investigated the electron-donating group on 2-bromoaryl derivatives 3l–o. These derivatives 3l–o underwent a smooth S-arylation process yielding benzo[b]thiolanes 1l–o in good to excellent yields (Table 2). However, the halo-substituted thioamides 3p,q gave the S-arylated products 1p,q in 35–42% yields (Table 2). Interestingly, the sterically hindered arylbromo derivative 3r gave rise to benzo[b]thiolane 1r in 92% yield (Table 2). Next, we changed the methyl group at the α-trisubstituted thioamide to ethyl, benzyl, and allyl groups. Thus, ethyl- and benzyl-substituted thioamide derivatives 3s,t yielded the corresponding benzo[b]thiolanes 1s,t in 85–95% yields (Table 2). However, the allyl derivative 3u gave only 52% yield of the S-arylated product 1u and took a longer reaction time (Table 2). The ester motif-containing trisubstituted thioamide 3v was studied under identical conditions. The ester-containing benzo[b]thiolane 1v was obtained in 89% yield in 3.5 h (Table 2). Next, we focused our attention on synthesizing enantiomerically pure benzo[b]thiolanes from chiral α-trisubstituted thioamides. The chiral α-trisubstituted thioamides 3w–y were prepared in moderate to good yields with 1:1 diastereoisomers (Table S1, Supporting Information). The thioamides 3w–y were subjected to optimized conditions affording 2-iminobenzo[b]thiolanes 1w–y in 66–75% yields (Table 2).
Table 2. Scope of the Copper-Catalyzed S-Arylation of α-Trisubstituted Thioamides 3b–ya.
Isolated yields.
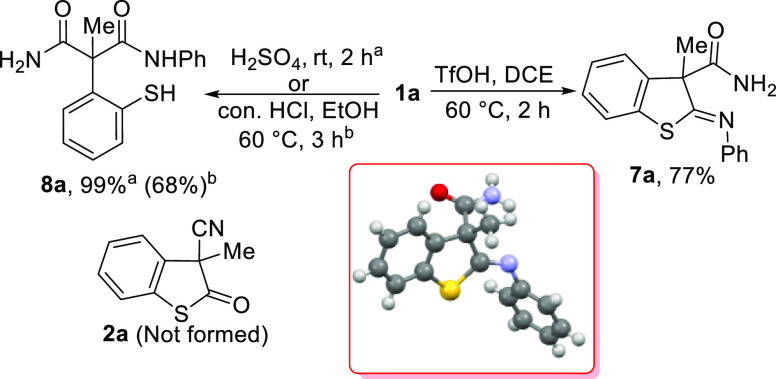
Finally, we attempted to synthesize S-analogues of 2-oxindole derivatives. Thus, newly synthesized 2-iminobenzo[b]thiolane 1a was subjected to hydrolysis conditions; to our surprise, the imine functionality was relatively rigid to hydrolysis. The 2-iminobenzo[b]thiolane 1a was subjected to 10 equivalents of triflic acid at 60 °C, resulting in the hydrolysis of the nitrile moiety. The imine functionality remained intact. The structure of 7a was also confirmed by single-crystal X-ray analysis (Scheme 1). However, 1a was subjected to concentrated H2SO4 under a neat condition, which resulted in the cleavage of the heterocycle and hydrolysis of nitrile and imine functionalities yielding 2-(2-mercaptophenyl)-2-methyl-N-phenylmalonamide 8a in a quantitative yield (Scheme 1). A similar result was observed in the presence of conc. HCl. However, 8a was obtained in 68% yield (Scheme 1). Furthermore, our attempts to reduce the imine moiety of 1a failed.23
Scheme 1. Hydrolysis of Benzo[b]thiolane 1a.
To understand the chemoselective S-arylation process mechanism, as aforementioned the S-arylation process proceeds through an ionic pathway instead of a radical path (Table 1, entry 17). The S-center of thioamide is more nucleophilic than the N-center. Hence, first, thioamide 3 forms a coordinate complex A with the copper salt. In a base, complex A is transformed to the copper(I)thiolate complex B, which is also stabilized by N-center coordination. Next, complex B undergoes an intramolecular oxidative addition affording the copper(III) complex C. This step is crucial, which is influenced by the nature of aryl halides and nitrogen substituents. Finally, the complex C undergoes reductive elimination to afford the S-arylated product 1 and regenerated the Cu(I) catalyst (Scheme 2). The oxidative addition of 2-chlorophenyl derivative 3z is slow and less efficient; hence, a lower yield of 1a was observed (Table 1, entry 15). Electron-deficient 2-bromoaryl thioamides 3p,q undergo a slow oxidative addition process; therefore, lower yields were obtained (Table 2). Electron-rich N-alkyl thioamides 3h–k form a strong coordinate complex B. Thus, these complexes require a longer reaction time to complete the oxidative process.
Scheme 2. Plausible Mechanism for the Formation of Benzo[b]thiolane 1.
Conclusions
We have demonstrated a simple approach for synthesizing unexplored 2-imino-3,3′-alkylcyanobenzo[b]thiolanes via the copper-catalyzed S-arylation of α-trisubstituted thioamides for the first time. This catalytic process is highly chemoselective. We have prepared a series of all-carbon quaternary-centered 2-iminobenzo[b]thiolanes in good to excellent yields. Interestingly, we found that the 2-imino moiety is very stable under mild acidic conditions, whereas the thiolane ring was cleaved under a neat acidic condition. Overall, we demonstrated that the Ullmann reaction is a powerful tool for synthesizing a new class of S-heterocycles without using any ligands. Currently, the application of 3,3′-disubstituted-2-iminobenzothiolanes is undergoing in our laboratory.
Experimental Section
General Information
All reactions were performed using the standard vial technique with a rubber septum. All solids were weighed in air. Dioxane, Cs2CO3, K3PO4, KOtBu, and K2CO3 were purchased from Aldrich, Acros, Merck, Spectrochem, or Alfa Aesar and used as received. CuI, CuBr, CuCl, Cu(OAc)2, and 1,10-phenanthroline were purchased from Aldrich. Dried DMF, DMSO, toluene, and Et3N were used. Isothiocyanates were synthesized from substituted anilines, and few isothiocyanates were purchased from Aldrich. All other reagents were purchased from common suppliers and used without further purification. Flash chromatography was performed using a Merck silica gel (230–400 mesh). Fractions were monitored by thin-layer chromatography (TLC) on precoated silica gel 60 F254 plates (Merck & Co.) and were visualized by a UV light. Nuclear magnetic resonance (NMR) spectroscopy data were recorded using Bruker ARX 400 and 700 spectrometers. 13C and 1H NMR spectra were recorded in CDCl3 and CD3SOCD3 referenced according to signals of deutero solvents. Electrospray ionization high-resolution mass spectrometry (ESI HR-MS) measurements were performed using a Bruker micrOTOF-Q II mass-spectrometer.
General Procedure for the Synthesis of Thioamides 3a–aa
To a stirring suspension of corresponding 2-(2-bromoaryl)propanenitriles24 (6.0 mmol, 1.0 equiv) in THF, NaHMDS (1.0 M in THF) was added dropwise at 0 °C. After being further stirred for 15 min at room temperature, a solution of isothiocyanates (6.6 mmol, 1.2 equiv) in THF was added to the reaction mixture at 0 °C followed by further stirring for 15–45 min at room temperature. After the starting materials (monitored by TLC) were completely consumed, the reaction mixture was quenched with a saturated NH4Cl solution and extracted with ethyl acetate (EtOAc). The combined organic layer was washed with water (3 × 25 mL) and brine (25 mL), dried over anhydrous Na2SO4, and concentrated under a reduced pressure. The crude products were purified by flash chromatography using hexane and EtOAc as the eluents.
2-(2-Bromophenyl)-2-cyano-N-phenylpropanethioamide (3a)
Yield: 75% (0.258 g); pale yellow solid; Rf: 0.35 in 30% EtOAc in hexanes; mp: 124–125 °C; IR (ν cm–1) =2974, 2362, 2243, 1530, 1406, 1344, 1160, 1036, 1004, 808, 754; 1H NMR (400 MHz, CDCl3) δ 9.04 (s, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.66 (d, J = 7.6 Hz, 1H), 7.56 (d, J = 8.0 Hz, 2H), 7.48 (t, J = 7.2 Hz, 1H), 7.42 (t, J = 8.0 Hz, 2H), 7.34–7.29 (m, 2H), 2.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 197.6, 138.1, 135.5, 135.2, 131.1, 129.4, 129.2, 128.0, 127.7, 124.6, 124.4, 119.7, 56.7, 29.6. HR-MS (ESI-TOF) m/z: cal. for C16H13BrN2S [M + H]+: 345.0056 and 347.0044; found: 345.0051 and 347.0035.
2-(2-Bromophenyl)-2-cyano-N-(4-methoxyphenyl)propanethioamide (3b)
Yield: 91% (0.340 g); pale yellow solid; Rf: 0.32 in 30% EtOAc in hexanes; mp: 127–129 °C; IR (ν cm–1) = 3628, 3306, 2352, 1732, 1698, 1656, 1564, 746; 1H NMR (400 MHz, CD3SOCD3) δ 10.73 (s, 1H), 7.77 (d, J = 7.6 Hz, 2H), 7.55 (t, J = 7.2 Hz, 1H), 7.40 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 3.76 (s, 3H), 2.27 (s, 3H). 13C NMR (100 MHz, CD3SOCD3) δ 199.1, 158.3, 136.4, 135.0, 132.7, 131.4, 131.0, 128.7, 127.3, 124.6, 120.7, 114.2, 57.5, 55.7, 27.8. HR-MS (ESI-TOF) m/z: cal. for C17H15BrN2OS [M + H]+: 375.0161 and 377.0146; found: 375.0166 and 377.0141.
2-(2-Bromophenyl)-2-cyano-N-(p-tolyl)propanethioamide (3c)
Yield: 73% (0.261 g); pale yellow solid; Rf: 0.30 in 30% EtOAc in hexanes; mp: 180–182 °C; IR (ν cm–1) = 3427, 2362, 1638, 1517, 1371, 1036; 1H NMR (400 MHz, CDCl3) δ 9.03 (s, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 7.6 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.43 (t, J = 8.4 Hz, 2H), 7.32 (d, J = 7.6 Hz, 1H), 7.22 (d, J = 8.0 Hz, 2H), 2.36 (s, 3H), 2.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 197.5, 137.8, 135.7, 135.5, 135.2, 130.9, 129.8, 129.4, 128.2, 124.6, 124.3, 119.8, 56.44, 28.7, 21.2. HR-MS (ESI-TOF) m/z: cal. for C17H15BrN2S [M + H]+: 359.0212 and 361.0192; found: 359.0227 and 361.0206.
2-(2-Bromophenyl)-2-cyano-N-(4-fluorophenyl)propanethioamide (3d)
Yield: 68% (0.261 g); pale yellow solid; Rf: 0.30 in 30% EtOAc in hexanes; mp: 139–141 °C; IR (ν cm–1) = 3308, 1700, 1507, 1413, 1217, 833, 769; 1H NMR (400 MHz, CDCl3) 7.74–7.69 (m, 2H), 7.54 (s, 1H), 7.50 (t, J = 7.6 Hz, 1H), 7.46–7.43 (m, 2H), 7.35 (t, J = 7.6 Hz, 1H), 7.06 (t, J = 8.4 Hz, 2H), 2.19 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.0, 160.1 (d, J = 245.2 Hz), 135.2, 134.1, 132.6 (d, J = 3.0 Hz), 131.1, 129.1, 128.4, 123.7, 122.7 (d, J = 8.0 Hz), 119.4, 115.9 (d, J = 22.5 Hz), 50.1, 24.2; 19F NMR (376 Hz, CDCl3) δ −116.23; HR-MS (ESI-TOF) m/z: cal. for C16H12BrFN2S [M + H]+: 384.9781 and 386.9751; found: 384.9770 and 386.9760.
2-(2-Bromophenyl)-2-cyano-N-(4-nitrophenyl)propanethioamide (3e)
Yield: 70% (0.271 g); Rf: 0.26 in 30% EtOAc in hexanes; mp: 185–187 °C; IR (ν cm–1) = 3303, 2927, 2362, 1336, 756, 667. 1H NMR (400 MHz, CD3SOCD3) δ 11.23 (s, 1H), 8.29 (d, J = 8.8 Hz, 2H), 7.80–7.78 (m, 4H), 7.59 (t, J = 7.2 Hz, 1H), 7.43 (t, J = 7.4 Hz, 1H), 2.31 (s, 3H). 13C NMR (100 MHz, CD3SOCD3) δ 200.8, 145.7, 145.6, 136.2, 135.1, 131.6, 131.1, 128.8, 126.6, 124.7, 124.6, 120.4, 58.0, 27.7. HR-MS (ESI-TOF) m/z: cal. for C16H12BrN3O2S [M + H]+: 389.9914 and 391.9895; found: 389.9906 and 391.9886.
2-(2-Bromophenyl)-2-cyano-N-(2-methoxyphenyl)propanethioamide (3f)
Yield: 75% (0.280 g); yellow solid; Rf: 0.32 in 30% EtOAc in hexanes; mp: 154–156 °C; IR (ν cm–1) = 3628, 3306, 2352, 1732, 1698, 1656, 1564, 746; 1H NMR (400 MHz, CDCl3) δ 9.71 (s, 1H), 8.91 (d, J = 8.4 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.6 Hz, 1H), 7.34 (t, J = 7.6 Hz, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.01 (t, J = 8.0 Hz, 1H), 6.91 (d, J = 8.0 Hz, 1H), 3.78 (s, 3H), 2.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 194.2, 150.1, 135.7, 135.2, 130.8, 129.4, 127.98, 127.80, 127.2, 125.1, 121.4, 120.5, 119.7, 110.5, 57.8, 56.1, 28.7. HR-MS (ESI-TOF) m/z: cal. for C17H15BrN2OS [M + H]+: 375.0161 and 377.0139; found: 375.0161 and 377.0141.
2-(2-Bromophenyl)-2-cyano-N-(2-(trifluoromethyl)phenyl)propanethioamide (3g)
Yield: 75% (0.309 g); yellow solid; Rf: 0.26 in 30% EtOAc in hexanes; mp: 200–202 °C; IR (ν cm–1) = 3207, 3006, 2349, 2253, 1505, 1450, 1319, 1125, 746, 675; 1H NMR (700 MHz, CDCl3) δ 8.87 (s, 1H), 8.00 (d, J = 8.4 Hz, 1H), 7.74 (d, J = 8.4 Hz, 1H), 7.70 (d, J = 7.7 Hz, 1H), 7.67–7.63 (m, 2H), 7.50 (t, J = 7.7 Hz, 1H), 7.45 (t, J = 7.7 Hz, 1H), 7.35 (t, J = 7.7 Hz, 1H), 2.36 (s, 3H). 13C NMR (175 MHz, CDCl3) δ 199.5, 135.9 (q, J = 1.22 Hz), 135.3, 135.2, 132.7, 131.2, 129.1, 128.8, 128.2, 128.0, 126.6 (q, J = 5.0 Hz), 125.0, 124.9 (q, J = 30.1 Hz), 123.3 (q, J = 271.7 Hz), 119.4, 57.1, 28.3; 19F NMR (376 Hz, CDCl3) δ −60.88. HR-MS (ESI-TOF) m/z: cal. for C17H12BrF3N2S [M + H]+: 412.9929 and 414.9903; found: 412.9918 and 414.9909.
N-Benzyl-2-(2-bromophenyl)-2-cyanopropanethioamide (3h)
Yield: 60% (0.215 g); pale yellow solid; Rf: 0.28 in 30% EtOAc in hexanes; mp: 148–150 °C; IR (ν cm–1) = 3253, 2359, 1588, 1558, 1513, 1373, 1202, 1029, 748, 638, 502; 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.39–7.29 (m, 5H), 4.87 (s, 1H), 4.88 (s, 1H), 2.28 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 198.2, 135.5, 135.3, 135.1, 130.8, 129.2, 129.0, 128.4, 128.3, 128.1, 124.5, 119.6, 55.5, 51.1, 28.7. HR-MS (ESI-TOF) m/z: cal. for C17H15BrN2S [M + H]+: 359.0212 and 361.0168; found: 359.0192 and 361.0192.
2-(2-Bromophenyl)-2-cyano-N-isopropylpropanethioamide (3i)
Yield: 56% (0.174 g); pale yellow solid; Rf: 0.30 in 30% EtOAc in hexanes; mp: 133–135 °C; IR (ν cm–1) = 3744, 3303, 2961, 2347, 1735, 1700, 1656, 1559, 833, 647; 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.0 Hz, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.28 (t, J = 7.8 Hz, 1H), 4.66–4.58 (m, 1H), 2.21 (s, 3H), 1.28 (d, J = 6.0 Hz, 3H), 1.27 (d, J = 6.0 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 196.5, 135.6, 134.9, 130.7, 129.2, 127.9, 124.5, 119.6, 55.4, 48.5, 28.7, 20.9, 20.5. HR-MS (ESI-TOF) m/z: cal. for C13H15BrN2S [M + H]+: 311.0212 and 313.0185; found: 311.0207 and 313.0192.
2-(2-Bromophenyl)-2-cyano-N-ethylpropanethioamide (3j)
Yield: 64% (0.190 g); yellow solid; Rf: 0.28 in 30% EtOAc in hexanes; mp: 122–124 °C; IR (ν cm–1) = 3628, 2934, 2362, 2339, 1611, 1561, 1438, 1420, 1004, 665. 1H NMR (400 MHz, CDCl3) δ 7.81 (s, 1H), 7.67 (d, J = 7.2 Hz, 1H), 7.59 (d, J = 7.2 Hz, 1H), 7.46 (t, J = 6.8 Hz, 1H), 7.30 (t, J = 7.2 Hz, 1H), 3.74 (q, J = 6.4 Hz, 2H), 2.24 (s, 3H), 1.30 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 197.8, 135.6, 135.0, 130.7, 129.2, 128.0, 124.5, 119.7, 55.4, 41.8, 28.7, 12.5. HR-MS (ESI-TOF) m/z: cal. for C12H13BrN2S [M + H]+: 297.0056 and 299.0037; found: 297.0058 and 299.0035.
2-(2-Bromophenyl)-N-(tert-butyl)-2-cyanopropanethioamide (3k)
Yield: 58% (0.188 g); yellow solid; Rf: 0.28 in 30% EtOAc in hexanes; mp: 128–130 °C. IR (ν cm–1) = 2969, 2934, 2867, 2238, 1651, 1472, 1130, 987, 756, 439; 1H NMR (700 MHz, CDCl3) δ 7.69 (d, J = 9.1 Hz, 1H), 7.59 (s, 1H), 7.56 (d, J = 9.1 Hz, 1H), 7.45 (t, J = 8.4 Hz, 1H), 7.31 (t, J = 8.4 Hz, 1H), 2.24 (s, 3H), 1.57 (s, 9H). 13C NMR (175 MHz, CDCl3) δ 196.1, 135.8, 134.9, 130.6, 129.3, 127.9, 124.7, 119.9, 56.9, 56.6, 28.9, 27.0. HR-MS (ESI-TOF) m/z: cal. for C14H17BrN2S [M + H]+: 325.0369 and 327.0355; found: 325.0377 and 327.0348.
2-(2-Bromo-4,5-dimethoxyphenyl)-2-cyano-N-phenylpropanethioamide (3l)
Yield: 73% (0.295 g); pale yellow solid; Rf: 0.32 in 30% EtOAc in hexanes; mp: 222–224 °C; IR (ν cm–1) = 3447, 2253, 1517, 1467, 1299, 1004, 741; 1H NMR (400 MHz, CD3SOCD3) δ 10.60 (s, 1H), 7.46–7.35 (m, 4H), 7.33 (s, 1H), 7.32–7.27 (m, 1H), 7.24 (s, 1H), 3.89 (s, 3H), 3.84 (s, 3H), 2.30 (s, 3H). 13C NMR (100 MHz, CD3SOCD3) δ 200.1, 150.3, 148.4, 139.8, 129.0, 127.7, 127.5, 126.2, 120.8, 117.9, 115.4, 114.6, 58.1, 56.5, 56.3, 28.2. HR-MS (ESI-TOF) m/z: cal. for C18H17BrN2O2S [M + H]+: 405.0267 and 407.0230; found: 405.0253 and 407.0247.
2-(6-Bromo-3,4-methylenedioxyphenyl)-2-cyano-N-phenylpropanethioamide (3m)
Yield: 72% (0.280 g); yellow solid; Rf: 0.31 in 30% EtOAc in hexanes; mp: 174–176 °C; IR (ν cm–1) = 3450, 2349, 1529, 1395, 1256, 1004, 735; 1H NMR (400 MHz, CDCl3) δ 9.07 (s, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.42 (t, J = 7.6 Hz, 2H), 7.39–7.27 (m, 2H), 7.27–7.19 (m, 1H), 7.13 (d, J = 8.8 Hz, 1H), 6.07 (s, 2H), 2.28 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 198.0, 149.2, 148.1, 138.0, 129.2, 128.4, 127.8, 124.5, 119.8, 116.2, 114.9, 109.4, 102.7, 56.6, 29.2. HR-MS (ESI-TOF) m/z: cal. for C17H13BrN2O2S [M + H]+: 388.9954 and 390.9917; found: 388.9938 and 390.9934.
2-(6-Bromo-2,3-dimethoxyphenyl)-2-cyano-N-phenylpropanethioamide (3n)
Yield: 73% (0.295 g); pale yellow solid; Rf: 0.31 in 30% EtOAc in hexanes; mp: 174–176 °C; IR (νcm–1) = 3450, 2240, 1723, 1518, 1456, 1290, 1012, 736; 1H NMR (400 MHz, CDCl3) δ 8.97 (s, 1H), 7.58 (d, J = 8.0 Hz, 2H), 7.48–7.32 (m, 3H), 7.29–7.24 (m, 1H), 6.83 (d, J = 8.8 Hz, 1H), 3.95 (s, 3H), 3.87 (s, 3H), 2.43 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 198.8, 153.3, 150.1, 138.4, 130.5, 129.4, 129.1, 127.6, 124.4, 121.0, 114.0, 113.6, 61.6, 56.1, 55.6, 29.4. HR-MS (ESI-TOF) m/z: cal. for C18H17BrN2O2S [M + H]+: 405.0267 and 407.0236; found: 405.0251 and 407.0247.
2-(6-Bromo-2,3-dimethoxyphenyl)-2-cyano-N-(p-tolyl)propanethioamide (3o)
Yield: 70% (0.293 g); yellow solid; Rf: 0.30 in 30% EtOAc in hexanes; mp: 146–148 °C; IR (ν cm–1) = 3447, 2237, 1732, 1517, 1467, 1299, 1004, 741, 656; 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.8 Hz, 1H), 7.21 (d, J = 8.0 Hz, 2H), 6.84 (d, J = 8.4 Hz, 1H), 3.96 (s, 3H), 3.89 (s, 3H), 2.44 (s, 3H), 2.35 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 198.9, 153.3, 150.0, 137.7, 135.8, 130.5, 129.7, 129.4, 124.4, 121.1, 113.9, 113.7, 61.6, 56.2, 55.6, 29.5, 21.3. HR-MS (ESI-TOF) m/z: cal. for C19H19BrN2O2S [M + H]+: 419.0423 and 421.0420; found: 419.0434 and 421.0403.
2-(2-Bromo-4-chlorophenyl)-2-cyano-N-phenylpropanethioamide (3p)
Yield: 65% (0.246 g); pale yellow solid; Rf: 0.31 in 30% EtOAc in hexanes; mp: 129–131 °C; IR (ν cm–1) = 3442, 2362, 2238, 1641, 1520, 670; 1H NMR (400 MHz, CDCl3) δ 9.26 (s, 1H), 7.71 (s, 1H), 7.60 (d, J = 7.6 Hz, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.51–7.39 (m, 3H), 7.34 (t, J = 7.2 Hz, 1H), 2.32 (s, 3H). 13C NMR (175 MHz, CDCl3) δ 196.9, 137.9, 136.2, 134.7, 134.5, 130.0, 129.2, 128.2, 127.9, 125.0, 124.3, 119.4, 55.6, 28.9. HR-MS (ESI-TOF) m/z: cal. for C16H12BrClN2S [M + H]+: 378.9666 and 380.9668; found: 378.9681 and 380.9645.
2-(2-Bromo-4-fluorophenyl)-2-cyano-N-phenylpropanethioamide (3q)
Yield: 72% (0.261 g); white solid; Rf: 0.30 in 30% EtOAc in hexanes; mp: 133–135 °C; IR (ν cm–1) = 3427, 2359, 2236, 1656, 1530, 1222, 670; 1H NMR (400 MHz, CDCl3) δ 9.21 (s, 1H), 7.61–7.57 (m, 3H), 7.48–7.40 (m, 3H), 7.33 (t, J = 7.6 Hz, 1H), 7.18 (t, J = 6.8 Hz, 1H), 2.32 (s, 3H). 13C NMR (175 MHz, CDCl3) δ 197.2, 162.5 (d, J = 253.4 Hz), 138.0, 132.0 (d, J = 3.5 Hz), 130.5 (d, J = 8.7 Hz), 129.2, 127.9, 125.1 (d, J = 9.4 Hz), 124.3, 122.5 (d, J = 24.7 Hz), 119.5, 115.1 (d, J = 21.0 Hz), 55.7, 29.0; 19F NMR (376 Hz, CDCl3) δ −109.71. HR-MS (ESI-TOF) m/z: cal. for C16H12BrFN2S [M + H]+: 384.9781 and 386.9769; found: 384.9788 and 386.9760.
2-(2-Bromo-3,5-di-tert-butylphenyl)-2-cyano-N-phenylpropanethioamide (3r)
Yield: 55% (0.251 g); pale yellow solid; Rf: 0.58 in 30% EtOAc in hexanes; mp: 178–180 °C; IR (ν cm–1) = 3338, 3262, 2962, 2871, 2360, 2238, 1710, 1500, 1462, 1393, 1237, 1071, 885, 646, 488; 1H NMR (700 MHz, CDCl3) δ 8.68 (s, 1H), 7.65 (s, 1H), 7.56 (s, 1H), 7.48 (d, J = 7.7 Hz, 2H), 7.41 (t, J = 7.7 Hz, 2H), 7.30 (t, J = 7.7 Hz, 1H), 2.38 (s, 3H), 1.58 (s, 9H), 1.38 (s, 9H). 13C NMR (175 MHz, CDCl3) δ 199.2, 150.4, 150.0, 138.2, 136.4, 129.1, 127.6, 127.1, 124.9, 124.4, 122.9, 120.5, 59.2, 38.1, 35.2, 31.2, 30.2, 29.9. HR-MS (ESI-TOF) m/z: cal. for C24H29BrN2S [M + H]+: 457.1306 and 459.1286; found: 457.1308 and 459.1288.
2-(2-Bromophenyl)-2-cyano-N-phenylpent-4-enethioamide (3s)
Yield: 60% (0.222 g); pale yellow solid; Rf: 0.5 in 30% EtOAc in hexanes; mp: 102–104 °C; IR (ν cm–1) = 3841, 3004, 2347, 1697, 1558, 1541, 1269, 753, 664; 1H NMR (400 MHz, CDCl3) δ 9.29 (s, 1H), 7.62–7.53 (m, 4H), 7.37 (t, 3H), 7.28–7.19 (m, 2H), 2.81–2.73 (m, 1H), 2.61–2.53 (m, 1H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 196.0, 137.9, 136.7, 136.2, 130.6, 129.7, 129.2, 127.9, 127.9, 124.4, 124.3, 118.7, 61.1, 33.8, 9.8. HR-MS (ESI-TOF) m/z: cal. for C17H16BrN2S [M + H]+: 358.0139 and 360.0119; found: 358.0139 and 360.0139.
2-(2-Bromophenyl)-2-cyano-N-3-diphenylpropanethioamide (3t)
Yield: 96% (0.404 g); yellow solid; Rf: 0.6 in 30% EtOAc in hexanes; mp: 150–152 °C; IR (ν cm–1) = 3745, 3004, 2986, 2349, 1697, 1469, 1257, 763, 755; 1H NMR (400 MHz, CDCl3) δ 8.68 (s, 1H), 7.65 (d, J = 8.0 Hz, 4H), 7.58 (d, J = 8.0 Hz, 3H), 7.40–7.36 (m, 3H), 7.31–7.24 (m, 6H), 7.21–7.17 (m, 1H), 7.08 (d, J = 7.6 Hz, 2H), 3.97 (d, J = 12.4 Hz, 1H), 3.72 (d, J = 12.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 196.0, 137.6, 136.4, 136.2, 133.5, 130.65, 130.57, 129.4, 129.0, 128.6, 128.4, 127.9, 127.8, 124.8, 124.5, 118.6, 61.1, 44.9. HR-MS (ESI-TOF) m/z: cal. for C22H17BrN2S [M + H]+: 443.0188 and 445.0168; found: 443.0183 and 445.0144.
2-(2-Bromophenyl)-2-cyano-N-phenylpent-4-enethioamide (3u)
Yield: 74% (0.274 g); yellow solid; Rf: 0.32 in 30% EtOAc in hexanes; mp: 125–127 °C; IR (ν cm–1) = 3343, 3274, 2359, 1595, 1467, 1374, 1076, 753, 641, 489; 1H NMR (400 MHz, CDCl3) δ 9.34 (s, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 7.6 Hz, 2H), 7.49–7.44 (m, 3H), 7.37–7.29 (m, 2H), 6.93–5.93 (m, 1H), 5.47–5.37 (m, 2H), 3.55 (dd, J = 13.0, 7.6 Hz, 1H), 3.36 (dd, J = 13.0, 6.9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 195.2, 137.9, 135.4, 135.2, 130.6, 130.1, 129.7, 129.2, 127.9, 127.9, 124.5, 124.4, 122.1, 118.5, 60.1, 44.3. HR-MS (ESI-TOF) m/z: cal. for C17H16BrNO2S [M + H]+: 393.0032 and 395.0011; found: 393.0037 and 395.0023.
Methyl 2-(2-bromophenyl)-2-methyl-3-(phenylamino)-3-thioxopropanoate (3v)
Yield: 70% (0.264 g); yellow solid; Rf: 0.32 in 30% EtOAc in hexanes; mp: 102–104 °C; IR (ν cm–1) = 3189, 3061, 2949, 2359, 1717, 1497, 1255, 1032, 741, 557, 460; 1H NMR (400 MHz, CDCl3) δ 12.49 (s, 1H), 7.82 (d, J = 7.6 Hz, 2H), 7.65–7.54 (m, 2H), 7.51–7.40 (m, 3H), 7.31 (t, J = 7.6 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 3.78 (s, 3H), 2.24 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 201.39, 177.6, 141.8, 139.2, 133.4, 129.4, 128.9, 128.8, 127.2, 126.9, 125.2, 123.8, 63.1, 53.8, 29.0. HR-MS (ESI-TOF) m/z: cal. for C17H16BrNO2S [M + H]+: 378.0158 and 380.0143; found: 378.0161 and 380.0138.
2-(2-Bromophenyl)-2-cyano-N-(1-phenylethyl)propanethioamide (3w)
Yield: 72% (0.268 g); yellow solid; Rf: 0.30 in 30% EtOAc in hexanes; mp: 132–134 °C; IR (ν cm–1) = 3357, 3287, 3061, 2931, 2359, 2239, 1509, 1392, 1049, 995, 545; diastereoselectivity ratio: 1:1 (only one diastereomer is given); 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H), 7.69 (d, J = 7.9 Hz, 1H), 7.57 (t, J = 8.0 Hz, 2H), 7.49–7.38 (m, 5H), 5.77–5.74 (m, 1H), 2.26 (s, 3H), 1.68 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 196.7, 140.6, 135.7, 135.2, 134.9, 130.7, 128.9, 128.1, 127.9, 126.4, 124.5, 119.6, 55.4, 55.5, 28.6, 19.3. HR-MS (ESI-TOF) m/z: cal. for C18H17N2SBr [M + H]+: 373.0369 and 375.0348; found: 373.1061 and 375.0361.
2-(2-Bromo-4,5-dimethoxyphenyl)-2-cyano-N-(1-phenylethyl)propanethioamide (3x)
Yield: 79% (0.342 g); pale yellow solid; Rf: 0.30 in 30% EtOAc in hexanes; mp: 94–96 °C; IR (ν cm–1) = 3289, 2934, 2841, 2359, 1596, 1390, 1262, 1172, 1022, 855, 667; diastereoselectivity ratio: 1:1 (only one diastereomer is given); 1H NMR (400 MHz) δ 7.93 (s, 1H), 7.39–7.35 (m, 5H), 7.095 (s, 1H), 7.03 (s, 1H), 5.75–5.71 (m, 1H), 3.879 (s, 6H), 2.21 (s, 3H), 1.64 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 197.0, 150.1, 148.5, 140.3, 128.9, 128.2, 127.5, 126.8, 119.8, 117.6, 114.9, 112.3, 56.4, 55.54, 55.50, 55.1, 28.8, 19.4. HR-MS (ESI-TOF) m/z: cal. for C20H21BrN2O2S [M + H]+: 433.0580 and 435.0568; found: 433.0583 and 435.0560.
2-(6-Bromo-3,4-methylenedioxyphenyl)-2-cyano-N-(1-phenylethyl)propanethioamide (3y)
Yield: 75% (0.312 g); yellow solid; Rf: 0.34 in 30% EtOAc in hexanes; mp: 142–144 °C; IR (ν cm–1) = 3353, 2902, 2359, 1725, 1657, 1480, 1389, 1125, 699, 430; diastereoselectivity ratio: 1:1 (only one diastereomer is given); 1H NMR (700 MHz, CDCl3) δ 7.79 (s, 1H), 7.44–7.29 (m, 5H), 7.06 (s, 1H), 7.01 (s, 1H), 6.04 (s, 2H), 5.72–5.70 (m, 1H), 2.17 (s, 3H), 1.62 (d, J = 7.0 Hz, 3H). 13C NMR (175 MHz, CDCl3) δ 197.2, 149.0, 147.9, 140.2, 129.0, 128.9, 128.2, 119.6, 116.0, 114.7, 109.1, 102.5, 55.5, 55.4, 55.0, 29.3, 19.4. HR-MS (ESI-TOF) m/z: cal. for C19H17BrN2O2S [M + H]+: 417.0267 and 419.0247; found: 417.0266 and 419.0245.
2-(2-Chlorophenyl)-2-cyano-N-phenylpropanethioamide (3z)
Yield: 74% (0.222 g); viscous liquid; Rf: 0.30 in 30% EtOAc in hexanes; IR (ν cm–1) = 3230, 2996, 2984, 2290, 1734, 1479, 1235, 1018, 735, 565; 1H NMR (400 MHz, CDCl3) δ 9.04 (s, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 8.0 Hz, 2H), 7.42 (d, J = 8.0 Hz, 1H), 7.36–7.33 (m, 4H), 7.24 (t, J = 8.0 Hz, 1H), 2.24 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 197.6, 138.0, 134.6, 134.4, 131.9, 130.9, 129.2, 128.9, 127.8, 127.6, 124.4, 119.8, 54.8, 28.2. HR-MS (ESI-TOF) m/z: cal. for C16H13ClN2S [M + H]+: 301.0561; found: 301.0548.
2-(2-Iodophenyl)-2-cyano-N-phenylpropanethioamide (3aa)
Yield: 70% (0.274 g); viscous liquid; Rf: 0.32 in 30% EtOAc in hexanes; IR (ν cm–1) = 3234, 3012, 2997, 2306, 1729, 1468, 1249, 1006, 729, 640; 1H NMR (400 MHz, CDCl3) δ 9.02 (s, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 8.0 Hz, 2H), 7.55 (t, J = 8.0 Hz, 1H), 7.47–7.43 (m, 2H), 7.37–7.33 (m, 1H), 2.43 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 197.4, 142.7, 138.0, 130.9, 129.7, 129.4, 129.2, 128.9, 127.8, 124.3, 119.8, 99.2, 59.2, 29.4. HR-MS (ESI-TOF) m/z: cal. for C16H13IN2S [M + H]+: 414.9736; found: 414.9743.
Procedure for Optimization of Benzo[b]thiolane 1a
An oven-dried 8 mL reaction vial was charged with copper salt (5 mol %), base (1.5 mmol), the respective thioamide 3a (0.5 mmol) in a solvent (2.0 mL). The mixture was stirred at 90 °C for 2.5–3.5 h. The reaction mixture was monitored by TLC. After the starting material was completely consumed, the reaction mixture was purified by flash chromatography using hexane and EtOAc as the eluents.
General Procedure for the Synthesis of Benzo[b]thiolanes 1
An oven-dried 8 mL reaction vial was charged with CuBr (5 mol %), Et3N (0.75 mmol), and respective thioamides 3a–y in 2.0 mL of DMF solvent, which was stirred at 90 °C for 2.5–12 h. The reaction mixture was monitored by TLC. After the starting material was completely consumed, the reaction mixture was purified by flash chromatography using hexane and EtOAc as the eluents.
3-Cyano-3-methyl-2-phenyliminobenzo[b]thiolane (1a)
Reaction time: 1.5 h; yield: 98% (0.129 g); pale yellow solid; Rf = 0.28 in 30% EtOAc in hexane; mp = 119–121 °C; IR (KBr, ν cm–1): 3449, 2364, 1653, 1448, 1113, 667; 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.2 Hz, 1H), 7.42 (t, J = 7.6 Hz, 2H), 7.36–7.30 (m, 2H), 7.26–7.21 (m, 2H), 7.05 (d, J = 8.0 Hz, 2H), 2.05 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 166.8, 150.2, 135.7, 135.3, 130.3, 129.4, 126.9, 125.9, 124.6, 123.1, 119.7, 119.4, 52.0, 28.8. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C16H12N2S: 265.0794; found: 265.0799.
3-Cyano-3-methyl-2-(4-methoxyphenyl)iminobenzo[b]thiolane (1b)
Reaction time: 1.5 h; yield: 91% (0.134 g); pale yellow solid; Rf = 0.33 in 30% EtOAc in hexane; mp = 143–145 °C; IR (KBr, ν cm–1): 2929, 2840, 2190, 1641, 1505, 1244, 838, 702; 1H NMR (400 MHz, CDCl3) δ 7.43 (t, J = 7.6 Hz, 2H), 7.24 (t, J = 8.0 Hz, 1H), 7.19–7.10 (m, 2H), 7.07 (d, J = 8.0 Hz, 2H), 6.94 (d, J = 8.8 Hz, 1H), 3.87 (s, 3H), 2.06 (s, 3H).13C NMR (100 MHz, CDCl3) δ 170.4, 155.6, 145.3, 135.6, 134.2, 130.6, 127.5, 125.4, 124.8, 123.4, 120.2, 118.9, 52.3, 28.98, 28.99. HR-MS (ESI-TOF), m/z: [M + H] + cal. for C17H14N2OS: 295.0900; found: 295.0908.
3-Cyano-3-methyl-2-(4-methylphenyl)iminobenzo[b]thiolane (1c)
Reaction time: 2 h; yield: 85% (0.129 g); brown viscous liquid; Rf = 0.29 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2950, 2365, 1270, 1018, 759, 665; 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.6 Hz, 1H), 7.38–7.29 (m, 2H), 7.26–7.23 (m, 3H), 6.99 (d, J = 8.0 Hz, 2H), 2.39 (s, 3H), 2.04 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.9, 147.6, 135.72, 135.69, 135.4, 130.2, 129.9, 126.9, 124.6, 123.1, 119.8, 119.5, 52.0, 28.8, 21.1. HR-MS (ESI-TOF), m/z: [M + Na]+ cal. for C17H14N2S: 301.0770; found: 301.0787.
3-Cyano-3-methyl-2-(4-fluorophenyl)iminobenzo[b]thiolane (1d)
Reaction time: 1.5 h; yield: 79% (0.111 g); pale yellow solid; Rf = 0.28 in 30% EtOAc in hexane; mp = 148–150 °C; IR (KBr, ν cm–1): 3335, 2150,1683, 1509, 1410, 1220, 838, 761; 1H NMR (400 MHz, CDCl3) δ 7.71–7.65 (m, 2H), 7.49–7.46 (m, 1H), 7.42–7.39 (m, 2H), 7.35–7.30 (m, 1H), 7.05–7.00 (m, 2H), 2.18 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.1, 160.2 (d, J = 244.2 Hz), 135.2, 134.1, 132.7 (d, J = 2.9 Hz), 131.1, 129.2, 128.5, 123.8, 122.8 (d, J = 8.3 Hz), 119.4, 115.9 (d, J = 22.7 Hz), 50.1, 24.2; 19F NMR (376 Hz, CDCl3) δ −116.19. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C16H11N2SF: 283.0700; found: 283.0732.
3-Cyano-3-methyl-2-(4-nitrophenyl)iminobenzo[b]thiolane (1e)
Reaction time: 1 h; yield: 97% (0.149 g); pale yellow solid; Rf = 0.28 in 30% EtOAc in hexane; mp = 147–149 °C; IR (KBr, ν cm–1): 3335, 2253, 1683, 1509, 1410, 1220, 838, 761; 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 8.4 Hz, 2H), 7.58 (d, J = 7.2 Hz, 1H), 7.42–7.38 (m, 2H), 7.29 (d, J = 7.6 Hz, 1H), 7.15 (d, J = 8.8 Hz, 2H), 2.08 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.4, 155.6, 145.3, 135.6, 134.1, 130.6, 127.5, 125.4, 124.8, 123.3, 120.2, 118.9, 52.3, 28.8. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C16H11N3O2S: 310.0645; found: 310.0617.
3-Cyano-3-methyl-2-(2-methoxyphenyl)iminobenzo[b]thiolane (1f)
Reaction time: 1.5 h; yield: 90% (0.132 g); pale yellow solid; Rf = 0.32 in 30% EtOAc in hexane; mp = 106–108 °C; IR (KBr, ν cm–1): 3469, 2837, 2240, 1651, 1589, 1490, 1254, 751, 712; 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.2 Hz, 1H), 7.36–7.26 (m, 2H), 7.23–7.17 (m, 2H), 7.01–6.96 (m, 2H), 6.92 (d, J = 7.6 Hz, 1H), 3.83 (s, 3H), 2.07 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.4, 149.6, 139.6, 136.0, 135.4, 130.2, 126.8, 126.7, 124.6, 123.0, 121.1, 119.8, 119.3, 112.3, 112.3, 55.9, 51.6, 28.8. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C17H14N2OS: 295.0900; found: 295.0914.
3-Cyano-3-methyl-2-(2-trifluoromethylphenyl)iminobenzo[b]thiolane (1g)
Reaction time: 2.5 h; yield: 75% (0.124 g); pale yellow solid; Rf = 0.25 in 30% EtOAc in hexane; mp = 106–108 °C; IR (KBr, ν cm–1): 3851, 2949, 2258, 1700, 1507, 1289, 665; 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.0 Hz, 1H), 7.62–7.56 (m, 2H), 7.39–7.30 (m, 3H), 7.26 (d, J = 7.6 Hz, 1H), 7.05 (d, J = 8.0 Hz, 1H), 2.08 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.1, 148.56, 148.55, 135.8, 134.6, 133.1, 130.5, 127.2, 126.8 (q, J = 9.5 Hz), 125.3, 124.8, 123.5 (q, J = 271.6 Hz), 120.8 (q, J = 30.8 Hz), 119.2, 118.9, 51.9, 28.4; 19F NMR (376 Hz, CDCl3) δ −61.44. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C17H11F3N2S: 333.0668; found: 333.0666.
3-Cyano-3-methyl-2-(benzyl)iminobenzo[b]thiolane (1h)
Reaction time: 6 h; yield: 65% (0.090 g); pale yellow solid; Rf = 0.28 in 30% EtOAc in hexane; mp = 84–86 °C; IR (KBr, ν cm–1): 3449, 2364, 1653, 1490, 1254, 751, 667; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 7.2 Hz, 1H), 7.43–7.28 (m, 8H), 4.71 (d, J = 15.6 Hz, 1H), 4.56 (d, J = 15.6 Hz, 1H), 1.97 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.2, 137.9, 136.0, 134.8, 130.2, 128.5, 127.6, 127.2, 126.9, 124.6, 123.2, 119.7, 61.1, 51.6, 28.8. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C17H14N2S: 279.0950; found: 279.0958.
3-Cyano-3-methyl-2-(isopropyl)iminobenzo[b]thiolane (1i)
Reaction time: 6 h; yield: 73% (0.084 g); brown viscous liquid; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 3547, 2934, 2238, 1651, 1130, 987, 756; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 7.2 Hz, 1H), 7.38–7.27 (m, 3H), 3.40–3.34 (m, 1H), 1.90 (s, 3H), 1.31 (d, J = 6.0 Hz, 3H), 1.25 (d, J = 6.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.8, 135.9, 135.2, 130.0, 126.6, 124.5, 123.1, 119.9, 59.7, 51.2, 28.8, 22.5, 22.4. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C13H14N2S: 231.0950; found: 231.0944.
3-Cyano-3-methyl-2-(ethyl)iminobenzo[b]thiolane (1j)
Reaction time: 12 h; yield: 79% (0.084 g); yellow viscous liquid; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 3452, 2979, 2349, 1656, 1470, 982, 756; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 8.0 Hz, 1H), 7.38–7.28 (m, 3H), 3.52–3.34 (m, 2H), 1.91 (s, 3H), 1.36 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 163.1, 136.0, 135.0, 130.1, 126.7, 124.5, 123.2, 119.8, 52.8, 51.3, 28.8, 14.7. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C12H12N2S: 217.0794; found: 217.0805.
3-Cyano-3-methyl-2-(tert-butyl)iminobenzo[b]thiolane (1k)
Reaction time: 6.5 h; yield: 77% (0.094 g); yellow viscous liquid; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 3452, 2979, 2336, 1656, 1512, 1470, 982, 756; 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.2 Hz, 1H), 7.33 (d, J = 7.6 Hz, 1H), 7.34–7.24 (m, 2H), 1.86 (s, 3H), 1.38 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 155.3, 136.7, 135.3, 129.9, 126.4, 124.4, 122.6, 120.3, 57.2, 53.6, 29.3, 28.1. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C14H16N2S: 245.1107; found: 245.1109.
3-Cyano-3-methyl-2-phenylimino-5,6-dimethoxybenzo[b]thiolane (1l)
Reaction time: 1 h; yield: 96% (0.155 g); brown viscous liquid; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2946, 2365, 2237, 1265, 1012, 810, 657; 1H NMR (400 MHz, CD3SOCD3) δ 7.46 (t, J = 7.6 Hz, 2H), 7.39 (s, 1H), 7.25 (t, J = 7.0 Hz, 2H), 7.14 (s, 1H), 7.04 (d, J = 8.0 Hz, 2H), 3.84 (s, 3H), 3.77 (s, 3H), 2.02 (s, 3H); 13C NMR (100 MHz, CD3SOCD3) δ 169.1, 151.4, 150.5, 149.2, 130.0, 126.8, 126.2, 125.4, 120.2, 119.8, 109.2, 107.4, 56.7, 56.5, 52.2, 27.9. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C18H16N2O2S: 325.1005; found: 325.1032.
3-Cyano-3-methyl-2-(phenylimino)-5,6-methylenedioxybenzo[b]thiolane (1m)
Reaction time: 1 h; yield: 92% (0.155 g); pale yellow solid; mp = 121–123 °C; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2952, 2815, 2340, 1628, 1539, 1195, 750, 660; 1H NMR (400 MHz, CDCl3) δ 7.42 (t, J = 7.8 Hz, 2H), 7.24 (t, J = 7.0 Hz, 1H), 7.05 (d, J = 8.0 Hz, 2H), 6.69 (s, 1H), 6.03 (s, 2H), 2.02 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 167.4, 150.1, 149.5, 147.5, 129.4, 127.7, 126.9, 125.9, 119.7, 119.4, 105.2, 103.6, 102.0, 52.1, 28.7. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C17H12N2O2S: 309.0692; found: 309.0631.
3-Cyano-3-methyl-2-phenylimino-6,7-dimethoxybenzo[b]thiolane (1n)
Reaction time: 2 h; yield: 79% (0.128 g); white solid; mp = 128–130 °C; Rf = 0.30 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2930, 2829, 2339, 1630, 1506, 1239, 826, 700; 1H NMR (700 MHz, CDCl3) δ 7.40 (t, J = 7.7 Hz, 2H), 7.20 (t, J = 7.7 Hz, 1H), 7.03 (d, J = 7.7 Hz, 2H), 6.92 (d, J = 8.4 Hz, 1H), 6.86 (d, J = 8.4 Hz, 2H), 4.10 (s, 3H), 3.88 (s, 3H), 2.08 (s, 3H); 13C NMR (175 MHz, CDCl3) δ 168.0, 151.5, 150.4, 146.7, 129.3, 129.0, 126.3, 125.7, 119.6, 117.6, 114.7, 60.9, 56.2, 51.2, 26.6. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C18H16N2O2S: 325.1005; found: 325.1004.
3-Cyano-3-methyl-2-(4-methylphenyl)imino-6,7-dimethoxybenzo[b]thiolane (1o)
Reaction time: 2 h; yield: 80% (0.135 g); pale yellow solid; mp = 124–126 °C; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2949, 2367, 1269, 1016, 811, 667; 1H NMR (400 MHz, CDCl3) δ 7.20 (d, J = 8.0 Hz, 2H), 6.96–6.85 (m, 4H), 4.09 (s, 3H), 3.88 (s, 3H), 2.36 (s, 3H), 2.06 (s, 3H); 13C NMR (175 MHz, CDCl3) δ 167.1, 151.4, 147.8, 146.7, 135.4, 129.8, 129.0, 126.5, 119.7, 119.6, 117.5, 114.6, 60.8, 56.2, 51.2, 26.6, 21.0. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C19H18N2O2S: 339.1162; found: 339.1161.
3-Cyano-3-methyl-2-phenylimino-6-chlorobenzo[b]thiolane (1p)
Reaction time: 1 h; yield: 42% (0.062 g); brown viscous liquid; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 3442, 2362, 1641, 1252, 970, 670; 1H NMR (400 MHz, CDCl3) δ 7.49–7.43 (m, 3H), 7.32–7.24 (m, 3H), 7.06 (d, J = 7.6 Hz, 2H), 2.05(s, 3H); 13C NMR (175 MHz, CDCl3) δ 165.6, 149.8, 137.2, 136.2, 129.5, 127.3, 126.1, 125.5, 123.1, 119.6, 118.9, 51.5, 28.7. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C16H11ClN2S: 299.0404; found: 299.0393.
3-Cyano-3-methyl-2-phenylimino-6-fluorobenzo[b]thiolane (1q)
Reaction time: 1 h; yield: 35% (0.049 g); white solid; mp = 106–108 °C; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 3427, 2359, 1656, 1222, 670; 1H NMR (400 MHz, CDCl3) δ 7.51–7.48 (m, 1H), 7.43–7.39 (m, 2H), 7.25–7.21 (m, 1H), 7.04–7.00 (m, 3H), 6.99–6.95 (m, 1H), 2.02 (s, 3H); 13C NMR (175 MHz, CDCl3) δ 165.4 (d, J = 234 Hz), 162.3, 149.9, 137.4 (d, J = 16.9 Hz), 131.5 (d, J = 8.0 Hz), 129.5, 126.2, 126.0 (d, J = 16.1 Hz), 119.7, 119.2, 114.3 (d, J = 40 Hz), 110.8 (d, J = 45.1 Hz), 51.3, 28.9; 19F NMR (376 Hz, CDCl3) δ −109.43. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C16H11FN2S: 283.0700; found: 283.0678.
3-Cyano-3-methyl-2-phenylimino-4,6-di-tertbutylbenzo[b]thiolane (1r)
Reaction time: 3 h; yield: 92%; pale yellow solid; mp = 192–194 °C; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2964, 2341, 1635, 1538, 1436, 1145, 1071, 743, 502, 442; 1H NMR (700 MHz, CDCl3) δ 7.44–7.42 (m, 4H), 7.22 (t, J = 7.7 Hz, 1H), 7.08 (d, J = 7.7 Hz, 2H), 2.04 (s, 3H), 1.36 (s, 9H), 1.35(s, 9H); 13C NMR (100 MHz, CDCl3) δ 167.2, 150.4, 144.9, 136.7, 129.3, 129.2, 125.6, 124.7, 120.0, 119.9, 119.2, 51.7, 35.8, 34.9, 31.3, 29.4, 29.3. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C24H28N2S: 377.2046; found: 377.2050.
3-Cyano-3-ethyl-2-phenyliminobenzo[b]thiolane (1s)
Reaction time: 2.5 h; yield: 95%; pale yellow solid; Rf: 0.6 in 30% EtOAc in hexane; mp = 85–86 °C; IR (KBr, ν cm–1) = 3738, 3006, 2989, 2849, 2253, 1763, 1279, 1264, 763, 656. 1H NMR (400 MHz, CDCl3) δ 7.50–7.48 (m, 1H), 7.41 (t, J = 7.8 Hz, 2H), 7.37–7.28 (m, 2H), 7.24–7.19 (m, 2H), 7.04–7.01 (m, 2H), 2.51–2.41 (m, 1H), 2.39–2.32 (m, 1H), 1.05 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 166.4, 150.4, 136.4, 134.2, 130.2, 129.5, 126.8, 125.9, 125.0, 123.1, 119.7, 119.0, 56.6, 35.9, 8.1. HR-MS (ESI-TOF), m/z [M + H]+ cal. for C17H14N2S: 279.0950; found: 279.0954.
3-Cyano-3-benzyl-2-phenyliminobenzo[b]thiolane (1t)
Reaction time: 2.5 h; yield: 85%; white solid; Rf: 0.7 in 30% EtOAc in hexanes; mp = 145–147 °C; 1H NMR (400 MHz, CDCl3) δ 7.33 (t, J = 7.6 Hz, 2H), 7.25–7.14 (m, 7H), 7.04 (d, J = 7.6 Hz, 1H), 6.95 (d, J = 7.6 Hz, 2H), 6.89 (d, J = 6.8 Hz, 2H), 3.60–3.51 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 166.2, 150.1, 136.5, 133.3, 132.5, 130.7, 130.3, 129.4, 128.2, 128.0, 126.3, 125.95, 125.86, 122.9, 119.8, 118.5, 57.1, 48.0. HR-MS (ESI-TOF), m/z [M + H]+ cal. for C22H16N2S: 341.1107; found: 341.1092.
3-Cyano-3-allyl-2-phenyliminobenzo[b]thiolane (1u)
Reaction time: 6 h; yield: 52% (0.075 g); viscous liquid; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 3447, 2956, 2248, 1652, 1465, 960, 742, 656; 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 8.0 Hz, 4H), 7.44 (t, J = 7.8 Hz, 2H), 7.39–7.31 (m, 2H), 7.25 (d, J = 7.2 Hz, 2H), 7.05 (d, J = 8.4 Hz, 2H), 5.81–5.70 (m, 1H), 5.24 (t, J = 15.2 Hz, 2H), 3.13 (d, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 165.9, 150.3, 136.1, 133.7, 130.2, 129.4, 129.1, 126.6, 125.9, 125.3, 123.0, 121.9, 119.7, 118.5, 55.7, 46.3. HR-MS (ESI-TOF), m/z: [M + Na]+ cal. for C18H14N2S: 313.0770; found: 313.0803.
3-Methyl ester-3-methyl-2-phenyliminobenzo[b]thiolane (1v)
Reaction time: 3.5 h; yield: 89% (0.132 g); brown viscous compound; Rf = 0.28 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2954, 2280, 1745, 1487, 1237, 959, 697; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 7.6 Hz, 1H), 7.37–7.27 (m, 4H), 7.08 (d, J = 8.8 Hz, 2H), 6.97 (d, J = 8.8 Hz, 2H), 3.85 (s, 3H), 2.04(s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.7, 157.8, 142.9, 136.6, 135.5, 130.2, 126.9, 124.6, 123.1, 121.6, 119.6, 144.5, 55.5, 52.2, 28.9. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C17H15N2OS: 298.0896; found: 298.0905.
3-Cyano-3-methyl-2-(1-phenylethyl)iminobenzo[b]thiolane (1w)
Reaction time: 1.5 h; yield: 75% (0.109 g); viscous liquid; Rf = 0.29 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 3061, 2972, 2850, 2240, 1651, 1352, 1026, 884, 578; diastereoselectivity ratio: 1:1 (only one diastereomer is given); 1H NMR (400 MHz, CDCl3) δ 7.52–7.45 (m, 3H), 7.41–7.35 (m, 3H), 7.32–7.28 (m, 3H), 4.44–4.38 (m, 1H), 2.00 (s, 3H), 1.66 (d, J = 6.4 Hz, 3H); 13C NMR (175 MHz, CDCl3) δ 162.8, 143.7, 135.9, 135.1, 130.1, 128.5, 127.2, 126.8, 126.4, 124.6, 123.2, 119.7, 66.9, 51.5, 29.1, 23.9. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C18H16N2S: 293.1107; found: 293.1135.
3-Cyano-3-methyl-2-(1-phenylethyl)imino-5,6-methylenedioxybenzo[b]thiolane (1x)
Reaction time: 2 h; yield: 75% (0.132 g); viscous liquid; Rf = 0.32 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2967, 2359, 1653, 1497, 1277, 1175, 1032, 699, 435; diastereoselectivity ratio: 1:1 (only one diastereomer is given); 1H NMR (400 MHz, CDCl3) δ 7.43–7.28 (m, 5H), 6.99 (s, 1H), 6.76 (s, 1H), 4.36–4.33 (m, 1H), 3.92 (s, 3H), 3.88 (s, 3H), 1.96 (s, 3H), 1.62 (d, J = 5.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 163.7, 150.9, 149.6, 143.7, 128.5, 127.2, 125.9, 120.1, 114.0, 107.5, 105.9, 67.3, 56.3, 51.9, 29.7, 29.0, 23.9. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C20H20N2O2S: 353.1318; found: 353.1301.
3-Cyano-3-methyl-2-(1-phenylethyl)imino-5,6-dimethoxybenzo[b]thiolane (1y)
Reaction time: 2.5 h; yield: 66% (0.126 g); viscous liquid; Rf = 0.30 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2958, 2365, 1637, 1285, 1167, 1022, 720, 585; diastereoselectivity ratio: 1:1 (only one diastereomer is given); 1H NMR (400 MHz, CDCl3) δ 7.46–7.28 (m, 5H), 6.79 (s, 1H), 6.74 (s, 1H), 6.01 (s, 2H), 4.36–4.33 (m, 1H), 1.95 (s, 3H), 1.63 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 155.9, 142.7, 136.6, 132.9, 132.4, 128.59, 128.58, 127.9, 127.7, 126.7, 126.2, 114.0, 113.9, 50.2, 50.1, 40.1, 34.2. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C19H16N2O2S: 337.1005; found: 337.1000.
Procedure for (Z)-3-Methyl-2-(phenylimino)-2,3-dihydrobenzo[b]thiophene-3-carboxamide (7a)
An oven-dried 8 mL reaction vial was charged with 1a (0.50 mmol) and TfOH (5 mmol) in 2.0 mL of DCE solvent, which was stirred at 60 °C for 2 h. The reaction mixture was monitored by TLC. After the starting material was completely consumed, the reaction mixture was quenched with saturated NaHCO3 solution and extracted with EtOAc. The combined organic layer was washed with water (3 × 25 mL), dried over anhydrous Na2SO4, and concentrated under a reduced pressure. The crude product was purified by flash chromatography using hexane and EtOAc as the eluents.
Reaction time: 2 h; yield: 77% (0.108 g); pale yellow solid; Rf = 0.28 in 30% EtOAc in hexane; mp = 121–123 °C; IR (KBr, ν cm–1): 2958, 2365, 1637, 1285, 1167, 1022, 720, 585; 1H NMR (400 MHz, CDCl3) δ 7.65–7.31 (m, 1H), 7.44 (t, J = 7.4 Hz, 2H), 7.30–7.24 (m, 4H), 7.04 (d, J = 7.2 Hz, 2H), 5.64 (s, 1H), 1.97 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 174.5, 172.3, 151.2, 139.4, 135.0, 129.5, 129.0, 127.0, 126.2, 125.6, 122.3, 119.8, 63.5, 28.4. HR-MS (ESI-TOF), m/z: [M + Na]+ cal. for C16H14N2OS: 305.0719; found: 305.0725.
Procedure for 2-(2-Mercaptophenyl)-2-methyl-N-phenylmalonamide (8a)
In an oven-dried round-bottom flask, 1a (0.5 mmol) was taken. To it, conc. H2SO4 was added (10 mL). Then, it was stirred at room temperature for 2 h (in the case of HCl, 1a was dissolved in EtOH (12 mL). To it, conc. HCl (1.5 mmol) was added. Then, it was stirred at 60 °C for 3 h). After the starting material (monitored by TLC) was completely consumed, the reaction was quenched with cold NaHCO3 solution and extracted with EtOAc. The combined organic layer was washed with water (3 × 25 mL), dried over anhydrous Na2SO4, and concentrated under a reduced pressure. The crude product was purified by flash chromatography using hexane and EtOAc as eluents.
Reaction time: 2 h; yield: 99% (0.148 g) [68% (0.102 g)]; viscous liquid; Rf = 0.25 in 30% EtOAc in hexane; IR (KBr, ν cm–1): 2960, 2100, 1549, 1290, 1158, 1122, 720, 585; 1H NMR (400 MHz, CDCl3) δ 7.66–7.65 (m, 1H), 7.44 (t, J = 7.8 Hz, 2H), 7.32–7.23 (m, 4H), 7.06 (d, J = 7.2 Hz, 2H), 5.83 (s, 1H), 1.99 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 174.5, 172.0, 151.0, 139.2, 134.9, 129.4, 128.9, 126.9, 126.1, 125.5, 122.2, 119.7, 63.4, 28. HR-MS (ESI-TOF), m/z: [M + H]+ cal. for C16H16N2O2S: 301.1000; found: 301.1010.
Crystal data for 1a in CH2Cl2/n-hexane: C16H12N2S, Mw = 264.34, triclinic, space group P-1, a = 8.1526(2) Å, b = 9.0963(2) Å, c = 10.4069(2) Å, α = 106.308(2)°, β = 104.374(2)°, γ = 103.637(2)°, V = 677.69(3) Å3, Z = 2, Dcalc = 1.295 g/cm3, T = 291 K, R1 = 0.0408(2645), wR2 = 0.1105(2813), and GOF = 1.046.
Crystal data for 7a in CH2Cl2/n-hexane: C16H14N2OS, Mw = 282.35, monoclinic, space group C 2/c, a = 18.6061(13) Å, b = 6.3779(3) Å, c = 24.5520(12) Å, α = 90°, β = 103.547(4)°, γ = 90°, V = 2832.5(3) Å3, Z = 8, Dcalc = 1.324 g/cm3, T = 293 K, R1 = 0.0149(2945), wR2 = 0.0276(3228), and GOF = 1.092.
Acknowledgments
The Department of Atomic Energy supported this work. We thank the Science and Engineering Research Board (SERB), New Delhi (Grant EMR/2017/005521) for the financial support. We thank Dr. Krishnan Venkatasubbaiah for solving one of the X-ray crystal data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03410.
The authors declare no competing financial interest.
Supplementary Material
References
- Selected papers; a Lu Y.; Gutgesell M. L.; Xiong R.; Zhao J.; Li Y.; Rosales I. C.; Hollas M.; Shen Z.; Gordon-Blake J.; Dye K.; Wang Y.; Lee S.; Chen H.; He D.; Dubrovyskyii O.; Zhao H.; Huang F.; Lasek W. A.; Tonetti A. D.; Thatcher R. J. G. Design and Synthesis of Basic Selective Estrogen Receptor Degraders for Endocrine Therapy Resistant Breast Cancer. J. Med. Chem. 2019, 62, 11301–11323. 10.1021/acs.jmedchem.9b01580. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kawamoto Y.; Tomino M.; Hiramatsu K.; Oyama Y.; Hayashi Y. Benzothiophene derivatives as phosphodiesterase 10A (PDE10A) inhibitors: Hit-to-lead studies. Bioorg. Med. Chem. Lett. 2019, 29, 1419–1422. 10.1016/j.bmcl.2019.03.021. [DOI] [PubMed] [Google Scholar]; c Pieroni M.; Azzali E.; Basilico N.; Parapini S.; Zolkiewski M.; Beato C.; Annunziato G.; Bruno A.; Vacondio F.; Costantino G. Accepting the Invitation to Open Innovation in Malaria Drug Discovery: Synthesis, Biological Evaluation, and Investigation on the Structure–Activity Relationships of Benzo[b]thiophene-2-carboxamides as Antimalarial Agents. J. Med. Chem. 2017, 60, 1959–1970. 10.1021/acs.jmedchem.6b01685. [DOI] [PubMed] [Google Scholar]; d Chalmers J. M.; Wang Y.; Novick S.; Sato M.; Bryant U. H.; Montrose-Rafizdeh C.; Griffin R. P.; Dodge A. J. Hydrophobic Interactions Improve Selectivity to ERα for Benzothiophene SERMs. ACS Med. Chem. Lett. 2012, 3, 207–210. 10.1021/ml2002532. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Berrade L.; Aisa B.; Ramirez J. M.; Galiano S.; Guccione S.; Moltzau R. L.; Levy O. F.; Nicoletti F.; Battaglia G.; Molinaro G.; Aldana I.; Monge A.; Perez-Silanes S. Novel Benzo[b]thiophene Derivatives as New Potential Antidepressants with Rapid Onset of Action. J. Med. Chem. 2011, 54, 3086–3090. 10.1021/jm2000773. [DOI] [PubMed] [Google Scholar]; f Qin Z.; Kastrati I.; Chandrasena R. E. P.; Liu H.; Yao P.; Petukhov P. A.; Bolton J. L.; Thatcher G. R. J. Benzothiophene Selective Estrogen Receptor Modulators with Modulated Oxidative Activity and Receptor Affinity. J. Med. Chem. 2007, 50, 2682–2692. 10.1021/jm070079j. [DOI] [PubMed] [Google Scholar]
- Mitsui C.; Okamoto T.; Yamagishi M.; Tsurumi J.; Yoshimoto K.; Nakahara K.; Soeda J.; Hirose Y.; Sato H.; Yamano A.; Uemura T.; Takeya J. High-Performance Solution-Processable N-Shaped Organic Semiconducting Materials with Stabilized Crystal Phase. Adv. Mater. 2014, 26, 4546–4551. 10.1002/adma.201400289. [DOI] [PubMed] [Google Scholar]
- Selected Benzothiophene synthesis; a Zhang D.; Cai J.; Du J.; Wang X.; He W.; Yang Z.; Liu C.; Fang Z.; Guo K. Oxidant- and Catalyst-Free Synthesis of Sulfonated Benzothiophenes via Electrooxidative Tandem Cyclization. J. Org. Chem. 2021, 86, 2593–2601. 10.1021/acs.joc.0c02679. [DOI] [PubMed] [Google Scholar]; b Matsuzawa T.; Hosoya T.; Yoshida S. Transition-Metal-Free Synthesis of N-Arylphenothiazines through an N- and S-Arylation Sequence. Org. Lett. 2021, 23, 2347–2352. 10.1021/acs.orglett.1c00515. [DOI] [PubMed] [Google Scholar]; c Wang H.; Wu Q.; Zhang J. D.; Li H. Y.; Li H. X. Photocatalyst- and Transition-Metal-Free Visible-Light-Promoted Intramolecular C(sp2)–S Formation. Org. Lett. 2021, 23, 2078–2083. 10.1021/acs.orglett.1c00235. [DOI] [PubMed] [Google Scholar]; d Sundaravelu N.; Nandy A.; Sekar G. Visible Light Mediated Photocatalyst Free C–S Cross Coupling: Domino Synthesis of Thiochromane Derivatives via Photoinduced Electron Transfer. Org. Lett. 2021, 23, 3115–3119. 10.1021/acs.orglett.1c00806. [DOI] [PubMed] [Google Scholar]; e Kumar Y.; Ila H. Synthesis of Substituted Benzo[b]thiophenes via Base-Promoted Domino Condensation–Intramolecular C–S Bond Formation. Org. Lett. 2021, 23, 1698–1702. 10.1021/acs.orglett.1c00085. [DOI] [PubMed] [Google Scholar]; f Cai T.; Shen F.; Ni Y.; Xu H.; Shen R.; Gao Y. Cascade Radical Annulation of 2-Alkynylthio(seleno)anisoles with Acetone or Acetonitrile: Synthesis of 3-Acetonethyl- or Cyanomethyl-Substituted Benzothio(seleno)phenes. J. Org. Chem. 2021, 86, 1002–1011. 10.1021/acs.joc.0c02444. [DOI] [PubMed] [Google Scholar]; g Zhang W.; Tao S.; Ge H.; Li Q.; Ai Z.; Li X.; Zhang B.; Sun F.; Xu X.; Du Y. Construction of 2-Arylbenzo[4,5]thieno[2,3-d]thiazole Skeleton via CuCl/S-Mediated Three-Component Reaction. Org. Lett. 2020, 22, 448–452. 10.1021/acs.orglett.9b04206. [DOI] [PubMed] [Google Scholar]; h Cheng B.; Li Y.; Wang T.; Zhang X.; Li H.; He Y.; Li Y.; Zhai H. Application of Pyridinium 1,4-Zwitterionic Thiolates: Synthesis of Benzopyridothiazepines and Benzothiophenes. J. Org. Chem. 2020, 85, 6794–6802. 10.1021/acs.joc.0c00374. [DOI] [PubMed] [Google Scholar]; i Matsuzawa T.; Hosoya T.; Yoshida S. One-step synthesis of benzo[b]thiophenes by aryne reaction with alkynyl sulfides. Chem. Sci. 2020, 11, 9691–9696. 10.1039/D0SC04450D. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhang J.; Zhuang Y.; Ma Y.; Yang X.; Szostak M. Palladium-Catalyzed Synthesis of Benzothiophenes via Cross-Dehydrogenative Coupling of 4-Arylthiocoumarins and Pyrones. Adv. Synth. Catal. 2019, 361, 5709–5714. 10.1002/adsc.201901058. [DOI] [Google Scholar]; k Xu D.; Qi X.; Duan M.; Yu Z.; Zhu L.; Shan C.; Yue X.; Bai R.; Lan Y. Thiolate–palladium(IV) or sulfonium–palladate(0)? A theoretical study on the mechanism of palladium-catalyzed C–S bond formation reactions. Org. Chem. Front. 2017, 4, 943–950. 10.1039/C6QO00841K. [DOI] [Google Scholar]; l Xie X.; Li P.; Shi Q.; Wang L. Visible-light-induced tandem cyclization of 2-alkynylanilines with disulfides: a convenient method for accessing benzothiophenes under transition-metal-free and photocatalyst-free conditions. Org. Biomol. Chem. 2017, 15, 7678–7684. 10.1039/C7OB01747B. [DOI] [PubMed] [Google Scholar]; m Masuya Y.; Tobisu M.; Chatani N. Org. Lett. 2016, 18, 4312–4315. 10.1021/acs.orglett.6b02055. [DOI] [PubMed] [Google Scholar]; n Tandem Thien- and Benzannulations of α-Alkenoyl-α-alkynyl Ketene Dithioacetals with Cyanoacetates: Synthesis of Functionalized Benzo[b]thiophenes. Org. Lett. 2015, 17, 1746–1749 10.1021/acs.orglett.5b00523. [DOI] [PubMed] [Google Scholar]; Selected Review; o Sundaravelu N.; Sangeetha S.; Sekar G. Metal-catalyzed C–S bond formation using sulfur surrogates. Org. Biomol. Chem. 2021, 19, 1459–1459. 10.1039/D0OB02320E. [DOI] [PubMed] [Google Scholar]
- a Godoi B.; Schumacher R. F.; Zeni G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011, 111, 2937–2980. 10.1021/cr100214d. [DOI] [PubMed] [Google Scholar]; b Takimiya K.; Osaka I.; Mori T.; Nakano M. Organic Semiconductors Based on [1]Benzothieno[3,2-b][1]benzothiophene Substructure. Acc. Chem. Res. 2014, 47, 1493–1502. 10.1021/ar400282g. [DOI] [PubMed] [Google Scholar]; c Yao H.; Ye L.; Zhang H.; Li S.; Zhang S.; Hou J. Molecular Design of Benzodithiophene-Based Organic Photovoltaic Materials. Chem. Rev. 2016, 116, 7397–7457. 10.1021/acs.chemrev.6b00176. [DOI] [PubMed] [Google Scholar]; d Wu B.; Yoshikai N. Recent developments in synthetic methods for benzo[b]heteroles. Org. Biomol. Chem. 2016, 14, 5402–5416. 10.1039/C6OB00219F. [DOI] [PubMed] [Google Scholar]
- a Romagnoli R.; Baraldi P. G.; Carrion M. D.; Cara C. L.; Preti D.; Fruttarolo F.; Pavani M. G.; Tabrizi M. A.; Tolomeo M.; Grimaudo S.; di Cristina A.; Balzarini J.; Hadfield J. A.; Brancale A.; Hamel E. Synthesis and Biological Evaluation of 2- and 3-Aminobenzo[b]thiophene Derivatives as Antimitotic Agents and Inhibitors of Tubulin Polymerization. J. Med. Chem. 2007, 50, 2273–2277. 10.1021/jm070050f. [DOI] [PubMed] [Google Scholar]; b Chonan T.; Wakasugi D.; Yamamoto D.; Yashiro M.; Oi T.; Tanaka H.; Ohoka-Sugita A.; Io F.; Koretsune H.; Hiratate A. Discovery of novel (4-piperidinyl)-piperazines as potent and orally active acetyl-CoA carboxylase 1/2 non-selective inhibitors: F-Boc and triF-Boc groups are acid-stable bioisosteres for the Boc group. Bioorg. Med. Chem. 2011, 19, 1580–1593. 10.1016/j.bmc.2011.01.041. [DOI] [PubMed] [Google Scholar]
- Dadiboyena S. Recent advances in the synthesis of raloxifene: A selective estrogen receptor modulator. Eur. J. Med. Chem. 2012, 51, 17–34. 10.1016/j.ejmech.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Selected 2-Aminobenzothiophene synthesis:; a Stacy G. W.; Villaescusa F. W.; Wollner T. E. 2-Aminobenzo[b]thiophene. An Aromatic Ring Tautomer1,2. J. Org. Chem. 1965, 30, 4074–4078. 10.1021/jo01023a021. [DOI] [Google Scholar]; b Grandclaudon P.; Lablache-Combier A. Addition of Cyclic Secondary Amines to Benzo[b]thiophene and 3-Methylbenzo[b]thiophene. J. Org. Chem. 1978, 43, 4379–4381. 10.1021/jo00416a032. [DOI] [Google Scholar]; c Solovyev A. Y.; Androsov D. A.; Neckers D. C. One-Pot Synthesis of Substituted 2-Aminobenzo[b]thiophenes. J. Org. Chem. 2007, 72, 3122–3124. 10.1021/jo062141a. [DOI] [PubMed] [Google Scholar]; d Singh P. P.; Yadav A. K.; Ila H.; Junjappa H. Novel Route to 2,3-Substituted Benzo[b]thiophenes via Intramolecular Radical Cyclization. J. Org. Chem. 2009, 74, 5496–5501. 10.1021/jo900615p. [DOI] [PubMed] [Google Scholar]; e Androsov D. A.; Solovyev A. Y.; Petrov M. L.; Butcher R. J.; Jasinski J. P. A convenient approach towards 2- and 3-aminobenzo[b]thiophenes. Tetrahedron 2010, 66, 2474–2485. 10.1016/j.tet.2010.01.069. [DOI] [Google Scholar]; f Hou C.; He Q.; Yang C. Direct Synthesis of Diverse 2-Aminobenzo[b]thiophenes via Palladium-Catalyzed Carbon–Sulfur Bond Formation Using Na2S2O3 as the Sulfur Source. Org. Lett. 2014, 16, 5040–5043. 10.1021/ol502381e. [DOI] [PubMed] [Google Scholar]; g Adib M.; Soheilizad M.; Rajai-daryasaraei S.; Mirzaei P. An Efficient Aromatization of 2-Amino-4,5,6,7-tetrahydrobenzo-[b]thiophene-3-carboxylates in Dimethyl Sulfoxide Catalyzed by p-Toluenesulfonic Acid. Synlett 2015, 26, 1101–1105. 10.1055/s-0034-1379998. [DOI] [Google Scholar]; h Krishnananthan S.; Smith D.; Traeger S. C.; Mathur A.; Li J. An efficient one-pot synthesis of substituted 2-aminobenzo-1-thiophene-3-carbonitriles. Tetrahedron Lett. 2015, 56, 3766–3768. 10.1016/j.tetlet.2015.04.048. [DOI] [Google Scholar]
- Two compounds are reported; a Soria-Castro S. M.; Bisogno F. R.; Peñéñory A. B. Versatile one-pot synthesis of benzo-fused thiacycles by copper catalysis. Org. Chem. Front. 2017, 4, 1533–1540. 10.1039/C6QO00776G. [DOI] [Google Scholar]; b Nandi C. G.; Singh S. M. p-TSA/Base-Promoted Propargylation/Cyclization of ß-Ketothioamides for the Regioselective Synthesis of Highly Substituted (Hydro)thiophenes. J. Org. Chem. 2016, 81, 5824–5836. 10.1021/acs.joc.6b00342. [DOI] [PubMed] [Google Scholar]
- Selected synthesis of 2-Iminothiolanes; a Wang H.; Yang W.; Liu H.; Wang W.; Li H. FeCl3 promoted highly regioselective [3 + 2] cycloaddition of dimethyl 2-vinyl and aryl cyclopropane-1,1-dicarboxylates with aryl isothiocyanates. Org. Biomol. Chem. 2012, 10, 5032–5035. 10.1039/c2ob25682g. [DOI] [PubMed] [Google Scholar]; b Goldberg A. F. G.; O’Connor N. R.; Craig R. A.; Stoltz B. M. Org. Lett. 2012, 14, 5314–5317. 10.1021/ol302494n. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tsunoi S.; Maruoka Y.; Suzuki I.; Shibata I. Catalytic [3 + 2] Cycloaddition through Ring Cleavage of Simple Cyclopropanes with Isocyanates. Org. Lett. 2015, 17, 4010–4013. 10.1021/acs.orglett.5b01905. [DOI] [PubMed] [Google Scholar]; d Huang X.-Y.; Ding R.; Mo Z.-Y.; Xu Y.-L.; Tang H.-T.; Wang H.-S.; Chen Y.-Y.; Pan Y.-M. Photocatalytic Construction of S–S and C–S Bonds Promoted by Acridinium Salt: An Unexpected Pathway To Synthesize 1,2,4-Dithiazoles. Org. Lett. 2018, 20, 4819–4823. 10.1021/acs.orglett.8b01970. [DOI] [PubMed] [Google Scholar]; e Kreft A.; Jones P. G.; Werz D. B. The Cyclopropyl Group as a Neglected Donor in Donor–Acceptor Cyclopropane Chemistry. Org. Lett. 2018, 20, 2059–2062. 10.1021/acs.orglett.8b00603. [DOI] [PubMed] [Google Scholar]
- Witalewska M.; Wrona-Piotrowicz A.; Makal A.; Zakrzewski J. Polycyclic Aromatic N-Ethoxycarbonyl Thioamide S-Oxides and Their Triflic Acid Promoted Cyclization to Fluorescent Thiophene Imine-Fused Arenes. J. Org. Chem. 2018, 83, 1933–1939. 10.1021/acs.joc.7b02867. [DOI] [PubMed] [Google Scholar]
- a Kita Y.; Egi M.; Ohtsubo M.; Saiki T.; Takada T.; Tohma H. Novel and efficient synthesis of sulfur-containing heterocycles using a hypervalent iodine(III) reagent. Chem. Commun. 1996, 2225–2226. 10.1039/cc9960002225. [DOI] [Google Scholar]; b Wada Y.; Fujioka H.; Kita Y. Synthesis of the Marine Pyrroloiminoquinone Alkaloids, Discorhabdins. Mar. Drugs 2010, 8, 1394–1416. 10.3390/md8041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fukuyama T.; Lu G. Stereocontrolled Total Synthesis of (±)-Gelsemine. J. Am. Chem. Soc. 1996, 118, 7426–7427. 10.1021/ja961701s. [DOI] [Google Scholar]; b Madin A.; O’Donnell C. J.; Oh T.; Old D. W.; Overman L. E.; Sharp M. J. Total Synthesis of (±)-Gelsemine. Angew. Chem., Int. Ed. 1999, 38, 2934–2936. . [DOI] [PubMed] [Google Scholar]; c Wearing X. Z.; Cook J. M. Enantiospecific, Stereospecific Total Synthesis of the Oxindole Alkaloid Alstonisine. Org. Lett. 2002, 4, 4237–4240. 10.1021/ol020170b. [DOI] [PubMed] [Google Scholar]; d Lin H.; Danishefsky S. J. Gelsemine: A Thought-Provoking Target for Total Synthesis. Angew. Chem., Int. Ed. 2003, 4, 36–51. 10.1002/anie.200390048. [DOI] [PubMed] [Google Scholar]; e Marti C.; Carreira E. M. Total Synthesis of (−)-Spirotryprostatin B: Synthesis and Related Studies. J. Am. Chem. Soc. 2005, 127, 11505–11515. 10.1021/ja0518880. [DOI] [PubMed] [Google Scholar]; f Yang J.; Wearing X. Z.; le Quesne P. W.; Deschamps J. R.; Cook J. M. Enantiospecific Synthesis of (+)-Alstonisine via a Stereospecific Osmylation Process. J. Nat. Prod. 2008, 71, 1431–1440. 10.1021/np800269k. [DOI] [PubMed] [Google Scholar]; g Ma S.; Han X.; Krishnan S.; Virgil C. S.; Stoltz M. B. Catalytic Enantioselective Stereoablative Alkylation of 3-Halooxindoles: Facile Access to Oxindoles with C3 All-Carbon Quaternary Stereocenters. Angew. Chem., Int. Ed. 2009, 48, 8037–8041. 10.1002/anie.200902943. [DOI] [PubMed] [Google Scholar]; h Kulkarni M. G.; Dhondge A. P.; Chavhan S. W.; Borhade A. S.; Shaikh Y. B.; Birhade D. R.; Desai M. P.; Dhatrak N. R. Total synthesis of (±)-coerulescine and (±)-horsfiline. Beilstein J. Org. Chem. 2010, 6, 876–879. 10.3762/bjoc.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Chen X.; Duan S.; Tao C.; Zhai S.; Qiu F. G. Total synthesis of (+)-gelsemine via an organocatalytic Diels–Alder approach. Nat. Commun. 2015, 6, 7204. 10.1038/ncomms8204. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhou Z.; Xu Y.; Zhu B.; Li P.; Hu G.; Yang F.; Xu S.; Zhang X. One-pot synthesis of 3-hydroxy-2-oxindoles via acyloin rearrangements of 2-hydroxy-indolin-3-ones generated in situ from 2-alkynyl arylazides. New J. Chem. 2020, 44, 20303–20307. 10.1039/D0NJ04588H. [DOI] [Google Scholar]
- a Qiao Z.; Liu H.; Xiao X.; Fu Y.; Wei J.; Li Y.; Jiang X. Efficient Access to 1,4-Benzothiazine: Palladium-Catalyzed Double C–S Bond Formation Using Na2S2O3 as Sulfurating Reagent. Org. Lett. 2013, 15, 2594–2597. 10.1021/ol400618k. [DOI] [PubMed] [Google Scholar]; b Urban S.; Beiring B.; Ortega N.; Paul D.; Glorius F. Asymmetric Hydrogenation of Thiophenes and Benzothiophenes. J. Am. Chem. Soc. 2012, 134, 15241–15244. 10.1021/ja306622y. [DOI] [PubMed] [Google Scholar]
- a Padmavathi V.; Padmaja A.; Reddy D. B. Selena and thiadiazole fused heterocycles. Indian J. Chem. 1999, 38B, 308–311. [Google Scholar]; b Mukherjee C.; Kamila S.; De A. Application of directed metalation in synthesis. Part 4: Expedient synthesis of substituted benzo[b]thiophene and naphthothiophene. Tetrahedron 2003, 59, 4767–4774. 10.1016/S0040-4020(03)00710-5. [DOI] [Google Scholar]; c Zweig J. E.; Newhouse T. R. Isomer-Specific Hydrogen Bonding as a Design Principle for Bidirectionally Quantitative and Redshifted Hemithioindigo Photoswitches. J. Am. Chem. Soc. 2017, 139, 10956–10959. 10.1021/jacs.7b04448. [DOI] [PubMed] [Google Scholar]; d Uhl E.; Thumser S.; Mayer P.; Dube H. Transmission of Unidirectional Molecular Motor Rotation to a Remote Biaryl Axis. Angew. Chem., Int. Ed. 2018, 57, 11064–11068. 10.1002/anie.201804716. [DOI] [PubMed] [Google Scholar]; e Kink F.; Collado M. P.; Wiedbrauk S.; Mayer P.; Dube H. Bistable Photoswitching of Hemithioindigo with Green and Red Light: Entry Point to Advanced Molecular Digital Information Processing. Chem. – Eur. J. 2017, 23, 6237–6243. 10.1002/chem.201700826. [DOI] [PubMed] [Google Scholar]
- a Jagodziński T. S. Thioamides as Useful Synthons in the Synthesis of Heterocycles. Chem. Rev. 2003, 103, 197–228. 10.1021/cr0200015. [DOI] [PubMed] [Google Scholar]; b Lebel H.Product Subclass 7: Thioamides in Science of Synthesis; Mayer R.; Scheithauer S., Eds.; Thieme: Germany, 2005, Vol. 22, pp. 141–143. [Google Scholar]
- Selected papers; a Wipf P.; Venkatraman S. A New Thiazole Synthesis by Cyclocondensation of Thioamides and Alkynyl(Aryl)Iodonium Reagents. J. Org. Chem. 1996, 61, 8004–8005. 10.1021/jo961681c. [DOI] [PubMed] [Google Scholar]; b Kikelj D.; Urleb U.. Variation 1 : From α-Functionalized Carboxylic Acid Derivatives and Aminothiocarbonyl Compounds. In Science of Synthesis; Schaumann E., Ed.; Thieme: Germany, 2002, Vol. 11, pp. 643–645; [Google Scholar]; c Ishiwata Y.; Togo H. Facile Preparation of Thiazoles from 1H-1-(1’-Alkynyl)-5-methyl-1,2,3- benziodoxathiole 3,3-Dioxide with Thioamides. Synlett 2008, 2637–2641. 10.1055/s-0028-1083439. [DOI] [Google Scholar]; d Romagnoli R.; Baraldi G. P.; Carrion D. M.; Cruz-Lopez O.; Lopez Cara C.; Basso G.; Viola G.; Khedr M.; Balzarini J.; Mahboobi S.; Sellmer A.; Brancale A.; Hamel E. 2-Arylamino-4-Amino-5-Aroylthiazoles. “One-Pot” Synthesis and Biological Evaluation of a New Class of Inhibitors of Tubulin Polymerization. J. Med. Chem. 2009, 52, 5551–5555. 10.1021/jm9001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected papers; a Ding Q.; He X.; Wu J. Synthesis of 2-Aminobenzothiazole via Copper(I)-Catalyzed Tandem Reaction of 2-Iodobenzenamine with Isothiocyanate. J. Comb. Chem. 2009, 11, 587–591. 10.1021/cc900027c. [DOI] [PubMed] [Google Scholar]; b Saha P.; Ramana T.; Purkait N.; Ali M. A.; Paul R.; Punniyamurthy T. Ligand-Free Copper-Catalyzed Synthesis of Substituted Benzimidazoles, 2-Aminobenzimidazoles, 2-Aminobenzothiazoles, and Benzoxazoles. J. Org. Chem. 2009, 74, 8719–8725. 10.1021/jo901813g. [DOI] [PubMed] [Google Scholar]; c Murru S.; Ghosh H.; Sahoo S. K.; Patel B. K. Intra- and Intermolecular C-S Bond Formation Using a Single Catalytic System: First Direct Access to Arylthiobenzothiazoles. Org. Lett. 2009, 11, 4254–4257. 10.1021/ol9017535. [DOI] [PubMed] [Google Scholar]; d Ma D.; Lu X.; Shi L.; Zhang H.; Jiang Y.; Liu X. Domino Condensation/S-Arylation/Heterocyclization Reactions: Copper-Catalyzed Three-Component Synthesis of 2-N-Substituted Benzothiazoles. Angew. Chem., Int. Ed. 2011, 123, 1150–1153. 10.1002/ange.201005787. [DOI] [PubMed] [Google Scholar]; e Zhang X.; Zeng W.; Yang Y.; Huang H.; Liang Y. Copper-Catalyzed Double C–S Bonds Formation via Different Paths: Synthesis of Benzothiazoles from N-Benzyl-2-iodoaniline and Potassium Sulfide. Org. Lett. 2014, 16, 876–879. 10.1021/ol403638d. [DOI] [PubMed] [Google Scholar]; f Dang P.; Zheng Z.; Liang Y. Copper-Catalyzed C(sp3)–S Bond and C(sp2)–S Bond Cross-Coupling of 2-(2-Iodobenzoyl) Substituted or 2-(2-Iodobenzyl) Substituted 1,2,3,4-Tetrahydroisoquinolines with Potassium Sulfide: Synthesis of Isoquinoline-Fused 1,3-Benzothiazine Scaffolds. J. Org. Chem. 2017, 82, 2263–2268. 10.1021/acs.joc.6b02943. [DOI] [PubMed] [Google Scholar]; g Yugandar S.; Konda S.; Ila H. Synthesis of Substituted Benzo[b]thiophenes via Sequential One-Pot, Copper-Catalyzed Intermolecular C–S Bond Formation and Palladium-Catalyzed Intramolecular Arene–Alkene Coupling of Bis(het)aryl/alkyl-1,3-monothiodiketones and o-Bromoiodoarenes. Org. Lett. 2017, 19, 1512–1515. 10.1021/acs.orglett.7b00273. [DOI] [PubMed] [Google Scholar]
- Selected papers; a Tarasova O. A.; Klyba L. V.; Vvedensky V. Y.; Nedolya N. A.; Trofimov B. A.; Brandsma L.; Verkruijsse H. D. One-Pot Syntheses of 2-N-Alkylamino-, 2-N-Phenylamino-2-N,N-Dialkylamino-, and 2-N-Alkyl-N-phenylaminothiophenes. Eur. J. Org. Chem. 1998, 253–256. . [DOI] [Google Scholar]; b Singh L. W.; Ila H.; Junjappa H. Polarized Ketene Dithioacetals. Part 65. Studies on Additions of 2-Aroyl-3-cyano-1,1-bis(methylthio)propenide Anions to Hetero-multiple Bonds. J. Chem. Soc. Perkin Trans. 1988, 2365–2368. 10.1039/p19880002365. [DOI] [Google Scholar]; c Lai L. L.; Reid D. H.; Wang F. L.; Liao L. F. An Unexpected Synthesis of Thiophene Derivatives by Thionation of N-Phenylacetylthio benzamides. Heteroat. Chem. 1994, 5, 479–486. 10.1002/hc.520050510. [DOI] [Google Scholar]
- Selected papers; a Kim Y. S.; Kwak S. H.; Gong Y. D. Application of Thio-Ugi Adducts for the Preparation of Benzo[b]thiophene and S-Heterocycle Library via Copper Catalyzed Intramolecular C–S Bond Formation. ACS Comb. Sci. 2015, 17, 365–373. 10.1021/acscombsci.5b00034. [DOI] [PubMed] [Google Scholar]; b Saraiah B.; Gautam V.; Acharya A.; Pasha M. A.; Hiriyakkanavar I. One-Pot Synthesis of 2-(Aryl/Alkyl)amino-3-cyanobenzo[b]thiophenes and Their Hetero-Fused Analogues by Pd-Catalyzed Intramolecular Oxidative C–H Functionalization/Arylthiolation. Eur. J. Org. Chem. 2017, 5679–5688. 10.1002/ejoc.201700963. [DOI] [Google Scholar]
- Villo P.; Kervefors A.; Olofsson B. Transition metal-free, chemoselective arylation of thioamides yielding aryl thioimidates or N-aryl thioamides. Chem. Commun. 2018, 54, 8810–8813. 10.1039/C8CC04795B. [DOI] [PubMed] [Google Scholar]
- Janni M.; Thirupathi A.; Arora S.; Peruncheralathan S. Chemoselective Ullmann coupling at room temperature: a facile access to 2-aminobenzo[b]thiophenes. Chem. Commun. 2017, 53, 8439–8442. 10.1039/C7CC03273K. [DOI] [PubMed] [Google Scholar]
- a Janni M.; Arora S.; Peruncheralathan S. Double heteroannulation of S,N-acetals: a facile access to quinolone derivatives. Org. Biomol. Chem. 2016, 14, 8781–8788. 10.1039/C6OB01568A. [DOI] [PubMed] [Google Scholar]; b Thirupathi A.; Janni M.; Peruncheralathan S. Copper Catalyzed Intramolecular N-Arylation of Ketene Aminals at Room Temperature: Synthesis of 2-Amino-3-cyanoindoles. J. Org. Chem. 2018, 83, 8668–8678. 10.1021/acs.joc.8b00816. [DOI] [PubMed] [Google Scholar]; c Bandyopadhyay D.; Thirupathi A.; Dhage N. M.; Mohanta N.; Peruncheralathan S. Nickel catalyzed site selective C–H functionalization of α-aryl-thioamides. Org. Biomol. Chem. 2018, 16, 6405–6409. 10.1039/C8OB01712C. [DOI] [PubMed] [Google Scholar]
- 1a was treated with LAH affording an intractable mixture of compounds, which could not characterized, whereas in NaBH4 in TFA, 1a was recovered completely.
- a Bradsher C. K.; Edgar J. K. 1-Substituted Benzocyclobutenes via Parham Cyclialkylation. J. Org. Chem. 1981, 46, 4600–4602. 10.1021/jo00335a068. [DOI] [Google Scholar]; b Guin J.; Varseev G.; List B. Catalytic Asymmetric Protonation of Silyl Ketene Imines. J. Am. Chem. Soc. 2013, 135, 2100–2103. 10.1021/ja312141b. [DOI] [PubMed] [Google Scholar]; c Yang X.-Y.; Lin H.-S.; Matsuo Y. Highly Selective Synthesis of Tetrahydronapthaleno[60]fullerenes via Fullerene-Cation-Mediated Intramolecular Cyclization. J. Org. Chem. 2019, 84, 16314–16322. 10.1021/acs.joc.9b02618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.