Abstract

In this study, we are interested in preparing Fe(III), Pd(II), and Cu(II) complexes from new thiazole derivatives. All syntheses were elaborately elucidated to estimate their molecular and structural formulae, which agreed with those of mononuclear complexes. The square-planer geometry of Pd(II) complex (MATYPd) was the starting point for its use as a heterocatalyst in preparing pyrazole-4-carbonitrile derivatives 4a–o using ultrasonic irradiation through a facile one-pot reaction. The simple operation, short-time reaction (20 min), and high efficiency (97%) were the special advantages of this protocol. Furthermore, this green synthesis strategy was advanced by examination of the reusability of the catalyst in four consecutive cycles without significant loss of catalytic activity. The new synthesis strategy presented remarkable advantages in terms of safety, simplicity, stability, mild conditions, short reaction time, excellent yields, and use of a H2O solvent. This catalytic protocol was confirmed by the density functional theory (DFT) study, which reflected the specific characteristics of such a complex. Logical mechanisms have been suggested for the successfully exerted essential physical parameters that confirmed the superiority of the Pd(II) complex in the catalytic role. Optical band gap, electrophilicity, and electronegativity features, which are essential parameters for the catalytic behavior of the Pd(II) complex, are based mainly on the unsaturated valence shell of Pd(II).
1. Introduction
Heterocyclic compounds of pyrazoles class are known by their importance in pharmaceutical targets and medicinal interest. Organic derivatives enriched by S and/or N atoms have a broad spectrum of biological activities such as antimicrobial, antioxidant,1,2 anti-HIV, anticancer,3 anticonvulsant,4 antimalarial, anti-inflammatory,5 and antidepressant.6 The ultrasonic state increases the rate of organic changes in mild conditions that otherwise require strict pressure and temperature conditions.7 Ultrasonic irradiation is also used to promote the formation, growth, and implosive collapse of bubbles in a liquid8 by various synthesis reactions. Initiated by cavitation, bubble collapse causes high stresses, extreme local heating, and very short lifetimes. Cavitation acts as a way of focusing the sound’s scattered energy.9,10 Ultrasound irradiation can cause several reactions by providing activation energy, in contrast to traditional heating that provides thermal energy in the macro system.10a Other benefits of ultrasound irradiation include high product yields, low reaction times, minimization of side products,10b and nontoxic and environment-friendly solvents,11 saving money and energy.
Previously variable methods involved synthesis of many pyrazole-4-carbonitrile derivatives via the one-pot multicomponent reaction (MCR) among arylaldehydes, malononitrile, and phenylhydrazine using appropriate catalysts.12 The significance of such compounds and relevance of such timely topics in organic synthesis were the use of ionic liquids13 and the need for green reaction approaches.14 The benefit of pyrazoles in drug designing has continuously trapped the pursuit for novel and advanced methods.15 Therefore, a novel protocol with a good and inexpensive catalyst demanding short reaction times is well desired.16,17
In any of the abovementioned previous studies, pyrazole-4-carbonitrile derivatives catalyzed by new Pd(II) complexes under mild conditions have not been reported. Coinciding with outlined strategies and continuation of our work, we intended to achieve another success in the catalytic history of Pd(II) complexes by synthesizing bioactive heterocyclic compounds via multicomponent reactions. Because of the the merits of being environmentally benign, readily accessible, and cost-effective, the Pd(II) complex seems to be a promising reusable catalyst in facile one-pot synthesis of pyrazole-4-carbonitrile derivatives 4a–o through a three-component coupling reaction (involving an aromatic aldehyde, malononitrile, and phenylhydrazine) under mild conditions. All products were isolated in distinguished yields within a short time. The catalytic reactivity of the selected complex was enhanced by the computational features of the DFT/B3LYP method.
2. Experimental Section
2.1. Chemicals and Techniques
All utilized materials and solvents implemented in this study were available from Sigma-Aldrich or Alfa Aesar and used as obtained. The characterization of the devices used and measurement conditions are stated in Supporting Information (part 1), while other conditions are listed in the discussion part, separately.
2.2. Synthesis of the MATY Ligand
2,4-Thiazolidinedione (10 mmol in 10 mL of hot EtOH) was reacted with a mixture of 4-anisaldehyde, malononitrile (10 mmol in 10 mL of hot EtOH), and piperidine (20 mmol). The resulting solution was refluxed for 2 h. The product was separated out by filtration and its purity was checked by TLC. MATY: 2-amino-6-oxo-3-(piperidinylamidino)-4-(4methoxyphenyl)-6,7-dihydro-pyrano[2, 3-d]-5,7thiazol derivative, molecular formula C19H22N4O3S (386), m.p.: 233 °C. Color: pale yellow. Anal. calcd (%): C 59.12, H 5.71, N 14.53; found (%): C 59.06, H 5.7, N 14.50. Solubility: ethanol. IR (KBr pellet, cm–1): 3402 ν (N–H), 3310–3220 ν (NH2), 1683 ν(C=O). 1H NMR δ in DMSO-d6: 9.88 (br, 1H, NH), 7.22–7.20 (d, 2H, ArH), 7.03 (s,2H,NH2), 6.94–6.92 (d, 2H, ArH), 4.57 (d, 1H, CH), 4.39 (d, 1H, NH), 3.75 (s, 3H, OCH3), 3.32–3.28 (t, 4H, 2CH2), and 1.56–1.45 (m, 6H, 3CH2) ppm. 13C NMR δ in DMSO-d6: 24.20, 25.86, 45.66, 51.18 (Al-C), 55.59 (OCH3), 56.38, 71.60, 114.63, 118.83, 129.59, 134.57 (Ar C), 152.47 (N–C–O, thiazol), 159.11 (N–C–O, pyran), 161.89 (N–C=NH), and 171.18 (C=O) (presented in Figures S1–S3).18
2.3. Synthesis of Metal Ion Complexes
An ethanolic solution of thiazole derivative (MATY) was reacted with an equimolar ratio of Fe(NO3)3 and Cu(OAc)2·H2O(1:1), while an equimolar ratio of MATY and Pd(OAc)2 was reacted with acetone solvent. The obtained solutions were heated and stirred under reflux for 2–3 h. Each precipitate was filtered off, and washed with hot EtOH and acetone (Scheme 1).18
Scheme 1. Synthetic strategy for the preparation of MATY ligand and its complexes.
[Cu(OAc)2(HL)(H2O)2], [MATYCu] (dark green): molecular formula C23H38N4O12SCu (657.5), decom.t.: 242 °C. Anal. calcd (%): C 41.92, H 5.77, N 8.55, found (%): C 41.97, H 5.77, N 8.51, Λm: 14.73 (Ω–1 cm2 mol–1), solubility: DMF, IR (KBr pellet cm–1): 3412 ν (N– H), 3320–3230 ν (NH2), 1681 ν(C=O), 441 (M–N).
[Fe(NO3)3(HL)(H2O)], [MATYFe] (pale brown): molecular formula C19H27N7O15SFe (680.8), decom.t: 265 °C. Anal. calcd (%): C 33.52, H 4.19, N 14.43, found (%): C 33.49, H 3.96, N 14.39, Λm: 24.85 (Ω–1 cm2 mol–1), solubility: DMF IR (KBr pellet cm–1): 3410 ν (N– H), 3324–3227 ν (NH2), 1684 ν(C=O), 487 (M–N).
[Pd(OAc)2(HL)].H2O, [ARPTPd] (orange): molecular formula C23H30N4O8SPd (628.4), decom.t: 285 °C. Anal. calcd (%): C 43.86, H 5.01, N 8.89, found (%): C 43.96, H 4.77, N 8.91, solubility: DMF, Λm: 5.12 (Ω–1 cm2 mol–1), IR (KBr pellet cm–1): 3429 ν (N– H), 3429 ν (NH2), 1682 ν(C=O), 454(M–N). 1 H NMR (δ, ppm), in DMSO-d6: 9.95 (s, 1H, NH), 7.56–7.38 (m, 4H, ArH), 7.24 (d, 2H, NH2), 6.94–6.92 (s, 1H, CH), 4.57 (s, 1H, NH, pip), 3.79 (s, 3H, OCH3), 3.30–3.27 (t, 4H, 2CH2), 1.93 (s, 6H, 2CH3), and 1.55-1.45 (m, 6H, 3CH2) (Figure S4).
2.4. Kinetic and Thermodynamic Parameters’ Calculations
To identify the thermal stability of the tested complexes under the impact of a constant heating rate, TGA was introduced. Besides, the metal percentage in the composition is also involved. Furthermore, the thermal system is kinetically governed and kinetic or thermodynamic factors (E, A, ΔS, ΔH and ΔG) can be assessed using the Coats–Redfern method.19,20
| 1 |
From the left part of the above equation and the 1/T value, A and E* can be attained from the intercept and slope, respectively. Then, the thermodynamic parameters (ΔH*, ΔS*, and ΔG*) can be calculated by applying the following relations. ΔS* = 2.303R log (Ah/KBT) and ΔH* = E*– RT and ΔG* = ΔH* – TΔS*.
2.5. Stoichiometry and Formation Constants of Complexes in Solution
By implementing Job’s method [involving molar ratio and continuous variation] to determine the stoichiometry and stability of the complexes in the solution state, which may agree with the values in their solid form. Homogenized and clear solutions prepared (M and L) were left to balance, and then the absorbance was taken21,22 and plotted versus either ([L]/[L]+[M]) or ([L]/[M]). From spectrophotometric analyses, the consistency parameter (Kf) of the complexes formed in solution was calculated by the continuous variation route, according to the relation18,23Kf = AAm/(1–A/Am)2C, where [Am], [A], and [C] are the absorbance at optimum consistency of the complex, the absorption value along both sides of the absorption curve, and the molar concentration of the metal, respectively.
2.6. Molecular Modeling Study
Using Materials Studio package,24 geometry optimization was executed for the ligand and its complexes to assess the mode of bonding and structural stability. This modeling was carried out by the DMOL3 program using the DFT method, which was adjusted at the DNP basis set.25 The method was used without constraints under the exchange-correlation functional of Becke3–Lee–Yang–Parr (B3LYP) using GGA and RPBE functions.26 The positive value of frequency is the indicator for the suitability of the optimized structures. Time-dependent DFT (TD-DFT) was proceeded by implementing a polarizable continuum model. Also, using the integral equation formalism variant (IEF-PCM), which was executed at the B3LYP level, we studied the properties of ground or excited state.27
2.7. Heterogeneous MATYPd Complex Catalyzed the Synthesis of Pyrazole-4-Carbonitrile Derivatives 4a–o
In a rounded flask, aquatic solutions of aromatic aldehyde 1 (1 mmol), malononitrile 2 (1 mmol), phenylhydrazine 3 (1 mmol), and MATYpd complex (0 mol %) were mixed in 30 mL of H2O under stirring (at 25 °C), and then at 20 kHz frequency and 40 W power at 80 °C; the sound was applied for the desired acceptable time (Scheme S2). After finishing the reaction according to the thin-layer chromatography test (TLC), the reaction mixture was allowed to cool to room temperature and the catalyst was filtered. The organic material was extracted with ethyl acetate (3×10 mL), and pyrazole-4-carbonitrile derivatives 4a–o combined with the organic phase were washed with water, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The resulting solid product was filtered and recrystallized from 10 mL of ethanol to give a pure product, which was first elucidated by its melting point and then characterized by IR and NMR spectra.
2.7.1. Catalyst Recovery and Reuse
Using a heterogeneous catalytic process, the MATYPd catalyst was easily separated by filtration and could be reused many times by the same efficacy approximately. The filtered complex was washed with ethanol and bi-distilled water. After drying at 90 °C (for 3 h), the catalyst was recycled for another run under typical reaction conditions.
2.7.2. Spectral Data for the Synthesized Derivatives
2.7.2.1. Compound 4a
White solid (M.P. = 160–162 °C), IR (KBr) cm–1 = 3481, 3340, 3083, 2327, 1590; 1H NMR (400 MHz, DMSO-d6) δ = 6.75 (t, J = 7.24 Hz, 1H, ArH), 7.07 (d, J = 7.92, 2H, ArH), 7.21 (t, J = 7.80, 2H, ArH), 7.28 (t, J = 7.32, 1H, ArH), 7.38 (t, J = 7.62 Hz, 2H, ArH), 7.64 (d, J = 8.18 Hz, 2H, ArH), 7.86 (s, 2H, NH2); 13C NMR (100 MHz, CDCl3) d (ppm) 156.61, 150.41, 146.09, 137.85, 135.77, 129.74, 128.05, 127.9, 126.64, 120.56, 113.25, 112.82; EIMS (m/z): 260 (M)+.
2.7.2.2. Compound 4b
White solid (M.P. = 107–109 °C), IR (KBr) cm–1 = 3314, 2366, 1595, 1244; 1H NMR (400 MHz, DMSO-d6) δ = 3.77 (s, 3H, OCH3), 6.71 (t, J = 7.22 Hz, 1H, ArH), 6.95 (d, J = 8.76, 2H, ArH), 7.03 (d, J = 8.00, 2H, ArH), 7.19 (t, J = 7.84, 2H, ArH), 7.57 (d, J = 8.76 Hz, 2H, ArH), 7.81 (s, 2H, NH2); 13C NMR (100 MHz, CDCl3): δ 160.03, 144.93, 143.44, 137.43, 128.10, 127.58, 127.10, 119, 114.11, 112.66, 99.98, 59.33, 55.35; EIMS (m/z) 290 (M)+.
2.7.2.3. Compound 4c
Brown solid (M.P. = 131–133 °C), IR (KBr) cm–1 = 3482, 3411, 3120, 2830, 2545, 2233, 1650; 1H NMR (400 MHz, CDCl3) d (ppm) 7.80–7.83 (m, 1H), 7.64 (s, 1H), 7.57–7.60 (m, 2H), 7.23–7.26 (m, 1H), 7.10 (d, J = 7.6, 2H), 7.01 (m, 1H), 6.89–6.93 (m, 2H), 6.85 (t, 1H) 3.84 (s, 3H); 13C NMR (100 MHz, CDCl3) d (ppm) 161.00, 143.90, 143.33, 137.51, 137.420, 129.04, 127.31, 128.44, 125.84, 118.92, 112.90, 114.02, 56.01; EIMS (m/z) 290 (M).
2.7.2.4. Compound 4d
White solid (M.P. = 121–123 °C), IR (KBr) cm–1 = 3291, 2344, 1593, 1255; 1H NMR (400 MHz, DMSO-d6) δ = 3.79 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 6.71 (t, J = 7.2 Hz, 1H, ArH), 6.99 (d, J = 8.1, 1H, ArH), 7.05 (t, J = 6.6, 3H, ArH), 7.21 (t, J = 7.8, 2H, ArH), 7.47 (s, 2H, NH2), 8.11 (s, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ 55.65, 60.82, 111.86, 112.03, 116.33, 118.71, 124.14, 129.07, 131.76, 145.19, 146.32, 152.64.
2.7.2.5. Compound 4e
Brown solid (M.P. = 142–144 °C), IR (KBr) cm–1 = 33055, 2348, 1601, 1254; 1H NMR (400 MHz, DMSO-d6) δ = 6.76 (t, J = 7.6 Hz, 1H, v), 7.11 (d, J = 8.3, 2H, ArH), 7.27–7.32 (m, 3H, ArH), 7.36 (t, J = 8.0, 1H, ArH), 7.45 (d, J = 7.9 Hz, 1H, ArH), 8.02 (d, J = 8.8 Hz, 1H, ArH), 8.23 (s, 2H, NH2); 13C NMR (100 MHz, CDCl3): δ 112.16, 119.27, 125.79, 127.33, 129.05, 129.14, 129.66, 131.14, 131.91, 132.89, 144.81; HRMS of [C16H12ClN4 + H]+ (m/z): 295.0866; Calcd.: 295.08.
2.7.2.6. Compound 4f
White solid (M.P. = 128–130 °C), IR (KBr) cm–1 =3461, 3382, 3131, 2522, 2251, 1662, 1H NMR; (400 MHz, CDCl3) δ = 7.67 (s, 2H) 7.65 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.3 Hz, 2H), 7.29–7.34 (m, 2H), 7.16 (d, J = 7.6 Hz, 2H), 6.94 (t, J = 7.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ = 154.25, 143.42, 142.11, 135.01, 130.00, 129.02, 127.23, 126.14, 119.40, 115.63.
2.7.2.7. Compound 4g
Yellow oil, IR (KBr) cm–1 = 3462, 3410, 3141, 2542, 2221, 1650; 1H NMR (400 MHz, CDCl3) δ = 7.88–7.97 (m, 2H), 7.65 (s, 1H), 7.57–7.60 (m, 2H), 7.25–7.27 (m, 2H), 7.13 (d, J = 7.7, 1H), 6.91–6.95 (m, 3H); 13C NMR (100 MHz, CDCl3) δ = 157.55, 144.91, 145.01, 136.22, 138.45, 130.58, 129.01, 128.32, 127.41, 119.30, 116.56, 115.67, 113.35.
2.7.2.8. Compound 4h
Rose solid (M.P. = 162–164 °C), IR (KBr) cm–1 = 3304, 2355, 1598; 1H NMR (400 MHz, DMSO-d6) δ = 6.76 (t, J = 7.3 Hz, 1H, ArH), 7.04 (d, J = 8.0, 2H, ArH), 7.22 (t, J = 7.8, 2H, ArH), 7.55 (q, J = 7.9, 4H, ArH), 7.79 (s, 2H, NH2); 13C NMR (100 MHz, CDCl3): δ = 112.01, 118.95, 120.63, 127.41, 129.09, 131.51, 134.10, 135.09, 145.11; HRMS of [C16H13BrN4 + 2H]+ (m/z): 340.144; Calcd.: 340.146.
2.7.2.9. Compound 4i
Brown solid (M.P. = 220–222 °C), IR (KBr) cm–1 = 3313, 2360, 1597, 1261; 1H NMR (400 MHz, DMSO-d6) δ = 6.77 (t, J = 6.8 Hz, 1H, ArH), 7.07 (d, J = 7.6, 2H, ArH), 7.20–7.24 (m, 3H, ArH), 7.36 (t, J = 7.5, 1H, ArH), 7.61 (s, 2H, NH2), 8.00 (d, J = 8.0 Hz, 1H), 8.15 (s, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ = 112.14, 119.26, 121.65, 126.15, 127.80, 129.15, 129.39, 132.90, 134.29, 144.81; HRMS of [C16H13BrN4 + 2H]+ (m/z): 340.115; Calcd.: 340.114.
2.7.2.10. Compound 4j
Red solid (M.P. = 165–167 °C), IR (KBr) cm–1 =3462, 3355, 3101, 2356, 1610, 1254, 1H NMR (400 MHz, CDCl3) δ = 8.27 (d, J = 7.7 Hz, 2H) 8.03 (s, 1H), 7.73–7.787 (m, 3H), 7.20–7.35 (m, 2H), 7.17 (d, J = 7.7 Hz, 2H), 6.98 (s, 1H); 13C NMR (100 MHz, CDCl3) δ = 156.50, 149.46, 145.326, 137.73, 135.30, 131.72, 130.92, 129.91, 122.78, 122.15, 123.47, 113.43, 112.32.
2.7.2.11. Compound 4k
Yellow solid (M.P. = 106–108 °C), IR (KBr) cm–1 = 3431, 3320, 3177, 2813, 2541, 2228, 1640; 1H NMR (400 MHz; CDCl3) δ = 7.810–7.84 (m, Arom., 4H), 7.65–7.85 (m, 2H), 7.63 (s, 1H), 7.25–7.30 (m, 2H), 7.16 (s, 2H), 3.15 (s, 6H); 13C NMR (100 MHz; CDCl3) δ = 151.01, 144.99, 129.289, 128.18, 123.01, 127.79, 115.14, 113.44, 59.01, 40.09.
2.7.2.12. Compound 4l
Rose powder (M.P. = 117–119 °C), IR (KBr) cm–1 = 3485, 3321, 3088, 2927, 2354, 1600, 1251; 1H NMR (400 MHz; CDCl3) δ = 7.71 (s, 2H), 7.58 (d, J = 7.7 Hz, 2H), 7.30–7.34 (m, 2H), 7.22 (d, J = 7.7 Hz, 2H), 7.15 (d, J = 7.8 Hz, 2H), 6.88 (d, J = 7.7 Hz, 1H), 2.44 (s, 3H); 13C NMR (100 MHz; CDCl3) δ = 154.20. 151.01, 145.13, 129.23, 128.15, 123.13, 127.85, 115.10, 113.41, 104.65, 21.90.
2.7.2.13. Compound 4m
Yellow solid (M.P. = 160 −162 °C), IR (KBr) cm–1 = 3579, 3489, 3340, 3120, 2351, 2211, 1600, 1212; 1H NMR (400 MHz; CDCl3) δ = 10.49 (s, 1H), 10.35 (s, 1H), 8.13 (s, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.24 (d, J = 7.7 Hz, 2H), 6.95 (d, J = 7.7 Hz, 2H), 7.12– 7.19 (m, 1H), 6.85–6.91 (m, 2H), 6.77 (t, J = 7.1 Hz, 1H); 13C NMR (100 MHz; CDCl3) δ = 156.51, 152.22, 150.51, 145.50, 138.03, 130.13, 129.99, 128.14, 125.40, 121.33, 120.25, 119.75, 116.84, 112.60.
2.7.2.14. Compound 4n
Brown solid (M.P. = 211–212 °C), IR (KBr) cm–1 = 3411, 2223, 1609, 1255; 1H NMR (400 MHz, DMSO-d6) δ = 9.70 (s, 1H, OH), 7.80 (s, 2H, NH2), 7.46 (d, J = 8.6 Hz, 2H, ArH), 7.18 (t, J = 7.7, 2H, ArH), 7.01 (d, J = 7.7, 2H, ArH), 6.78 (d, J = 8.6, 2H, ArH), 6.69 (t, J = 7.3 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ 111.70, 112.01, 115.52, 118.10, 126.85, 127.11, 137.05, 145.65, 157.67; HRMS of [C16H13N4O + H]+ (m/z): 277.084; Calcd.: 277.085.
2.7.2.15. Compound 4o
Yellow solid (M.P. = 180–182 °C), IR (KBr) cm–1 = 3311, 2354, 1600, 1248; 1H NMR (400 MHz, DMSO-d6) δ = 9.12 (s, 1H, OH); 8.77 (d, J = 8.8 Hz, 2H, ArH), 8.60 (s, 2H, NH2), 7.53-7.62 (m, 4H, ArH), 7.30 (t, J = 7.8, 2H, ArH), 7.13 (d, J = 7.7, 2H, ArH), 6.80 (t, J = 7.3 Hz, 1H, ArH), 13C NMR (100 MHz, CDCl3) δ = 111.90, 118.98, 125.02, 125.35, 126.52, 127.61, 128.92, 129.25, 131.10, 135.10, 145.19; IR (KBr, cm–1): HRMS of [C24H17N4+H]+ (m/z): 361.023; Calcd.: 361.020.
3. Results and Discussion
3.1. Preliminary Properties
The analytical data obtained suggested the chemical formulae of Cu(II), Fe(III), and Pd(I)-thiazole complexes. The most fitted ratio was 1L:1M for all colored complexes. All complexes were stable in air, nonhygroscopic, and insoluble in common solvents, but soluble in DMSO or DMF. Conductivity measurements exhibited values in the 5.12–24.85 (Ω–1 cm2 mol–1) range, which correspond to nonelectrolyte complexes.28
3.2. Infrared (IR) Spectroscopy
The IR spectra of the thiazole ligand (MATY) and its complexes were taken into account while using the KBr pellet technique over the 400–4000 cm–1 region to obtain information about the binding mode within the complexes (Figure S1). The MATY ligand presented a characteristic band of ν(NH) at 3402 cm–1. However, in MATYCu, MATYFe, and MATYPd complexes, the ν(NH) vibration was shifted to 3412, 3410, and 3429 cm–1, which indicates the coordination of the (NH) group with metal ions. Furthermore, the ligand spectrum exhibited bands at 3310–3220 cm–1 for ν(NH2) vibrations, which shifted to 3220, 3330, 3224, 3227, and 3429 cm–1 in the chelates, respectively. The peak at 1683 cm–1, which was assigned to the ν(C=O) vibration, was shifted to 1681, 1684, and 1682 cm–1 in the complexes, respectively. Moreover, the formation of MATYCu, MATYFe, and MATYPd complexes was determined by the presence of weak bands below 500 cm–1 corresponding to ν(M–O) and ν(M–N) vibrations.18 υas(OAc) and υs(OAc) in MATYCu and MATYPd complexes were assigned at 1500, 1270 cm–1 and 1480, 1270 cm–1, respectively, while υas(NO3) and υs(NO3) were assigned at 1530 and 1310 cm–1, respectively. The gap between two vibrations for both the acetate and nitrate groups (Δ = υas – υs) points to a monodentate mode of bonding for the acetate or nitrate anion.29
3.3. NMR Spectra
1 H NMR spectral data for MATY (Figure S3) and its MATYPd complex (Figure S4) were obtained in DMSO-d6 solvent. Signals of −NH and −NH2 protons were monitored to follow the effective synthesis of Pd(II) complex from [Pd(OAC)2] salt.31 A downfield shift of their signals was observed from 9.88 and 7.03 ppm in the MATY spectrum to 9.95 and 7.24 ppm in the MATYPd spectrum, respectively.18,30 Also, the 13C NMR spectrum of the ligand (Figure S2) displayed signals at 24.20, 25.86, 45.66, 51.18 (alph-C), 55.59 (OCH3), 56.38, 71.60, 114.63, 118.83, 129.59, 134.57, 152.47 (N–C–O, thiazol), 159.11 (N–C–O, pyran), 161.89 (N–C=NH), and 171.18 (C=O).
3.4. Electronic Spectroscopy
UV–Vis spectra of the thiazole ligand and its MATYCu, MATYFe, and MATYPd complexes were determined in DMF at 25 °C (Figure S5). The ligand spectrum exhibited an intense band at 295 nm, which is attributed to the n−π* transition inside C=N or C=O groups. For MATYCu, MATYPd, and MATYFe spectra, this band was slightly shifted to 310, 294, and 297 nm, respectively (Table S1). Also, the peaks at 397, 402, and 446 nm were attributed to the LMCT transitions, respectively. Besides, these complexes exhibited an intensity band around 730, 536, and 461 nm, assigned to the d–d transition, respectively. An irregular octahedral configuration was proposed for MATYFe and MATYCu complexes, whereas a square-planar geometry was proposed for the MATYPd complex.18,32
Using UV–Vis spectra, the optical band gap (Eg) can be calculated to measure the magnitude of separation between the valence band and the bottom of the conduction band. The lowest quantum (hυ) used to raise an electron from its level and leave behind a positive hole despite the attraction forces in-between is the Eg value. A minimized band gap leads to overlapping of the conduction band with the valence band and the electrons have a high elasticity for excitation. The optical band gap that seems to be the separation between LUMO and HOMO levels is an indicator of the activation energy values and semiconductor-like behavior.33 The Eg value of a known semiconductor such as Si is 1.14 eV, which is considered low enough to clarify the extent of electron flexibility that is effective in catalytic behavior also. The values estimated for the studied complexes were 2.45, 2.54, and 2.65 eV for MATYPd, MATYFe, and MATYCu complexes, respectively. The value of the Pd(II)-thiazole complex reflects its effective catalytic role under lower activation energy as well as its promising semiconductor-like behavior. The values were estimated by using the following simple relations:33 α = 1/d ln A (1), where d is the cell width; 2 – αhυ = A(hυ – Eg)m (2), where α is the absorption coefficient and A is the energy-independent constant. The direct or indirect transition was controlled by an m value of 0.5 or 2, respectively; consequently, α values can be estimated from relation 1 and utilized in (αhυ)2. Then, a relation was drawn between (αhυ)2and hυ (Figure 1), extrapolating the line for the first transition band to interact with the x-axis [(αhυ)2 = 0]; Eg value is the interacting point. Finally, the lower value recorded for the MATYPd complex denotes its specific properties for solar cell, catalytic, and semiconductor uses.34
Figure 1.
Optical band gap for (a) Cu(II), (b) Fe(III), and (c) Pd(II) complexes.
3.5. Thermogravimetric and Kinetic Analysis
TGA curves for the tested complexes were obtained at a constant heating rate (5 °C min–1) and under air atmosphere in the 25–800 °C range. Thermal data hypotheses are displayed in Table S2 and Scheme S1 to prove the proposed formulae and discriminate the connecting H2O molecules. All complexes exhibited the whole distortion up to four degradation steps extended to ≈ 750 °C. The high thermal stability was documented after the first stage. This decrease indicates the involvement of crystal H2O molecules.35
In each TGA curve, the kinetics and thermodynamic factors were evaluated over all degradation stages. The objective of this analysis is to evaluate the level of stability within the sphere of coordination and also the kinetic performance of the degradation reaction. The thermodynamic parameters are computed and listed in Table S2. In Coats–Redfern equations, fraction degradation functions (α) at specific temperatures (t) were determined to measure the target parameters under a continual heating rate. The data lead directly to the following observations: the high activation energy (E*, −ve) results signify a strong grade of bonding for such fragment; the values of (ΔS*) reflect the high-complexity degree in a nonspontaneous reaction.18,36,37
3.6. Stoichiometry, Formation Constants, and pH Profile of the Complexes
By applying spectrophotometric methods (continuous variation and molar ratio), the most fitted stoichiometry of each metal chelate in solution was estimated (Figures S6 and S7). The stoichiometry was 1:1, in agreement with that proposed in solid form. The highest absorbance at a stable ligand mole fraction (X) was recorded at 0.5, which refers to the 1M:1L ratio in all complexes, as shown by the continuous variation curves (Figure S6). Furthermore, the graphs were drawn based on the molar ratio method (Figure S7), indicating the same ratio. Also, the formation constants (Kf) for such complexes were evaluated. The Kf values obtained (Table S3) designate the high stability of metal chelates in solution. Kf values are in the following order: MATYFe > MATYPd > MATYCu complex. Also, Gibb’s free energy (ΔG*) and stability constant (pK) values have been determined. The -ve sign of Gibb’s free energy suggests the spontaneity of the formation reactions.1874,75 Owing to the impact of pH changes, the complexes (Figure 2) exhibited stability over a wide pH range up to 4–10. This stability range permits the safe applicability of such complexes under restricted conditions such as acidity.
Figure 2.
pH effect on the synthesized MATY complexes at [complex] = 10 M in aqueous medium at 25 °C.
3.7. Synthesis of Polysubstituted Pyrazole-4-Carbonitrile Derivatives 4a–o
The catalytic activity of Pd(II) complex was evaluated towards the synthesis of bioactive pyrazole-4-carbonitrile derivatives with ultrasonic irradiation conditions. In this context, a facile one-pot condensation reaction for three-component (1 mmol) of aromatic aldehyde 1, malononitrile 2 and phenylhydrazine 3,was designated as a model reaction. Through present experiments, improvement in condensation reaction was systemically studied based on the influence of catalyst dose, reaction-solvent, as well as different active Lewis acid catalysts.
In absence of catalyst and ultrasonic irradiation, the trace of the product was got at a longer reaction time. While ultrasonic irradiation was only used without a catalyst, the product was more efficient at a longer reaction time. But there was improved yield in the presence of the Pd(II) complexes, catalyst and ultrasonic irradiation environment, which appeared excellent after a short reaction period. In the presence of Pd(II) complexes and ultrasonic irradiation environment, the yield of products was improved as clarified in Scheme 2. Series of aromatic aldehydes undergo electrophilic substitutions reactions are successfully synthesized in excellent yields as illustrated in Scheme 2.
Scheme 2. Pyrazole-4-Carbonitrile Derivatives 4a–o Time of Reaction (min) and Yield (%).

3.7.1. Effect of Catalyst Loading
The relationship between the catalyst amount and product yield is shown in Table 1. In particular, loading of the catalyst from 3 to 10 mol % improved the yield of the product from 16 to 97% (Table 1). Improvement of the yield by a continual increase of catalyst amount (MATYPd) can be rationalized based on the following factors: (i) the possibility for the interaction of the Pd atom with reactants to form an intermediate with five coordinates due to the presence of available sites; (ii) increasing the amount of catalyst will lead to increase in the contact surface with reactants and facilitate their collision. Notably, upon further increase of the loaded catalyst from 10 to 11 mol %, the yields and reaction times did not change significantly (Table 1). Thus, 10 mol % is the optimal amount of catalyst needed.
Table 1. Amounts of MATYPd Catalyst for the Synthesis of Pyrazole-4-Carbonitrile Derivatives 4a–ob.
| entry | catalyst (mol %) | yield (%)a | entry | catalyst (mol %) | yield (%)a |
|---|---|---|---|---|---|
| 1 | 3 | 16 | 5 | 8 | 84 |
| 2 | 5 | 38 | 6 | 9 | 93 |
| 3 | 6 | 57 | 7 | 10 | 97 |
| 4 | 7 | 77 | 8 | 11 | 97 |
Isolated yields based on 4a.
Reaction conditions: 1a (1 mmol), 2 (1. mmol), and 3 (1 mmol) in water were heated at 80 °C under ultrasonic irradiation condition for 15 min.
3.7.2. Effect of Solvents
To handle the procedure more easily, we then continued to optimize the model process mentioned above by detecting the efficiency of the classical solvents chosen as the medium of reaction for comparison (Table 2). The efficiency of solvents was evaluated with the model reaction 4a. As indicated in Table 2, the polar protic solvents (MeOH, EtOH, AcOH, and H2O) were much better than aprotic solvents (DCM, DMF, THF, CH3CN, and CHCl3). The data reflect the much better solubility of the reactants in polar solvents. From Table 2, it is evident that the use of water as a solvent is obviously the best choice for the synthesis of pyrazole-4-carbonitrile derivatives 4a, which proceeded rapidly with the highest yield. This solvent is preferable because it is considered green, safe, and cheap in comparison with organic solvents.
Table 2. Effect of Solvents on Synthesis of Pyrazole-4-Carbonitrile Derivatives 4a–o.
| solventb | time (min) | yield (%)a |
|---|---|---|
| DCM | 120 | 40 |
| DMF | 120 | 47 |
| THF | 120 | 53 |
| CH3CN | 120 | 42 |
| CHCl3 | 120 | 49 |
| MeOH | 40 | 76 |
| ACOH | 40 | 75 |
| EtOH | 15 | 92 |
| H2O | 15 | 97 |
Isolated yields based on 4a.
Reaction conditions: 1a (1 mmol), 2 (1 mmol), 3 (1 mmol), and catalyst (0.1 mmol) in water were heated at 80 °C under ultrasonic irradiation condition for 15 min.
3.7.3. Effect of Various Lewis Acid Catalysts
Recently, Pd(II) complexes have received considerable attention as a mild Lewis acid catalyst for an array of organic transformations. By the reaction of benzaldehyde 1, malononitrile 2, and phenylhydrazine 3 in the absence of catalyst and ultrasonic irradiation at the same condition, the trace product was obtained (Table 3, entry 1). While ultrasonic irradiation was only used without a catalyst, the product was more efficient at a longer reaction time (Table 3, entry 2). Various types of Lewis acids and Lewis bases such as AlCl3, MgCl2, FeCl3.6H2O, I2, ZnBr2, CuCl2, CuO, Pd(OAc)2, TiCl4, PTSA, Et3N, and TBABrc were tested in the selected reaction conditions. It was confirmed that the iron-soluble porphyrin catalyst was much better in comparison with all other Lewis acids due to its stability in water (Table 3, entries 3–14). When MATYCu, MATYFe, and MATYPd were tested (Table 3, entries 15–17), MATYPd was found to be the most effective catalyst, which afforded the desired product 4a by 97% yield (Table 3, entry 17). Use of MATYPd complex without ultrasonic irradiation at the same condition gave a lower yield (Table 3, entry 18).
Table 3. Use of Different Lewis Acids and Lewis Bases by Ultrasonic Irradiation (US) for the Reaction 4ab.
| entry | catalyst (mol %) | conditionsb | yield (%)a |
|---|---|---|---|
| 1 | no catalyst | water, 1 day | trace |
| no US | |||
| 2 | no catalyst | water, 1 h | 49 |
| 3 | AlCl3 (10) | water, 15 min | 42 |
| 4 | MgCl2 (10) | water, 15 min | 52 |
| 5 | FeCl3·6H2O (10) | water, 15 min | 43 |
| 6 | I2 (10) | water, 15 min | 51 |
| 7 | ZnBr2 (10) | water, 15 min | 54 |
| 8 | CuCl2 (10) | water, 15 min | 57 |
| 9 | CuO (10) | water, 15 min | 54 |
| 10 | Pd(OAc)2 (10) | water, 15 min | 73 |
| 11 | TiCl4 (10) | water, 15 min | 49 |
| 12 | PTSA (10) | water, 15 min | 44 |
| 13 | Et3N (10) | water, 15 min | 67 |
| 14 | TBABrc (10) | water, 15 min | 64 |
| 15 | MATYCu (10) | water, 15 min | 84 |
| 16 | MATYFe (10) | water, 15 min | 89 |
| 17 | MATYpd (10) | water, 15 min | 97 |
| 18 | MATYpd (10) | water, 15 min | 88 |
| no US |
Isolated yields based on 4a.
Reaction conditions: 1a (1 mmol), 2 (1 mmol), 3 (1 mmol), and catalyst (0.1 mmol) in water were heated at 80 °C under ultrasonic irradiation condition for 15 min.
Tetrabutylammonium bromide.
3.7.4. Recycling of the Suggested Catalyst
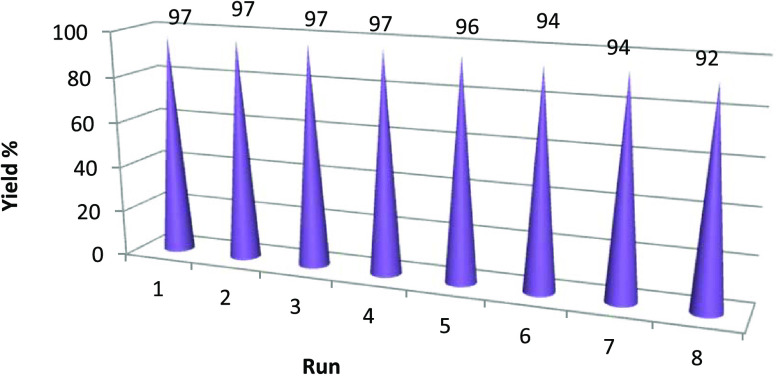
The green and economic aspects of this synthetic protocol was further studied by examining the possibility of reusing the catalyst in the next runs of synthesis for derivatives. To do this, the progress of the model reaction at optimum conditions and in the presence of the MATYPd catalyst was repeated five times for synthesis of compound 4a, and there was found an inevitable loss of catalyst during the recovery process. The results summarized (Figure 3) show that the catalyst was reused for four consecutive cycles without a significant decrease in its activity, but during the next runs (5–8), a low catalytic activity was shown under the same conditions. IR spectra for the investigated MATYPd catalyst before and after the catalytic reaction are presented in Figure 4. It was noted that there is no notable change in the spectra of the investigated MATYPd catalyst after its recycling from the reaction medium.
Figure 3.
Recyclability of the MATYPd catalyst in the model reaction.
Figure 4.
IR spectra of the MATYPd catalyst before and after the investigated catalytic reaction.
3.7.5. Suggested Mechanism for the Investigated Catalytic Reaction
We proposed a plausible mechanism for the heterogeneous catalytic procedure to synthesize pyrazole-4-carbonitrile derivatives under the influence of the MATYPd catalyst using ultrasonic irradiation at mild conditions as depicted in Scheme 3. Enolization of malononitrile was improved in the presence of the catalyst and water, and the nucleophilic character of methylene carbon increased through a hydrogen bond.38 As a result, the Knoevenagel condensation and Michael addition produced intermediates I and II, respectively. Phenylhydrazine functioned as both a Brønsted base and a nucleophile in this catalyst-free system. Subsequently, annulation, tautomerization, and aromatization of intermediate II (B) yielded the final product.
Scheme 3. Suggested mechanism for the synthesis of pyrazole-4-carbonitrile derivatives and the catalytic role of MATYPd by ultrasonic irradiation.
3.8. Supporting Computations
3.8.1. Quantitative Structural Activity Relationships (QSAR)
QSAR parameter scan be done for the complexes under consideration using Hyper Chem (8.1) software after adjusting the geometry optimization requirements. The steps were started by adding H-atoms over the selected molecule, using the semiempirical (AM1) and force-field Molecular Mechanics (MM+) setups, and after that starting the energy minimization process. This structural optimization was executed without any restrictions according to the Polake–Ribiere conjugated gradient algorithm method.39 The parameters computed were surface area, hydration energy, reactivity, polarizability, and partition coefficient (log p), in order to clarify the features of these solid complexes (Table 4). Reactivity, polarizability, and surface area were the indicators for the surface properties of the solid compounds, which were significant in catalytic efficiency. According to their values, the properties of the Cu(II) complex were shown to be commonly superior to those of the Pd(II) or Fe(III) complexes. This superiority may be not effective, due to the octahedral configuration of the Cu(II) complex blocking perfectly the metal active sites and reducing the probability for extra coordination. However, the Pd(II) complex, due to its square-planar geometry, has a good chance for extra coordination and exceeded the number to five, which may have happened during the catalytic steps.
Table 4. QSAR Parameters for the Tested Complexes.
| complexes |
|||
|---|---|---|---|
| parameters | Fe(III) | Pd(II) | Cu(II) |
| surface area (grid) (Å) | 638.49 | 719.35 | 761.51 |
| volume(Å) | 1319.54 | 1329.55 | 1338.0 |
| hydration energy (kcal/mol) | –31.40 | –13.75 | –21.01 |
| log p | –8.16 | –0.87 | –1.85 |
| reactivity (Å) | 134.15 | 131.91 | 134.31 |
| polarizability (Å) | 50.00 | 50.55 | 50.57 |
3.8.2. Global Reactivity and Electrostatic Potential Maps
The DFT method was implemented in the DMOL3 program using the Becke3–Lee–Yang–Parr (B3LYP) exchange-correlation functional under the DNP basis set due to its flexibility and consistency. This study was used to optimize the geometries of MATY and its complexes to realize their comparative features and confirm the MATY binding mode. Significant computational parameters were extracted (Table 5) to discriminate between them regarding their catalytic efficiency. An electrostatic potential map was drawn for all optimized structures to clarify the attacking behavior of the functional groups.40 The best geometry obtained for the MATY ligand points to the perfect positions of the coordinating sites (N20 and N19). Also, the geometry of the complexes showed normal bond lengths, and the bonds do not suffer any unfavorable strain (Figure 5). This emphasizes the mode of coordination proposed from practical analyses. Electrophilicity (ω), absolute softness (σ), global softness (S), and electronegativity (χ) indexes were calculated to have a clear view about some properties (Table 5).40 The softness property appeared to be distinguished for the MATYPd complex. This reflects the elasticity of such a complex, which is preferable for interaction with biological systems. The high electrophilicity and electronegativity values of the MATYPd complex denote its susceptibility for acquiring electrons from any donor species. This capacity depends on the unsaturated electronic configuration of the Pd atom, which pushed for extra coordination to reach saturation (18 e–1).41,42 The lower energy-gap values (ΔE = ELUMO – EHOMO) of the complexes, particularly for the MATYPd complex, indicate the ease of electronic transitions and low activation energy barrier, which are preferred in a catalytic application. Other molecular parameters were computed to measure the stability of the complexes under study (Table 6). The MATYPd complex is less stable due to its high energy content, which suggests its ability for extra bonding to complete the electron count of the palladium valence shell. Frontier orbitals were established on optimized geometries, to recognize the groups that are responsible for coordination and electronic transitions (Figure 6). With respect to the MATY ligand, the HOMO level appeared to be focused on the coordinating zone (N20 and N19), while the LUMO level appeared extended over the whole molecule. However, such frontier orbitals appeared focused on the ligand in the MATYCu complex, but concentrated around the Pd and Fe atoms in their complexes.43
Table 5. Estimated Physical Parameters (eV) according to Frontier Energy Gaps.
| compound | EH | EL | EH – EL | EL – EH | x | μ | η | S (eV–1) | ω | σ (eV) |
|---|---|---|---|---|---|---|---|---|---|---|
| MATY | –0.1868 | –0.1100 | –0.0768 | 0.0768 | 0.1484 | –0.1484 | 0.0384 | 0.0192 | 0.2868 | 26.0417 |
| MATYCu | –0.1798 | –0.1444 | –0.0354 | 0.0354 | 0.16211 | –0.1621 | 0.0177 | 0.0089 | 0.7415 | 56.4334 |
| MATYFe | –0.0619 | –0.0270 | –0.0349 | 0.0349 | 0.04443 | –0.0444 | 0.0175 | 0.0087 | 0.0565 | 57.2902 |
| MATYPd | –0.1463 | –0.1198 | –0.0265 | 0.0265 | 0.13308 | –0.1331 | 0.0133 | 0.0066 | 0.6683 | 75.4717 |
Figure 5.
Optimized geometry of the investigated (a) MATY ligand, and (b) MATYCu, (c) MATYPd, and (d) MATYFe complexes.
Table 6. Molecular Parameters of the MATY Ligand and Its Complexes.
| computed
values |
||||
|---|---|---|---|---|
| compounds | MATYFe | MATYPd | MATYCu | MATY |
| total energy (Ha) | –1580.591392 | –2416.144023 | –2204.070170 | –2642.515338 |
| sum of atomic energies (Ha) | –1572.1156181 | –2403.6396360 | –2192.2986133 | –2630.9706630 |
| kinetic energy (Ha) | –10.7121247 | –18.0085026 | –17.2035576 | –20.4028446 |
| electrostatic energy (Ha) | –3.9931612 | –2.9883189 | –2.4260780 | 0.5258222 |
| exchange-correlation energy (Ha) | 3.6028155 | 4.8463313 | 4.5024046 | 4.7095150 |
| spin polarization energy (Ha) | 2.6266963 | 3.6461037 | 3.3556744 | 3.6228328 |
| binding energy (Ha) | –8.4757741 | –11.6576227 | –11.3715566 | –11.5446746 |
| dipole moment (Debye) | 5.4926 | 4.4641 | 6.4713 | 16.6801 |
Figure 6.
HOMO and LUMO levels for (a) the MATY ligand, and (b) MATYCu, (c) MATYPd, and (d) MATYFe complexes.
Electrostatic potential maps (MEP) were established (Figure 7) to clarify the electronic distribution over the functional groups. Nucleophilic, electrophilic, and neutrality features could be easily indicate by such maps. Red, blue, and green colors were the indicators used for discrimination between the three features, respectively. The nucleophilic property of N19 and N20 was clearly noticed and mainly improved in the complexes due to M → L charge transfer.44,45
Figure 7.
MEP maps for (a) the MATY ligand, and (b) MATYCu, (c) MATYPd, and (d) MATYFe complexes.
3.8.3. Theoretical Bases for Catalytic Behavior
The catalytic behavior of the MATYPd complex is estimated based on the following features:
-
(1)
The reduced band-gap value (ΔE = ELUMO – EHOMO = 0.0265 eV) estimated denotes the flexibility of the valence electrons and lower required activation energy for a catalytic role.
-
(2)
The unsaturated valence shell of the central atom in a complex (16 e–1) reflects its instability and reactivity toward acquiring electrons to reach saturation (18 e–1), which may be attributed to the extra-coordinate bond.
Consequently, a computational study assumes importance in supporting the catalytic mechanism, which is based mainly on the logical hypotheses regarding how the catalytic processes have happened. The suggested reaction pathway has proceeded through the formation of five conformers (A–E). Such conformers were optimized by the DFT/B3LYP method under the 6-31G++basis set to confirm this mechanism opportunity through the stability of the assumed conformers and the possibility for their formation (Scheme 4). The MATYPd catalyst (E = −6938 au) has a lower energy content, which is increased after its interaction (Compound A) with starting materials such as benzaldehyde and malononitrile (E = −2112.8 au). Four sequenced conformers appeared with the following formation energies: −836.82, −835.30, −836.85, and −835.69 au for conformers B–E, respectively. These values reflect their closeness to each other in stability degree, which gives credibility for the suggested mechanism pathway.
Scheme 4. Profile of the activated intermediates during the synthesis of pyrazole-4-carbonitrile derivatives that were catalyzed by the MAYTPd complex by ultrasonic irradiation.
4. Conclusion
Three thiazole complexes were prepared and characterized to establish their chemical forms. The ligand behaved as a neutral bidentate toward the mononuclear central atom (1:1 molar ratio) within the complexes. Octahedral geometry was suggested for Fe(III) and Cu(II) complexes, while Pd(II) complex (MATYPd) appeared to have a square-planar geometry. Pd(II) complex was successfully developed by a facile and efficient method for synthesis of pyrazole-4-carbonitrile derivatives using ultrasonic irradiation. This was achieved by reaction of aromatic aldehyde 1 (1 mmol), malononitrile 2, and phenylhydrazine 3 in the presence of catalyst by ultrasonic irradiation at mild conditions. The catalytic activity of MATYPd in a three-component reaction approach for the aromatization of pyrazole-4-carbonitrile derivatives was achieved by the green way (in H2O). The higher catalytic activity of the complex is ascribed to its high acidity and water tolerance. Also, the superiority of using MATYPd toward the synthesis of pyrazoles is compared with other Lewis acids and Lewis bases. All reactions were carried out in H2O within 15–30 min to afford the products with high to excellent yields. Computational parameters asserted the properties of the Pd(II) complex, which may be effective in catalytic application because of its reduced optical band gap and electrophilicity. The mechanism of the catalytic process was suggested and supported by the DFT/B3LYP method. This simple, economical, and green procedure may be applied to industry in the future.
Acknowledgments
The authors thank the Taif University Researchers Supporting Project Number (TURSP-2020/158), Taif University, Taif, Saudi Arabia. Also, the authors are deeply grateful to Sohag University in Egypt for supporting and facilitating this study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02811.
Reagents and instrumentation used in investigated study; thermogravimetric scheme for degradation of degradation steps for prepared complexes; IR, NMR, electronic spectra, Jop’s and molar ratio figures; Tables S1–S3 for electronic spectra, degradation steps and stability constants for complex formation (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Renuka N.; Kariyappa A. K. Synthesis and biological evaluation of novel formyl-pyrazoles bearing coumarin moiety as potent antimicrobial and antioxidant agents. Bioorg. Med. Chem. Lett. 2013, 23, 6406–6409. 10.1016/j.bmcl.2013.09.053. [DOI] [PubMed] [Google Scholar]
- Kalaria P. N.; Shailesh P. S.; Dipak K. R. Synthesis, identification and in vitro biological evaluation of some novel 5-imidazopyrazole incorporated pyrazoline and isoxazoline derivatives. New J. Chem. 2014, 38, 2902–3002. 10.1039/c4nj00244j. [DOI] [Google Scholar]
- a El-Metwaly N.; Katouah H.; Aljuhani E.; Alharbi A.; Alkhatib F.; Aljohani M.; Alzahrani S.; Alfaifi M. Y.; Khedr A. M. Synthesis and elucidation for new nanosized Cr (III)-pyrazolin complexes; crystal surface properties, antitumor simulation studies beside practical apoptotic path. J. Inorg. Organomet. Polym. 2020, 30, 4142–4154. 10.1007/s10904-020-01561-2. [DOI] [Google Scholar]; b El-Metwaly N.; Althagafi I.; Khedr A. M.; Al-Fahemi J. H.; Katouah H. A.; Hossan A. S.; Al-Dawood A. Y.; Al-Hazmi G. A. complexes-dyes and their usage in dyeing cotton to be special bandage for cancerous wounds. J. Mol. Struct. 2019, 1194, 86–103. 10.1016/j.molstruc.2019.05.080. [DOI] [Google Scholar]; c Althagafi I.; El-Metwaly N. M.; Farghaly T. Characterization of new Pt (IV)–thiazole complexes: Analytical, spectral, molecular modeling andmolecular docking studies and applications in two opposing pathways. Appl. Organomet. Chem. 2019, 33, e5099 10.1002/aoc.5099. [DOI] [Google Scholar]
- Yang J.; Gharagozloo P.; Yao J.; Ilyin V. I.; Carter R. B.; Nguyen P.; Robledo S.; Woodward R. M.; Hogenkamp D. J. 3-(4-Phenoxyphenyl) pyrazoles: a novel class of sodium channel blockers. J. Med. Chem. 2004, 47, 1547–1552. 10.1021/jm030498q. [DOI] [PubMed] [Google Scholar]
- El-Metwaly N.; Farghaly T. A.; Elghalban M. G. Synthesis, analytical and spectral characterization for new VO (II)-triazole complexes; conformational study beside MOE docking simulation features. Appl. Organomet. Chem. 2020, 34, e5505 10.1002/aoc.5505. [DOI] [Google Scholar]
- Bailey D. M.; Hansen P. E.; Hlavac A. G.; Baizman E. R.; Pearl J.; DeFelice A. F.; Feigenson M. E. 3, 4-Diphenyl-1H-pyrazole-1-propanamine antidepressants. J. Med. Chem. 1985, 28, 256–260. 10.1021/jm00380a020. [DOI] [PubMed] [Google Scholar]
- a Eddingsaas N. C.; Suslick K. S. Light from sonication of crystal slurries. Nature 2006, 444, 163. 10.1038/444163a. [DOI] [PubMed] [Google Scholar]; b Khaligh N. G.; Shirini F. N-Sulfonic acid poly (4-vinylpyridinium) hydrogen sulfate as an efficient and reusable solid acid catalyst for one-pot synthesis of xanthene derivatives in dry media under ultrasound irradiation. Ultrason. Sonochem. 2015, 22, 397–403. 10.1016/j.ultsonch.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Suslick K. S. The chemical effects of ultrasound. Sci. Am. 1989, 260, 80–87. 10.1038/scientificamerican0289-80. [DOI] [Google Scholar]
- Allahyari S.; Haghighi M.; Ebadi A.; Hosseinzadeh S. Ultrasound assisted co-precipitation of nanostructured CuO–ZnO–Al2O3 over HZSM-5: effect of precursor and irradiation power on nanocatalyst properties and catalytic performance for direct syngas to DME. Ultrason. Sonochem. 2014, 21, 663–673. 10.1016/j.ultsonch.2013.09.014. [DOI] [PubMed] [Google Scholar]
- a Ramazani A.; Rouhani M.; Joo S. W. Catalyst-free sonosynthesis of highly substituted propanamide derivatives in water. Ultrason. Sonochem. 2016, 28, 393–399. 10.1016/j.ultsonch.2015.08.019. [DOI] [PubMed] [Google Scholar]; b Xu H.; Zeiger B. W.; Suslick K. S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. 10.1039/C2CS35282F. [DOI] [PubMed] [Google Scholar]
- a Jones R. A.; Bean G. P.. The Chemistry of Pyrroles, 1st ed.; Academic: London, 1977; pp 1–5. [Google Scholar]; b Sundberg R. J.Comprehensive Heterocyclic Chemistry; In Katritzky A. R.; Rees C. W.; Bird C. W.; Cheeseman G. W. H., Eds.; Pergamon: Oxford, 1984; Vol. 4, pp 313–376. [Google Scholar]; c Sundberg R. J.Comprehensive Heterocyclic Chemistry II; In Katritzky A. R.; Rees C. W., Eds.; Pergamon: Oxford, 1996; Vol. 2, p 149. [Google Scholar]
- a Maddila S.; Rana S.; Pagadala R.; Kankala S.; Maddila S.; Jonnalagadda S. B. Synthesis of pyrazole-4-carbonitrile derivatives in aqueous media with CuO/ZrO2 as recyclable catalyst. Catal. Commun. 2015, 61, 26–30. 10.1016/j.catcom.2014.12.005. [DOI] [Google Scholar]; b Srivastava M.; Rai P.; Singh J.; Singh J. An environmentally friendlier approach—ionic liquid catalysed, water promoted and grinding induced synthesis of highly functionalised pyrazole derivatives. RSC Adv. 2013, 3, 16994–16998. 10.1039/c3ra42493f. [DOI] [Google Scholar]; c Kiyani H.; Bamdad M. Sodium ascorbate as an expedient catalyst for green synthesis of polysubstituted 5-aminopyrazole-4-carbonitriles and 6-amino-1, 4-dihydropyrano [2,3-c] pyrazole-5-carbonitriles. Res. Chem. Intermed. 2018, 44, 2761–2778. 10.1007/s11164-018-3260-0. [DOI] [Google Scholar]; d Srivastava M.; Rai P.; Singh J.; Singh J. Efficient iodine-catalyzed one pot synthesis of highly functionalised pyrazoles in water. New J. Chem. 2014, 38, 302–307. 10.1039/C3NJ01149F. [DOI] [Google Scholar]; e Liu P.; Pan Y. M.; Xu Y. L.; Wang H. S. PTSA-catalyzed Mannich-type–cyclization–oxidation tandem reactions: one-pot synthesis of 1,3,5-substituted pyrazoles from aldehydes, hydrazines and alkynes. Org. Biomol. Chem. 2012, 10, 4696–4698. 10.1039/c2ob25487e. [DOI] [PubMed] [Google Scholar]
- a Neochoritis C. G.; Zhao T.; Doming A. Tetrazoles via multicomponent reactions. Chem. Rev. 2019, 119, 1970–2042. 10.1021/acs.chemrev.8b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Safaei H. R.; Khastkhoda T.; Shekouhy M. A New Highly Efficient Method for the Catalyst-Free Synthesis of Conjoined Twins 1, 6-Dioxaspiro Derivatives Through Double Reaction of Carbon Dioxide in One Reaction. ChemistrySelect 2018, 3, 6273–6278. 10.1002/slct.201801082. [DOI] [Google Scholar]; c Elhamifar D.; Kazempoor S.; Karimi B. Amine-functionalized ionic liquid-based mesoporous organosilica as a highly efficient nanocatalyst for the Knoevenagel condensation. Catal. Sci. Technol. 2016, 6, 4318–4326. 10.1039/C5CY01666E. [DOI] [Google Scholar]; d Mirbagheri R.; Elhamifar D.; Norouzi M. Propylamine-containing magnetic ethyl-based organosilica with a core–shell structure: an efficient and highly stable nanocatalyst. New J. Chem. 2018, 42, 10741–10750. 10.1039/C8NJ01674G. [DOI] [Google Scholar]; e Norouzi M.; Elhamifar D. Magnetic yolk-shell structured methylene and propylamine based mesoporous organosilica nanocomposite: A highly recoverable and durable nanocatalyst with improved efficiency. Colloids Surf., A 2021, 615, 126226 10.1016/j.colsurfa.2021.126226. [DOI] [Google Scholar]
- a Salehi N.; Mirjalili B. B. F. Green Synthesis of Pyrano [2, 3-c] pyrazoles and Spiro [indoline-3, 4′-pyrano [2, 3-c] pyrazoles] Using Nano-silica Supported 1, 4-Diazabicyclo [2.2. 2] octane as a Novel Catalyst. Org. Prep. Proced. Int. 2018, 50, 578–587. 10.1080/00304948.2018.1537748. [DOI] [Google Scholar]; b Carvalho R. B.; Joshi S. V. Solvent and catalyst free synthesis of 3, 4-dihydropyrimidin-2 (1 H)-ones/thiones by twin screw extrusion. Green Chem. 2019, 21, 1921–1924. 10.1039/C9GC00036D. [DOI] [Google Scholar]; c McDonald E.; Keith J.; Paul A. B.; Drysdale M. J.; Workman P. Discovery and development of pyrazole-scaffold Hsp90 inhibitors. Curr. Top. Med. Chem. 2006, 6, 1193–1203. 10.2174/156802606777812086. [DOI] [PubMed] [Google Scholar]; d Norouzi M.; Elhamifar D.; Mirbagheri R. Phenylene-based periodic mesoporous organosilica supported melamine: an efficient, durable and reusable organocatalyst. Microporous Mesoporous Mater. 2019, 278, 251–256. 10.1016/j.micromeso.2018.11.040. [DOI] [Google Scholar]; e Mofatehnia P.; Ziarani G. M.; Elhamifar D.; Badiei A. A new yolk-shell hollow mesoporous nanocomposite, Fe3O4@ SiO2@ MCM41-IL/WO42-, as a catalyst in the synthesis of novel pyrazole coumarin compounds. J. Phys. Chem. Solids 2021, 155, 110097 10.1016/j.jpcs.2021.110097. [DOI] [Google Scholar]; f Mousavi F.; Elhamifar D.; Kargar S. Copper/IL-containing magnetic nanoporous MCM-41: A powerful and highly stable nanocatalyst. Surf. Interfaces 2021, 25, 101225 10.1016/j.surfin.2021.101225. [DOI] [Google Scholar]
- a Higgins S. J. Conjugated polymers incorporating pendant functional groups—synthesis and characterisation. Chem. Soc. Rev. 1997, 26, 247–257. 10.1039/CS9972600247. [DOI] [Google Scholar]; b Yamaguchi S.; Tamao K. Cross-coupling reactions in the chemistry of silole-containing π-conjugated oligomers and polymers. J. Organomet. Chem. 2002, 653, 223–228. 10.1016/S0022-328X(02)01152-X. [DOI] [Google Scholar]; c Shiner C. M.; Lash T. D. Porphyrins with exocyclic rings. Part 21: Influence of pyrrolic and carbocyclic ring alkyl substituents on the synthesis of porphyrins bearing six-membered exocyclic rings. Tetrahedron 2005, 61, 11628–11640. 10.1016/j.tet.2005.10.019. [DOI] [Google Scholar]; d Hantzsch A. Neue bildungs weise von pyrrol derivaten. Ber. Dtsch. Chem. Ges. 1890, 23, 1474–1476. 10.1002/cber.189002301243. [DOI] [Google Scholar]; e Matiychuk V. S.; Martyak R. L.; Obushak N. D.; Ostapiuk Y. V.; Pidlypnyi N. I. 3-aryl-2-chloropropanals in Hantzsch synthesis of pyrroles. Chem. Heterocycl. Compd. 2004, 40, 1218–1219. 10.1023/B:COHC.0000048299.17625.7f. [DOI] [Google Scholar]; f Chen J.; Wu H.; Zheng Z.; Jin C.; Zhang X.; Su W. An approach to the Paal–Knorr pyrroles synthesis catalyzed by Sc (OTf) 3 under solvent-free conditions. Tetrahedron Lett. 2006, 47, 5383–5387. 10.1016/j.tetlet.2006.05.085. [DOI] [Google Scholar]
- a El-Remaily M. A. E. A. A. A.; Elhady O. M. Iron (III) porphyrin Complex FeTSPP as an efficient catalyst for synthesis of tetrazole derivatives via [2 + 3] cycloaddition reaction in aqueous medium. Appl. Organomet. Chem. 2019, 33, 4989 10.1002/aoc.4989. [DOI] [Google Scholar]; b El-Remaily M. A. E. A. A. A.; Elhady O. M. Cobalt (III)-porphyrin Complex CoTCPP as an efficient and recyclable homogeneous catalyst for synthesis of tryptanthrin in water. Tetrahedron Lett. 2016, 57, 435–437. 10.1016/j.tetlet.2015.12.052. [DOI] [Google Scholar]; c El-Remaily M. A. E. A. A. A.; Hamad H. A. Synthesis and characterization of highly stable superparamagnetic CoFe2O4 nanoparticles as a catalyst for novel synthesis of thiazolo [4,5-b]quinolin-9-one derivatives in aqueous medium. J. Mol. Catal., A 2015, 404–405, 148–155. 10.1016/j.molcata.2015.04.023. [DOI] [Google Scholar]; d El-Remaily M. A. E. A. A. A.; Abu-Dief A. M.CuFe2O4 nanoparticles: an efficient heterogeneous magnetically Separable catalyst for Synthesis of some novel propynyl-1H imidazoles derivatives. Tetrahedron 2015, 71, 2579–2584. 10.1016/j.tet.2015.02.057. [DOI] [Google Scholar]; e El-Remaily M. A. A. Synthesis of Pyranopyrazoles Using Magnetic Fe3O4 Nanoparticles as Efficient and Reusable Catalyst. Tetrahedron 2014, 70, 2971–2975. 10.1016/j.tet.2014.03.024. [DOI] [Google Scholar]; f El-Remaily M. A. E. A. A. A. Bismuth Triflate highly an efficient catalyzed for Synthesized of bio-active coumarin compounds via one-pot multi-component reactions protocol. Chin. J. Catal. 2015, 36, 1124–1130. 10.1016/S1872-2067(14)60308-9. [DOI] [Google Scholar]; g Soliman A. M. M.; Mohamed S. K.; El-Remaily M. A. A.; Abdel-Ghany H. Synthesis and biological activity of Dihydroimidazole and 3,4-dihydrobenzo[4,5]imidazo[1,2-a][1,3,5] triazins. Eur. J. Med. Chem. 2012, 47, 138–142. 10.1016/j.ejmech.2011.10.034. [DOI] [PubMed] [Google Scholar]; h El-Remaily M. A. E. A. A. A.; Mohamed S. K. Eco- Friendly synthesis of guanidinyltetrazole compounds and 5- substituted 1H-tetrazoles in water under microwave irradiation. Tetrahedron 2014, 70, 270–275. 10.1016/j.tet.2013.11.069. [DOI] [Google Scholar]; i El-Remaily M. A. E. A. A. A.; Soliman A. M. M. Epichlorohydrin cross-linked β-cyclodextrin polymer a promising method in industry for the synthesis of 2-phenylbenzthiazole in water. J. Sulfur Chem. 2016, 37, 70. 10.1080/17415993.2015.1089874. [DOI] [Google Scholar]; j Ni S.; El-Remaily M. A. E. A. A. A.; Johan F. Carbocation Catalyzed Bromination of Alkyl Arenes, a Chemoselective sp3 vs. sp2 C_H functionalization. Adv. Synth. Catal. 2018, 360, 4197–4204. 10.1002/adsc.201800788. [DOI] [Google Scholar]
- a El-Remaily M. A. E. A. A. A.; Abu-Dief A. M.; Elhady O. M. Green synthesis of TiO2 nanoparticles as an efficient heterogeneous catalyst with high reusability for synthesis of 1,2-dihydroquinoline derivatives. Appl. Organomet. Chem. 2019, 33, 5005 10.1002/aoc.5005. [DOI] [Google Scholar]; b El-Remaily M. A. E. A. A. A.; Abu-Dief A. M.; Rafat M. E. A robust Synthesis and Characterization for Superparamagnetic CoFe2O4 Nanoparticles as an Efficient and Reusable Catalyst for Synthesis of some Heterocyclic rings in aqueous media. Appl. Organomet. Chem. 2016, 30, 1022–1029. 10.1002/aoc.3536. [DOI] [Google Scholar]; c El-Remaily M. A. E. A. A. A.; Soliman A. M. M.; Elhady O. M. Green Method for the Synthetic Ugi Reaction by Twin Screw Extrusion without a Solvent and Catalyst. ACS Omega 2020, 5, 6194–6198. 10.1021/acsomega.0c00369. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ahmed E. A.; Soliman A. M. M.; Ali A. M.; El-Remaily M. A. A. Boosting the catalytic performance of zinc linked amino acid complex as an eco-friendly for synthesis of novel pyrimidines in aqueous medium. Appl. Organomet. Chem. 2021, 35, e6197 10.1002/aoc.6197. [DOI] [Google Scholar]; e El-Remaily M. A. E. A. A. A.; Elhady O. M. Green Bio-organic and Recoverable Catalyst Taurine (2-aminoethanesulfonic acid) for Synthesis of Bio-active Compounds 3,4-Dihydropyrimidin Derivatives in Aqueous Medium. ChemistrySelect 2020, 5, 12098–12102. 10.1002/slct.202002575. [DOI] [Google Scholar]; f El-Remaily M. A. E. A. A. A.; Hamad H. A.; Hamad H. A.; Elhady O. M. Appl. Organomet. Chem. 2021, 35, e6238. [Google Scholar]; g Shokr E. K.; Kamel M. S.; Abdel-Ghany H.; El-Remaily M. A. A. Optoelectronic characteristics of as-deposited, annealed and I2–Treated thin films of newly synthesized organic dye based on pyrrolo [2, 3-b] pyrrole. Curr. Opin. Green Sustainable Chem. 2021, 4, 100090 10.1016/j.crgsc.2021.100090. [DOI] [Google Scholar]; h Hamad H. A.; Nageh H.; El-Bery H. M.; Kasry A.; Carrasco-Marín F.; Elhady O. M.; Soliman A. M. M.; El-Remaily M. A. A. Unveiling the exceptional synergism-induced design of Co-Mg-Al layered triple hydroxides (LTHs) for boosting catalytic activity toward the green synthesis of indol-3-yl derivatives under mild conditions. J. Colloid Interface Sci. 2021, 599, 227–244. 10.1016/j.jcis.2021.04.083. [DOI] [PubMed] [Google Scholar]; i Shokr E. K.; Kamel M. S.; Abdel-Ghany H.; El-Remaily M. A. A. Optical characterization and effects of iodine vapor and gaseous HCl adsorption investigation of Novel Synthesized Organic dye Based on Thieno [2,3-b]thiophene. Optik 2021, 243, 167385 10.1016/j.ijleo.2021.167385. [DOI] [Google Scholar]
- a Abu-Dief A. M.; El-Metwaly N. M.; Alzahrani S. O.; Alkhatib F.; Abualnaja M. M.; El-Dabea T.; El-Remaily M. A. E. A. A. Synthesis and characterization of Fe (III), Pd (II) and Cu (II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J. Mol. Liq. 2021, 326, 115277 10.1016/j.molliq.2021.115277. [DOI] [Google Scholar]; b Alzahrani S. O.; Abu-Dief A. M.; Alkamis K.; Alkhatib F.; El-Remaily M. A. A.; El-Metwaly N. M.; et al. Synthesis and structural elucidation for new pyranothiazolecomplexes: Biological screening and effects on DNA throughin-vitro and in-silico approaches. J. Mol. Liq. 2021, 332, 115844 10.1016/j.molliq.2021.115844. [DOI] [Google Scholar]; c Abu-Dief A. M.; El-Metwaly N. M.; Alzahrani S. O.; Alkhatib F.; Abumelha H. M.; El-Dabea T.; El-Remaily M. A. A. Structural, conformational and therapeutic studies on new thiazole complexes: drug-likeness and MOE-simulation assessments. Res. Chem. Intermed. 2021, 47, 1979–2002. 10.1007/s11164-020-04380-9. [DOI] [Google Scholar]; d El-Remaily M. A. E. A. A. A.; Soliman Khalifa M. A.; El-Metwaly N. M.; Alsoliemy M.; El-Dabea T.; Abu-Dief A. M. Rapidly, highly yielded and green synthesis of dihydrotetrazolo[1,5-a]pyrimidine derivatives in aqueous media using recoverable Pd (II) thiazole catalyst accelerated by ultrasonic: Computational studies. Appl. Organomet. Chem. 2021, 35, e6320 10.1002/aoc.6320. [DOI] [Google Scholar]
- a Taakeyama T.; Quinn F. X.. Thermal Analysis Fundamentals and Applications to Polymer Science; John Wiley and Sons: Chichester, 1994. [Google Scholar]; b Abdel-Rahman L. H.; Abu-Dief A. M.; Moustafa H.; Abdel-Mawgoud A. A. H. Design and nonlinear optical properties (NLO) using DFT approach of new Cr (III), VO (II), and Ni (II) chelates incorporating tri-dentate imine ligand for DNA interaction, antimicrobial, anticancer activities and molecular docking studies. Arab. J. Chem. 2020, 13, 649–670. 10.1016/j.arabjc.2017.07.007. [DOI] [Google Scholar]; c Coats A. W.; Redfern P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. 10.1038/201068a0. [DOI] [Google Scholar]
- Alkhatib F.; Hameed A.; Sayqal A.; Bayazeed A. A.; Alzahrani S.; Al-Ahmed Z. A.; Zaky R.; El-Metwaly N. M.; et al. Green-synthesis and characterization for new Schiff-base complexes; spectroscopy, conductometry, Hirshfeld properties and biological assay enhanced by in-silico study. Arab. J. Chem. 2020, 13, 6327–6340. 10.1016/j.arabjc.2020.05.033. [DOI] [Google Scholar]
- Abd El-Lateef H. M.; Abu-Dief A. M.; Mohamed M. A. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017, 1130, 522–542. 10.1016/j.molstruc.2016.10.078. [DOI] [Google Scholar]
- Abd El-Lateef H. M.; Abu-Dief A. M.; Abdel-Rahman L. H.; Sañudo E. C.; Aliaga-Alcalde N. Electrochemical and theoretical quantum approaches on the inhibition of C1018 carbon steel corrosion in acidic medium containing chloride using some newly synthesized phenolic Schiff bases compounds. J. Electroanal. Chem. 2015, 743, 120–133. 10.1016/j.jelechem.2015.02.023. [DOI] [Google Scholar]
- Abdel-Rahman L. H.; Abu-Dief A. M.; Ismael M.; Mohamed M. A.; Hashem N. A. Synthesis, structure elucidation, biological screening, molecular modeling and DNA binding of some Cu (II) chelates incorporating imines derived from amino acids. J. Mol. Struct. 2016, 1103, 232–244. 10.1016/j.molstruc.2015.09.039. [DOI] [Google Scholar]
- Modeling and Simulation Solutions for Chemicals and Materials Research, Materials Studio (version 5.0); Accelrys Software Inc.: San Diego, USA, www.accelrys.com, 2009.
- Frisch M. J.; Trucks G. W.; Pople J. A.. Gaussian 09, revision B.2; Gaussian, Inc.: Pittsburgh, PA, 2009.
- Hehre W. J.; Radom L.; Schlyer P. V. R.; Pople J. A.. Ab Initio Molecular Orbital Theory; Wiley: New York, 1986. [Google Scholar]
- Kessi A.; Delley B. Density functional crystal vs. cluster models as applied to zeolites. Int. J. Quantum Chem. 1998, 68, 135–144. . [DOI] [Google Scholar]
- a Abdel-Rahman L. H.; Adam M. S. S.; Abu-Dief A. M.; Moustafa H.; Basha M. T.; Aboraia A. S.; Al -Farhan B. S.; El -Sayed A. H. Synthesis, theoretical investigations, biocidal screening, DNA binding, in vitro cytotoxicity and molecular docking of novel Cu (II), Pd (II) and Ag (I) complexes of chlorobenzylidene Schiff base: Promising antibiotic and anticancer agents. Appl. Organomet. Chem. 2018, 32, e4527 10.1002/aoc.4527. [DOI] [Google Scholar]; b Abu-Dief A. M.; El-khatib R. M.; Aljohani F. S.; Alzahrani S. O.; Mahran A.; Khalifa M. E.; El-Metwaly N. M. Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J. Mole Struct. 2021, 1242, 130693 10.1016/j.molstruc.2021.130693. [DOI] [Google Scholar]; c Alsalme A. M.; Nafady A.; Abdel-Rahman L. H.; Abu-Dief A. M.; Atlam F. M.; Hassan Abdel-Mawgoud A. A.; Alothman A. A. Chemical, physical and biological properties of Pd(II), V(IV)O, and Ag(I) complexes of N3 tridentate pyridinebased Schiff base ligand. J. Coord. Chem. 2020, 73, 3150–3173. 10.1080/00958972.2020.1842378. [DOI] [Google Scholar]
- a Aljohani E. T.; Shehata M. R.; Alkhatib F.; Alzahrani S. O.; Abu-Dief A. M. Development and structure elucidation of new VO2+, Mn2+, Zn2+, and Pd2+ complexes based on azomethine ferrocenyl ligand: DNA interaction, antimicrobial, antioxidant, anticancer activities, and molecular docking. Appl. Organomet. Chem. 2021, 35, e6154 10.1002/aoc.6154. [DOI] [Google Scholar]; b Abdel-Rahman L. H.; Adam M. S.; Abu-Dief A. M.; El-Sayed Ahmed H.; Nafady A. Non-Linear Optical Property and Biological Assays of Therapeutic Potentials Under In Vitro Conditions of Pd(II), Ag(I) and Cu(II) Complexes of 5-Diethyl amino-2-({2-[(2-hydroxy-Benzylidene)-amino]-phenylimino}-methyl)-phenol. Molecules 2020, 25, 5089 10.3390/molecules25215089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Rahman L. H.; Abu-Dief A. M.; Mostafa H.; Hamdan S. K. Ni (II) and Cu (II) complexes with ONNO asymmetric tetradentate Schiff base ligand: synthesis, spectroscopic characterization, theoretical calculations, DNA interaction and antimicrobial studies. Appl. Organomet. Chem. 2017, 31, e3555 10.1002/aoc.3555. [DOI] [Google Scholar]
- Abu-Dief A. M.; Abdel-Rahman L. H.; Abdel-Mawgoud A. A. H. A robust in vitro anticancer, antioxidant and antimicrobial agents based on new metal-azomethine chelates incorporating Ag (I), Pd (II) and VO (II) cations: Probing the aspects of DNA interaction. Appl. Organomet. Chem. 2020, 34, e5373 10.1002/aoc.5373. [DOI] [Google Scholar]
- Al-Hazmi G. A.; Abou-Melha K. S.; El-Metwaly N. M.; Althagafi I.; Shaaban F.; Zaky R. Green synthesis approach for Fe (III), Cu (II), Zn (II) and Ni (II)-Schiff base complexes, spectral, conformational, MOE-docking and biological studies. Appl. Organomet. Chem. 2019, 34, e5403 10.1002/aoc.5403. [DOI] [Google Scholar]
- a Getsoian A. B.; Zhai Z.; Bell A. T. Band-gap energy as a descriptor of catalytic activity for propene oxidation over mixed metal oxide catalysts. J. Am. Chem. Soc. 2014, 136, 13684–13697. 10.1021/ja5051555. [DOI] [PubMed] [Google Scholar]; b Mott N. F.; Davis E. A.. Electrochemical Process. In Non-Crystalline Materials; Calendron Press: Oxford, 1979. [Google Scholar]
- Karipcin F.; Dede B.; Caglar Y.; Hur D.; Ilican S.; Caglar M.; Sahin Y. A new dioxime ligand and its trinuclear copper (II) complex: Synthesis, characterization and optical properties. Opt. Commun. 2007, 272, 131–137. 10.1016/j.optcom.2006.10.079. [DOI] [Google Scholar]
- Vinodkumar C.; Muraleedharan Nair M.; Radhakrishnan P. Thermal studies on lanthanide nitrate complexes of 4-n-(2′-furfurylidene) aminoantipyrine. J. Therm. Anal. Calorim. 2000, 61, 143–149. 10.1023/A:1010120909987. [DOI] [Google Scholar]
- Katouah H.; Hameed A. M.; Alharbi A.; Alkhatib F.; Shah R.; Alzahrani S.; Zaky R.; El-Metwaly N. M. Green Synthesis Strategy for New Schiff-Base Complexes: Characterization, Conductometry, In Vitro Assay Confirmed by In Silico Approach. ChemistrySelect 2020, 5, 10256–10268. 10.1002/slct.202002388. [DOI] [Google Scholar]
- Shah S. S.; Parmar R. G. Extractive spectrophotometric determination of Nickel (II) with 2-hydroxy-4n-butoxy-5-bromo acetophenone oxime. Der. Pharma Chem. 2011, 3, 318–321. [Google Scholar]
- a Irannejad-Gheshlaghchaei N.; Zare A.; Sajadikhah S. S.; Banaei A. A novel dicationic ionic liquid as a highly effectual and dual-functional catalyst for the synthesis of 3-methyl-4-arylmethylene-isoxazole-5 (4 H)-ones. Res. Chem. Intermed. 2018, 44, 6253–6266. 10.1007/s11164-018-3488-8. [DOI] [Google Scholar]; b Abshirini Z.; Lotfifar N.; Zare A. A Highly Effectual and Rapid Protocol for the Synthesis of 5-Amino-1, 3-diaryl-1 H-pyrazole-4-carbonitriles Using 1, 3-Disulfonic Acid Imidazolium Trifluoroacetate as a Dual-Functional Catalyst. Org. Prep. Proced. Int. 2020, 52, 428–433. 10.1080/00304948.2020.1780884. [DOI] [Google Scholar]
- Morad M.; Habeebullah T. M.; Althagafi I.; Asghar B. H.; Bayazeed A. A.; Bawazeer T. M.; Al-Solimy A. M.; El-Metwaly N. Copper–acetanilide complexes: synthesis, characterization, crystal structure, computational analysis and their application as heterogeneous catalysts for biodiesel synthesis from frying waste oils. Res. Chem. Intermed. 2020, 46, 4543–4562. 10.1007/s11164-020-04220-w. [DOI] [Google Scholar]
- Shah R.; Katouah H.; Sedayo A. A.; Abualnaja M.; Aljohani M. M.; Saad F.; Zaky R.; El-Metwaly N. M. Practical and computational studies on novel Schiff base complexes derived from green synthesis approach: conductometry as well as in-vitro screening supported by in-silico study. J. Mol. Liq. 2020, 319, 114116 10.1016/j.molliq.2020.114116. [DOI] [Google Scholar]
- Olasunkanmi L. O.; Obot I. B.; Ebenso E. E. Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4, 5-dihydropyrazol-3-yl] phenyl} methanesulfonamides on mild steel in 1 M HCl: experimental and theoretical studies. RSC Adv. 2016, 6, 86782–86797. 10.1039/C6RA11373G. [DOI] [Google Scholar]
- El Adnani Z.; Mcharfi M.; Sfaira M.; Benzakour M.; Benjelloun A. T.; Touhami M. E. DFT theoretical study of 7-R-3methylquinoxalin-2 (1H)-thiones (RH; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Corros. Sci. 2013, 68, 223–230. 10.1016/j.corsci.2012.11.020. [DOI] [Google Scholar]
- Al-Wasidi A. S.; Al-Jafshar N. M.; Al-Anazi A. M.; Refat M. S.; El-Metwaly N. M.; Ibrahim H. K.; Abd El-Fattah W.; Naglah A. M.; Al-Omar M. A.; Kalmouch A. In Methanolic Solvent Synthesis of New Mn(II), Co(II), Ni(II) and Cu(II) Schiff Base of Aromatic β Amino Acids: Spectroscopic, Thermal, Molecular Docking and Antimicrobial Studies. Sci. Adv. Mater. 2020, 12, 1137–1148. 10.1166/sam.2020.3784. [DOI] [Google Scholar]
- Al-nami S. Y.; Aljuhani E.; Althagafi I.; Abumelha H. M.; Bawazeer T. M.; Al-Solimy A. M.; Al-Ahmed Z. A.; Al-Zahrani F.; El-Metwaly N. Synthesis and Characterization for new nanometer Cu (II) complexes, conformational study and molecular docking approach compatible with promising in vitro screening. Arab. J. Sci. Eng. 2021, 46, 365–382. 10.1007/s13369-020-04814-x. [DOI] [Google Scholar]
- Alkhatib F.; Hameed A.; Sayqal A.; Bayazeed A. A.; Alzahrani S.; Al-Ahmed Z. A.; Zaky R.; El-Metwaly N. M.; et al. Green-synthesis and characterization for new Schiff-base complexes; spectroscopy, conductometry, Hirshfeld properties and biological assay enhanced by in-silico study. Arab. J. Chem. 2020, 13, 6327–6340. 10.1016/j.arabjc.2020.05.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.