Abstract
Background
Resistance to ceftazidime-avibactam was reported, and it is important to investigate the mechanisms of ceftazidime/avibactam resistance in K. pneumoniae with mutations in blaKPC.
Results
We report the mutated blaKPC is not the only mechanism related to CZA resistance, and investigate the role of outer porin defects, efflux pump, and relative gene expression and copy number of blaKPC and ompk35/36. Four ceftazidime/avibactam-sensitive isolates detected wild type blaKPC-2, while 4 ceftazidime/avibactam-resistant isolates detected mutated blaKPC (blaKPC-51, blaKPC-52, and blaKPC-33). Compared with other ceftazidime/avibactam-resistant isolates with the minimal inhibitory concentration of ceftazidime/avibactam ranging 128–256 mg/L, the relative gene expression and copy number of blaKPC was increased in the isolate which carried blaKPC-51 and also showed the highest minimal inhibitory concentration of ceftazidime/avibactam at 2048 mg/L. The truncated Ompk35 contributes rare to ceftazidime/avibactam resistance in our isolates. No significant difference in minimal inhibitory concentration of ceftazidime/avibactam was observed after the addition of PABN.
Conclusions
Increased gene expression and copy number of mutated blaKPC can cause high-level ceftazidime/avibactam resistance.
Keywords: Ceftazidime/avibactam resistance, Minimal inhibitory concentration, Mutated blaKPC, Outer membrane protein, Electroporation
Background
Carbapenem-resistant Enterobacerales (CRE), especially carbapenem-resistant Klebsiella pneumonia (CRKP) have emerged as a major public health concern worldwide. In China, the production of K. pneumoniae carbapenemases (KPCs) is the predominant mechanism of carbapenem resistance and is frequently linked to a highly successful K. pneumoniae sequence type 11(ST11) clone [1]. The existing antibiotics treating infections caused by KPC-producing K. pneumoniae (KPC-Kp) have limited efficacy, and novel antibiotics are urgently needed.
Avibactam is a non-β-lactam, β-lactamase inhibitor that inhibits the activities of Ambler class A and C β-lactamases and some Ambler class D enzymes. Ceftazidime/avibactam (CZA) has been considered a promising β-lactam-β-lactamase inhibitor combination with activity against serine β-lactamases, including KPCs [2]. However, CZA resistance has been reported in patients after short periods of CZA exposure [3–17], and also in patients with no history of CZA therapy [18–23]. Mechanisms of CZA resistance have reported in several studies, including specific mutations in the blaKPC gene [4], specific mutations in the blaCTX-M gene [17], porin deficiency combined with high ceftazidime hydrolysis [19, 20], or porin inactivation with increased expression of the blaKPC gene [21, 22]. The mechanism most often associated with the emergence of CZA resistance after treatment has been observed to be mutations in the blaKPC gene encoding for KPC enzymes [4–12]. And the most common amino acid substitution of KPC was D179Y in KPC-2 (KPC-33) [6] and KPC-3 (KPC-31) [4].
CZA has been approved by the China State Drug Administration on May 21, 2019, for the treatment of complex intra-abdominal infections (cIAI), hospital-acquired pneumonia (HAP), and for the treatment of gram-negative bacterial infections in adults with limited therapeutic options: K. pneumoniae, E. cloacae, E. coli, Proteus mirabilis, and Pseudomonas aeruginosa. Before the wide use of CZA, CZA-resistant isolates carrying wild type KPC-2 have been reported in China [19, 23]. Besides, we reported our experience in treating ten lung transplant recipients (9 with KPC-Kp infections, and 1 with Pseudomonas aeruginosa infection) with CZA at the China-Japan friendship hospital [24]. CZA resistant KPs with mutated blaKPC were recovered from 4 patients after CZA treatment [25]. Plasmid transfer and blaKPC cloning showed the mutated blaKPC in these isolates were associated with CZA resistance. We observed that the minimal inhibitory concentration (MIC) for CZA of KPC-51-producing K. pneumoniae clinical isolate was the highest (2048 mg/L) in our study, while the CZA MIC for the KPC-51-harbouring E. coli DH5a transformant was only 8 mg/L. We believed that the mutated blaKPC was only partly contributing to the resistance of CZA in this isolate, and other resistance mechanisms should be further investigated. Besides, though increased gene expression and copy number of blaKPC and/or porins defects were reported associated with CZA resistance, this finding was often reported in isolates with wild type blaKPC, and rarely reported in isolates with mutated blaKPC. The role of blaKPC expression and porins in CZA resistant isolates with mutated blaKPC is not clear, especially in isolates with different resistant levels.
Results
Bacterial isolates
After 13–22 days of CZA treatment in lung transplant patients, CZA resistance was found in 4 isolates. These 4 CZA resistant isolates (1B, 3B, 7B, 8B) and 4 baseline isolates (1A, 3A, 7A, 8A) recovered before CZA treatment from the same patients were analyzed in the present study. All 8 isolates produced KPC, and other beta-lactamases were detected (Table 1). Four baseline isolates carrying wild type blaKPC-2 were susceptible to CZA, while the blaKPC of corresponding CZA-resistant isolates were mutated after CZA treatment. KPC-33(D179Y), KPC-51(D179N, Y241H, H274N), and KPC-52(D179Y, valine insertion after 262 position) were observed in 8B, 1B, and 7B, respectively. Among the 1228 reads covering KPC in the next-generation sequencing data of isolate 3B, 349 reads (28.4%) belonged to KPC-2, and 879 reads (71.6%) belonged to KPC-33. The transformed E.coli isolates carrying mutated blaKPC manifested increased CZA MICs compared with the WT blaKPC-2 transformant. The CZA MIC of 3B, 7B, and 8B were 256, 256, and 128 mg/L, and the CZA MIC in corresponding transformed E. coli isolates harboring the same blaKPC variants were 2, 32, and 2 mg/L, respectively [25]. It is worth noting that the CZA MIC of KPC-51-producing K. pneumoniae clinical isolate 1B was the highest (2048 mg/L) in our study, while the CZA MIC for the KPC-51-harbouring E. coli DH5a transformant was only 8 mg/L. Other beta-lactamases detected in isolates from the same patient were identical.
Table 1.
Characteristics of isolates recoverd from the same patients before (A) and after (B) ceftazidime/avibactam exposure
| MIC | Porin sequcence modifications | ||||||
|---|---|---|---|---|---|---|---|
| KPN clinical isolate | E.coli DH5a(clone) | ||||||
| Strain | KPCa | β-lactamase | CZAa | CZA + 25 mg/ml PABN | CZAa | Ompk35b | Ompk36c |
| 1A | KPC-2 | TEM,SHV-64,CTX-M-65 | 2 | d | truncated at 62 aa | GD insertion at 136–137 aa | |
| 3A | KPC-2 | TEM,SHV-11,CTX-M-65 | 4 | truncated at 62 aa | GD insertion at 136–137 aa | ||
| 7A | KPC-2 | SHV-64,OXA-10,DHA-1 | 4 | truncated at 62 aa | GD insertion at 136–137 aa | ||
| 8A | KPC-2 | SHV-64,DHA-1 | 4 | 4 | ≤0.125 | truncated at 62 aa | GD insertion at 136–137 aa |
| 1B | KPC-51 | TEM,SHV-64,CTX-M-65 | 2048 | 2048 | 8 | truncated at 62 aa | GD insertion at 136–137 aa |
| 3B | KPC-33,KPC-2 | TEM,SHV-11,CTX-M-65 | 256 | 512 | 2 | truncated at 62 aa | GD insertion at 136–137 aa |
| 7B | KPC-52 | SHV-64,OXA-10,DHA-1 | 256 | 512 | 32 | truncated at 62 aa | GD insertion at 136–137 aa |
| 8B | KPC-33 | SHV-64,DHA-1 | 128 | 128 | 2 | truncated at 62 aa | GD insertion at 136–137 aa |
Notes
a Data were from the reported paper [25].
b Predicted translational modification of Ompk35 were based on the reference sequence (NCBI reference sequence WP_135730820.1) from K. pneumoniae ATCC 13883.
c Predicted translational modification of Ompk36 were based on the reference sequence (GenBank accession number AEW62399.1) from K. pneumoniae ATCC 13883.
d The MIC of CZA + 25 mg/mL PABN were not detected in isolates 1A,3A, and 7A.
Abbreviations: CZA ceftazidime/avibactam
Outer membrane porin gene sequence analysis
Compared with the sequences of wild type ompk35 (NCBI reference sequence WP_135730820.1) and ompk36 (GenBank accession number AEW62399.1) genes from the reference strain K. pneumoniae ATCC13883, the ompk35 sequence of all 8 isolates had a deletion after 85 bp, which caused a premature stop codon after amino acid position 62. And the Ompk36 in all 8 isolates had a glycine and aspartic acid duplication at amino acid 136 (136–137 GD insertion) (Table 1). Different sequences of Ompk35 and Ompk36 were not found between the baseline isolates (A) and CZA resistant isolates (B) recovered from the same patient.
Functional restoration of OmpK35 and Ompk36
The blunt vectors harboring functional wild-type Ompk35 or Ompk36 were transferred into selected isolates with different KPC variants by electroporation. The profiles of outer membrane proteins in all clinical isolates and transformants were analyzed by SDS-PAGE (Fig. 1). The truncated Ompk35 in clinical isolates were not shown, while the restoration of lost Ompk35 in corresponding transformants was confirmed in the profiles. The mutated Ompk36 did not influence the profiles of outer membrane proteins in clinical isolates. As a control, the empty blunt vector was also transferred into clinical isolates. The existence of wild type Ompk36 and empty blunt vector in corresponding transformants can not be reflected by SDS-PAGE, but it was confirmed using PCR and sequencing. Compared with clinical isolates with different KPC variants (8A, 1B, 3B, 7B, 8B), no significant reduction in CZA MICs was observed for selected isolates with the restoration of functional wild type Ompk35 or Ompk36 (Table 2). CZA MIC differences between clinical isolates, transformants with wild type Ompk35, transformants with wild type Ompk36, and transformants with original blunt vector were no more than 2-fold.
Fig. 1.
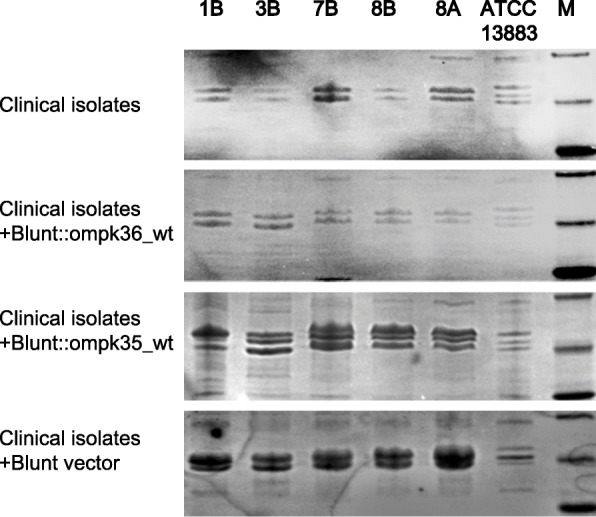
Outer membrane protein profiles of selected representative clinical isolates and corresponding transformants. M: protein markers of 45, 35 and 25 kDa. The bands showed in ATCC13883 were Ompk35, Ompk36 and OmpkA
Table 2.
Effects of restoration of wild type Ompk35 and Ompk36 into clinical KPC-KP isolates
| Strain | Description | MIC (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| CZA | CAZ | IMP | MEM | ATM | FEP | ||
| 8A | clinical isolate 8A | 8 | ≥64 | ≥16 | ≥16 | ≥64 | ≥32 |
| KPM30 | 8A + Blunt vector | 4 | ≥64 | ≥16 | ≥16 | ≥64 | ≥32 |
| KPM02 | 8A + Blunt::ompk35_wt | 4 | ≥64 | ≥16 | ≥16 | 16 | ≥32 |
| KPM06 | 8A + Blunt::ompk36_wt | 8 | ≥64 | ≥16 | ≥16 | ≥64 | ≥32 |
| 1B | clinical isolate 1B | 2048 | ≥64 | 1 | 4 | ≥64 | ≥32 |
| KPM21 | 1B + Blunt vector | 2048 | ≥64 | 0.5 | 4 | ≥64 | ≥32 |
| KPM10 | 1B + Blunt::ompk35_wt | 2048 | ≥64 | 1 | ≤0.25 | ≥64 | ≥32 |
| KPM11 | 1B + Blunt::ompk36_wt | 2048 | ≥64 | 0.5 | 0.5 | ≥64 | ≥32 |
| 3B | clinical isolate 3B | 512 | ≥64 | ≥16 | ≥16 | ≥64 | ≥32 |
| KPM23 | 3B + Blunt vector | 512 | ≥64 | ≥16 | ≥16 | ≥64 | ≥32 |
| KPM01 | 3B + Blunt::ompk35_wt | 512 | ≥64 | ≥16 | ≥16 | ≥64 | ≥32 |
| KPM05 | 3B + Blunt::ompk36_wt | 512 | ≥64 | ≥16 | ≥16 | ≥64 | ≥32 |
| 7B | clinical isolate 7B | 512 | ≥64 | 2 | 2 | ≥64 | ≥32 |
| KPM27 | 7B + Blunt vector | 512 | ≥64 | 2 | 2 | ≥64 | ≥32 |
| KPM04 | 7B + Blunt::ompk35_wt | 512 | ≥64 | 1 | 2 | ≥64 | ≥32 |
| KPM08 | 7B + Blunt::ompk36_wt | 512 | ≥64 | 1 | 2 | ≥64 | ≥32 |
| 8B | clinical isolate 8B | 256 | ≥64 | 2 | 4 | ≥64 | ≥32 |
| KPM28 | 8B + Blunt vector | 256 | ≥64 | 1 | 4 | ≥64 | ≥32 |
| KPM03 | 8B + Blunt::ompk35_wt | 256 | ≥64 | 2 | 0.5 | 16 | 2 |
| KPM07 | 8B + Blunt::ompk36_wt | 256 | ≥64 | 1 | 4 | ≥64 | ≥32 |
Abbreviations: CZA ceftazidime/avibactam; CAZ ceftazidime; IMP imipenem; MEM meropenem; ATM aztreonam; FEP cefepime
Gene expression and copy number of blaKPC, and ompk35/36
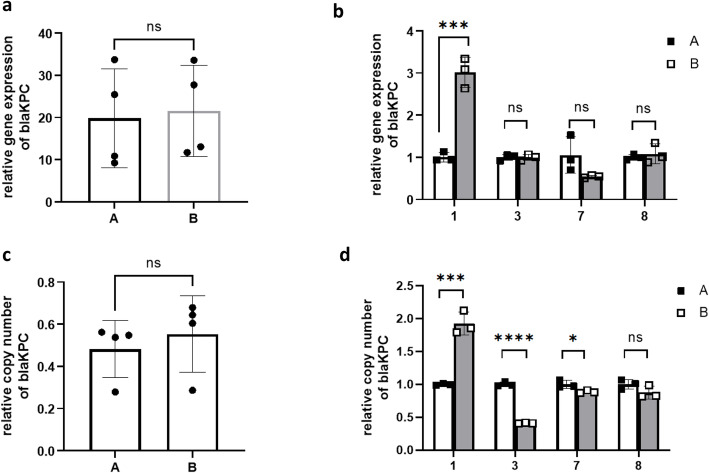
There was no significant difference in relative gene expression and copy number of blaKPC, or ompk35/36 between the baseline isolates (A) and CZA-resistant isolates (B) (Fig. 2a, c, Fig. 3a, c, Fig. 4a, c). When we compared the baseline isolate and the CZA-resistant isolate recovered from the same patient, the relative gene expression of blaKPC in 1B was higher than 1A (Fig. 2b), while no significant difference was observed in isolates of other patients. The relative copy number of blaKPC in 1B was higher than 1A, the relative copy number of blaKPC in 3B and 7B were lower than 3A and 7A, and no significant difference in relative copy number was observed between 8A and 8B (Fig. 2d).
Fig. 2.
Relative gene expression (ab)and copy number (cd)of blaKPC in the baseline isolates (A) and CZA resistant isolates (B)
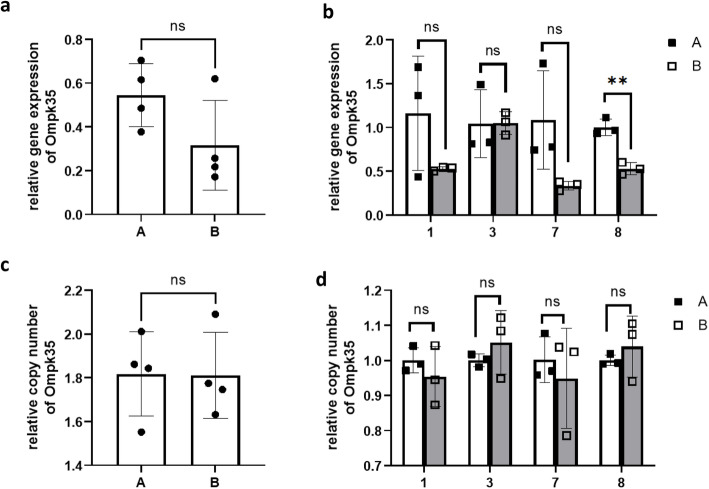
Fig. 3.
Relative gene expression (ab)and copy number (cd)of Ompk35 in the baseline isolates (A) and CZA resistant isolates (B)
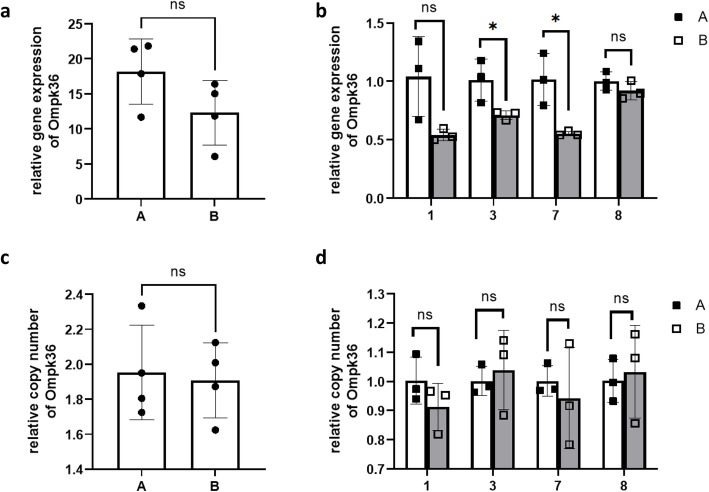
Fig. 4.
Relative gene expression (ab)and copy number (cd)of Ompk36 in the baseline isolates (A) and CZA resistant isolates (B)
No significant difference of gene expression and copy number of ompk35/36 were observed between 1A and 1B (Fig. 3b, d, Fig. 4b, d), while decreased ompk35 gene expression was found in CZA-resistant isolate with blaKPC-52 (8B) (Fig. 3b), and decreased ompk36 gene expression was found in other CZA-resistant isolates with blaKPC-33 (3B, and7B) (Fig. 4b).
The role of the AcrAB efflux pump
After the addition of PABN, CZA MICs of all selected isolates were not decreased by more than 2-fold (Table 1). This indicated that efflux is not a major mechanism for resistance to CZA.
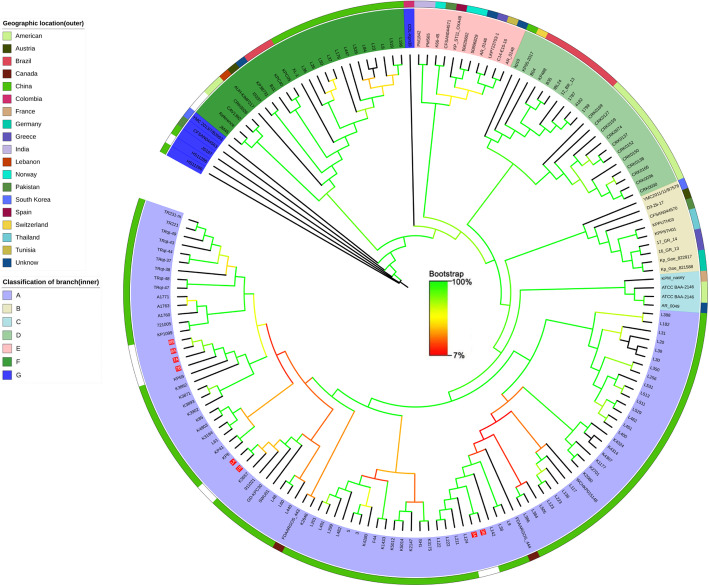
Core genome phylogenetic analysis of isolated K. pneumoniae
Core genome phylogenetic analysis was performed to compare the 8 isolates from this study based on the results of WGS analysis and 157 of K. pneumonia strains filtered by MLST from NCBI (until July 2019). As shown in Fig. 5, all of the 8 isolates were located in a large branch (light purple, A-class) from the phylogenetic tree as a whole, which was almost from China except for 2 Canadian sources. Ten isolated strains can be divided into three groups. Groups 1 (isolate 3A, 3B) was the closest phylogenetic tree branch (L122, L222, L211, and L124) which were all isolated from Hangzhou China.
Fig. 5.
Maximum likelihood phylogeny based on SNPs in the core genomes of the K. pneumoniae ST11 strains isolated worldwide
Discussion
In our report, we demonstrate a new mechanism of high-level CZA resistance in a KPC-producing K. pneumoniae strain in a lung transplant recipient, which is that high-level resistance to CZA is due to increased gene expression and copy number of the mutated blaKPC. Though increased blaKPC expression and copy number and/or Ompk defects were reported associated with increased CZA MICs [6, 21, 26], this result was often reported in isolates with wild type KPC, and the role of blaKPC expression and porins in isolates with mutated blaKPC is not clear. Our results have supplemented this evidence. In isolates with mutated blaKPC, the mutated blaKPC may play a major role in CZA resistance, and the increased gene expression and copy number of the mutated blaKPC could cause high-level CZA resistance, while the truncated Ompk35 may rarely contribute to the increased MIC of CZA.
Resistance to CZA has been reported in KPC-producing K. pneumoniae following treatment of CZA. Shields et al. first reported the evolution of CZA resistance during the treatment of K. pneumoniae infections in three patients from the United States in 2016 [3]. The treatment-emergent CZA resistance was subsequently described in Greece [27], Italy [9, 15, 16], Finland [10], Germany [12], and Spain [28] since then. And we recently reported four CZA-resistant KPC-KP recovered from lung transplant recipients after 13–22 days of CZA treatment in China [25].
KPC-31 [4] and KPC-33 [6] were the first two reported mechanisms associated with CZA resistance during treatment. Besides, KPC-35 [8], KPC-36 [15], KPC-41 [14], KPC-44 [10], KPC-48 [28], and KPC-57 [27] were also reported to be associated with acquired CZA resistance. We showed that CZA resistance in our 4 CZA-resistant isolates is due to mutations of blaKPC, and that two novel KPC variants (KPC-51, KPC-52) are also associated with CZA resistance [25]. The CZA MIC of the KPC-51-producing K. pneumoniae clinical isolate was the highest (2048 mg/L) in our study, while the CZA MIC for the KPC-51 E. coli DH5a transformant was only 8 mg/L. That indicated the variant KPC (KPC-51) is not the only mechanism of increased CZA MIC.
The role of mutated porins is not clear for CZA resistance. Modifications of Ompk35 and Ompk36, accompanied with various beta-lactamases, lead to carbapenem resistance, while do not influence CZA [29, 30]. However, several reports showed Ompk35/36 defects were associated with a high MIC of CZA. In the first reported CZA-resistant KPC-KP from a patient without CZA treatment [18], a nonfunctional Ompk35 (a premature stop codon at amino acid position 63) and a T333N mutation in Ompk36, were associated with impacted CZA susceptibility [22]. Castanheira et al. described a CZA-resistant K. pneumoniae displayed a premature stop codon in Ompk35 and decreased expression of Ompk36 [31]. Furthermore, Shields et al. reported mutations in Ompk36 were associated with CZA MICs [32]. Shen et al. showed that the restoration of functional Ompk35 resulted in a 2–4 fold decreased in the MICs of CZA for selected CZA-resistant isolates, indicating that the nonfunctional Ompk35 was related to CZA resistance [19]. Cui et al. reported the same ompk35 and ompk36 gene mutations were detected in reduced CZA susceptible strains and the CZA susceptible strains, which indicated that ompk35/36 mutations only partially contribute to the reduced susceptibility of CZA in the study [33]. Similarly, we observed that a truncated Ompk35 and a GD insertion at amino acid position 136–137 in Ompk36 in all our isolates, including 4 CZA-resistant isolates and 4 CZA-susceptible isolates.
The reports of K.pneumoniae harboring mutated KPC combined with porins defects were rare. A novel KPC variant (KPC-36) and porins defects were discovered in a CZA-resistant K.pneumoniae ST1519 (MIC = 16 mg/L) [15]. In another CZA-resistant K.pneumoniae (MIC = 64 mg/L) harboring KPC-53, porins defects could be detected [34].
We hypothesize that the different KPC variants combined with Ompk35/36 defects could lead to the different levels of CZA MIC, and the KPC-51 coupled with porins defects may lead to the highest MIC of CZA. However, after the restoration of functional Ompk35 or Ompk36, no significantly decreased CZA MICs were observed in CZA-resistant isolates. We believed that the mutated blaKPC was the most important mechanism in our isolates, and the mutations of Ompk35 or Ompk36 contribute no or rare to CZA resistance. The role of mutated porins for CZA should be further investigated.
The expression and copy number of blaKPC were often associated with reduced susceptibility to CZA. Overexpression of the blaKPC gene is a potential mechanism of CZA resistance in wild type blaKPC isolates. Nelson et al. reported that porins alteration combined with increased blaKPC-3 gene copy number and gene expression can cause CZA resistance [22]. And the relative blaKPC-2 copy numbers and relative expression of blaKPC-2 in the reduced susceptibility group were significantly higher than those in the susceptibility group [19, 23, 33]. For mutated blaKPC, the report of KPC variants combined with gene expression and copy number is rare. As we writing this article, one study [34] showed the increased blaKPC-53 gene dosage (two copies) coupled with porins alterations may lead to high-level CZA resistance (MIC = 64 mg/L). But there was only one strain with increased blaKPC-53 gene dosage was reported in the study, and the comparison between isolates with different KPC variants was not investigated.
Our results showed that the relative gene expression and copy number of blaKPC in isolate with the highest CZA MIC was higher than baseline isolate carrying wild type blaKPC-2, while this phenomenon did not appear in other CZA-resistant isolates. These results indicate that the mutated blaKPC was the dominant mechanism of CZA resistance in our isolates, and when combined with increased gene expression and copy number of blaKPC could lead to the higher level MIC of CZA.
This study is limited by its small patient population. CZA resistance was discovered before the widespread use of CZA in China. More research needs to be done, especially in data collected after the CZA treatment.
Conclusions
In summary, we found that mutated blaKPC is not the only mechanism related to CZA resistance, the increased gene expression and copy number of mutated blaKPC can cause high-level CZA resistance. With the use of CZA, more CZA resistant isolates have been reported worldwide, and more mechanisms of CZA resistance need to be explored. In consideration of the rapid acquisition of CZA resistance after therapy, our founding suggests that it is crucial to monitor the MIC of CZA in KPC-KP.
Methods
Isolates
Eight previously described isolates [25], including 4 baseline isolates recovered before CZA treatment and 4 corresponding CZA-resistant isolates recovered after CZA treatment, were analyzed in the present study. K. pneumoniae ATCC13883, K. pneumoniae ATCC700603, E.coli ATCC25922, and Salmonella enteric serotype Braenderup H9812 were used as reference isolates.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing performed using the VITEK-2 compact system (bioMerieux, Marcy-l’Etoile, France). Broth microdilution susceptibility testing of CZA was performed, and the result was interpreted according to the guidelines established by the Clinical and Laboratory Standards Institute (CLSI, M100, 2019). Avibactam was tested at a fixed concentration of 4 μg/ml in combination with increasing concentrations of ceftazidime. The reference strains E. coli ATCC25922, and K. pneumoniae ATCC700603 were used as controls.
Detection of genes encoding β-lactamases, and outer membrane proteins
PCR detection for the presence of beta-lactamase genes encoding carbapenemases (blaKPC, blaNDM-1, blaVIM, blaIMP, and blaOXA-48), ESBL associated genes (blaCTX-M, blaSHV, and blaTEM), and plasmid-borne AmpC beta-lactamases (blaACC, blaDHA, and blaCMY) were performed as described previously [35]. Outer membrane protein genes were amplified by PCR as described previously [36]. PCR amplicons were sequenced and compared with sequences available in the GenBank database using BLAST searches.
Quantitative real-time PCR (qRT-PCR) determinating gene expression and copy number of blaKPC and ompk35/36
The gene expression and copy number of blaKPC and ompk35/36 of 4 baseline isolates (A) and 4 CZA-resistant isolates (B) were examined as described previously [36–38], and the reference genes were listed in Table 3. Total RNA at mid-logarithmic growth phase bacterial cultures were obtained using RNeasy Mini Kit (Qiagen, Germany) and treated with RNase-Free DNase Set (Qiagen) in accordance with the manufacturer’s protocol. RT-PCR was performed using the QuantiTect SYBR Green RT-PCR Kit (Qiagen) on a QuantiStudio 12 K Flex system (Thermo Fisher Scientific). DNA was extracted by QIAamp DNA Mini Kit (Qiagen), and copy numbers were measured using the QuantiFast SYBR Green PCR Kit (Qiagen) on a QuantiStudio 12 K Flex system. K. pneumoniae house-keeping gene rpoB was used to normalize the gene expression and copy number of blaKPC and ompk35/36. Statistical analyses were performed using GraphPad Prism 9.
Table 3.
Primers used in this study
Cloning of ompK36 and ompK35 genes
The coding sequences of wild type ompk35 and ompk36 genes from reference isolate K. pneumoniae ATCC 13883 were amplified using primer pairs bluntOmpk35 (forward, 5′-AATGATGAAGCGCAATATTCT-3′; reverse, 5′- CGAAGGGGTGTACTGCAGATTA-3′) and bluntOmpk36 (forward, 5′- CATGAAAGTTAAAGTACTGTC-3′; reverse, 5′- TTATGCAGCTTGCAACTTAGAA-3′), respectively, and subsequently cloned into pEASY-Blunt vectors (Transgen Biotech Co., China). After nucleotide sequence verification, the kanamycin resistance gene of recombinant plasmids was replaced by the apramycin resistance gene using PCR. As a control, the kanamycin resistance gene in an empty vector was also replaced by the apramycin resistance gene. The recombinant plasmids were selected on Luria-Bertani (LB) agar containing apramycin at a concentration of 50 μg/ml.
Preparation of competent cells and electroporation
The competent cells of K. pneumoniae clinical isolates were prepared using 10% glycerol as previously described [39]. The mixture of 50ul electrocompetent cells and 5ul plasmid was transferred into a 2 mm electroporation cuvette and electroporated using MicroPuler System (Bio-Rad) at 2.5 kV. The cells were plated onto Luria-Bertani (LB) agar containing apramycin at 50 mg/L. The plates were incubated at 37 °C overnight, and the successful clone was identified using PCR and sequencing. The MICs of the recombinant strains were determined in the presence of IPTG at 100 μM [22].
Isolation and analysis of outer membrane components
Outer membrane proteins were isolated according to Carlone’s rapid procedure [40], and analyzed with SDS-PAGE.
Efflux pump inhibitor tests
MICs of CZA in combination with PABN (phenylalanine-arginine beta-naphthylamide) (25 mg/mL) [19, 41], a pump inhibitor, were determined. A fourfold decrease in MIC after the addition of PABN was considered significant.
Core genome phylogenetic analysis
WGS of all 8 isolates was carried out using the Illumina NovaSeq system in our previous study [25], and the assembled genome sequence has been deposited on NCBI with BioProject ‘PRJNA588110’. SNP analysis was performed using snippy software and the JM45 sequence was used for reference sequences. 8313 SNV sites were identified and the Modelfinder was used to find the best model. A phylogenetic tree was built by Iqtree [42, 43] with the best model (HKY + F + ASC + R4) and the number of bootstraps was set to 1000 times.
Acknowledgments
We thank Pro. Hui Wang, Jiankang Zhao, Xiaohui Zou, Shuai Liu, Xiaoxuan Feng, and Longyang Jin for their assistance.
Abbreviations
- cIAI
complex intra-abdominal infections
- CRE
Carbapenem-resistant enterobacerales
- CRKP
Carbapenem-resistant Klebsiella pneumonia
- CZA
Ceftazidime/avibactam
- HAP
Hospital-acquired pneumonia
- KPCs
K. pneumoniae carbapenemases
- KPC-Kp
KPC-producing K. pneumoniae
- LB
Luria-Bertani
- MIC
Minimal inhibitory concentration
- PABN
Phenylalanine-arginine beta-naphthylamide
- qRT-PCR
Quantitative real-time PCR
- ST11
Sequence type 11
Authors’ contributions
Study concept and design: LxS, HbL, YmL, and BC. Acquisition of data: LxS, HbL, and QW. Statistical analysis of data: LxS. Drafting of the manuscript: LxS. Critical revision of the manuscript for important intellectual content: YmL and BC. Study supervision: BC. All authors read and approved the study.
Funding
This study was supported by the National Key R&D Program of China (2017YFC1309301, 2017YFC1309300), the CAMS Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1–003), and the National Science Grant for Distinguished Young Scholars (81425001/H0104) (Bin Cao). The funding bodies played no role in the design of the study, collection, analysis, and interpretation of data, and in writing the manuscript.
Availability of data and materials
Illumina sequence reads for the sequenced isolates in this study have been deposited in the NCBI sequence read archive are available in the sequence read archive under accessions ‘SRR10394536’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394536), ‘SRR10394605’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394605), ‘SRR10394910’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394910), ‘SRR10394933’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394933), ‘SRR10397948’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397948), ‘SRR10397950’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397950), ‘SRR10397951’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397951), ‘SRR10397953’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397953).
Declarations
Ethics approval and consent to participate
Permission for using the raw data and the isolates for research purposes was approved by the Ethics Committee of the China-Japan Friendship Hospital (2019–164-K113). Because of the retrospective nature of this study, informed consent is not needed. The data used in this study was anonymised before its use.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yingmei Liu, Email: lym13601063223@126.com.
Bin Cao, Email: caobin_ben@163.com.
References
- 1.Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, et al. Epidemiology of carbapenem-resistant enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2):e01882–17. [DOI] [PMC free article] [PubMed]
- 2.van Duin D, Bonomo RA. Ceftazidime/avibactam and Ceftolozane/Tazobactam: second-generation beta-lactam/beta-lactamase inhibitor combinations. Clin Infect Dis. 2016;63(2):234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for Carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis. 2016;63(12):1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61(3):e02097–16. [DOI] [PMC free article] [PubMed]
- 5.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K pneumoniae: a case report and review of literature. Open Forum Infect Dis. 2017;4(3):ofx101. doi: 10.1093/ofid/ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, McConville TH, et al. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother. 2018;62(3):e02101–17. [DOI] [PMC free article] [PubMed]
- 7.Athans V, Neuner EA, Hassouna H, Richter SS, Keller G, Castanheira M, et al. Meropenem-vaborbactam as salvage therapy for ceftazidime-avibactam-resistant Klebsiella pneumoniae bacteremia and abscess in a liver transplant recipient. Antimicrob Agents Chemother. 2019;63(1):e01551–18. [DOI] [PMC free article] [PubMed]
- 8.Hemarajata P, Humphries RM. Ceftazidime/avibactam resistance associated with L169P mutation in the omega loop of KPC-2. J Antimicrob Chemother. 2019;74(5):1241–1243. doi: 10.1093/jac/dkz026. [DOI] [PubMed] [Google Scholar]
- 9.Gaibani P, Campoli C, Lewis RE, Volpe SL, Scaltriti E, Giannella M, Pongolini S, Berlingeri A, Cristini F, Bartoletti M, Tedeschi S, Ambretti S. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J Antimicrob Chemother. 2018;73(6):1525–1529. doi: 10.1093/jac/dky082. [DOI] [PubMed] [Google Scholar]
- 10.Raisanen K, Koivula I, Ilmavirta H, Puranen S, Kallonen T, Lyytikainen O, et al. Emergence of ceftazidime-avibactam-resistant Klebsiella pneumoniae during treatment, Finland december 2018. Euro Surveill. 2019;24(19):1900256. [DOI] [PMC free article] [PubMed]
- 11.Venditti C, Nisii C, D'Arezzo S, Vulcano A, Capone A, Antonini M, Ippolito G, Di Caro A. Molecular and phenotypical characterization of two cases of antibiotic-driven ceftazidime-avibactam resistance in Bla KPC-3-harboring Klebsiella pneumoniae. Infect Drug Resist. 2019;12:1935–1940. doi: 10.2147/IDR.S207993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottig S, Frank D, Mungo E, Nolte A, Hogardt M, Besier S, Wichelhaus TA. Emergence of ceftazidime/avibactam resistance in KPC-3-producing Klebsiella pneumoniae in vivo. J Antimicrob Chemother. 2019;74(11):3211–3216. doi: 10.1093/jac/dkz330. [DOI] [PubMed] [Google Scholar]
- 13.Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62(5):e02497–17. [DOI] [PMC free article] [PubMed]
- 14.Mueller L, Masseron A, Prod'Hom G, Galperine T, Greub G, Poirel L, et al. Phenotypic, biochemical and genetic analysis of KPC-41, a KPC-3 variant conferring resistance to ceftazidime-avibactam and exhibiting reduced carbapenemase activity. Antimicrob Agents Chemother. 2019;63(12):e01111–19. [DOI] [PMC free article] [PubMed]
- 15.Gaibani P, Ambretti S, Campoli C, Viale P, Re MC. Genomic characterization of a Klebsiella pneumoniae ST1519 resistant to ceftazidime/avibactam carrying a novel KPC variant (KPC-36) Int J Antimicrob Agents. 2020;55(1):105816. doi: 10.1016/j.ijantimicag.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Antonelli A, Giani T, Di Pilato V, Riccobono E, Perriello G, Mencacci A, Rossolini GM. KPC-31 expressed in a ceftazidime/avibactam-resistant Klebsiella pneumoniae is associated with relevant detection issues. J Antimicrob Chemother. 2019;74(8):2464–2466. doi: 10.1093/jac/dkz156. [DOI] [PubMed] [Google Scholar]
- 17.Both A, Buttner H, Huang J, Perbandt M, Belmar Campos C, Christner M, Maurer FP, Kluge S, Konig C, Aepfelbacher M, et al. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J Antimicrob Chemother. 2017;72(9):2483–2488. doi: 10.1093/jac/dkx179. [DOI] [PubMed] [Google Scholar]
- 18.Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother. 2015;59(10):6605–6607. doi: 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Z, Ding B, Ye M, Wang P, Bi Y, Wu S, Xu X, Guo Q, Wang M. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2017;72(7):1930–1936. doi: 10.1093/jac/dkx066. [DOI] [PubMed] [Google Scholar]
- 20.Galani I, Antoniadou A, Karaiskos I, Kontopoulou K, Giamarellou H, Souli M. Genomic characterization of a KPC-23-producing Klebsiella pneumoniae ST258 clinical isolate resistant to ceftazidime-avibactam. Clin Microbiol Infect. 2019;25(6):763 e765–763 e768. doi: 10.1016/j.cmi.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Humphries RM, Hemarajata P. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother. 2017;61(6):e00537–17. [DOI] [PMC free article] [PubMed]
- 22.Nelson K, Hemarajata P, Sun D, Rubio-Aparicio D, Tsivkovski R, Yang S, et al. Resistance to ceftazidime-avibactam is due to transposition of KPC in a porin-deficient strain of Klebsiella pneumoniae with increased efflux activity. Antimicrob Agents Chemother. 2017;61(10):e00989–17. [DOI] [PMC free article] [PubMed]
- 23.Zhang P, Shi Q, Hu H, Hong B, Wu X, Du X, Akova M, Yu Y. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin Microbiol Infect. 2020;26(1):124 e121–124 e124. doi: 10.1016/j.cmi.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Sun L, Guo L, Cao B, Liu Y, Zhao L, Lu B, Li B, Chen J, Wang C. Clinical outcomes of ceftazidime-avibactam in lung transplant recipients with infections caused by extensively drug-resistant gram-negative bacilli. Ann Transl Med. 2020;8(3):39. doi: 10.21037/atm.2019.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Chen W, Li H, Li L, Zou X, Zhao J, Lu B, Li B, Wang C, Li H, Liu Y, Cao B. Phenotypic and genotypic analysis of KPC-51 and KPC-52, two novel KPC-2 variants conferring resistance to ceftazidime/avibactam in the KPC-producing Klebsiella pneumoniae ST11 clone background. J Antimicrob Chemother. 2020;75(10):3072–3074. doi: 10.1093/jac/dkaa241. [DOI] [PubMed] [Google Scholar]
- 26.Coppi M, Di Pilato V, Monaco F, Giani T, Conaldi PG, Rossolini GM. Ceftazidime-avibactam resistance associated with increased bla KPC-3 gene copy number mediated by pKpQIL Plasmid derivatives in sequence type 258 Klebsiella pneumoniae. Antimicrob Agents Chemother. 2020;64(4):e01816–19. [DOI] [PMC free article] [PubMed]
- 27.Galani I, Karaiskos I, Angelidis E, Papoutsaki V, Galani L, Souli M, Antoniadou A, Giamarellou H. Emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in KPC-2-producing Klebsiella pneumoniae of sequence type 39 during treatment. Eur J Clin Microbiol Infect Dis. 2021;40(1):219–224. doi: 10.1007/s10096-020-04000-9. [DOI] [PubMed] [Google Scholar]
- 28.Cano A, Guzman-Puche J, Garcia-Gutierrez M, Caston JJ, Gracia-Ahufinger I, Perez-Nadales E, Recio M, Natera AM, Marfil-Perez E, Martinez-Martinez L, et al. Use of carbapenems in the combined treatment of emerging ceftazidime/avibactam-resistant and carbapenem-susceptible KPC-producing Klebsiella pneumoniae infections: report of a case and review of the literature. J Glob Antimicrob Resist. 2020;22:9–12. doi: 10.1016/j.jgar.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Pulzova L, Navratilova L, Comor L. Alterations in outer membrane permeability favor drug-resistant phenotype of Klebsiella pneumoniae. Microb Drug Resist. 2017;23(4):413–420. doi: 10.1089/mdr.2016.0017. [DOI] [PubMed] [Google Scholar]
- 30.Wong JLC, Romano M, Kerry LE, Kwong HS, Low WW, Brett SJ, Clements A, Beis K, Frankel G. OmpK36-mediated Carbapenem resistance attenuates ST258 Klebsiella pneumoniae in vivo. Nat Commun. 2019;10(1):3957. doi: 10.1038/s41467-019-11756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanheira M, Mendes RE, Sader HS. Low frequency of ceftazidime-avibactam resistance among enterobacteriaceae isolates carrying blaKPC Collected in U.S. hospitals from 2012 to 2015. Antimicrob Agents Chemother. 2017;61(3):e02369–16. [DOI] [PMC free article] [PubMed]
- 32.Shields RK, Clancy CJ, Hao B, Chen L, Press EG, Iovine NM, Kreiswirth BN, Nguyen MH. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum beta-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae. Antimicrob Agents Chemother. 2015;59(9):5793–5797. doi: 10.1128/AAC.00548-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui X, Shan B, Zhang X, Qu F, Jia W, Huang B, Yu H, Tang YW, Chen L, Du H. Reduced ceftazidime-avibactam susceptibility in KPC-producing Klebsiella pneumoniae from patients without ceftazidime-avibactam use history - a multicenter study in China. Front Microbiol. 2020;11:1365. doi: 10.3389/fmicb.2020.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Pilato V, Aiezza N, Viaggi V, Antonelli A, Principe L, Giani T, et al. KPC-53, a KPC-3 variant of clinical origin associated with reduced susceptibility to ceftazidime-avibactam. Antimicrob Agents Chemother. 2020:65(1):e01429–20. [DOI] [PMC free article] [PubMed]
- 35.Li B, Yi Y, Wang Q, Woo PC, Tan L, Jing H, Gao GF, Liu CH. Analysis of drug resistance determinants in Klebsiella pneumoniae isolates from a tertiary-care hospital in Beijing, China. PLoS One. 2012;7(7):e42280. doi: 10.1371/journal.pone.0042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doumith M, Ellington MJ, Livermore DM, Woodford N. Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J Antimicrob Chemother. 2009;63(4):659–667. doi: 10.1093/jac/dkp029. [DOI] [PubMed] [Google Scholar]
- 37.Ruzin A, Visalli MA, Keeney D, Bradford PA. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2005;49(3):1017–1022. doi: 10.1128/AAC.49.3.1017-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitchel B, Rasheed JK, Endimiani A, Hujer AM, Anderson KF, Bonomo RA, Patel JB. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2010;54(10):4201–4207. doi: 10.1128/AAC.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Wang S, Chen W, Song L, Zhang Y, Shen Z, et al. CRISPR-Cas9 and CRISPR-assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl Environ Microbiol. 2018;84(23):e01834–18. [DOI] [PMC free article] [PubMed]
- 40.Carlone GM, Thomas ML, Rumschlag HS, Sottnek FO. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986;24(3):330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XZ, Plesiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin Microbiol Rev. 2015;28(2):337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2018;35(2):518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Illumina sequence reads for the sequenced isolates in this study have been deposited in the NCBI sequence read archive are available in the sequence read archive under accessions ‘SRR10394536’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394536), ‘SRR10394605’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394605), ‘SRR10394910’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394910), ‘SRR10394933’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10394933), ‘SRR10397948’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397948), ‘SRR10397950’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397950), ‘SRR10397951’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397951), ‘SRR10397953’(https://www.ncbi.nlm.nih.gov/search/all/?term=SRR10397953).