Abstract
Background
A specific 3-dimensional intrachromosomal architecture of core stem cell factor genes is required to reprogram a somatic cell into pluripotency. As little is known about the epigenetic readers that orchestrate this architectural remodeling, we used a novel chromatin RNA in situ reverse transcription sequencing (CRIST-seq) approach to profile long noncoding RNAs (lncRNAs) in the Oct4 promoter.
Results
We identify Platr10 as an Oct4 - Sox2 binding lncRNA that is activated in somatic cell reprogramming. Platr10 is essential for the maintenance of pluripotency, and lack of this lncRNA causes stem cells to exit from pluripotency. In fibroblasts, ectopically expressed Platr10 functions in trans to activate core stem cell factor genes and enhance pluripotent reprogramming. Using RNA reverse transcription-associated trap sequencing (RAT-seq), we show that Platr10 interacts with multiple pluripotency-associated genes, including Oct4, Sox2, Klf4, and c-Myc, which have been extensively used to reprogram somatic cells. Mechanistically, we demonstrate that Platr10 helps orchestrate intrachromosomal promoter-enhancer looping and recruits TET1, the enzyme that actively induces DNA demethylation for the initiation of pluripotency. We further show that Platr10 contains an Oct4 binding element that interacts with the Oct4 promoter and a TET1-binding element that recruits TET1. Mutation of either of these two elements abolishes Platr10 activity.
Conclusion
These data suggest that Platr10 functions as a novel chromatin RNA molecule to control pluripotency in trans by modulating chromatin architecture and regulating DNA methylation in the core stem cell factor network.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13059-021-02444-6.
Keywords: Stem cell, Pluripotency, Long noncoding RNA, Intrachromosomal loop, Oct4, Sox2, DNA methylation
Introduction
Terminally differentiated cells can be reprogrammed into a pluripotent stage known as induced pluripotent stem cells (iPSCs) by using a cocktail of stem cell transcription factors Oct4-Sox2-Klf4-c-Myc (OSKM) [1], small chemical compounds [2, 3], or by nuclear transfer [4]. However, these reprogramming processes are extremely inefficient and time-consuming, hindering potential clinical applications of iPSCs for regenerative medicine [5].
It is now clear that there are strong epigenetic barriers that must be overcome before cells achieve full pluripotency. The initiation of cell reprogramming towards pluripotency requires appropriate expression of the core stem cell factor network [6, 7]. The specific chromatin architecture surrounding key pluripotency gene loci, such as Oct4, Sox2, and Nanog, is the culmination of critical epigenetic steps involved in the regulation of cell remodeling [8]. Self-renewal of pluripotent stem cells also requires the formation of a specific long-range interchromosomal and intrachromosomal interacting network.
To explore the mechanisms underlying reprogramming, we compared promoter DNA binding and chromatin architecture between iPSCs that have completed reprogramming and cells we referred to as “non-iPSCs,” which expressed lentiviral OSKM factors, but failed to complete reprogramming [9]. We found that the virally expressed OSKM factors bound to their target genes in both groups of cells. However, in non-iPSCs, the target genes could not be activated to achieve pluripotency, partially due to the lack of a promoter-enhancer intrachromosomal loop architecture [9]. Formation of this intrachromosomal loop is a critical epigenetic barrier that must be overcome for the induction of pluripotency. In addition, the maintenance of pluripotency also requires a specific, higher-order genomic architecture consisting of long-range chromatin interactions [10]. However, the molecular factors that orchestrate this pluripotency-specific intrachromosomal network are still uncharacterized.
Recent studies suggest that long noncoding RNAs (lncRNAs) are important structural components of three-dimensional nuclear architecture [11, 12]. In the nucleus, lncRNAs can regulate gene transcription at different functional steps in cis or in trans through multiple epigenetic mechanisms, including binding to regulatory elements (promoters and enhancers), inter-and intrachromosomal interactions, histone modifications, DNA methylation, chromatin remodeling [13], and post-transcriptional protein ubiquitination and degradation [14]. Recently, we used a chromatin RNA in situ reverse transcription sequencing (CRIST-seq) approach to map the lncRNA network within regulatory elements of key stemness genes [15]. In the present study, we have employed this approach to discover which lncRNAs interact with the Oct4 promoter. Activation of this master stem cell factor is absolutely required for the establishment and maintenance of pluripotency [9]. We hypothesized that lncRNAs embedded in this chromatin structure might actively participate in the control of the Oct4 promoter.
Using this approach, we have identified 27 differentially transcribed RNA candidates that interacted with the Oct4 and Sox2 promoters [15]. Among them, Platr10 is a lncRNA that is co-expressed with Oct4 and other pluripotency factors [16]. In this report, we focus on the mechanisms underlying the role of Platr10 in reprograming. We demonstrate that Platr10 is primarily located in the nucleus, where it regulates pluripotency by binding to multiple stem cell core factors, including Oct4, Sox2, Klf4, and c-Myc, four factors that have been used to induce pluripotent reprogramming. Mutation assays demonstrate that Platr10 contains an Oct4 binding element as well as a TET1 binding element, both of which are required for the regulation of stem cell pluripotency and reprogramming.
Results
Profiling pluripotency-associated lncRNAs by CRIST-seq
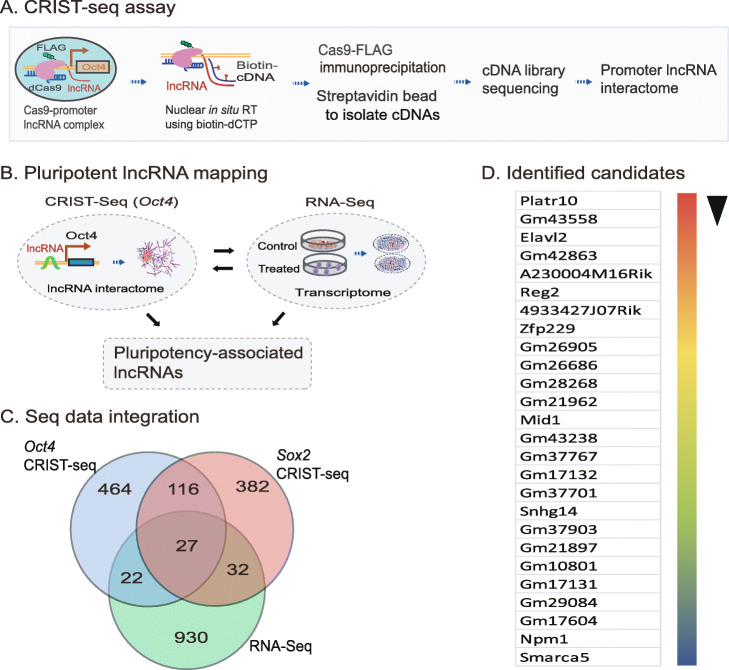
To identify epigenetic pathways that coordinate chromatin remodeling, we focused on lncRNAs that interact with the Oct4 promoter, a core stem cell factor that is essential for pluripotency maintenance. We hypothesized that components that interact with the Oct4 promoter, particularly those lncRNAs that are specifically transcribed during reprogramming, would participate in the regulation of pluripotency. We used a CRIST-Seq approach [15] to profile lncRNAs that interact with the Oct4 promoter (Fig. 1A). This assay combines the simplicity of nuclear in situ RNA biotin labeling with the specificity of CRISPR Cas9 gene targeting. The assay includes (1) targeting of the promoter complex by Cas9 gRNAs, (2) RNA in situ labeling by reverse transcription with biotin-dCTP, (3) pull-down of the locus and its associated cDNAs by Cas9-FLAG immunoprecipitation, (4) purification of the promoter-associated cDNAs from genomic DNAs by streptavidin beads, and (5) Illumina cDNA library sequencing (Fig. S1).
Fig. 1.
Profiling of Oct4 promoter-interacting lncRNAs by CRIST-seq. A Schematic diagram of the chromatin-lncRNA in situ reverse transcription trap sequencing (CRIST-Seq) assay. dCas9: Catalytically inactive CRISPR Cas9; FLAG: a tag octapeptide having the sequence motif DYKDDDDK that is attached to the N-terminal of Cas9; Oct4-gRNA: Cas9 guiding RNAs that target the Oct4 promoter. In iPSCs, Cas9-gRNA binds to the Oct4 promoter through a mechanism of base pairing between the gRNA and target DNA. After fixation, the Oct4 promoter-interacting RNAs were reverse transcribed into cDNAs in the isolated nuclei with biotin-dCTP. The Cas9 Oct4 promoter biotin-cDNA complex was immunoprecipitated by a Cas9-FLAG antibody, and biotin-cDNAs were further purified from genomic DNAs by biotin-streptavidin bead purification. The CRIST-captured cDNAs were profiled by Illumina library sequencing to identify the RNA components that regulate pluripotency. B Profiling pluripotency-associated lncRNAs by the combined CRIST-seq and RNA-Seq datasets. The Oct4-interacting lncRNAs identified by CRIST-seq were integrated with the dataset of RNA transcriptome sequencing. The combination of these two datasets identifies lncRNAs that not only interact with the Oct4 promoter but are also expressed differentially in reprogramming. C Integration of the RNA-Seq and CRIST-Seq datasets. RNA-Seq was initially used to identify the upregulated RNAs (>2-fold, p<0.05) in reprogramming. The upregulated RNAs were then integrated into the Oct4 and Sox2 CRIST-Seq datasets using a VENN program. The CRIST-Seq data were adjusted over the values of the IgG control and Cas9-gCT control. A cut-off threshold of peak enrichment FPKM>50 was arbitrarily set to select CRIST-Seq RNAs for VENN analysis. Integration of three datasets generated a list of 27 pluripotency-associated RNA candidates. D A list of 27 pluripotency-associated RNA (PALR) candidates identified by RNA-Seq and CRIST-Seq. The RNA candidates are ranked based on the RNA expression-fold from high (red) to low (blue) between fibroblasts (FBC) and iPSCs
To profile the Oct4-associated lncRNAs, two Cas9 gRNAs were designed from the Oct4 promoter (Fig. S2) and cloned into a Cas9-FLAG lentiviral vector (Fig. S1B). After lentivirus infection and puromycin selection, iPSCs were cross-linked with formaldehyde to fix the Oct4 promoter-RNA chromatin structure. The chromatin-associated RNAs were then in situ reverse transcribed in the nucleus into cDNAs using biotin-dCTP. The Cas9-FLAG-Oct4-cDNA complex was immunoprecipitated by an anti-FLAG antibody. After reversal of the crosslinks, the biotin-labeled cDNAs were purified with streptavidin beads and used for Illumina library sequencing to identify the Oct4-interacting RNA network [15].
We used quantitative PCR to examine the specificity of CRIST targeting (Figs. S1C-S1E). We detected the enrichment of CRIST signals at the targeting site (pOct4), where the two gRNAs are located. At the same locus, no enrichment was detected for a random gRNA control (gCT) or a Cas9 vector control (Vector). Similarly, we did not detect CRIST enrichment at the 5′-control site (5′-Ct), which is 13.9 kb distant from the pOct4 target site. In addition, no Cas9-gRNA enrichment was detected at the off-target control site that is 33.8 kb upstream of the housekeeping gene GAPDH. Taken together, these data demonstrate the specificity of the CRIST approach to target the Oct4 promoter.
We then mapped the RNA network in the Oct4 promoter. Two controls were performed in parallel with Oct4 CRIST sequencing, including a random Cas9 gRNA control (gCT) and an IgG immunoprecipitation control (IgG). To define the specific binding of RNAs, CRIST-Seq signal intensities were normalized over that of the non-targeting Cas9 gCT control and the IgG control using parameters of fold change ≥2 and p value < 0.05.
LncRNAs are critical components of the regulatory chromatin complex. Like chromatin factors, some lncRNAs may regulate multiple gene targets. Thus, we proposed to identify those lncRNAs that bind to multiple pluripotent target genes, assuming that they would play more important roles in pluripotency than those that bind only to a single gene target. Thus, we integrated the above Oct4 CRIST-seq data with the CRIST-seq data targeting Sox2 [15], a second core stem cell transcription factor that is essential for the maintenance of pluripotency (Fig. S3).
We reasoned that a key lncRNA candidate should also become activated during reprogramming. Therefore, we collected cells at different stages of reprogramming [9, 17]. RNA-Seq was performed to identify RNAs that are differentially expressed in association with reprogramming. RNA transcriptome sequencing identified differentially expressed lncRNAs (>2 fold) between control fibroblasts and iPSCs [18]. To identify the pluripotency-associated lncRNA candidates, we integrated the Oct4 and Sox2 CRIST lncRNA data with the RNA-Seq data (Fig. 1B, C). By combining these datasets, we identified 27 RNA candidates that not only interacted with the Oct4 and Sox promoters, but are also differentially transcribed during reprogramming into pluripotency (Fig. 1D) [15].
CRIST-seq identifies Platr10 as an essential lncRNA for pluripotency
The lncRNA NONMMUT043505 (Platr10) showed the largest fold increment when fibroblasts were reprogrammed into iPSCs. This lncRNA is located on chromosome 3, and RNA-seq data showed that it is specifically expressed in iPSCs (Fig. S4). Further analysis showed that this lncRNA was comprised of two variants (Fig. S5). Neither variant contains a large open reading frame. Variant 1, which contains 4 exons, is the major transcript, while variant 2 contains three exons; the latter variant matches with Platr10, one of 32 pluripotency-associated lncRNAs (Platrs) identified by Bergmann et al. [16]. Using a weighted co-expression analysis, they showed that Platrs were clustered tightly with the expression Oct4 and other pluripotency factors. We thus focused on Platr10 and examined its underlying mechanisms in pluripotency.
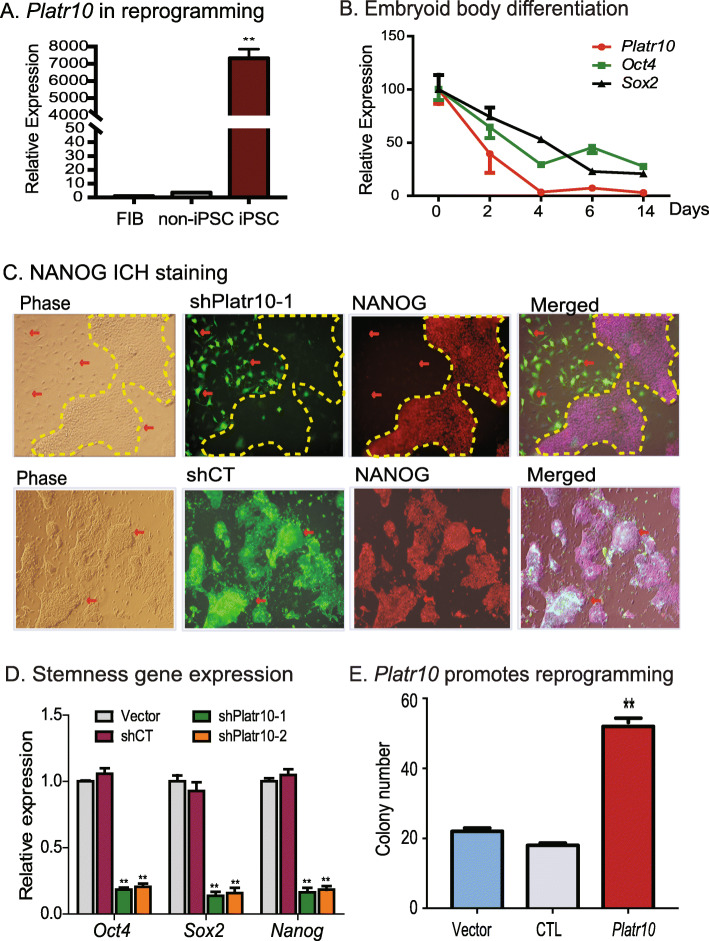
We first quantitated the transcriptional abundance of Platr10 in cells collected at different stages of reprogramming, including fibroblast control, fully reprogrammed cells (iPSCs), and “non-iPSCs” that expressed the lentiviral OSKM factors but failed to complete reprogramming. Platr10 expression correlated with pluripotency status, as it was silenced in fibroblasts and transcribed at only a very low level in non-iPSCs. The expression of Platr10 was greatly increased in cells that were fully reprogrammed (iPSCs) (Fig. 2A). Using sodium bisulfite sequencing, we found that the Platr10 gene was epigenetically regulated by DNA methylation (Fig. S6). The CpG islands in the Platr10 promoter were hypermethylated in fibroblasts but unmethylated in iPSCs, suggesting an epigenetic regulation of this lncRNA in reprogramming.
Fig. 2.
Platr10 is required for the maintenance of pluripotency. A Reactivation of Platr10 in reprogramming. Skin fibroblasts were reprogrammed using lentiviral Oct4-Sox2-Kilf4-c-Myc (OSKM). Cells were collected at different stages of reprogramming and the expression of Platr10 was measured by RT-PCR. FIB, fibroblasts; iPSC, induced pluripotent stem cells; non-iPCS (un-reprogrammed cells), cells that express the four viral OSKM factors, but fail to complete reprogramming. β-Actin was used as the PCR control. Throughout the manuscript, the data are presented as the mean ± SD from three independent experiments unless they are specifically defined. **p < 0.01 as compared with FIB and non-iPSCs. B Platr10 expression is associated with Sox2 and Oct4 expression during embryoid body (EB) differentiation. iPSCs were collected at different stages of EB formation for quantitative PCR. C Requirement for Platr10 in the maintenance of pluripotency. shPlatr10-1, shRNA vector that targets Platr10 lncRNA; shCT, random shRNA control; Vector, lentiviral vector control. Platr10 was knocked down by shRNA lentiviruses in E14 cells. Cells transfected with lentiviruses carrying a random shRNA (gCT) were used as the control. The lentivirus-transfected cells were tracked by the co-expressed copGFP. Pluripotency status was examined by immunohistochemical (IHC) staining of stem cell marker NANOG. Note that the exit of iPSCs from pluripotency in the shRNA-copGFP expressing cells is accompanied by altered cell morphology and the loss of NANOG protein (red arrow). The cell islands that escape lentiviral shPlatr10 transfection are marked by a yellow dotted line. These cells maintain the same stem cell pluripotency as the iPSCs. C Platr10 is essential for optimal activity of core stem cell factor genes in iPSCs. After lentiviral transfection, iPSCs were selected by puromycin. The mixed stable cells were collected for qPCR quantitation. **p < 0.01 as compared with the Vector and shCT controls. D Platr10 enhances cell reprogramming. MEF cells were transfected with the lentiviruses carrying Platr10, the empty vector (Vector), and CTL (lncRNA control containing Platr10 antisense RNA). After doxycycline (DOX) induction, iPSC colonies were detected using an alkaline phosphatase (AP) staining kit and were quantitated as iPSC colonies per microscope field. **p < 0.01 as compared with the Vector and CTL controls
We also collected cells during embryoid body differentiation. Using quantitative PCR, we found that Platr10 became significantly downregulated during embryoid body differentiation, in parallel with core stem cell factors Oct4, Sox2, and Nanog (Fig. 2B). We then examined the function of Platr10 by transfecting E14 cells with two shRNA lentiviruses (Fig. S7A, shPlatr10-1 and shPlatr10-2). Both shRNA lentiviruses significantly knocked down Platr10 lncRNA as compared with the random control (shCT) and vector control (Fig. S7B). We examined if Platr10 knockdown would affect cell morphology of iPSCs. The activity of the CMV promoter-copGFP was much weaker in iPSCs than it was in differentiated cells. In the random shRNA control group (shCT), the copGFP-positive cells maintained the same cell morphology as pluripotent stem cells (Fig. 2C, bottom panels). However, knockdown of Platr10 dramatically altered cell morphology (top panels, red arrows, shPlatr10-1). The Platr10-deficient cells became enlarged and flat, appearing more like fibroblasts.
We then examined the pluripotency of treated iPSCs by immunohistochemical staining of pluripotency-associated marker protein NANOG. The shCT control cells showed extensive expression of the pluripotency-associated marker protein NANOG (Fig. 2C, bottom panel 3). After Platr10 shRNA knockdown, iPSCs became differentiated and lost the pluripotency-associated marker NANOG (top panel; unmarked regions, red arrow). Interestingly, in the shPlatr10 group, there are some “island” cells that escaped lentiviral infection and did not express the shPlatr10-copGFP track marker. These cells served as the “escaped” control and still maintained the original compact shape of iPSCs and expressed NANOG (yellow marked areas without copGFP fluorescence).
Using qPCR, we found that knockdown of Platr10 was associated with downregulation of the three core stem cell factor genes Oct4, Sox2, and Nanog. In the control groups, treatment with a random control shRNA (shCT) and the vector control (Vector) did not affect the activity of core stem cell factor genes (Fig. 2D).
We further examined the role of Platr10 in pluripotent reprogramming using a DOX-inducible system [19]. OG2 MEF cells were first transfected with lentiviruses carrying the Platr10 cDNA, DsRed control (CTL), and empty vector (Vector). After puromycin selection, cells were incubated in reprogramming media containing the doxycycline (DOX) inducer and then stained for pluripotent marker NANOG. Compared with the vector (Vector) and DsRed (CTL) controls, ectopic expression of Platr10 was associated with increased reprogramming of MEF cells into pluripotency as quantitated by alkaline phosphatase (AP) staining (Fig. 2E) and NANOG foci (Fig. S7C).
We also validated the role of Platr10 using CRISPR Cas9 editing. For this, we constructed two targeting vectors carrying the dual SpCas9-NmCas9 cassette [20] and four gRNAs to target the Platr10 promoter and 3′-downstream region, respectively (Fig. S8A). The donor vector was constructed to include two Platr10 arms for homologous recombination, copGFP/Puro for the positive selection, and TK for the negative selection. Using this dual Cas9 approach, we successfully deleted the region covering the Platr10 promoter and coding region in E14 cells. The homozygous deletion of Platr10 was confirmed by DNA sequencing. As expected, Platr10-deleted cells (GFP-positive) showed a change in morphology (Fig. S8B) and exhibited loss of pluripotent marker NANOG immunostaining (Fig. S8C). As the control, the random Cas9 gRNAs did not alter the immunostaining signal of NANOG in E14 cells. Taken together, these data suggest that Platr10 is critical for the maintenance of pluripotency.
Platr10 binds to the Oct4 promoter using a 50 bp OBE element
We then explored the mechanisms underlying the role of Platr10 in pluripotency. The CRIST-seq IGV analysis also revealed that Platr10 bound to the Oct4 promoter using a consensus 50 bp fragment located in exon 3 (5′-GACAAAAAATGGAGCAGACTGAAGGAAAGGCCATCCAGAGACTGTCCCAC-3′) (Fig. S9). Using a cellular fractionation assay, we showed that Platr10 was primarily localized to the nucleus (Figs. S10A-10B). The nuclear Platr10 was primarily in the chromatin-bound form (Fig. S10C). An RNA fluorescent in situ hybridization (FISH) assay also validated the nuclear localization of Platr10 lncRNA (Fig. S10D).
Platr10 binds to multiple stem cell core factor genes
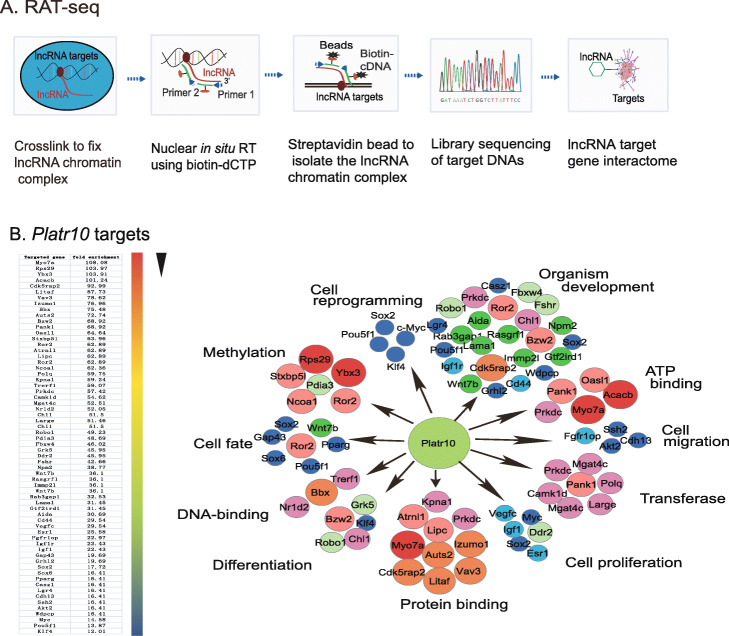
We hypothesized that Platr10 might control reprogramming by regulating a target gene network that is associated with pluripotency. To test this hypothesis, we utilized RNA reverse transcription-associated trap sequencing (RAT-seq) [21, 22] to identify a gene network coordinated by Platr10 (Figs. 3A). After crosslinking the chromatin structure, Platr10 was reverse transcribed in situ under a more stringent condition using three Platr10-specific complementary primers (Table S1) and biotin-dCTP. The biotin-labeled Platr10 chromatin complex was pulled down with streptavidin beads. Target gene DNAs were eluted, and a DNA library was constructed for Illumina sequencing. The Platr10 RAT-seq data were adjusted over the random oligonucleotide RAT-seq data. Using parameters of fold change difference >2 and p value < 0.05, with false discovery rate (FDR) <0.1, we identified 416 Platr10 target genes. Ontology analysis showed that Platr10 bound to gene targets belonging to pathways that are closely related to stem cell maintenance and differentiation (Fig. 3B).
Fig. 3.
Genome wide mapping of the Platr10 target gene network by RAT-seq. A Schematic diagram of the RNA reverse transcription-associated trap sequencing (RAT-seq) assay. Platr10 lncRNA was labeled with biotin-dCTP in situ reverse transcription using three Platr10-specific complementary primers. The biotin-Platr10 chromatin complex was isolated by streptavidin beads and the Platr10-binding target DNAs were isolated for Illumina library sequencing. This RAT-seq generates a Platr10 genome wide target DNA network. B Platr10 target gene interacting network. The Platr10 target pathway genes were mapped by gene ontology analysis. Platr10 binds to all four stem cell-associated factors that have been used to reprogram somatic cells: Oct4, Sox2, Klf4, and c-Myc
Platr10 interacts with multiple core stem cell factor genes, including Oct4, Sox2, Klf4, and c-Myc (Fig. S11). These four transcription factors have been commonly used to reprogram somatic cells into pluripotency. These interactions were not detectable in a RAT-seq dataset from the RAT-seq control library (RAT-CT), in which two random oligonucleotides, instead of Platr10-specific antisense oligonucleotides, were used for the RAT assay. Neither did we detect the RAT-seq signal of stemness gene binding for the lncRNA control Palr35, which was also differentially expressed in reprogramming, with abundant transcripts in iPSCs. These data suggest that Platr10 may actively and specifically participate in reprogramming by regulating pluripotency-associated transcriptional factor genes.
We further validated the Platr10-Oct4 interaction in shPlatr10-treated E14 cells. Using RAT-qPCR, we detected a higher Platr10-Oct4 interaction signal in control E14 cells (shCT) (Fig. S12A). After Platr10 knockdown, however, only the background interaction signal was detected in shPlatr10-1 and shPlatr10-2 groups. Similarly, a very low background RAT signal was detected in the control lncRNA Palr35, which was also differentially expressed in reprogramming, but was not found in the list of CRIST-seq. We also validated this Platr10-Oct4 promoter interaction using a ChIRP (Chromatin Isolation by RNA Purification) assay (Fig. S12B). This interaction was abolished in the shPlatr10 groups.
Platr10 orchestrates pluripotency-specific intrachromosomal looping
Epigenetic remodeling plays a key role in cell-fate conversion. During chromatin remodeling, the genome undergoes major epigenetic alterations to reacquire the euchromatin characteristic of pluripotent cells. By comparing local chromatin structure of the OCT4 locus, we previously showed that there is a pluripotency-associated intrachromosomal loop in iPSCs that juxtaposes a downstream enhancer to the gene’s promoter, enabling activation of endogenous stemness genes to achieve reprogramming [9]. Since Platr10 binds to the same regulatory elements that are known to be involved in chromatin three-dimensional structure, we hypothesized that Platr10 might be a critical factor that orchestrates intrachromosomal looping for reprogramming.
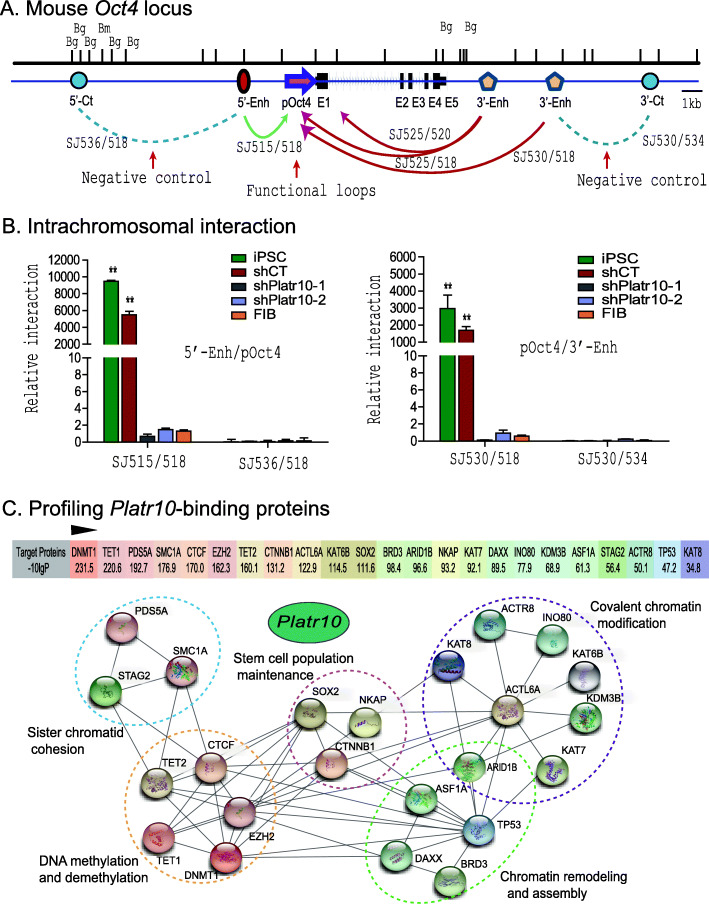
We used chromatin conformation capture (3C) [23] to compare intrachromosomal looping between iPSCs and Platr10 knockdown cells. Cells were fixed with 1% formaldehyde, digested with restriction enzymes BamH1/BglII, and then ligated with T4 DNA ligase. After reversal of the crosslinks and DNA purification, the chromatin interaction was detected by specific 3C primers located in the promoter and the enhancers of Oct4 (Fig. 4A).
Fig. 4.
Platr10 lncRNA is required for the formation of intrachromosomal looping in the Oct4 locus. A Location of 3C primers used to detect the interaction between the Oct4 promoter and enhancer. Enh, enhancers; pOct4, Oct4 promoter; 5’-Ct, the 5’ upstream control region of Oct4; 3’-Ct, The 3’ downstream control region of Oct4; E1-E5, exons; Bm, BamH1; Bg, Bgl2. Arrows: intrachromosomal interactions. B Knockdown of Platr10 abolishes the intrachromosomal interaction loop. shCT, negative control shRNA; shPlatr10, shRNA that targets Platr10 lncRNA; iPSC, induced pluripotent stem cell; FIB, fibroblasts. Primer sets that detect the presence of looping are marked in red. The 3C interaction was quantitated by qPCR and was standardized over the 3C control Ercc3 gene. For comparison, the relative 3C interaction was calculated by setting the 5’ or 3’ control as 1. **p < 0.01 as compared with the shPlatr10 treatment and FIB controls. C Profiling Platr10-binding proteins. The Platr10-binding protein factors were mapped by RNA pulldown MS sequencing using biotin-labeled Platr10 sense lncRNA in E14 cells. The Platr10 antisense RNA was used as the control. The binding signal was calculated as the protein enrichment ratio (the PEAKS score, −10logP) after adjusting over that of the antisense control. The protein interaction network was constructed using the String database web tool (https://string-db.org/)
As previously reported [9], we detected reprogramming-associated intrachromosomal interaction products in fully reprogrammed iPSCs: the SJ515/SJ518 loop between the 5′ upstream enhance and the promoter, and the SJ530/SJ518 loop between the 3′ enhancer and the promoter (Fig. 4B). However, shRNA knockdown of Platr10 abolished these intrachromosomal interaction signals and caused the iPSCs to exit from pluripotency. In the control group that was transfected with the shRNA control (shCT), these intrachromosomal loops remained intact. As expected, none of these 3-dimensional interactions were detected in fibroblasts.
We sequenced the 3C products and confirmed the presence of the ligated BamHI or BamHI/BglII sites, which were flanked by the sequences from the promoter and enhancers of Oct4, respectively (Fig. S13). These data suggest that Platr10 is critical for the maintenance of intrachromosomal interactions that are known to be associated with reprogramming and the maintenance of pluripotency [9].
To examine how Platr10 lncRNA coordinates this pluripotency-associated intrachromosomal looping, we mapped the Platr10-binding proteins using RNA pulldown-mass spectrometry (MS) protein sequencing. We found that DNA demethylase TET1 was high on the list of the MS-identified proteins (Fig. 4C), although Platr10 also interacted with many proteins in other pathways, including chromatin modification and remodeling (Fig. S14A).
Platr10 recruits TET1 DNA demethylase
CpG DNA demethylation in the Oct4 promoter is required for nuclear reprogramming. In order to initiate reprogramming, the methylated CpGs in Oct4 must be demethylated to initiate transcription in somatic cells. TET proteins, a group of Fe(II)/2-oxoglutarate-dependent dioxygenases, have recently been identified as critical factors that induce the oxidation-deamination mechanism underlying active DNA demethylation in mammals [24]. Knockdown of Tet1 induces DNA hypermethylation in the Nanog promoter accompanied by defects in self-renewal in ESCs [25]. TET1-initated DNA demethylation is essential for the initiation of reprogramming [26].
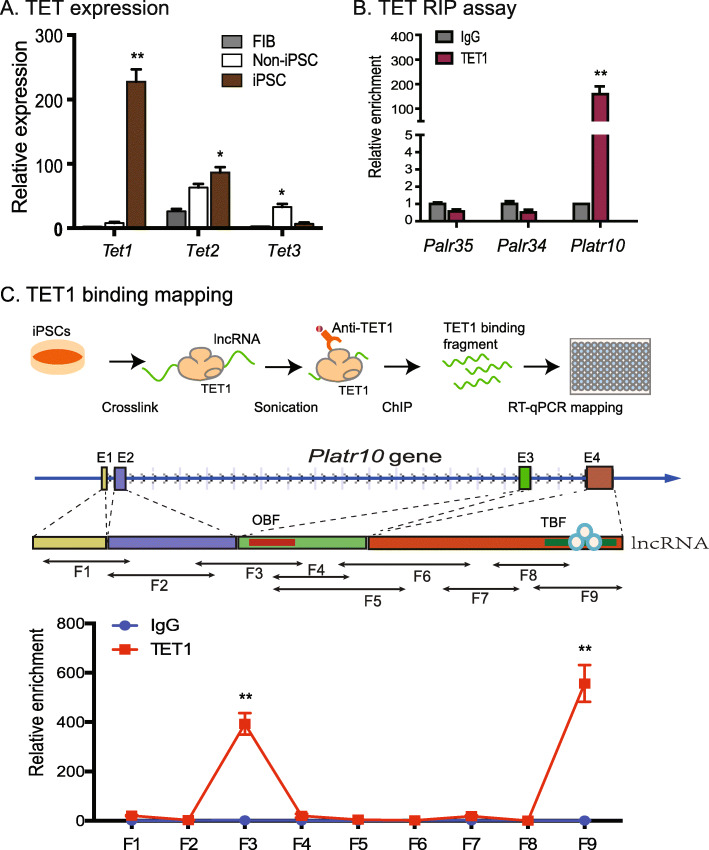
We used quantitative PCR to measure the transcript abundance for TET family genes. Among the TET family members, TET1 exhibited differential expression during reprogramming, with high abundance in iPSCs (Fig. 5A). We then asked if the binding of Platr10 to the Oct4 promoter would guide this demethylation process in reprogramming. We used an RNA-chromatin immunoprecipitation (RIP) method to pull down TET complexes in iPSCs. The pulled-down RNAs were reverse transcribed and quantitated by PCR using primers for Platr10. Using this assay, we detected enrichment of Platr10 in the TET1 antibody-precipitated complexes (Fig. 5B), suggesting the interaction of the lncRNA with DNA demethylases. No similar interaction was detected in the IgG control group. We also examined if the TET1-pulldown complex contained two control lncRNAs (Palr35, Palr34) that are also activated in reprogramming. We did not detect either of these two lncRNAs in the TET1 complex (Fig. 5B). We also used a second CLIP assay to validate the Platr10-TET1 binding in E14 cells (Fig. S14B). As expected, we found that TET1 bound to Platr10 in E14 cells. This binding was abolished after shPlatr10 treatment.
Fig. 5.
Platr10 coordinates DNA methylation in the Oct4 promoter by recruiting TET1 demethylase. A Differential expression of TET family genes during reprogramming. Cells were collected at different stages of reprogramming and the expression of the three TET demethylases was measured by RT-PCR. FIB, fibroblasts; non-iPSC, cells that ectopically express OSKM cocktail factors, but fail to complete reprogramming; iPSC, reprogrammed pluripotent stem cells. *p < 0.05, **p < 0.01 as compared with other two groups. B Interaction of Platr10 with TET1 DNA demethylase enzyme by RNA-chromatin immunoprecipitation (RIP). After formaldehyde crosslinking, the TET1-lncRNA chromatin complex was immunoprecipitated with a TET1-specific antibody. After de-crosslinking, the immunoprecipitated RNAs were reverse-transcribed. The TET-interacting lncRNAs were measured by PCR. IgG was used as the antibody control. Input: aliquot DNAs collected during the RIP assay. Note that lncRNA controls Palr35 and Palr34 did not interact with TET1, even though these two lncRNAs were also differentially activated in pluripotent reprogramming. C Identification of the TET1 binding fragment by RIP mapping. Top panel: Schematic diagram of RIP mapping. iPSC cells were fixed and were subject to a more stringent sonication treatment in order to break the Platr10 lncRNA regions that are not a part of the TET1 binding site. After immunoprecipitation with a TET1 antibody, the TET1 interacting Platr10 lncRNA fragments were mapped by quantitative PCR using overlapping primers (middle panel). For comparison, the value of the IgG control was set as 1. **p < 0.01 as compared with other PCR fragments. The F3 and F9 show a strong binding of TET1
We further examined the TET1/Oct4 interaction in E14 cells (Fig. S15A). For this, we collected E14 cells treated with shPlatr10 and shCT. Using a TET1-specific antibody, we detected the TET1-Oct4 interaction in E14 shCT control cells. However, treatment of E14 cells with two Platr10 shRNAs significantly reduced the ChIP signal at the Oct4 promoter locus (Fig. S15B). These data suggest a critical role of Platr10 lncRNA in the binding of TET1 to the Oct4 promoter.
After confirming the role of Platr10 in the TET1-Oct4 binding, we used a RIP in situ mapping assay to identify the specific fragment of Platr10 that interacts with TET1 (Fig. 5C). After crosslinking, iPSCs were lysed and the chromatin fraction was subjected to a longer sonication to fracture unbound RNAs. The TET1-binding RNAs were immunoprecipitated by a TET1 antibody and were reverse transcribed. The TET1-interacting regions were mapped by overlapping PCR (Fig. 5C, top panel). Using this approach, we identified two regions that showed enriched TET1 binding signals (Fig. 5C, bottom panel). The F3 region overlapped with the Oct4 binding element (OBE) identified from the RAT-seq dataset. The second TET1 binding site F9 was located at the 3′ end of Platr10.
Mapping of the TET1-binding element in Platr10
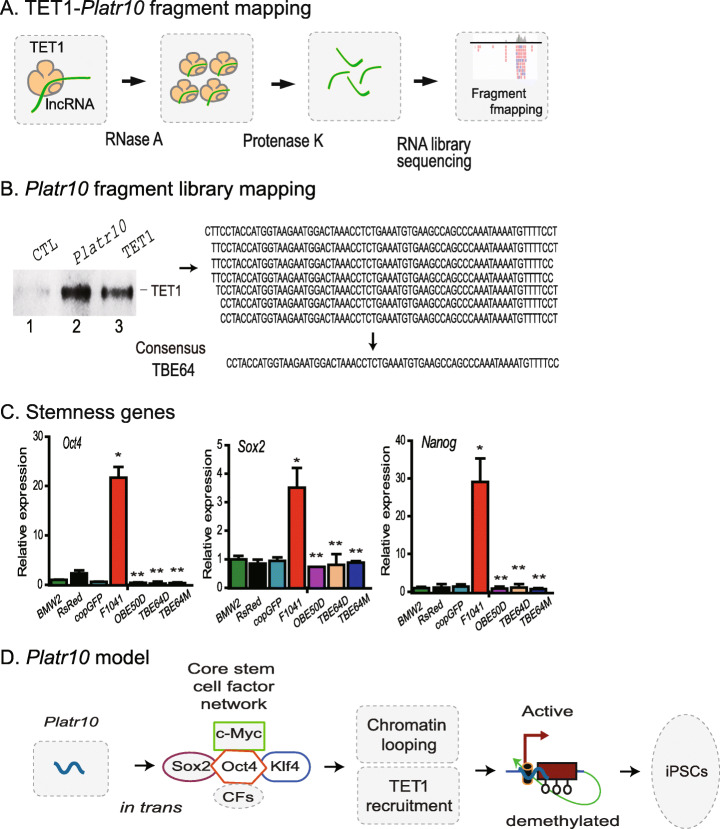
We then used an RNase A mapping approach to define the specific TET1 binding element. Platr10 lncRNA was synthesized in vitro using T7 RNA polymerase and biotin-CTP. The biotin-labeled lncRNA was incubated with TET1 recombinant protein. The mixture was treated with RNase A to remove the unbound lncRNA. The TET1-interacting lncRNA fragment was recovered with streptavidin beads, ligated with short RNA library adaptors, reverse transcribed using an adaptor primer, and cloned into pJet vector for DNA sequencing (Fig. 6A). We mapped the TET1 binding element to the 3′-end (Fig. 6B, right panel), which is identical to the locus as defined by the nuclear RIP assay. Considering the presence of some shorter Platr10 transcripts from 3′-RACE, a consensus 58 bp fragment was used for further studies. After streptavidin pulldown, the Platr10-interacting proteins were eluted and analyzed by Western blot. Using a TET1-specific antibody, we confirmed that Platr10 specifically interacted with TET1.
Fig. 6.
Mutation of the Oct4 binding element and TET1 binding elements abolishes the function of Platr10 lncRNA. A Diagram of TET1 binding element (TBE) mapping. The biotin-labeled Platr10 full-length lncRNA was incubated with TET1 recombinant protein. After binding, RNase A was used to remove the free Platr10 lncRNA fragments. After treatment with proteinase K, the TET1 protected Platr10 lncRNA fragment was purified and was used for RNA library cloning. DNA sequencing was then performed to map the TET1 binding elements. B Identification of the TET1 binding element. Left panel: Western blot detection of the Oscrl8-TET1 interaction. After binding, the Platr10-Tet1 complex was pulled-down by streptavidin beads and was subject to Western blot analysis. Platr10, biotin-labeled Platr10 full-length lncRNA; CTL, biotin-labeled Platr10 antisense lncRNA control; TET1, recombinant protein. Right panel: read sequences of the TET1 binding library. TBE58: consensus TET1 binding element. C Requirement of the OBE and TBE elements in Platr10 lncRNA. Fibroblasts (BMW2) were transfected with lentiviruses carrying full-length Platr10 or Platr10 mutants that lack either the OBE or TBE. After puromycin selection, mixed cells were collected for qPCR analyses of the endogenous core stem cell factor genes. *p < 0.01 as compared with controls; **p < 0.01 as compared with the full length Platr10 lncRNA. The function of Platr10 was abrogated in the OBE50D, TBE58D, and TBE58M groups. D Putative model of Platr10 in maintaining pluripotency. Open circle under the promoter: unmethylated CpG islands; TET1: DNA demethylases. In un-reprogrammed cells, such as fibroblasts, the Oct4 promoter is fully methylated and is transcriptionally inactive. During reprogramming, Platr10 becomes activated. By binding to the core stem cell gene network, Platr10 orchestrates an intrachromosomal loop, juxtaposing the enhancers close to the promoter. In addition, Platr10 also recruits TET1 and induces DNA demethylation in the promoter. By activating pluripotency-associated genes, the lncRNA promotes reprogramming and keeps stem cells from differentiation.
We then performed mutation assays to characterize the function of the identified Platr10 elements. The expression constructs included the full Platr10 (F1041), deletion of a 50 bp Oct4 binding element (OBE50D), deletion of a 58 bp TET1 binding element (TBE58D), and mutation of the 58 bp TBE with a random sequence (TBE58M) (Figs. S16A-S16B). To characterize the role of these elements, we stably transfected the expression vectors in fibroblasts. After puromycin selection, stable clones were collected to examine the expression of the core stem cell factor genes Oct4, Sox2, and Nanog (Fig. 6C). Neither the lentiviral DsRed vector control nor the copGFP control altered the activity of the three endogenous stem cell factor genes. However, ectopic expression of the full Platr10 (F1041) activated these core stem cell factor genes. Deletion of the Oct4 binding element (OBE50) abrogated Platr10 activity. Deletion or mutation of the TET1 binding element abolished Platr10 activity as well.
We also examined the role of Platr10 in an Oct4 promoter-luciferase reporter system. The activity of the 4.2 kb Oct4 promoter was measured using a luciferase kit. As compared with the vector control, the full length Platr10 (F1041) activated the Oct4 promoter. In the OBE50D group, deletion of the Oct4 binding element completely abolished the function of Platr10. However, deletion and mutation of the TET1 binding element did not affect the function of Platr10 in this luciferase co-transfection assay (Fig. S16C). By comparing these in vitro and in vivo data, it appears likely that other regulatory elements may also be required to optimally activate the Oct4 promoter in vivo. Alternatively, the promoter plasmid DNAs used in the luciferase assay were un-methylated and might not need the participation of TET1 demethylase.
We further examined the role of Platr10 in a LIF-withdrawal model. Upon LIF withdrawal, E14 cells formed stem cell spheroids slowly when seeded at very low density. However, the full-length Platr10 was able to partially correct the LIF withdrawal-induced defect, including spheroid formation and Nanog expression. This ability, however, was abolished in Platr10 mutants that lack the 3′-fragment (Fig S17). After Platr10 knockdown, the Oct4 promoter became demethylated in parallel with the loss of pluripotency. In addition, expression of Platr10 partially reversed the altered DNA demethylation in the Oct4 promoter in LIF-withdrawal E14 cells (Fig. S18). Taken together, these data suggest that both the Oct4 binding element and the TET1 binding element are critical for the optimal function of Platr10 lncRNA in activating endogenous core stem cells factor genes.
Discussion
This study demonstrates a previously undisclosed trans role for a lncRNA in the establishment and maintenance of stem cell pluripotency. It has been more than a decade since the discovery that somatic cells can be reprogrammed into pluripotent status by OKSM factors [1]. However, we still know very little about how this reprogramming is initiated. In particular, it is unclear why the majority of cells, even though they express the viral reprogramming factors, fail to convert into pluripotent cells. Recent studies suggest that during reprogramming, chromatin architecture must be appropriately remodeled, so that core stem cell genes occupy preferred territories and are organized in a pluripotency-specific network consisting of specific intra/inter-chromosomal loops [10, 27]. Using CRIST-seq, we profiled the RNAs that interact with the Oct4 promoter. In conjunction with the RNA-seq data, we identified Platr10 as a critical pluripotency-associated lncRNA. Loss- and gain-of-function assays confirmed that Platr10 is essential for optimal maintenance of pluripotency. Knockdown of Platr10 induces iPSCs to exit from pluripotency. The Platr10-deficient iPSCs lose the potential of self-renewal and become differentiated. Overexpression of Platr10, on the other hand, triggers the activation of core stem cells factor genes, and enhances the efficiency of pluripotent reprogramming. Most importantly, we demonstrate that Platr10 is transcribed specifically during reprogramming. In the nucleus, it regulates pluripotency in trans by helping to organize a 3-dimensional chromatin interaction network and by recruiting TET1, a DNA demethylase that removes the epigenetic barriers of DNA methylation in the core stem cell gene promoter (Fig. 6D). However, it is not clear if chromatin looping and the methylation status at the Oct4 locus impact each other. The data from RNA pulldown-MS protein sequencing suggest that Platr10 binds multiple factors involved in covalent chromatin modification and chromatin remodeling, including SMC1a, CTCF, and Cohesin subunit SA-2 (Stag2). Thus, it is possible that by binding to these chromatin factors, Platr10 lncRNA may coordinate both the methylation status and chromatin looping at the Oct4 locus. Even more interestingly, the RNA pulldown MS protein sequencing data demonstrate that in addition to TET1, Platr10 also binds to DNA methyltransferase DNMT1. This finding suggests that by binding to DNMT1, Platr10 may also regulate stem cell fate by actively coordinating DNA demethylation in many other target genes as identified by RAT-seq. Further studies are needed to address these critical issues.
At the Oct4 gene locus, for example, the intrachromosomal looping between the promoter and enhancer regulatory regions is specific for pluripotent stem cells. These intrachromosomal interactions have been demonstrated by several technologies, including 3C, Hi-C, and ChIA-PET [28]. This topological structure helps bring distal regulatory elements, like enhancers, into physical proximity with gene target promoters, thereby activating them to initiate cellular reprogramming [9]. In this study, we showed that Platr10 was enriched in areas containing these regulatory elements, including the promoter, and 5′- and 3′-enhancers, where pluripotent transcription factors such as Oct4, Sox2, Nanog, and other chromatin factors are found [8, 9, 29]. Unlike many transcription factors that bind to target genes within a short stretch of DNA, Platr10 covers huge areas in the promoter, enhancer, or exons. Our RAT-seq data also show that Platr10 binds to many targets that do not belong to the accepted classes of stemness genes. Currently, we are not sure whether those target genes are also involved in the establishment and maintenance of pluripotency. It is likely that Platr10 may have multiple functions in addition to its role of regulating pluripotent genes.
We have demonstrated that Platr10 is essential for the maintenance of intrachromosomal looping at the Oct4 locus. Knockdown of Platr10 abolishes this intrachromosomal loop structure and causes the exit of iPSCs from pluripotency. In addition to Oct4, the RAT-seq data also demonstrate that Platr10 binds to many other pluripotency-associated genes, including Sox2, Klf4, and c-Myc. These four transcription factors have been widely used to reprogram somatic cells. During reprogramming, Platr10 is actively transcribed and acts in concert with other chromatin factors to coordinate a topological architecture network that is necessary to initiate pluripotency. The Hi-C assay has been used to map the chromatin architecture in human [30] and mouse [31] ESCs. However, the resolution of Hi-C is too low to delineate the promoter-enhancer intrachromosomal loop at the Oct4 locus. Thus, it will be important to delineate this lncRNA-chromatin interaction in greater detail using a high-resolution approach, like Micro-C, enhanced Hi-C, and Capture-C [32].
Currently, we know very little about how Paltr10 recognizes its target genes, like Oct4. By searching the lncRNA-DNA interaction prediction website (LongTarget: http://lncrna.smu.edu.cn/show/DNATriplex) [33], we could not find an interaction using the base matching mechanism between Platr10 and the Oct4 promoter. To explore this issue, we have performed RNA pulldown-MS sequencing for Platr10 lncRNA. The MS sequencing data show that in addition to TET1, Platr10 also binds to a number of proteins that are involved in covalent chromatin modification, chromatin remodeling, and chromosome cohesion. Thus, it is possible that this lncRNA-chromatin interaction requires the participation of other chromatin factors, like CTCF and Cohesin. In addition, the RNA pulldown-MS sequencing data suggests that Platr10 binds to P53. Thus, it is possible that the nuclear Platr10 may enhance reprogramming, at least partially, by regulating the P53 pathway.
Platr10 lncRNA also contains a short TBE fragment at its 3′ terminus, which can interact with TET1 demethylase. In fibroblasts, we show that viral expression of the full-length Platr10 upregulates the core stem cell factor genes Oct4, Sox2, and Nanog. When this TBE element was deleted or replaced with a 58 bp random RNA, the Platr10 mutants completely lose activity. These data suggest that the binding of TET1 demethylase may be an important step to activate core stem cell factor genes during the initiation of pluripotent reprogramming. It should be noted that in addition to its activity to catalyze hydroxylation of 5-methylcytosine to generate 5-hydroxymethylcytosine in DNA, recent studies show that TET1 and TET2 are also critical for RNA hydroxymethylation [34]. The TET family has a C-terminal RNA-binding domain that binds to thousands of RNA targets, including Platr10. It is not known if all TET1-interacting RNA molecules have a consensus motif like the Platr10 TBE.
In addition to the TET family, a recent study by Holmes et al. [35] showed that SOX2 protein also bound to multiple RNA molecules in mECSs. Their formaldehyde cross-linked immunoprecipitation (fRIP) approach mapped 1010 SOX2-binding RNA genes, while the CLIP-seq approach showed 54 enriched genes, with 41 genes overlapping in the two approaches. By analyzing their geo data, it can be shown that Platr10 appears in both fRIP-seq and CLIP-seq databases. Using RNA pulldown-MS protein sequencing, we also detected the binding of Platr10 with multiple stemness markers, including SOX2. Thus, it would be interesting to examine if in addition to the role in coordinating intrachromosomal loop at the Oct4 locus, Platr10 lncRNA may regulate pluripotency through modulating the activity of these pluripotent markers at the post-transcriptional level.
Overall, our work demonstrates a critical role for the lncRNA Platr10 in both the induction of cell reprogramming and the maintenance of pluripotent status in iPSCs. As a lncRNA, Platr10 does not encode a known pluripotent factor. However, it regulates pluripotency using unique mechanisms that cannot be fulfilled by pluripotency-associated protein transcription factors. Specifically, it helps maintain a pluripotency-specific chromatin architecture in the Oct4 promoter. By binding to the Oct4 locus, it facilitates a functional juxtaposition between the two Oct4 regulatory regions. With this intrachromosomal loop, the distal enhancer element is moved in close juxtaposition to the promoter, activating this stem cell core factor gene. After binding to Oct4, this lncRNA recruits the DNA demethylase TET1, which induces demethylation of the Oct4 promoter as an essential step to initiate reprogramming. By coordinating the necessary chromatin architecture and DNA demethylation, Platr10 may play an essential role in initiating pluripotency during reprogramming (Fig. 6D). As Platr10 is involved in the regulation of pluripotency, it will also be interesting to expand this study by exploring the role of this lncRNA in a knockout mouse model.
Materials and methods
Cell lines and cell culture
E14 mouse embryonic stem cells were purchased from ATCC and were cultured in the ESC medium containing KnockOut DMEM, 15% fetal calf serum, l-glutamine, non-essential amino acids, penicillin/streptomycin, and 2-mercaptoethanol and supplemented with LIF. Mouse embryonic fibroblasts (MEFs) were cultured from fetal mice, and MBW2 fibroblast-like cells were cultured from M. spretus-Balb/c F1 mouse bone marrow mesenchymal stem cells [17], and were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acid (NEAA), and 1% antibiotics (penicillin-streptomycin) at 37°C in an atmosphere containing 5% CO2.
Construction of CRIST targeting vectors
CRIST-Seq was performed to identify lncRNAs that bind to the Oct4 and Sox2 promoters (Fig. S1). Specifically, we constructed the Cas9-Oct4 gRNA vector by cloning two Oct4 promoter gRNAs (Fig. S2B) into the lentiCRISPR-EGFP sgRNA 2 vector that contains the catalytically inactive dCas9-FLAG [36, 37]. The U6-gRNA1-T5-H1-gRNA2 T5 cassette was synthesized by joining the H1 promoter with two oligonucleotides that contain the guiding RNA (gRNA) from the Oct4 promoter (Oct4-gRNA1: 5′-GAACATTCAATGGATGTTTT-3′ and Oct4-gRNA2: 5′-GTGTGAGGGGATTGGGGCTC-3′), respectively. The expression cassette was inserted downstream of the U6 promoter in the CRIST targeting vector. The same strategy was used to construct the Cas9-Sox2 gRNA vector. The Sox2 promoter guiding RNAs include: Sox2-gRNA1: 5′-GGGGTTGAGGACACGTGCTG-3′ and Sox2-gRNA2: 5′-GAGCCAATATTCCGTAGCAT-3′). A CRIST-Seq control vector was constructed using a Cas9 random gRNA (Cas9-gCT): GTGCGTTGTTAGTACTAATC.
Cell reprogramming
Reprogramming of mouse fibroblasts into iPSCs was performed by lentiviral Oct4-Sox2-Klf4-c-Myc-GFP (OSKMN) as previously described [9]. Briefly, fibroblasts in 6-well plates were infected with lentiviruses in the presence of polybrene (8 μg/ml). Three days after infection, the cells were digested and transferred to 100-mm dishes on mitomycin C-inactivated MEF feeder cells. The media were replaced with ES medium (DMEM/F12 supplemented with 20% KSR, 10 ng/ml leukemia inhibitory factor (LIF, Sigma), 10 ng/ml β-FGF (PeproTech), 0.1 mM β-mercaptoethanol, L-glutamine, and 1×10-4 M non-essential amino acids [38]. The selected iPSC colon cells had been fully characterized previously by immunostaining stem cell markers, alkaline phosphatase (AP) staining, karyotype analysis, and teratoma formation [9]. The fibroblast-like cells that expressed OSKMN but were not reprogrammed were termed “non-iPSCs” and used in parallel with iPSCs in the study [9, 18].
CRIST-Seq to map the Oct4-interacting lncRNAs
A CRIST-seq approach was used to map the Oct4 promoter-interacting lncRNAs as descried [15]. Briefly, iPSCs were transfected with the dCas9-gRNA lentiviruses. After transfection, cells were selected by puromycin and were collected for CRIST immunoprecipitation [39, 40]. To isolate the Oct4 promoter-interacting lncRNA, cells were cross-linked with 2% formaldehyde and were lysed with cell lysis buffer (10 mM Tris [pH 8.0], 10 mM NaCl, 0.2% NP-40, 1x protease inhibitors). Nuclei were collected by centrifugation and were suspended in 1× reverse transcription buffer in the presence of 0.3% sodium dodecyl sulfate (SDS) and incubated at 37°C for 1 h. Triton X-100 was then added to a final concentration of 1.8% to sequester the residual SDS. An aliquot of nuclei (3×106) was reverse transcribed at 37°C for 30 min in a 20-μl reaction containing 1μl random hexamer, 1μl 10 mM dNTP, 1μl 0.4 mM dCTP -biotin, 1μl RT enzyme, 0.5μl RNase inhibitors, 1μl 0.1M DTT, 4μl 5× cDNA synthesis buffer, 1μl Maxima Reverse Transcriptase, and RNase-free water to 20μl. The reaction was stopped by adding 4μl 0.5M EDTA. After nuclear lysis, the chromatin complex was subjected to sonication for 180 s (10 s on and 10 s off) on ice using a Branson sonicator with a 2-mm microtip at 40% output control and 90% duty cycle settings. The biotin-labeled cDNA/Cas9 complex was immunoprecipitated with anti-FLAG antibodies (#MA1-91878, Thermo Fisher, IL, or #F7425, Sigma, MO). The DNAs were released after cross-linking reversal and proteinase K treatment and were precipitated with ethanol. The biotin-labeled cDNAs were further purified from genomic DNAs with M-280 streptavidin beads (Invitrogen, CA). After purification, the Oct4 promoter-interacting cDNAs were quantitated by PCR using target gene primers (Table S1).
For Illumina cDNA sequencing, the second-strand cDNA was synthesized using a Stratagene cDNA Synthesis kit (Agilent Technologies, CA). The double-strand cDNAs were digested by Dpn I for library construction by ligating with the NEBNext adaptors (NEBNext® ChIP-Seq Library Prep Master Mix Set for Illumina). Triplicate library DNAs were sent to Shanghai Biotechnology (Shanghai) for Illumina sequencing.
For CRIST-Seq control, we performed a CRIST assay using a random gRNA (gCT) and constructed the control library for sequencing using the same protocol. An anti-IgG antibody was used as the background control for immunoprecipitation [18].
CRIST-Seq data analyses
After CRIST sequencing, the adapter sequences were removed from the raw data using Illumina annotated adapter sequences with parameter ILLUMINACLIP: 2:30:10 and the low-quality data were filtered using Fastx software (http://hannonlab.cshl.edu/fastx_toolkit/index.html). After filtering, clean reads were mapped to the mouse genome (genome version: mm10, GRCm38.p4) for mRNAs and lncRNAs using TopHat (version:2..0.9) software [41]. The mapped RNA reads were quantitated using Cuffling (version:2.1.1). Gene counts were normalized to the values of fragments per kilobase of transcript per million fragments mapped (FPKM). The resulting coverage tracks (bedgraph file) were visualized in UCSC genome browser. The peak was called and annotated with RIPSeeker. The called peaks that overlapped with the IgG control enriched regions were removed. To define the specific binding of RNAs, the CRIST-Seq signal intensities were further normalized over that of the non-targeting Cas9 gCT control with the DiffBind package using parameters of fold change difference ≥2 and p value < 0.05, with false discovery rate (FDR) <0.1. The adjusted CrIST-Seq data were then used for mapping the Oct4 and Sox2 RNA interactions [18].
RNA-Seq to identify differentially expressed lncRNAs in reprogramming
Total RNA was isolated from fibroblasts and iPSCs [9] using TRIzol (Invitrogen, Carlsbad, CA). The indexed libraries were prepared using Illumina’s TruSeq RNA Sample Prep Kit v2. Paired-end sequencing in triplicate was performed by Shanghai Biotechnology (Shanghai, PRC) using a HiSeq4000 (Illumina). The RNA-Seq yielded 145 million raw reads for iPSC and 148 million raw reads for fibroblasts. After Seqtk filtering, a total of 120 million clean reads for fibroblasts and 124 million clean reads for iPSCs were mapped to the mouse genome (genome version: mm10, GRCm38.p4 (ftp://ftp.ensembl.org/pub/release-83/fasta/mus_musculus/dna/Mus_musculus.GRCm38.dna.primary_assembly.fa.gz) for mRNAs and lncRNAs using the STAR software. Gene counts were normalized to the values of reads per kilobase of transcript per million mapped reads (RPKM). Cuffdiff was used to calculate the differentially expressed RNAs when the fold-change was > 2 and p < 0.05 with an unpaired two-sided t test [18].
Integration of RNA-Seq and CRIST-Seq data
Reprogramming-associated RNA candidates were identified by RNA-Seq using a cutoff threshold of >2-fold, p<0.05. CRIST-Seq RNAs were selected by peak enrichment FPKM>50 as a cut-off threshold after adjusting over the IgG control and Cas9-gCT control. The RNA-Seq data were merged with the Oct4 and Sox2 CRIST-Seq data using VENN program (http://bioinformatics.psb.ugent.be/webtools/Venn/). Venn diagrams were used to visualize the overlap RNAs between the datasets. The overlapping RNAs identified by the datasets were chosen for further functional characterization.
Characterization of Platr10 lncRNA
During the cloning of full length Platr10 cDNA, our PCR products indicated that the lncRNA underwent alternative splicing. Therefore, we characterized Platr10 lncRNA by cDNA 5′- and 3′-RACE following the method as previously described [21]. For the 3′-end racing, total iPSC RNAs were reverse transcribed using Maxima Reverse Transcriptase with a poly T primer: SJ773 5′-CTGCGTAATACGACTCACTATAGGAGACAGGCTCGAGTTTTTTTTTTTTTTTTTT-3′. The first racing PCR was performed using 3′-RACE primer SJ771: 5′-CTGCGTAATACGACTCACTATAGG-3′ and a 5′-Platr10-specific primer SJ938: 5′-CTGTTGAGC CAGGCAGCTG-3′. The PCR DNAs were diluted 500-fold and were used for the second nested PCR using 3′-RACE primer SJ772: 5′-GACTCACTATAGGAGACAGGCTCGA-3′ and a 5′-Platr10-specific primer SJ937: 5′-AGCTGGAGGAAGTGTGTCAC-3′. The racing PCR bands were cut and cloned into pJet vector for sequencing.
For the 5′-racing, the iPSC RNAs were reverse-transcribed using Maxima Reverse Transcriptase with random hexamer oligonucleotides for 30 min. The poly G primer SJ774: 5′-CCAGATTCAGGACTGTCGACATCGAATTCGGGG-3′ was added into the mixture and the reaction was continued for another 30 min. After 10-fold dilution, cDNAs were amplified with 5′-adapter RACE primer SJ775: 5′-GATTCAGGACTGTCGACATCGA-3′ and Platr10-specific primer SJ935: 5′-TCATGTCCGGGTTCGAGCCT-3′. The PCR bands were cloned into pJet vector for sequencing.
RAT-Seq assay to map the genome-wide interacting target genes for Platr10
A RAT assay was modified to map the genome-wide interacting target genes for Platr10 [42]. Specifically, cells were cross-linked with 2% formaldehyde and lysed with cell lysis buffer (10 mM Tris [pH 8.0], 10 mM NaCl, 0.2% NP-40, 1x protease inhibitors). Nuclei were suspended in 1× reverse transcription buffer in the presence of 0.3% sodium dodecyl sulfate (SDS) and incubated at 37°C for 1 h. Triton X-100 was then added to a final concentration of 1.8% to sequester the SDS. Gene strand-specific reverse transcription was performed using four Platr10-specific complementary primers in the presence of biotin-dCTP. After 50 min of reverse transcription of Platr10 lncRNA with Maxima Reverse Transcriptase (Thermo Fisher Scientific, CA) at 62C, the reaction was stopped by adding 4μl 0.5M EDTA. After nuclear lysis, the chromatin complex was subjected to sonication for 180 s (10 s on and 10 s off) on ice with a Branson sonicator with a 2-mm microtip at 40% output control and 90% duty cycle settings. The biotin-labeled cDNA/chromatin DNA complex was pulled down with biotin-streptavidin magic beads (Invitrogen, CA). After reversing the cross-links and washing with 10 mg/ml proteinase K at 65°C overnight and treatment with 0.4 μg/ml RNase A for 30 min at 37°C, the genomic DNA that interacted with Platr10 was extracted and digested by Dpn I, and ligated with the NEBNext adaptors (NEBNext® ChIP-Seq Library Prep Master Mix Set for Illumina) to construct the library. Triplicate library DNAs were sent to Shanghai Biotechnology for Illumina sequencing.
For RAT-Seq control, we performed a RAT assay by replacing Platr10 complementary primers with random primers and constructed a control library for sequencing using the same protocol.
RAT-Seq data analyses
After RAT sequencing, the low-quality reads were filtered using Fastx (version:0.0.13) software (http://hannonlab.cshl.edu/fastx_toolkit/index.html). Clean reads were mapped to the mouse genome (genome version: mm10) using the Bowtie (version:0.12.8) software with default parameters. Enriched regions of the genome were identified by comparing the RAT-Seq peaks to input samples using MACS2 (version:2.1.1), and q value of 0.05 was used as the initial cutoff threshold to minimize peak caller bias. The upstream 2k of the transcription start sites and the downstream 5k of the transcription termination region were defined as the gene regions. The significant GO terms of biological processes with a p value < 0.05 were selected. We also used the MEME suite [43] for the discovery and analysis of the peaks’ sequence motifs. The resulting coverage tracks (bedgraph file) were visualized in the UCSC genome browser. To reduce the background, the RAT-Seq data were further normalized over the peaks of the control RAT-Seq data that were generated by using random oligonucleotide primers in the RAT assay. Differential binding analysis was performed with the DiffBind package using parameters of fold-change difference >2 and p value < 0.05, with false discovery rate (FDR) <0.1. The adjusted RAT-Seq data were used for mapping the lncRNA target gene interaction network [44, 45].
RT-PCR
Total RNA was extracted by TRIzol reagent (Sigma, MO) from cells and stored at −80°C. RT-PCR reaction was performed with a Bio-Rad Thermol Cycler. The target amplification was performed by PCR of 1 cycle at 95°C for 5 min; 33 cycles at 95°C for 20s, 62°C for 15s, and 72°C for 15 s; and 1 cycle at 72 °C for 10 min.
Quantitative real-time PCR was performed using SYBR GREEN PCR Master (Applied Biosystems, USA) as previously described [9, 46]. The threshold cycle (Ct) values of target genes were assessed by quantitative PCR in triplicate using a sequence detector (ABI Prism 7900HT; Applied Biosystems) and were normalized over the Ct of the β-ACTIN control.
RNA fish
RNA FISH was performed by a modification of the method as previously described [47]. The RNA FISH probe was prepared as antisense single-strand DNA (ssDNA) by asymmetric PCR. Briefly, single-strand DNA probes were synthesized by Klen-Taq DNA polymerase using Platr10 cDNA as the template, PCR primer JH4022: 5′-CACTGCTGGTTTGGAGCTCCAT-3′ and JH4023 5′-TGGGACAGTCTCTGGATGGCCT-3′ in a ratio of 1:50, with dig labeling dNTP MIX (Roche:11277065910). PCR probe products were purified by electrophoresis on 2% agarose gel and eluted in 20 μl TE buffer. For hybridization, 0.1 μg ssDNA probe and 10 μg salmon sperm DNA (Boehringer, Meylan, France) were precipitated with ethanol and suspended in 10 μl RNA hybridization buffer (2xSSC, 10% dextran sulfate, 0.2mg/mL BSA (Invitrogen, CA), and 2mM VCR, 10% formamide). Slides were counterstained with DAPI and FISH signals. Images were captured and merged to confirm subcellular localization.
Knockdown of Platr10 by shRNA lentiviruses
Platr10 was knocked down by two separate shRNA lentiviruses. The shRNA vector was constructed by cloning four shRNAs into a pGreenPuro vector (#SI505A-1, SBI, CA) under the control of the H1 and U6 promoters. Vector 1 (shPaltr10-1) contained shRNA #1: 5′-TTCTGTGTATCTGTTGAGCCAG-3′ and #3: 5′-CTGCCAGCATCTGACTAAGATA-3′. Vector 2 (shPlatr10-2) contained shRNA #2: 5′-CCTGCTGCCTGTCAATCCAAAT-3′, and #4: 5′-CCTGCTGCCTGTCAATCCAAAT-3′ (Table S1). The promoter-shRNA cassettes were ligated by PCR and were ligated into the EcoR1/BamH1 site in the pGreenPuro vector. The copGFP reporter in the vector was used to track the lentiviral transfection in iPSCs. Two random shRNAs (5′-GCAGCAACTGGACACGTGATCTTAA-3′ and 5′-TGAAATGTACTGCGCGTGGAGACTA-3) were cloned in the same vector as the assay control (shCT).
Genomic deletion of Platr10 by CRISPR Cas9 editing
The Platr10 gene was deleted in E14 cells using CRISPR Cas9 editing following the protocol in our lab [48]. Briefly, two Cas9 targeting vectors were constructed based on the dual SpCas9-NmCas9 cassette reported by Bolukbasi et al. [20]. Four gRNAs were designed from the Platr10 promoter and 3′-downstream region, respectively (Table S1). As the control, random Cas9 gRNAs were used to construct the Cas9 gCT vector. For homologous recombination, a donor vector was constructed to carry two Platr10 arm. The copGFP/Puro cassette was used for the positive selection, and the TK for the negative selection. After electroporation, E14 cells were selected by puromycin and ganciclovir, respectively, as previously described [49]. The copGFP-positive cells were collected, and homozygous deletion of Platr10 was confirmed by PCR and DNA sequencing.
Platr10 promotes DOX-OSKM reprogramming
Platr10 cDNA was cloned into pCMV-DsRed-Puro vector, and lentiviruses were packaged in 293 cells. Control lentiviruses carried the pCMV-DsRed-Puro empty vector (Vector) and the pCMV-DsRed-Puro CTL (800 bp random sequence). OG2 MEFs were first transfected with the Platr10 and control lentiviruses and were selected by puromycin. MEFs were reprogrammed following the method as described [19]. Briefly, 15,000 lentivirus-transfected MEFs were seeded in 12-well plates and were cultured in KSR iPS medium containing 2 μg/ml doxycycline (DOX). The medium was changed every other day. The iPSC colonies were immunostained with Rabbit anti-NANOG Antibody (A300-397A, Bethyl, 1:500 dilution) and alkaline phosphatase (AP) kit (AP100R-1, System Biosciences).
Immunohistochemical staining of stem cell markers
Immunofluorescent staining was used to examine stem cell markers in iPSC colonies [50]. Briefly, cells were fixed by 4% paraformaldehyde/PBS for 10–15 min and rinsed with PBS, then permeabilized and blocked with 0.1% Triton X-100/PBS containing 3% BSA for 30 min. After incubation with primary antibodies for 1 h at room temperature or overnight at 4C, the samples were washed three times with PBS and then incubated with secondary antibody for 1 h. The following antibodies were used in the immunostaining: rabbit anti-NANOG (1:100 dilution, Santa Cruz) and rabbit anti-OCT4 (1:100 dilution, Millipore). The cell samples were subsequently incubated with Cy3- or Alexa Fluor 488-labeled secondary antibodies for 1 h. After washing three times with PBS, samples were counterstained with Hoechst 33258 (Invitrogen). Alternatively, the pluripotency of stem cells was examined by Fluorescent Mouse ES/iPS Cell Characterization kit (Cat.#SCR077, Millipore, MA) following the protocol provided by the manufacturer. Fluorescence images were acquired with a Zeiss AxioCam Camera.
Embryoid body differentiation
For embryoid body (EB) formation, iPSCs or E14 cells were dispersed by collagenase IV (Invitrogen), and cell clumps were transferred to 60-mm dishes in ES medium without LIF. After being maintained in floating culture for 3 days, EB were seeded in 0.1% gelatin-coated 6-well plates in DMEM/F12 containing 20% FBS for spontaneous differentiation. Cells were collected at different time points and used for gene expression analysis using quantitative PCR.
Platr10 RNA pull-down mass spectrometry analysis
Platr10-binding proteins were mapped by RNA pull-down mass spectrometry sequencing (Shanghai Jixue Technology Co., Shanghai). Sense biotin-Platr10 lncRNA was synthesized by T7 RNA polymerase using Biotin RNA Labeling Mix, treated with RNase-free DNase I, and purified with RNeasy Mini Kit (Qiagen). Antisense bitoin-Platr10 RNA was used as the control. Both strands were used to pull-down target proteins. Differential protein bands were excised and analyzed by mass spectrometry. Fragment sequences from MS were performed using PEAKS 7 at a false discovery rate (FDR) threshold of <5%. Platr10-bindnig proteins were mapped after adjusting over the signal of the antisense RNA as the PEAKS score (−10logP).
RIP mapping of the TET1 binding element
A lncRNA-affinity binding precipitation assay (RIP) [51] was performed to examine the binding of TET proteins with Platr10 lncRNA. Briefly, iPSCs were fixed with 1% formaldehyde, treated with DNase I, and sonicated using a Branson sonicator. Sonicated samples were immunoprecipitated with antibodies against TET1 and TET3 (Abcam, MA). IgG was used as the experimental control. The precipitated RNA was released, and cDNA was synthesized. After proteinase K treatment, the TET-binding cDNAs were detected by PCR.
To map the TET1 binding fragment, a longer sonication was used by extension to 30 min. The samples immunoprecipitated by the IgG antibody were used as the RIP control. The RIP samples were quantitated by overlapping qPCR primers (Table S1). The Ct values were normalized over the input and compared with the IgG control. It was assumed that the TET1 binding site would be protected from sonication and would have a greater chance of being amplified by qPCR mapping.
Characterization of the TET1 binding element
An RNase A protection assay [52] was used to identify the specific Platr10 lncRNA sequence that interacts with TET1. The full length Platr10 lncRNA was synthesized using HiScribeTM T7 Quick High Yield RNA Synthesis Kit (NEB, MA) with biotin-CTP. The biotin-Platr10 lncRNA was purified using Streptavidin Magnetic Beads (Pierce™ Magnetic RNA-Protein Pull-Down Kit, Thermo, USA) following the protocol provided by the manufacturer. For TET1 binding mapping, biotin-Platr10 lncRNA (5 ng/μl) was incubated with TET1 recombinant protein (150 nM) in 150 μl Buffer (20 mM HEPES pH 7.5, 50 mM NaCl, 2 mM MgCl2, and 2 mM DTT) at 22°C for 10 min. After binding, 2 ng/μl RNase A was added to the reaction mixture and was incubated at 22°C for 5 min to remove the RNAs that were unbound or free from the TET1 binding. After RNase treatment, 500 μl TRIzol (Thermo, MA) was added to quench the digestion, and RNAs were purified following the manufacturer’s protocol. An RNA library was prepared using NEBNext Multiplex Small RNA Library Prep Set for Illumina (E7300, NEB, MA). RNA library products were purified by gel extraction and were cloned into a pJet vector for DNA sequencing. The sequencing reads were aligned with the Platr10 lncRNA sequence to locate the consensus TET1 binding motif. The RNA motif structure of TBE was obtained by submitting the 58 bp consensus sequence to the RNA structure prediction website: https://rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/ResultsPages/20180916.191929-72078a8d/Results.html.
Quantitation of intrachromosomal looping by chromosome conformation capture (3C)
The 3C assay was performed to determine long range intrachromosomal interactions [53]. Briefly, fibroblasts and iPSCs were cross-linked with 2% formaldehyde and lysed with cell lysis buffer. An aliquot of nuclei (2×106) was digested with 800 U BamH I/Bgl II at 37°C overnight. Chromatin DNA was diluted with NEB ligation buffer and ligated with 4000 U of T4 DNA ligase. After reversing the cross-links, DNA was purified and used for PCR amplification using primers that are derived from different regions of the Oct4 locus. The 3C PCR products were cloned and sequenced to validate the intrachromosomal interaction by checking for the presence of the BamH I/Bgl II ligation site. Each intrachromosomal loop had its own negative control site. The 3C interaction was quantitated by qPCR and was standardized over the 3C ligation control for the housekeeping gene Ercc3. For comparison, the relative 3C interaction was calculated by setting the 5′ or 3′ control as 1.
DNA methylation analysis in the gene promoter
Genomic DNAs were extracted from fibroblasts that express the full length Platr10 and its mutants and were treated with sodium bisulfate. Methylation PCR was performed, and PCR DNAs were cloned into pJet vector for sequencing. After treatment with sodium bisulfate, unmethylated cytosines were converted to uracils, which can be distinguished from the methylated cytosines by sequencing.
Mutation of Platr10 lncRNA
To define the function of Platr10 lncRNA, we constructed a series of lentiviral vectors that express the full length Platr10 and its mutants. The full-length Platr10 was amplified from iPSC cDNAs using primers JH4399 5′-CGCGTCGATATCCTCGAGGGAGCCTACACGTGGTCACCTG-3′ and JH6209 5′-GAATCGAAGAATTCGCATGGCAGCATGAAGGCAGACAT-3′. The OBE50D vector was synthesized by overlapping PCR using primers JH4399 and JH6212 5′-GGAAGAATCACAAGTCTGTGTTTCCTTCTCCGGTATGAAT-3′ for PCR fragment 1, and JH6213 5′-AGGAAACACAGACTTGTGATTCTTCCCATCTGCAGA-3 and JH6209 for PCR fragment 2. To construct TBE58D vector, the Platr10 insert was amplified by primers JH4399 and JH6214 5′-GAATCGAAGAATTCGTCGACAGGAAAACATTTTATTTGGGCTGGC-3′. The TBE58M vector was constructed by a two-step PCR using primers JH4399 with primer JH6210 5′-CAGTATCTGATCTACTATGTAGCATAAGATCATCAGTCCAGCATGGCAGCATGAAGGCAGACAT-3′ and JH6211 5′-GAATCGAAGAATTCCAGAGGATCATCCCACTTCAGTATCTGATCTACTATGTAGCAT-3. All PCR inserts were cloned into a lenti-DsRed vector by EcoRV/EcoR1.
The lentiviruses were packaged in 293T and were used to transfect fibroblasts. After transfection, cells were treated with puromycin, and stable cells were used for the measurement of gene expression of Oct4, Sox2, and Nanog.
The Oct4 promoter-luciferase assay
The function of Platr10 in activating the Oct4 promoter was first examined in 293T cells by using a dual-luciferase reporter assay. A 3.9 kb genomic DNA fragment covering the Oct4 promoter and part of the exon 1 sequence was amplified by PCR using primers: SJ559 5′-TATCGATAGGTACCGTCTGTGAGGAGGTGGCTGAACT-3′ and SJ560 5′-atcgcagatCTCGAGCTCCTCGGGAGTTGGTTCCAC-3′. The DNA fragment was cloned into pGL3 vector by Kpn1/Xho1.
For the luciferase assay, cells were seeded at a density of 5 × 104 cells/well in 96-well plates. The lentiviral expression vectors, including Platr10 full length lncRNAs (F947), OBE50D, TBE58D, and TBE58M were co-transfected with a pOct4-luciferase plasmid and Renilla luciferase control plasmid (Promega) using Lipofectamine 3000 (Invitrogen, CA). The empty lentiviral vector was used as the control. Forty-eight hours after transfection, firefly and Renilla luciferase activities were measured with the dual-luciferase reporter system (Promega) using a luminometer (Turner Biosytem, CA). The relative activity of the Oct4 promoter was calculated by standardized setting the untreated control cells as 1. All luciferase assays were repeated three times.
Statistical analysis
The data were expressed as mean ± SD and were analyzed using SPSS software (version 16.0; SPSS, Inc., IL). Student’s t test or one-way ANOVA (Bonferroni test) was used to compare statistical differences for variables among treatment groups. Results were considered statistically significant at p<0.05.
Supplementary Information
Additional file 1:. Supplementary files include the Methods and additional Extended Data.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0106902), National Natural Science Foundation of China (31430021, 81874052, 81672275, 31871297, 81670143, 81900701, 81900327), the Key Project of Chinese Ministry of Education grant (311015), the National Basic Research Program of China (973 Program)(2015CB943303), Nation Key Research and Development Program of China grant (2016YFC13038000), Research on Chronic Noncommunicable Diseases Prevention and Control of National Ministry of Science and Technology (2016YFC1303804), National Health Development Planning Commission Major Disease Prevention and Control of Science and Technology Plan of Action, Cancer Prevention and Control (ZX-07-C2016004), Natural Science Foundation of Jilin Province (20150101176JC, 20180101117JC, 20130413010GH), Provincial Science Fund of Jilin Province Development and Reform Commission (2014N147 and 2017C022), and California Institute of Regenerative Medicine (CIRM) grant (RT2-01942); and the Biomedical Research Service of the U.S. Department of Veterans Affairs (BX002905).
Review history
The review history is available as Additional file 2.
Peer review information
Tim Sands was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team
Availability of data and material
As previously reported [18], the CRIST-seq, RAT-Seq, and RNA-seq data generated in this study have been deposited in NIH GEO databases. The CRIST-seq data are available under accession number of GSE107945 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107945) [54]. The RAT-seq data are available under accession number of GSE101765 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101765) [55]. The RNA-seq data are available under accession number of GSE116605 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116605) [56]. The image data have been deposited in the Mendeley database (https://data.mendeley.com/datasets).
Authors’ contributions
ZD, XW, YW, and JL designed and performed assays for the study. YL conducted Platr10-binding protein MS sequencing. SZ performed the CRIST-seq assay. LZ, HL, CW, JC, HL, DL, NC, IC, YZ, ZY, CF, SL, BJ, ZW, and HZ performed cell reprogramming and cell culture studies. YH, WY, GG, JL, PK, FA, and RC contributed to bioinformatics analyses of RNA-seq, CRIST-seq, and RAT-seq data. JFH, ARH, JC, SZ, BQ, SG, RC, PK, MAE, and FA contributed to the study design and supported the study. JFH wrote the manuscript and ARH edited the manuscript. The authors read and approved the final manuscript.
Declarations
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhonghua Du, Xue Wen, Yichen Wang, and Lin Jia contributed equally to this work.
Contributor Information
Andrew R. Hoffman, Email: arhoffman@stanford.edu
Jiuwei Cui, Email: cuijw@jlu.edu.cn.
Ji-Fan Hu, Email: jifan@stanford.edu, Email: hujifan@jlu.edu.cn.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Liu B, Xu J, Wang J, Wu J, Shi C, Xu Y, Dong J, Wang C, Lai W, Zhu J, Xiong L, Zhu D, Li X, Yang W, Yamauchi T, Sugawara A, Li Z, Sun F, Li X, Li C, He A, du Y, Wang T, Zhao C, Li H, Chi X, Zhang H, Liu Y, Li C, Duo S, Yin M, Shen H, Belmonte JCI, Deng H. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell. 2017;169(2):243–257. doi: 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 4.Wolf DP, Morey R, Kang E, Ma H, Hayama T, Laurent LC, Mitalipov S. Concise review: embryonic stem cells derived by somatic cell nuclear transfer: a horse in the race? Stem Cells. 2017;35(1):26–34. doi: 10.1002/stem.2496. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimi B. Reprogramming barriers and enhancers: strategies to enhance the efficiency and kinetics of induced pluripotency. Cell Regen (Lond) 2015;4:10. doi: 10.1186/s13619-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young RA. Control of the embryonic stem cell state. Cell. 2011;144(6):940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2(2):151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apostolou E, Ferrari F, Walsh RM, Bar-Nur O, Stadtfeld M, Cheloufi S, Stuart HT, Polo JM, Ohsumi TK, Borowsky ML, Kharchenko PV, Park PJ, Hochedlinger K. Genome-wide chromatin interactions of the Nanog locus in pluripotency, differentiation, and reprogramming. Cell Stem Cell. 2013;12(6):699–712. doi: 10.1016/j.stem.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Jiao W, Sun L, Fan J, Chen M, Wang H, Xu X, Shen A, Li T, Niu B, Ge S, Li W, Cui J, Wang G, Sun J, Fan X, Hu X, Mrsny RJ, Hoffman AR, Hu JF. Intrachromosomal looping is required for activation of endogenous pluripotency genes during reprogramming. Cell Stem Cell. 2013;13(1):30–35. doi: 10.1016/j.stem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Sexton T, Cavalli G. The 3D genome shapes up for pluripotency. Cell Stem Cell. 2013;13(1):3–4. doi: 10.1016/j.stem.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Werner MS, Ruthenburg AJ. Nuclear fractionation reveals thousands of chromatin-tethered noncoding RNAs adjacent to active genes. Cell Rep. 2015;12(7):1089–1098. doi: 10.1016/j.celrep.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chedin F. Nascent connections: R-loops and chromatin patterning. Trends Genet. 2016;32(12):828–838. doi: 10.1016/j.tig.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan Y, Xiao X, He Z, Luo Y, Wu C, Li L, Song X. Long noncoding RNA OCC-1 suppresses cell growth through destabilizing HuR protein in colorectal cancer. Nucleic Acids Res. 2018;46(11):5809–5821. doi: 10.1093/nar/gky214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Wang Y, Jia L, Du Z, Wen X, Wang C, Hao Y, Yu D, Zhou L, Chen N, et al. Profiling the long noncoding RNA interaction network in the regulatory elements of target genes by chromatin in situ reverse transcription sequencing. Genome Res. 2019;29(9):1521–1532. doi: 10.1101/gr.244996.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergmann JH, Li J, Eckersley-Maslin MA, Rigo F, Freier SM, Spector DL. Regulation of the ESC transcriptome by nuclear long noncoding RNAs. Genome Res. 2015;25(9):1336–1346. doi: 10.1101/gr.189027.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai Y, Chen X, Yu D, Li T, Cui J, Wang G, Hu JF, Li W. Histone deacetylase inhibitor valproic acid promotes the induction of pluripotency in mouse fibroblasts by suppressing reprogramming-induced senescence stress. Exp Cell Res. 2015;337(1):61–67. doi: 10.1016/j.yexcr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Du Z, Jia L, Wang Y, Wang C, Wen X, Chen J, Zhu Y, Yu D, Zhou L, Chen N, et al. Combined RNA-seq and RAT-seq mapping of long noncoding RNAs in pluripotent reprogramming. Sci Data. 2018;5(1):180255. doi: 10.1038/sdata.2018.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang Q, Li W, Benda C, Huang Z, Ahmed T, Liu P, Guo X, Ibanez DP, Luo Z, Zhang M, et al. NCoR/SMRT co-repressors cooperate with c-MYC to create an epigenetic barrier to somatic cell reprogramming. Nat Cell Biol. 2018;20(4):400–412. doi: 10.1038/s41556-018-0047-x. [DOI] [PubMed] [Google Scholar]
- 20.Bolukbasi MF, Liu P, Luk K, Kwok SF, Gupta A, Amrani N, Sontheimer EJ, Zhu LJ, Wolfe SA. Orthogonal Cas9-Cas9 chimeras provide a versatile platform for genome editing. Nat Commun. 2018;9(1):4856. doi: 10.1038/s41467-018-07310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Li W, Sun Y, Yu D, Wen X, Wang H, Cui J, Wang G, Hoffman AR, Hu JF. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014;42(15):9588–9601. doi: 10.1093/nar/gku549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Ge S, Qian G, Li W, Cui J, Wang G, Hoffman AR, Hu JF. Restoration of IGF2 imprinting by polycomb repressive complex 2 docking factor SUZ12 in colon cancer cells. Exp Cell Res. 2015;338(2):214–221. doi: 10.1016/j.yexcr.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295(5558):1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156(1-2):45–68. doi: 10.1016/j.cell.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466(7310):1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagci H, Fisher AG. DNA demethylation in pluripotency and reprogramming: the role of tet proteins and cell division. Cell Stem Cell. 2013;13(3):265–269. doi: 10.1016/j.stem.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Ji X, Dadon DB, Powell BE, Fan ZP, Borges-Rivera D, Shachar S, Weintraub AS, Hnisz D, Pegoraro G, Lee TI, Misteli T, Jaenisch R, Young RA. 3D chromosome regulatory landscape of human pluripotent cells. Cell Stem Cell. 2016;18(2):262–275. doi: 10.1016/j.stem.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novo CL, Javierre BM, Cairns J, Segonds-Pichon A, Wingett SW, Freire-Pritchett P, Furlan-Magaril M, Schoenfelder S, Fraser P, Rugg-Gunn PJ. Long-range enhancer interactions are prevalent in mouse embryonic stem cells and are reorganized upon pluripotent state transition. Cell Rep. 2018;22(10):2615–2627. doi: 10.1016/j.celrep.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu JF, Hoffman AR. Chromatin looping is needed for iPSC induction. Cell Cycle. 2014;13(1):1–2. doi: 10.4161/cc.27017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon JR, Jung I, Selvaraj S, Shen Y, Antosiewicz-Bourget JE, Lee AY, Ye Z, Kim A, Rajagopal N, Xie W, Diao Y, Liang J, Zhao H, Lobanenkov VV, Ecker JR, Thomson JA, Ren B. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518(7539):331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonev B, Mendelson Cohen N, Szabo Q, Fritsch L, Papadopoulos GL, Lubling Y, Xu X, Lv X, Hugnot JP, Tanay A, Cavalli G. Multiscale 3D genome rewiring during mouse neural development. Cell. 2017;171(3):557–572. doi: 10.1016/j.cell.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerkovic I, Cavalli G. Understanding 3D genome organization by multidisciplinary methods. Nat Rev Mol Cell Biol. 2021;22(8):511–528. doi: 10.1038/s41580-021-00362-w. [DOI] [PubMed] [Google Scholar]
- 33.He S, Zhang H, Liu H, Zhu H. LongTarget: a tool to predict lncRNA DNA-binding motifs and binding sites via Hoogsteen base-pairing analysis. Bioinformatics. 2015;31(2):178–186. doi: 10.1093/bioinformatics/btu643. [DOI] [PubMed] [Google Scholar]
- 34.Lan J, Rajan N, Bizet M, Penning A, Singh NK, Guallar D, Calonne E, Li Greci A, Bonvin E, Deplus R, Hsu PJ, Nachtergaele S, Ma C, Song R, Fuentes-Iglesias A, Hassabi B, Putmans P, Mies F, Menschaert G, Wong JJL, Wang J, Fidalgo M, Yuan B, Fuks F. Functional role of Tet-mediated RNA hydroxymethylcytosine in mouse ES cells and during differentiation. Nat Commun. 2020;11(1):4956. doi: 10.1038/s41467-020-18729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes ZE, Hamilton DJ, Hwang T, Parsonnet NV, Rinn JL, Wuttke DS, Batey RT. The Sox2 transcription factor binds RNA. Nat Commun. 2020;11(1):1805. doi: 10.1038/s41467-020-15571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Hu JF, Wang H, Cui J, Gao S, Hoffman AR, Li W. CRISPR Cas9-guided chromatin immunoprecipitation identifies miR483 as an epigenetic modulator of IGF2 imprinting in tumors. Oncotarget. 2017;8(21):34177–34190. doi: 10.18632/oncotarget.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Guo R, Du Z, Bai L, Li L, Cui J, Li W, Hoffman AR, Hu JF. Epigenetic targeting of granulin in hepatoma cells by synthetic CRISPR dCas9 Epi-suppressors. Mol Ther Nucleic Acids. 2018;11:23–33. doi: 10.1016/j.omtn.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu XQ, Pan XH, Wang W, Chen Q, Pang RQ, Cai XM, Hoffman AR, Hu JF. Transient in vitro epigenetic reprogramming of skin fibroblasts into multipotent cells. Biomaterials. 2009;31:2779–2787. doi: 10.1016/j.biomaterials.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, Hu JF, Qiu X, Ling J, Chen H, Wang S, Hou A, Vu TH, Hoffman AR. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex-2 intrachromosomal loop. Mol Cell Biol. 2008;28(20):6473–6482. doi: 10.1128/MCB.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, Zhong B, Chen M, Yang G, Li Y, Wang H, Wang G, Li W, Cui J, Hoffman AR, Hu J. Epigenetic reprogramming reverses the malignant epigenotype of the MMP/TIMP axis genes in tumor cells. Int J Cancer. 2014;134(7):1583–1594. doi: 10.1002/ijc.28487. [DOI] [PubMed] [Google Scholar]
- 41.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen N, Yan X, Zhao G, Lv Z, Yin H, Zhang S, Song W, Li X, Li L, Du Z, et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018;19(1):218. doi: 10.1186/s13059-018-1594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machanick P, Bailey TL. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27(12):1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia L, Wang Y, Wang C, Du Z, Zhang S, Wen X, et al. Oplr16 serves as a novel chromatin factor to control stem cell fate by modulating pluripotency-specific chromosomal looping and TET2-mediated DNA demethylation. Nucleic Acids Res. 2020;48 in press. [DOI] [PMC free article] [PubMed]
- 45.Cao H, Xu D, Cai Y, Han X, Tang L, Gao F, Qi Y, Cai D, Wang H, Ri M, Antonets D, Vyatkin Y, Chen Y, You X, Wang F, Nicolas E, Kapranov P. Very long intergenic non-coding (vlinc) RNAs directly regulate multiple genes in cis and trans. BMC Biol. 2021;19(1):108. doi: 10.1186/s12915-021-01044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li T, Chen H, Li W, Cui J, Wang G, Hu X, Hoffman AR, Hu J. Promoter histone H3K27 methylation in the control of IGF2 imprinting in human tumor cell lines. Hum Mol Genet. 2014;23(1):117–128. doi: 10.1093/hmg/ddt405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y, Liu S, Li X, Zhou L, Meng Y, Li Y, Li L, Jiao B, Bai L, Yu Y, et al. Aberrant shuttling of long noncoding RNAs during the mitochondria-nuclear crosstalk in hepatocellular carcinoma cells. Am J Cancer Res. 2019;9(5):999–1008. [PMC free article] [PubMed] [Google Scholar]
- 48.Nie Y, Zhou L, Wang H, Chen N, Jia L, Wang C, Wang Y, Chen J, Wen X, Niu C, Li H, Guo R, Zhang S, Cui J, Hoffman AR, Hu JF, Li W. Profiling the epigenetic interplay of lncRNA RUNXOR and oncogenic RUNX1 in breast cancer cells by gene in situ cis-activation. Am J Cancer Res. 2019;9(8):1635–1649. [PMC free article] [PubMed] [Google Scholar]
- 49.Pian L, Wen X, Kang L, Li Z, Nie Y, Du Z, Yu D, Zhou L, Jia L, Chen N, et al. Targeting the IGF1R pathway in breast cancer using antisense lncRNA-mediated promoter cis competition. Mol Ther Nucleic Acids. 2018;12:105–117. doi: 10.1016/j.omtn.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen M, Zhang H, Wu J, Xu L, Xu D, Sun J, He Y, Zhou X, Wang Z, Wu L, Xu S, Wang J, Jiang S, Zhou X, Hoffman AR, Hu X, Hu J, Li T. Promotion of the induction of cell pluripotency through metabolic remodeling by thyroid hormone triiodothyronine-activated PI3K/AKT signal pathway. Biomaterials. 2012;33(22):5514–5523. doi: 10.1016/j.biomaterials.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1123. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmad S, Mu X, Yang F, Greenwald E, Park JW, Jacob E, Zhang CZ, Hur S. Breaching self-tolerance to alu duplex RNA underlies MDA5-mediated inflammation. Cell. 2018;172(4):797–810. doi: 10.1016/j.cell.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Jia L, Wang Y, Du Z, Zhou L, Wen X, Li H, Zhang S, Chen H, Chen N, et al. Genome-wide interaction target profiling reveals a novel Peblr20-eRNA activation pathway to control stem cell pluripotency. Theranostics. 2020;10(1):353–370. doi: 10.7150/thno.39093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S, Du Z, Zhu Y, Chen J, Jia L, Wang Y, Wang C, Hoffman AR, Cui J, Hu JF: Profiling lncRNAs in the regulatory elements of target genes by chromatin in situ reverse transcription trap sequencing. Datasets. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107945. (2019)
- 55.Du Z, Zhu Y, Celik I, J. C, Wang Y, Jia L, Wang C, Li W, Hoffman AR, Cui J, Hu JF: Genome-wide target profiling of pluripotency-associated long noncoding RNAs by RAT-Seq. Datasets. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101765. (2018)
- 56.Du Z, Wen X, Li W, Cui J, Hu JF: Combined RNA-seq and RAT-seq mapping of long noncoding RNAs in pluripotent reprogramming. Datasets. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116605. (2018) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1:. Supplementary files include the Methods and additional Extended Data.
Data Availability Statement
As previously reported [18], the CRIST-seq, RAT-Seq, and RNA-seq data generated in this study have been deposited in NIH GEO databases. The CRIST-seq data are available under accession number of GSE107945 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107945) [54]. The RAT-seq data are available under accession number of GSE101765 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101765) [55]. The RNA-seq data are available under accession number of GSE116605 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116605) [56]. The image data have been deposited in the Mendeley database (https://data.mendeley.com/datasets).