Abstract
Background
Dystonia is a known neurological complication of certain medications; however, the mechanism behind such effects is often undetermined. Similarly, the clinical pharmacogenomic effects associated with various alleles of the cytochrome P450 family of proteins, and their role in acute dystonic reactions, are also presently unknown.
Case presentation
We describe a woman presenting with acute dystonic reactions to ondansetron, prochlorperazine, and metoclopramide followed by persistent focal dystonia. A similar family history was reported in her siblings and her father to prochlorperazine, drugs all metabolized by the cytochrome P450 2D6 (CYP2D6) enzyme. Pharmacogenomic testing indicated the patient was heterozygous for the intermediate metabolizer *41 allele (CYP2D6 2988G>A, NM_000106.6:c.985+39G>A, rs28371725). Her father was homozygous for this CYP2D6 *41 allele, and consequently, her siblings were obligate carriers.
Conclusions
The metabolism of ondansetron, metoclopramide, or prochlorperazine in patients with the *41 CYP2D6 allele has not been studied. In this family, clinical evidence implicates the *41 CYP2D6 allele as causing extrapyramidal adverse pharmacologic reactions. Patients with a family history of medication-induced dystonia involving these medications should be considered for pharmacogenomic testing, and patients carrying the *41 CYP2D6 allele should consider reduction or avoidance of CYP2D6-mediated medications to minimize the potential risk of adverse extrapyramidal effects.
Keywords: CYP2D6, Pharmacogenetic, Ondansetron, Metoclopramide, Prochlorperazine, Dystonia, Neurogenetics
Background
Acute dystonia, a disorder of abnormal muscle tone leading to spasms and/or abnormal posture, can occur after exposure to certain medications, typically dopamine receptor antagonists [1]. Symptoms can appear following the initial dose, can be focal, segmental, or generalized, and often involve the ocular muscles, face, jaw, neck, tongue, and trunk [1]. In severe cases, this can be life threatening. The mechanism behind these effects is not fully understood but, in some cases, may be precipitated by impairments in drug metabolism, such as by the cytochrome P450 family of proteins. However, the full clinical spectrum of pharmacogenomic effects associated with various alleles of the cytochrome P450 proteins, and their contribution to complications such as dystonia, is also presently unknown.
Case presentation
We describe a 39-year-old white non-Hispanic woman of Ashkenazi descent who presented with focal dystonia characterized by bilateral blepharoclonus on eyelid closure, sequelae from a prior right-sided Bell’s palsy, and mildly elevated tone in both arms. She had suffered an acute dystonic reaction immediately following intravenous administration of ondansetron in her early twenties, including retrocollis and oculogyric crisis, as well as a later episode of acute akathisia in response to prochlorperazine, and, most recently, akathisia occurring within 1 hour of 10 mg of metoclopramide being given orally, followed by persistence of the current symptoms. There is a family history of similar reactions on the paternal side. Her father had severe akathisia in response to prochlorperazine, as did her brother. Her sister had also experienced akathisia after an epidural during pregnancy.
This history and presentation were concerning for a pharmacogenomic effect, supported by the observation that these drugs are all metabolized by cytochrome P450 2D6 (CYP2D6), expressed in the liver and central nervous system. This enzyme is known to interact with a large number of clinical drugs and has a known relationship with extrapyramidal syndromes related to antipsychotics [2].
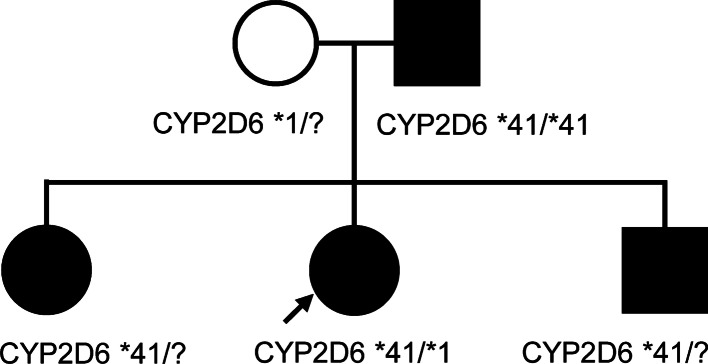
Pharmacogenomic testing was performed for CYP2D6 and indicated the patient was heterozygous for the *41 allele (CYP2D6 2988G>A, NM_000106.6:c.985+39G>A, rs28371725), an intronic polymorphism associated with aberrant splicing of CYP2D6 leading to the skipping of exon 6 with a corresponding reduction in activity [3]. This variant is responsible for the intermediate metabolizer phenotype in the majority of people of European descent [4]. Testing of other family members indicated her father was homozygous for the CYP2D6 *41 allele in the gene, and consequently, her sister and brother were obligate carriers (Fig. 1).
Fig. 1.
Pedigree. Proband (arrow) and patient genotypes are indicated. ? = not determined, listed genotype inferred from known familial relationships
Conclusions
There is limited information on the relationship between the *41 allele and dystonia. In one study of the drug risperidone, intermediate metabolizers with the *41 allele showed increased levels of the drug and its metabolites (nearly twofold versus poor, or CYP2D6 loss-of-function metabolizers, which show threefold) with an increased risk of developing dystonia and parkinsonism adverse effects [5]. There are presently no studies assessing the *41 intermediate metabolizer allele in patients taking ondansetron, metoclopramide, or prochlorperazine; however, patients homozygous for inactive loss-of-function alleles (*4 or *5) in CYP2D6 have been reported with acute dystonic reactions to metoclopramide [6, [7]. In this family, the available clinical evidence points to the *41 allele being the cause of their extrapyramidal adverse pharmacologic reactions despite showing reduced activity rather than being loss-of-function. Future studies should address correlations between genotype, symptom onset, and/or severity with dosage. In this family, we cannot exclude additional genetic or environmental modifiers affecting the overall metabolism of these medications. For example, it has been proposed that elevated estrogen levels during pregnancy can exacerbate dystonic reactions to metoclopramide in poor CYP2D6 metabolizers [7], which may have contributed to the dystonic reaction seen in our patient’s sister.
We recommend that patients with a family history of medication-induced dystonia involving ondansetron, metoclopramide, or prochlorperazine be considered for pharmacogenomic testing, and that patients carrying the *41 CYP2D6 allele should consider reduction or avoidance of CYP2D6-mediated medications to minimize the potential risk of adverse extrapyramidal effects.
Acknowledgements
The authors thank the patient and her family for their participation in this study. This study was approved by the Institutional Review Board of the University of California, Los Angeles, and all subjects provided informed consent for their participation. All genetic testing was performed commercially by CLIA-certified laboratories.
Full financial disclosures of all authors for the past year: BLF reports funding from the National Ataxia Foundation and NIH. DYW has no financial disclosures to make.
Authors’ contributions
DYW: writing of the first draft of the manuscript, review and critique, and literature search. BLF: manuscript review and critique, clinical care, neurological examinations, literature search, and genetic analyses. All authors read and approved the final manuscript.
Funding
The authors have no sources of funding for the study.
Availability of data and materials
Data generated during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the University of California, Los Angeles, and all subjects provided informed consent for their participation
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mehta SH, Morgan JC, Sethi KD. Drug-induced movement disorders. Neurol Clin. 2015;33(1):153–174. doi: 10.1016/j.ncl.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): Clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5(1):6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- 3.Toscano C, Klein K, Blievernicht J, Schaeffeler E, Saussele T, Raimundo S, et al. Impaired expression of CYP2D6 in intermediate metabolizers carrying the *41 allele caused by the intronic SNP 2988G>A: evidence for modulation of splicing events. Pharmacogenet Genomics. 2006;16(10):755–766. doi: 10.1097/01.fpc.0000230112.96086.e0. [DOI] [PubMed] [Google Scholar]
- 4.Raimundo S, Toscano C, Klein K, Fischer J, Griese EU, Eichelbaum M, et al. A novel intronic mutation, 2988G>A, with high predictivity for impaired function of cytochrome P450 2D6 in white subjects. Clin Pharmacol Ther. 2004;76(2):128–138. doi: 10.1016/j.clpt.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli I, Kastelic M, Koprivšek J, Kores-Plesničar B, Mrhar A, Dolžan V, et al. A population pharmacokinetic evaluation of the influence of CYP2D6 genotype on risperidone metabolism in patients with acute episode of schizophrenia. Eur J Pharm Sci. 2010;41(2):289–298. doi: 10.1016/j.ejps.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 6.van der Padt A, van Schaik RHN, Sonneveld P. Acute dystonic reaction to metoclopramide in patients carrying homozygous cytochrome P450 2D6 genetic polymorphisms. Neth J Med. 2006;64(5):160–162. [PubMed] [Google Scholar]
- 7.Wee Chua E, Harger SP, Kennedy MA. Metoclopramide-induced acute dystonic reactions may be associated with the CYP2D6 poor metaboliser status and pregnancy-related hormonal changes. Front Pharmacol. 2019;10(JULY):2–6. doi: 10.3389/fphar.2019.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.