Abstract
Pregnancy is a physiological state of continuous adaptation to changing maternal and fetal nutritional needs, including a reduction of maternal insulin sensitivity allowing for appropriately enhanced glucose availability to the fetus. However, excessive insulin resistance in conjunction with insufficient insulin secretion results in gestational diabetes mellitus (GDM), greatly increasing the risk for pregnancy complications and predisposing both mothers and offspring to future metabolic disease. Here, we report a signaling pathway connecting pregnancy-associated plasma protein A (PAPPA) with adipose tissue expansion in pregnancy. Adipose tissue plays a central role in the regulation of insulin sensitivity, and we show that, in both mice and humans, pregnancy caused remodeling of adipose tissue evidenced by altered adipocyte size, vascularization, and in vitro expansion capacity. PAPPA is known to be a metalloprotease secreted by human placenta that modulates insulin-like growth factor (IGF) bioavailability through prolteolysis of IGF binding proteins (IGFBPs) 2, 4, and 5. We demonstrate that recombinant PAPPA can stimulate ex vivo human adipose tissue expansion in an IGFBP-5– and IGF-1–dependent manner. Moreover, mice lacking PAPPA displayed impaired adipose tissue remodeling, pregnancy-induced insulin resistance, and hepatic steatosis, recapitulating multiple aspects of human GDM. In a cohort of 6361 pregnant women, concentrations of circulating PAPPA are inversely correlated with glycemia and odds of developing GDM. These data identify PAPPA and the IGF signaling pathway as necessary for the regulation of maternal adipose tissue physiology and systemic glucose homeostasis, with consequences for long-term metabolic risk and potential for therapeutic use.
INTRODUCTION
Gestational diabetes mellitus (GDM) is the most common complication in pregnancy, affecting about 5 to 9% of U.S. pregnancies and 2 to 25% of pregnancies worldwide (1, 2). The large variation in reported incidence is likely due to the broad diagnostic criteria that define GDM as any degree of hyperglycemia first recognized in pregnancy. This can include individuals who had impaired glucose tolerance or overt type 2 diabetes (T2DM) before pregnancy, and given the increasing incidence of obesity and consequent metabolic abnormalities, the size of this population has increased substantially. Nevertheless, glucose intolerance first develops in many individuals during pregnancy and resolves upon delivery, identifying GDM as a metabolic disease state dependent on pregnancy. As many as 50% of women diagnosed with GDM develop T2DM within 5 years (3–5). The strong association between pregnancy and subsequent T2DM may be attributable to unmasking of underlying susceptibility to T2DM by pregnancy or to a direct effect of pregnancy to enhance susceptibility to the disease. Moreover, exposure to the hyperglycemic in utero environment of women with GDM results in increased risk of obesity, metabolic syndrome, and other cardiometabolic disorders in the offspring (6–10). Therefore, GDM is a determining factor in transgenerational metabolic risk susceptibility, and understanding its etiology is crucial for developing appropriate preventive and therapeutic strategies.
The precise mechanisms underlying the development of GDM are not clear. Pregnancy is a state of rapid metabolic adaptation required to meet the nutritional needs of both mother and fetus during pregnancy and preparation of maternal tissues for the lactation period. An increase in total adipose mass is characteristic of normal pregnancy (11–14), with about 30% of recommended weight gained composed of fat mass. In the nonpregnant state, a direct mechanistic link between adipose tissue function and systemic glucose homeostasis is supported by numerous lines of evidence. These include a strong epidemiological correlation between subcutaneous adipose tissue size and mitigation of metabolic and cardiometabolic risk (15–20), extreme insulin resistance and hyperglycemia in congenital and acquired lipodystrophies (21–23), mitigation of insulin resistance in mice by genetic expansion of subcutaneous adipose tissue (24–27), and direct effects of adipose tissue on glycemic control (28–31). All these lines of evidence converge on the notion that an impaired capacity of adipose tissue to retain lipids leads to systemic lipotoxicity and development of insulin resistance.
Given the key role of adipose tissue in the pathogenesis of insulin resistance and T2DM, a similar role in the pathogenesis of GDM is possible. There is increasing evidence that excessive visceral adipose tissue expansion (32), larger adipocyte size (33, 34), and increased adipose tissue inflammation (35) are associated with insulin resistance in human pregnancy and contribute to GDM risk. Abnormalities in the mechanisms that promote normal expansion of adipose tissue depots during pregnancy may be responsible for development of excessive insulin resistance, but these mechanisms have yet to be fully defined (35).
Insulin-like growth factor-1 (IGF-1) and 2 play a central role in tissue growth through their interactions with the insulin and IGF-1 receptors (36). IGFs circulate at high concentrations relative to other peptide growth factors and are also produced locally to mediate paracrine effects (37–41). An important mechanism for regulation of the activities of IGF-1 and IGF-2 is their tight interaction with one of six different binding proteins, IGFBPs 1 to 6 (42). IGFBPs bind IGFs and prevent their clearance, thereby increasing their local concentration. However, IGFBPs also impair productive binding of IGFs to their receptors. In order for IGF signaling to occur, IGFBPs must be inactivated by proteolytic degradation (43).
The pregnancy-associated plasma protein A (PAPPA) was the first protease identified to cleave IGFBP4 and IGFBP5 to increase IGF bioavailability (44, 45). This protease derives its name from its discovery in the serum of pregnant women, as both the total concentration and proteolytic activity of PAPPA progressively increase by over 1000-fold during pregnancy. In nonpregnant human females, a substantial amount of circulating PAPPA is derived from the uterus (46), but the marked elevation seen during pregnancy is due to its production by the placenta (47). There is an established correlation between low circulating PAPPA concentration and pregnancy abnormalities, and a single low value for PAPPA is highly correlated with fetal aneuploidy (48, 49), leading to its adoption for screening chromosomal abnormalities in early pregnancy (50). PAPPA is conserved in mice, where its expression is highest in subcutaneous adipose tissue (51).
The IGF and IGFBP signaling axis has been implicated in regulating adipose tissue growth (52–55), and expression of subcutaneous and omental IGFBP4 and IGFBP5 is increased in human pregnancy (34, 35). The induction of IGFBPs in adipose tissue coupled with the progressive increase in placenta production of PAPPA during human pregnancy suggested the possibility that a placenta-adipose tissue signaling axis could play a role in the adaptation of adipose tissue to pregnancy, with consequent modulation of metabolic homeostasis. Here, we leveraged in vitro model systems to quantify human adipose tissue expandability and used mouse knockout (KO) models and retrospective population data to further investigate the hypothesis that cleavage of adipose tissue IGFBPs by PAPPA induces normal adipose tissue expansion in pregnancy, and that abnormalities in this mechanism may contribute to the development of GDM.
RESULTS
Adaptations of adipose tissue during pregnancy in humans
To characterize the changes that occur in adipose tissue during pregnancy, we compared total gene expression of subcutaneous (SQ) and omental (OM) adipose tissues obtained during Cesarean section from normoglycemic pregnant subjects with those of nondiabetic, nonpregnant subjects obtained during gastric bypass surgery as described previously (34, 56). We reanalyzed this dataset (data file S1) using hierarchical clustering of the 1200 genes displaying the highest median absolute deviation among the four groups. This analysis resulted in four distinct clusters, segregated by both depot and pregnancy state (Fig. 1A). Among the genes, defining these clusters was a set that was highly enriched during pregnancy in both subcutaneous and omental depots (Fig. 1A). To identify the specific genes and pathways modified by pregnancy in both depots, we performed differential expression analysis (Fig. 1, B and C, and data file S2). IGFBP5 was highly up-regulated by pregnancy, increasing by more than 60-fold and 20-fold in subcutaneous and omental adipose depots, respectively (Fig. 1, B and C). Pathway enrichment analysis identified a central regulatory arm of the IGF signaling axis, the growth hormone receptor signaling pathway, as regulated by pregnancy (Fig. 1D and data file S3).
Fig. 1. Adaptations of human adipose tissue to pregnancy.
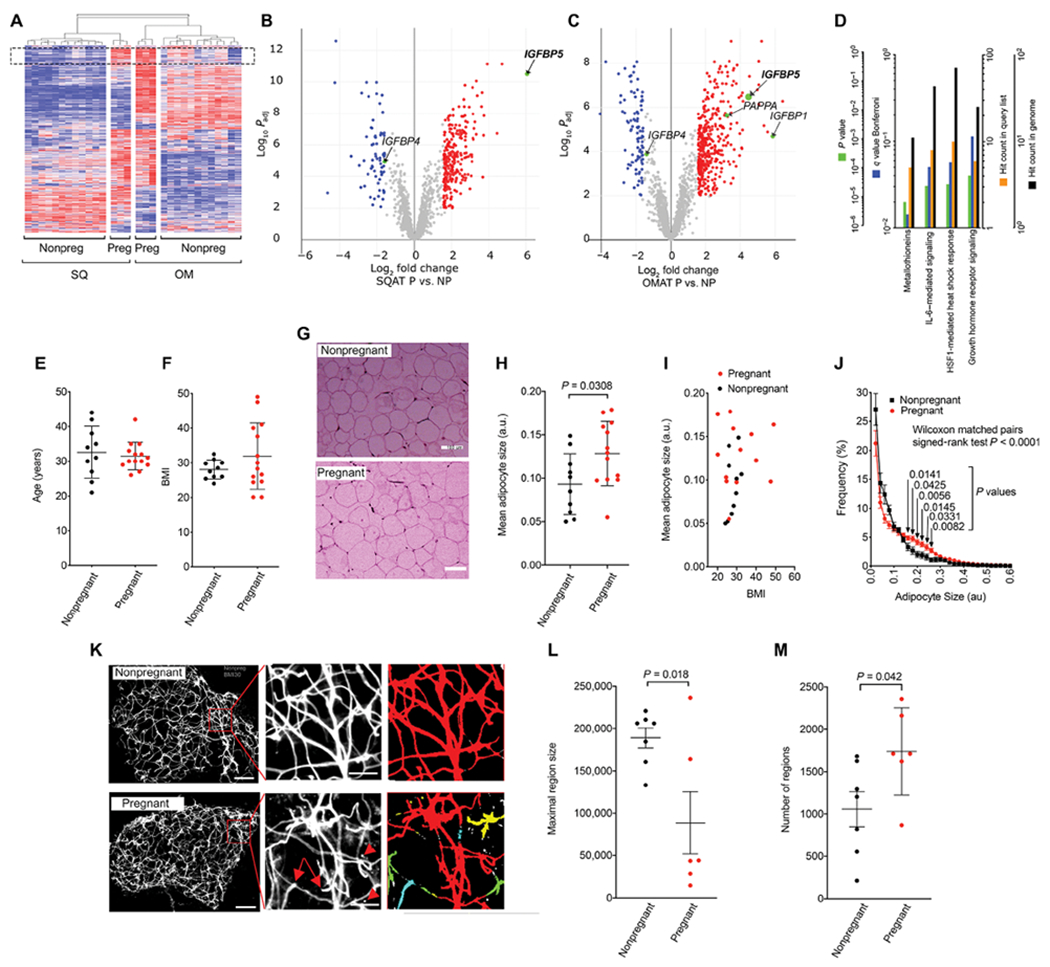
(A) Hierarchical clustering of genes expressed in subcutaneous (SQ) or omental (OM) adipose tissue of nonpregnant (NP) or pregnant (P) women. (B and C) Volcano plots of gene expression modulated by pregnancy in both depots. IGFBP-encoding genes detected are highlighted. (D) KEGG enrichment analysis of genes modulated by pregnancy in both depots. (E and F) Scatter plots of age and BMI of cohort of nonpregnant and pregnant women from whom samples were obtained for histological analysis. (G) Representative hematoxylin and eosin (H&E) stains of subcutaneous adipose tissue from a nonpregnant (above) and a pregnant (below) subject with equivalent BMIs of 26. Scale bars, 100 μm. (H and I) Mean adipocyte size in nonpregnant and pregnant women cohorts, and adipocyte size as a function of BMI in both cohorts. a.u., arbitrary units. (J) Frequency size distributions from H&E stains of adipose tissue. Adipocyte was measured in 5 to 10 slides from each subject, and the mean and SEM of each subject depicted in the plots. Difference of histograms, Wilcoxon matched pairs test; P values for differences at each size range, multiplicity-adjusted (Sidak) Studen’s t tests. (K) Whole mount isolectin staining of adipose tissue from a nonpregnant (above) and a pregnant (below) subject with similar BMIs (BMI = 30). Scale bars, 200 μm. Arrows in magnified areas in middle panels indicate vessel discontinuity, and arrowheads isolectinpositive cells separated from vessel structures, only seen in images from pregnant subjects. Scale bars, 50 μm. Left panels are pseudocolored images where each color comprises a continuous region defined using ImageJ connected regions algorithm. (L and M) Maximal region size and number of regions are measured in 5 to 10 whole mount images from each subject, and the mean and SEM of each subject are depicted in the plots. Statistical significance of the difference between pregnant and nonpregnant was calculated using Studen’s t tests.
To examine whether pregnancy induces structural alterations in adipose tissue, we obtained a separate cohort of samples by needle biopsy of subcutaneous adipose tissue from weight-matched nonpregnant women (Table 1). The mean age and body mass index (BMI) were not different between the cohorts (Fig. 1, E and F), but mean adipocyte size was altered by pregnancy (Fig. 1, G to I). The major change was an increase in mean size (Fig. 1H), which was not correlated with BMI in either nonpregnant or pregnant cohorts (Fig. 1I). The increase in mean adipocyte size was attributable to adipocyte hypertrophy as evidenced by an increased number of larger adipocytes seen in frequency distribution or adipocyte sizes (Fig. 1J). The number of large adipocytes increased without a concomitant decreases in the number of small adipocytes, which would be expected if the effect was due only to hyperplasia of existing cells. The maintenance of the pool of small adipocytes is consistent with compensatory hyperplasia during pregnancy.
Table 1.
Characteristics of subject population for adipose tissue analysis.
| Group | P value | ||||
|---|---|---|---|---|---|
| Nonpregnant (N = 10) | Pregnant (N = 13) | ||||
| N | % | N | % | ||
| Age (mean, SD) | 31.7 | 7.1 | 31.2 | 4.1 | 0.978 |
| Race | 0.480 | ||||
| White | 11 | 91.7% | 10 | 76.9% | |
| Asian | 0 | 0.0% | 1 | 7.7% | |
| Black or African-American | 0 | 0.0% | 2 | 15.4% | |
| Other race | 1 | 8.3% | 0 | 0.0% | |
| Hispanic, Latino, or Spanish origin | 0.071 | ||||
| No, not of Hispanic, Latino, or Spanish origin | 9 | 75.0% | 11 | 84.6% | |
| Yes, Puerto Rican | 0 | 0.0% | 2 | 15.4% | |
| Yes, another Hispanic, Latino, or Spanish origin | 3 | 25.0% | 0 | 0.0% | |
| Currently live with a smoker | 0.999 | ||||
| No | 10 | 83.3% | 10 | 76.9% | |
| Yes | 2 | 16.7% | 3 | 23.1% | |
| Ever smoked | 0.999 | ||||
| No | 8 | 66.7% | 8 | 61.5% | |
| Yes | 4 | 33.3% | 5 | 38.5% | |
| Current smoker | 0.999 | ||||
| No | 11 | 91.7% | 12 | 92.3% | |
| Yes | 1 | 8.3% | 1 | 7.7% | |
Adipose tissue is densely vascularized, and its expansion is preceded by neoangiogenesis (57–59). Activation of neoangiogenesis is accompanied by changes in vessel integrity due to tight junction instability (60–63). To determine whether pregnancy induces changes in the microvasculature consistent with neoangiogenesis, we stained 1-mm3 fragments of adipose tissue obtained from nonpregnant or pregnant women with isolectin, which marks microvessels within the tissue. We observed qualitative changes in the microvasculature consisting of decreased homogeneity of vessel diameter and discontinuity and tortuosity of vessel structure in biopsies from pregnant women (Fig. 1K). To quantify these observations, we applied an imaging algorithm to measure connectivity between regions. The microvasculature from pregnant women displayed a decrease in region size per image and an increased in total regions per image (Fig. 1, K to M), indicating vessel fragmentation. These results indicate that human adipose tissue adapts to pregnancy with a robust increase in expression of IGFBP5, together with hypertrophy, hyperplasia, and changes in microvasculature consistent with enhanced angiogenesis.
Human adipose tissue expandability is increased in pregnancy and stimulated by PAPPA
To directly explore whether PAPPA cleavage of IGFBPs could mediate the observed pregnancy-induced adaptations of adipose tissue, we used an in vitro system that measures adipose tissue expandability. When small fragments of human adipose tissue are embedded in Matrigel and cultured in microvascular endothelial cell growth medium-2 (EGM2-MV) medium, endothelial and mesenchymal progenitor cells emerge and proliferate, gradually covering a larger area around the explant. The area covered by the sprouting cells is a surrogate measure of adipose tissue expandability, varying as a function of depot of origin and physiological state of the donor (56). We found that explants from subcutaneous adipose tissue from pregnant women displayed greater expandability compared to those from nonpregnant women (Fig. 2, A and B), consistent with our previous findings of increased hyperplasia. To explore whether PAPPA can directly influence adipose tissue expandability, we cultured subcutaneous adipose tissue explants from pregnant women in the presence of recombinant human PAPPA (Fig. 2C). We found a dose-dependent stimulatory effect of recombinant PAPPA to increase expandability over time (Fig. 2D). This effect was seen at a concentrations within the range of that seen in normal pregnancies [1413 ng/ml at 13 weeks; (64)]. To further test the hypothesis that the effects of PAPPA are mediated through the IGFBP/IGF signaling pathway, we examined the effects of a specific inhibitor of the IGF-1 receptor tyrosine kinase activity, 7-[cis-3-(1-azetidinylmethyl) cydobutyl]-5-[3-(phenylmethoxy) phenyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine, dihydrochloride (NVP-AEW541) (65, 66), and found a profound inhibition of sprouting (Fig. 2E), consistent with a role for IGF-1 signaling in white adipose tissue development (67). To determine whether the effects of PAPPA to increase expandability were mediated through its interaction with IGFBP5, we examined the effects of recombinant IGFBP5 and PAPPA. We observed a dose-dependent inhibition of sprouting by recombinant IGFBP5, which was reversed by the presence of PAPPA (Fig. 2F). Together, these results are consistent with a direct effect of IGF-1 receptor signaling, and its modulation by IGFBP5 and PAPPA, to regulate adipose tissue expandability.
Fig. 2. PAPPA stimulates human adipose tissue expandability in vitro.
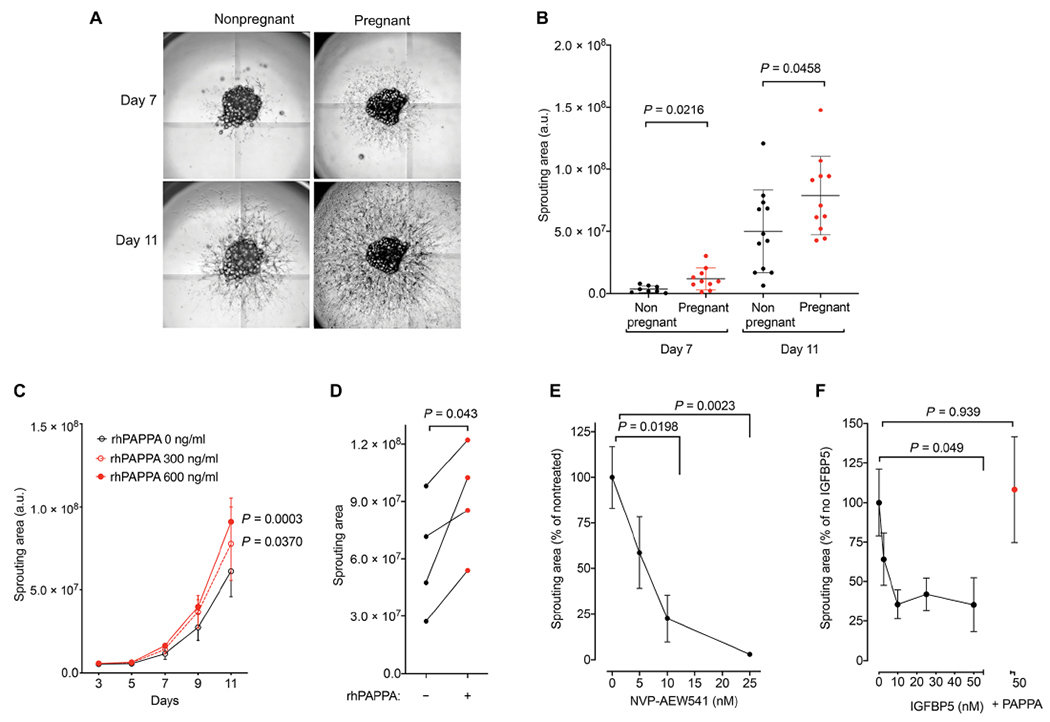
(A) Representative images of sprouts emerging from adipose tissue explants embedded in 96-mm wells obtained from normoglycemic nonpregnant or pregnant women. Images were taken at 7 (top) and 11 (bottom) days of culture. (B) Quantification of sprouting area from AT explants at indicated time points. Between 10 and 30 explants were embedded for each subject, and each symbol represents the mean sprouting area of all explants per subject. Means and SEM of nonpregnant (n = 12) and pregnant (n = 11) subjects are plotted. Unpaired, two-tailed Studen’s t tests. (C) Mean and SEM of the sprouting area of 5 to 10 explants from four separate subjects (n = 4 for each time point) treated in the absence or presence of the indicated concentration of recombinant human PAPPA (rhPAPPA) for the times shown. Statistical significance of differences between doses at each time point was calculated using repeated measures two-way ANOVA with Dunnet’s correction for multiple comparisons. (D) Explants from four pregnant women (n = 5 to 10 explants per subject) were cultured in the absence or presence of recombinant human PAPPA [rhPAPPA (1200 ng/ml)]. Two-tailed paired Studen’s t tests. (E) Mean and SEM of the sprouting area of n = 10 explants cultured in the presence of the indicated concentration of the IGF-1 receptor inhibitor NVP-AEW541. Statistical comparisons between no inhibitor and each dose were made using one-way ANOVA with Dunnet’s correction for multiple comparisons. (F) Mean and SEM of the sprouting area of n = 10 explants cultured in the presence of the indicated concentration of recombinant human IGFBP5 in the absence or presence of rhPAPPA. Statistical comparisons between no inhibitor and each dose were made using one-way ANOVA with the Dunnet’s correction for multiple comparisons.
PAPPA ablation in mice prevents pregnancy-associated adipose tissue remodeling
We studied mice in which the Pappa gene was ablated (KO) to directly test the role of PAPPA in remodeling adipose tissue during pregnancy in vivo. Pappa KO mice have been described as proportional dwarfs, being viable and fertile but exhibiting only 60% of wild-type (WT) size (68). Pregnancy induced a similar proportional increase in body weight in WT littermate controls and KO mice (Fig. 3A), which was attributable to an increase in lean body mass with no change in fat mass (Fig. 3, B and C), consistent with studies of pregnancy in C57BL6 mice (69). As a percent of total (lean + fat mass), lean and fat mass were affected by pregnancy to a similar extent between WT and KO mice (Fig. 3, D and E).
Fig. 3. Adaptations of adipose tissue to pregnancy in mice require Pappa.
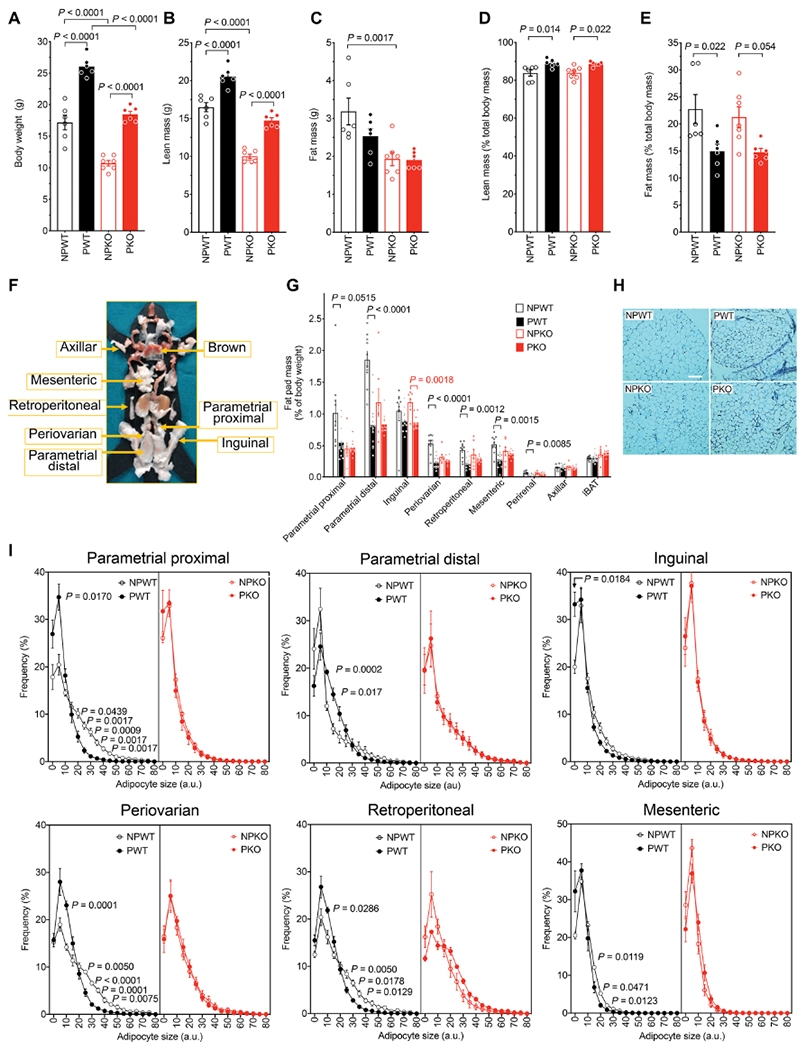
(A) Body weight, total lean mass (B), and total fat mass (C) from nonpregnant or pregnant wild-type (NPWT, n = 6; PWT, n = 6) or nonpregnant or pregnant PappA KO mice (NPKO, n = 7; PKO, n = 6). Lean mass percent (D) and fat mass percent (E) were calculated by normalizing to lean + fat mass. For (A) to (E), statistical significance was assessed using ordinary one-way ANOVA corrected with the Sidak test for multiple comparisons. (F) Representative figure of the anatomical localization for the fat pads analyzed [adapted from (70)]. (G) Mass of individual fat depots from NPWT (n=10), PWT (n = 13), NPKO (n = 7), and PKO (n = 11), expressed as % body weight. Statistical significance of differences between nonpregnant and pregnant state within each depot was measured using multiplicity (Holm-Sidak) adjusted Studen’s t tests. (H) Representative image of H&E staining displaying the proximal parametrial fat pads. Scale bar, 100 μm. (I) Frequency distribution of adipocyte sizes measured in H&E-stained sections (~10 images per depot per mouse) from n = 6 mice per group. Statistical significance of differences in frequency at each bin between nonpregnant and pregnant states measured using multiplicity (Holm-Sidak) adjusted Studen’s t tests.
To examine pregnancy-induced adipose tissue adaptations and the role of PAPPA, we dissected and analyzed all discernable depots in WT and KO pregnant and nonpregnant mice, following published guidelines (Fig. 3, F and G) (70). In WT mice, pregnancy caused a decrease in the mass of all depots with the exception of the axillary depot and interscapular brown adipose tissue, consistent with measurements of decreased total fat mass (Fig. 3E). In contrast, in KO mice, pregnancy did not alter the mass of adipose depots, with the exception of the inguinal depot (Fig. 3G). To examine detailed changes in adipose depots in response to pregnancy, we quantified the size distribution of adipocytes in tissue sections from each fat pad (Fig. 3, H and I). In WT mice, pregnancy was accompanied by a decrease in adipocyte size and an accumulation of small-sized adipocytes in the parametrial, inguinal, periovarian, retroperitoneal, and mesenteric depots, consistent with enhanced lipid mobilization and adipocyte hyperplasia. These changes in adipocyte size distribution did not occur in fat depots from KO mice. These results demonstrate that pregnancy-induced changes in major adipose depots are dependent on PAPPA. To determine whether the pregnancy-induced alterations seen in adipose tissue occur only in the late stages of pregnancy or are established during the entire pregnancy period, we compared days 8 and 16 of pregnancy. Pregnancy-induced changes in adipocyte size were similar at both time points (fig. S1), supporting a role of PAPPA in adipose tissue development throughout pregnancy.
To explore the mechanisms by which PAPPA might mediate adipose tissue remodeling, we measured the expression of genes in the IGF signaling pathway in depots from pregnant and nonpregnant WT and Pappa KO mouse depots. Depot, genotype, and pregnancy-dependent changes were analyzed by three-way analysis of variance (ANOVA). All genes analyzed varied by depot (Fig. 4), and Igf-2 (Fig. 4B), Igfbp-2 (Fig. 4C), and Igfbp-4 (Fig. 4D) were affected by pregnancy. Igfbp-2 was the gene most strongly up-regulated by pregnancy, mostly in the inguinal and axillary depots, but this up-regulation did not occur in mice lacking Pappa. In contrast, Igf-2 was up-regulated by pregnancy in both inguinal and axillary depots of Pappa KO mice to a greater extent than in WT mice.
Fig. 4. Gene expression reveals compensation for Pappa deficiency in specific depots.
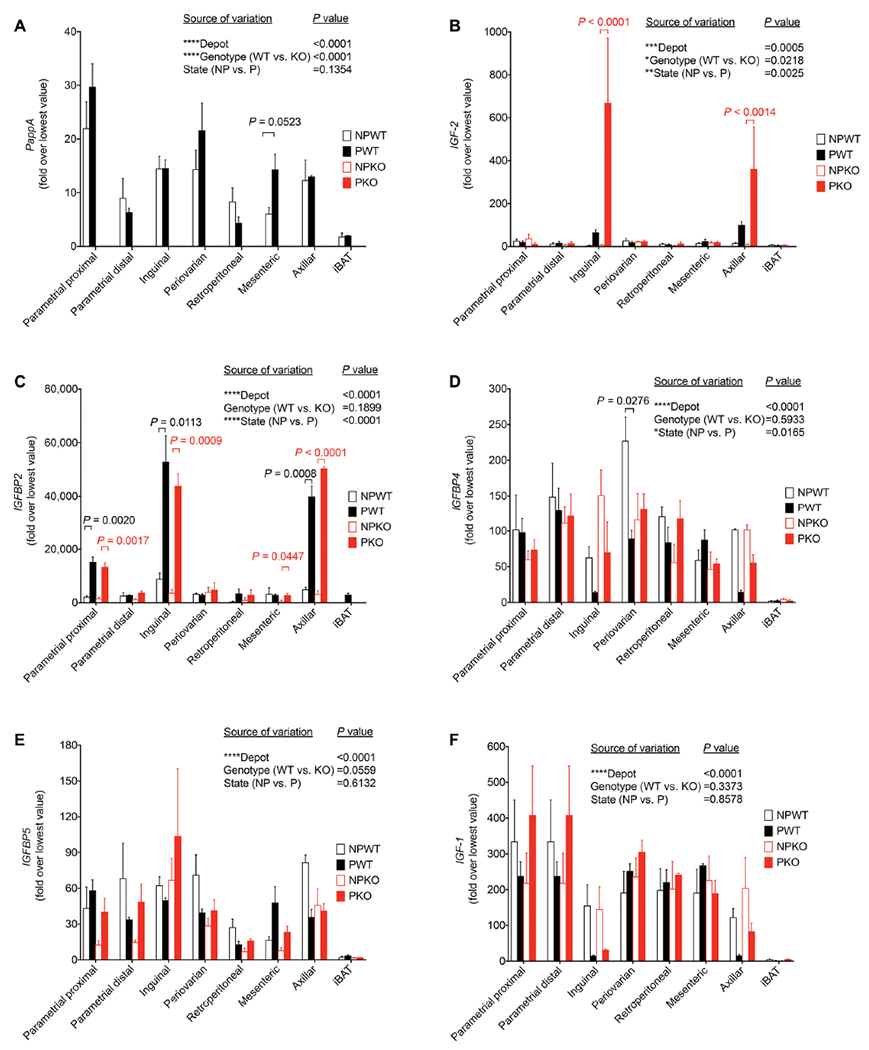
Real-time quantitative polymerase chain reaction for PappA (A), Igf-2 (B), Igfbp-2 (C), Igfbp-4 (D), Igfbp-5 (E), and Igf-1 (F) in fat depots from nonpregnant or pregnant wild-type (NPWT n = 3, PWT n = 3) or nonpregnant or pregnant Pappa KO mice (NPKO n = 3, PKO n = 3). Fold expression values were calculated by normalization to the lowest expression value in the dataset for each gene. For each gene, minimum and maximum Ct values were 26 and 29 for Pappa, 22 and 35 for Igf-2, 22 and 37 for Igfbp-2, 20 and 27 for Igfbp-4 20 and 28 for Igfbp-5, and 18 and 26 for Igf-1. Graphs show mean and SEM of n = 3 mice per group, where the value for each mouse was the mean of three technical replicates. Statistical significance of the differences between fat depot, genotype, and pregnant state for each gene was calculated using three-way ANOVA corrected for multiple comparisons using the Tukey’s test. In (A) to (C), statistical significance of differences in gene expression between states within individual depots was measured using multiplicity-adjusted t tests.
PAPPA ablation causes gestational insulin resistance
We next studied the metabolic consequences of the abnormalities in adipose tissue remodeling induced by PAPPA loss. In nonpregnant mice, fasting blood glucose was higher in Pappa KO compared to WT mice (Fig. 5A). Pregnancy decreased fasting blood glucose in both genotypes (Fig. 5A) but had no effect on fasting insulin values (Fig. 5B). To directly measure insulin sensitivity, we conducted insulin tolerance tests. Insulin sensitivity was decreased in KO compared to WT mice (Fig. 5C), and this effect was more pronounced in pregnant mice (Fig. 5D). To facilitate comparison between conditions, we analyzed glucose excursions as a percent of basal fasting blood glucose and found that pregnancy-induced insulin resistance was greater in KO mice compared to WT (Fig. 5E). Pregnancy also enhanced insulin secretion, but this was not further enhanced in KO mice (Fig. 5F), indicating that loss of PAPPA aggravates pregnancy-induced insulin resistance and may also affect β cell insulin secretion.
Fig. 5. Pappa deficiency results in insulin resistance during pregnancy.
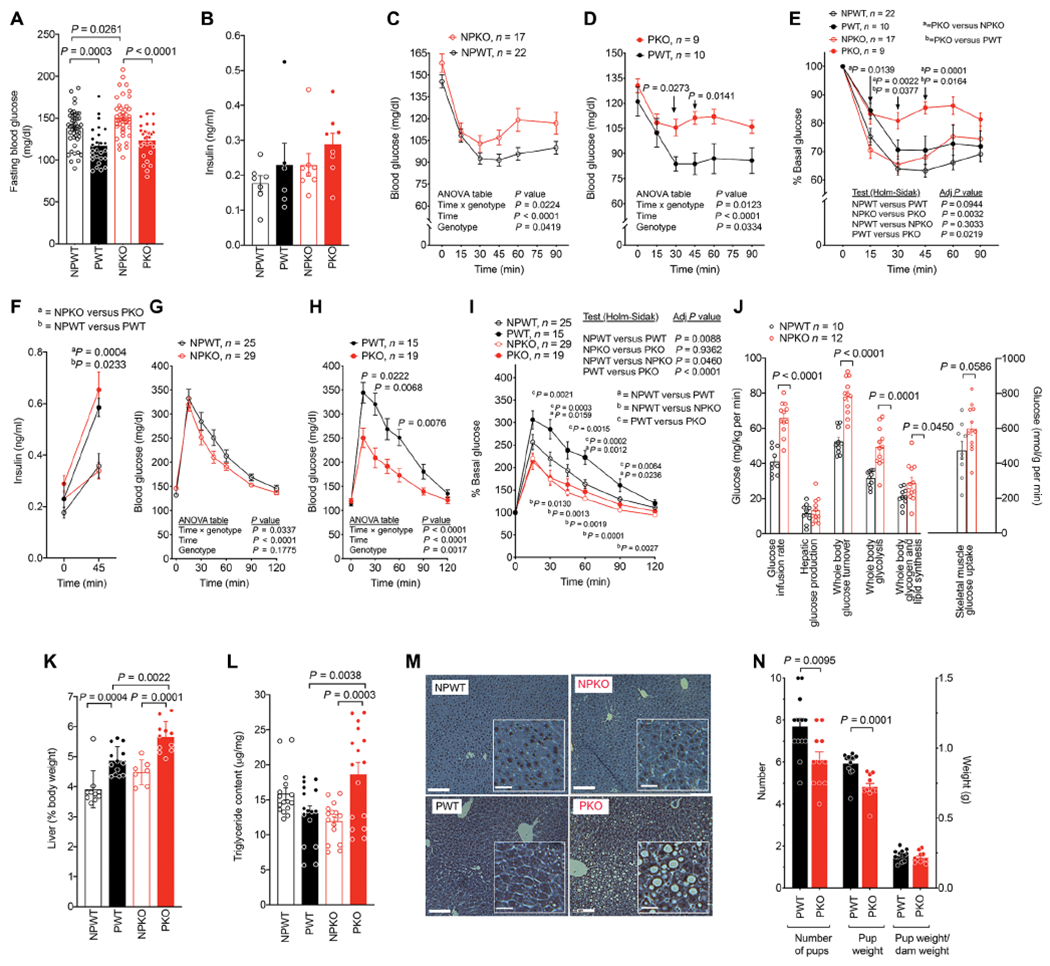
(A) Blood glucose after a 6-hour fast of nonpregnant wild-type (NPWT, n = 47), pregnant wild-type (PWT, n = 26), nonpregnant Pappa KO mice (NPKO, n = 46), or pregnant Pappa KO mice (PKO, n = 28) mice at 10 to 12 weeks of age. (B) Plasma insulin after a 4.5-hour fast of nonpregnant wild-type (NPWT, n = 7), pregnant wild-type (PWT, n = 6), nonpregnant Pappa KO mice (NPKO, n = 8), or pregnant Pappa KO mice (PKO, n = 8) mice at 12 weeks of age. Comparisons between groups in (A) and (B) were made using one-way ANOVA corrected for multiple comparisons using the Sidak test. (C to E) Insulin tolerance tests (0.65 units of insulin/kg body weight) after a 4.5-hour fast of nonpregnant wild-type (NPWT, n = 22), pregnant wild-type (PWT, n = 10), nonpregnant Pappa KO mice (NPKO, n = 17), or pregnant Pappa KO mice (PKO, n = 9). Panels (C) and (D) are raw values, and panel (E) is the percent of basal value. Variance between groups in (C) and (D) was assessed using one-way ANOVA corrected for multiple comparisons using the Holm-Sidak test and in (E) using repeated measures ANOVA corrected for multiple comparisons using the Holm-Sidak test. Significance of the differences at each time point was calculated using multiplicity adjusted t tests. (F) Plasma insulin before (t = 0) and after (t = 45 min) administration of glucose (2 g/kg body weight) after a 6-hour fast to NPWT (n = 7), PWT (n = 6), NPKO (n = 9), and PKO (n = 8) mice. Statistical significance was assessed using ordinary one-way ANOVA corrected with the Sidak test for multiple comparisons. (G to I) Glucose tolerance test (2 g of glucose/kg body weight) after 6-hour fast of NPWT (n = 25), PWT (n = 15), NPKO (n = 29), and PKO (n = 19) mice. Panels (G) and (H) are raw values, and panel (I) is the percent of basal value. Variance between groups in (G) and (H) was assessed using one-way ANOVA corrected for multiple comparisons using the Holm-Sidak test and in (I) using repeated measures ANOVA corrected for multiple comparisons using the Holm-Sidak test, and differences at each time point measured using multiplicity adjusted t tests. (J) Glucose values and muscle glucose uptake during hyperinsulinemic-euglycemic clamps for each parameter measured (x axis) for NPWT (n = 10) and NPKO (n = 12) mice. Bars indicate mean and SEM, and symbols are the values for each individual animal. Statistical significance of the differences for each parameter was calculated using two-tailed, unpaired Studen’s t tests and exact P values are shown. (K and L) Liver mass (K) and triglyceride (L) content from NPWT (n = 10), PWT (n = 13), NPKO (n = 7), and PKO (n = 11) mice. Variance between groups was assessed using one-way ANOVA corrected for multiple comparisons using the Sidak test (M). Representative images of H&E liver sections. Scale bars, 20 μm. Insets contain magnified sections of each image. Scale bars, 5 μm. (N) Average number of pups and average weight per pup from 13 liters from PWT and 11 liters from PKO mice. Shown are means and SEM, and statistical difference between groups was calculated using two-tailed unpaired Studen’s t tests.
Paradoxically, despite the differences observed in insulin tolerance tests, the absence of Pappa was accompanied by increased glucose disposal in both nonpregnant and pregnant mice (Fig. 5, G and H). Analysis of glucose excursions as a percent of basal fasting blood glucose indicated that Pappa KO enhanced glucose tolerance in both nonpregnant and pregnant mice (Fig. 5I). Enhanced glucose tolerance in the face of the insulin resistance seen in Pappa KO mice could possibly be explained by enhanced activity of insulin-independent glucose consumption mechanisms, such as those associated with exercise, or glucose consumption by the fetus. Muscle oxidative metabolism has been previously reported to be increased in Pappa KO mice (71), consistent with this possibility. To determine potential mechanisms of enhanced glucose disposal in Pappa KO mice, we conducted hyperinsulinemic euglycemic clamps. KO mice required higher glucose infusion rates and displayed enhanced whole body glucose turnover along with enhanced skeletal muscle glucose uptake and increased whole body glycolysis (Fig. 5J). Nevertheless, there was no decrease in hepatic glucose production, which would be expected if the enhanced glucose turnover was due to increased insulin action. These results are consistent with an unusual metabolic state of enhanced peripheral glucose uptake in the presence of insulin resistance in the Pappa KO mice.
Multiple studies have pointed to excessive lipolysis stemming from adipose tissue insulin resistance as an important cause of hepatosteatosis and increased hepatic glucose production (28, 31, 72, 73). To determine whether Pappa deficiency is accompanied by changes in the liver, we analyzed triglyceride content and histological appearance. Liver weight increased in response to pregnancy in both WT and KO mice, but livers from KO pregnant mice were larger than those from WT pregnant mice (Fig. 5K). This increased weight could be attributed to increased triglycerides (Fig. 5L) reflected by the presence of large lipid droplets (Fig. 5M) and only observed in pregnant KO mice. These results are consistent with adipose tissue insulin resistance induced by pregnancy in Pappa KO mice. To examine the consequences of dysregulated metabolism on fetal development, we measured the size of liters and pup weights. Liters from Pappa KO mice were smaller, as was mean pup weight. However, these values normalized as a function of dam weight were not different between genotypes (Fig. 5N). These results suggest that major alterations in fetal development are unlikely to underlie the observed adipose tissue and metabolic changes elicited by Pappa deficiency.
PAPPA values are directly associated with glycemic control in human pregnancy
We next explored whether PAPPA, acting through the IGFBP/IGF axis in adipose tissue, is important for the regulation of glucose homeostasis during pregnancy in humans. We examined the relationship between circulating values of PAPPA and glycemia in a cohort of 6361 women (Table 2) and performed a categorical analysis of serum PAPPA values in pregnant subjects stratified as having normal glucose tolerance, abnormal glucose tolerance, or GDM. PAPPA values were lower in both abnormal glucose tolerance and GDM groups compared to normal glucose tolerance (Fig. 6A). We separated all PAPPA serum values in the population into quartiles (Fig. 6B) and calculated the odds of abnormal glucose tolerance and GDM occurring as a function of PAPPA quartile. Compared to the quartile with highest PAPPA concentration (1763 to 16,498 ng/ml), the odds of AGT increased in all remaining quartiles, and the odds of GDM were higher for the lowest PAPPA quartile (Fig. 6C).
Table 2. Characteristics of subject population for retrospective study.
NGT, normal glucose tolerance; AGT, abnormal glucose tolerance.
| Total (N = 6361) | GDM status | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NGT (N = 5703) | AGT (N = 298) | GDM (N = 360) | |||||||
| Age (mean ± SD) | 29.8 ± 5.7 | 29.5 ± 5.7 | 31.8 ± 4.9 | 32.4 ± 4.9 | <0.001 | ||||
| Gestational age (mean + SD) | 12.5 ± 0.6 | 12.5 ± 0.6 | 12.5 ± 0.7 | 12.5 ± 0.5 | 0.652 | ||||
| N | % | N | % | N | % | N | % | ||
| BMI categories | <0.001 | ||||||||
| < 18.5 | 198 | 3.1% | 186 | 3.3% | 6 | 2.0% | 6 | 1.7% | |
| 18.5 to <25.0 | 3042 | 47.8% | 2823 | 49.5% | 117 | 39.3% | 102 | 28.3% | |
| 25.0 to <30.0 | 1757 | 27.6% | 1574 | 27.6% | 83 | 27.9% | 100 | 27.8% | |
| > = 30.0 | 1364 | 21.4% | 1120 | 19.6% | 92 | 30.9% | 152 | 42.2% | |
| Race | <0.001 | ||||||||
| Asian | 527 | 8.4% | 416 | 7.4% | 50 | 17.1% | 61 | 17.2% | |
| Black | 473 | 7.5% | 418 | 7.4% | 17 | 5.8% | 38 | 10.7% | |
| Caucasian/white | 4049 | 64.2% | 3692 | 65.3% | 181 | 61.8% | 176 | 49.6% | |
| Hispanic/Latino | 577 | 9.2% | 520 | 9.2% | 21 | 7.2% | 36 | 10.1% | |
| Other | 680 | 10.8% | 612 | 10.8% | 24 | 8.2% | 44 | 12.4% | |
| Cigarette smoker | 0.133 | ||||||||
| No | 5724 | 93.3% | 5122 | 93.1% | 276 | 94.5% | 326 | 95.0% | |
| Smoker | 411 | 6.7% | 378 | 6.9% | 16 | 5.5% | 17 | 5.0% | |
| Parity | 0.002 | ||||||||
| Nulliparous | 2763 | 43.4% | 2519 | 44.2% | 107 | 35.9% | 137 | 38.1% | |
| Multiparous | 3598 | 56.6% | 3184 | 55.8% | 191 | 64.1% | 223 | 61.9% | |
Fig. 6. Association between PAPPA and glycemia in pregnancy.
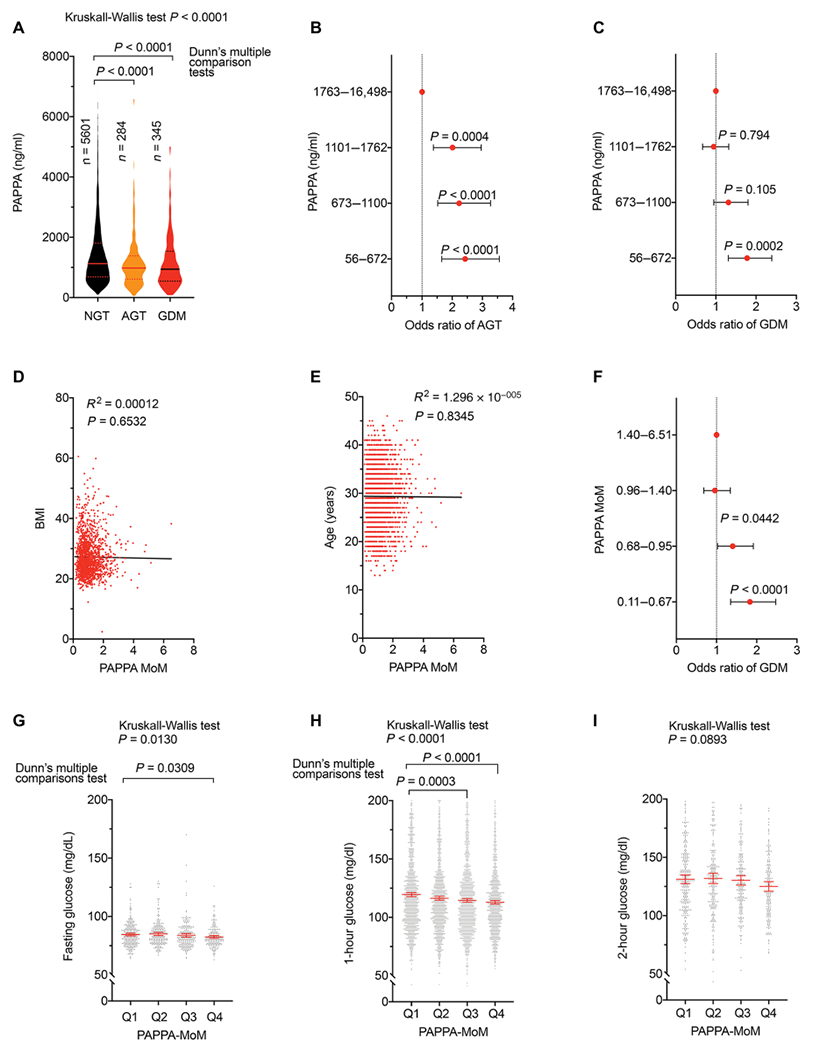
(A) Violin plots of PAPPA values at 10 to 14 weeks of gestation in women categorized with normal glucose tolerance (NGT n = 5601), abnormal glucose tolerance (AGT n = 284), and gestational diabetes mellitus (GDM n = 345) at week 24 to 28 of gestation. Statistical significance was estimated using ordinary one-way ANOVA corrected for multiple comparisons using the Sidak test. Median and quartile values are indicated. (B and C) Odds ratio and 95% confidence interval (CI) for AGT (B) or GDM (C). (D) Linear regression between PAPPA MoM and BMI (n = 1620). (E) Linear regression between PAPPA MoM and age (n = 3465). (F) Odds ratio and 95% CI of GDM at different quartiles of PAPPA MoM. For (B), (C), and (F), statistical significance between comparisons to the highest quartile was done using Fisher’s exact test. (G to I) Fasting (n = 932) (G), 1 hour (n = 5294) (H), and 2 hours (n = 909) (I) glucose values at each PAPPA MoM quartile. Analysis of variance was performed using the Kruskall-Wallis test, and difference between the first quartile (Q1) and others was calculated using the Dunn’s multiple comparison test.
PAPPA concentration varies as a function of gestational age, as well as with factors associated with increased diabetes risk such as BMI, ethnicity, and smoking status. For this reason, laboratory values are also reported as multiples of the median (MoM), which represents the net value of PAPPA represented as a function of the median value for that particular laboratory. As expected, we found no association between PAPPA MoM and BMI (Fig. 6D) or age (Fig. 6E) in our cohort. Nevertheless, the odds of GDM increased in the two lowest PAPPA MoM quartiles compared to the highest quartile (Fig. 6F). These results indicate that the associations between low PAPPA and abnormal glucose tolerance and gestational diabetes are more likely due to a direct relationship between PAPPA and glycemic control mechanisms, rather than being mediated by other factors associated with diabetes risk. To further test the hypothesis that PAPPA is associated with systemic glucose metabolism independent of disease state, we compared mean blood glucose values between serum PAPPA MoM quartiles only in subjects with normal glucose tolerance (Fig. 6, G to I). Mean fasting glucose was lower in the highest (Q4) PAPPA MoM quartile compared to that in the lowest (Q1) quartile (Fig. 6G), and the mean 1-hour glucose was lower in the Q3 and Q4 PAPPA MoM quartiles compared to that in Q1 (Fig. 6H). No differences between quartiles were seen when comparing 2-hour glucose values (Fig. 6I). The association between higher circulating PAPPA with lower glucose values within the normal range is consistent with a mechanism in which circulating PAPPA directly enhances glucose tolerance during pregnancy, and disruption of this mechanism can contribute to development of GDM.
DISCUSSION
The results presented here demonstrate that, in both mice and humans, pregnancy induces adipose tissue adaptations involving changes in adipocyte size and capillary structure that could underlie changes in insulin sensitivity and systemic glucose homeostasis characteristic of gestation. Our findings suggest that PAPPA is a key signaling molecule involved in these adipose tissue adaptations. Acting on IGFBPs, this protease may establish a pregnancy-specific signaling axis in adipose tissue that can couple fetal growth to maternal fuel homoeo-stasis. Although our findings in the human population are correlative, they are consistent with experimental results in vitro indicating a direct effect of the PAPPA, IGFBP, and IGF-1 axis in mediating adipose tissue expandability. They are also consistent with the metabolic phenotype of Pappa KO mice, in which pregnancy-induced changes in adipocyte size are abrogated, and which display systemic metabolic abnormalities consistent with adipose tissue insulin resistance. The very large >100-fold increase in PAPPA serum concentration and activity during human pregnancy, compared to nearly undetectable circulating activity outside of gestation, supports the notion of this pathway being a pregnancy-specific adaptation.
PAPPA was initially described as one of three proteins produced by the human endometrium that increase markedly after decidualization and pregnancy (74, 75). Of these, only PAPPA increases progressively from very early (6 to 10 weeks) gestation to term (47). The development of sensitive assays for PAPPA early on revealed correlations with pregnancy abnormalities (76–78). A single low value for PAPPA was highly correlated with Down syndrome pregnancies (48, 49), leading to its adoption for screening chromosomal abnormalities in early pregnancy, although the mechanisms behind these low values are unclear. Cloning of the PAPPA gene led to its identification as a zinc-binding metalloprotease (79) and functional studies to its subsequent identification as the single protease responsible for cleavage of IGFBP4 (80) and capable of cleavage of IGFBP5 (81). The key role of PAPPA in IGFBP proteolysis and IGF signaling is evidenced by its effects on embryonic development; in humans, PAPPA values in early pregnancy correlate with fetal size at term (82–84), and in mice, KO of Pappa results in proportional dwarfism (68). In addition to its role in fetal development, total and proteolytically active human PAPPA concentrations progressively rise in serum throughout pregnancy (50). Although our cross-sectional studies results are restricted to a specific point in time, the observed inverse correlation between circulating PAPPA and serum glucose concentrations could be consistent with longitudinal mediation of maternal peripheral tissue adaptations to fetal growth, provided that PAPPA and glucose concentrations maintained similar trajectories. Further longitudinal studies will be required to further explore this hypothesis.
In the case of diabetes, a correlation between low first trimester PAPPA and GDM risk has been reported in some (85–87), though not all studies (88, 89). In our retrospective study of over 6000 women, we found that PAPPA concentration in the lower quartiles was strongly correlated with development of GDM. It has been recognized that circulating PAPPA concentrations at the three trimesters of pregnancy are affected by diverse maternal factors and medical history (90), including weight, age, smoking, race, and diabetes status. Because these factors lower the sensitivity and accuracy of single measure PAPPA for aneuploidy screening, algorithms used by clinical laboratories to report PAPPA MoM correct for these factors. Nevertheless, in our study, we find that PAPPA MoM values in the lowest quartile are still associated with increased odds of development of GDM, supporting the hypothesis PAPPA concentration is an independent risk factor for the development of gestational metabolic disease.
Mechanistically, our results support a model in which low concentrations of PAPPA result in impaired proteolysis of adipose tissue IGFBPs, which are up-regulated in adipose depots during pregnancy. In turn, IGF signaling decreases, impairing pregnancy-induced increases in adipocyte number and size, as well as tissue vascularization. Inadequate adipose tissue adaptation leads to enhanced insulin resistance and impaired glucose tolerance. This mechanistic view is supported by our finding of direct stimulation of human adipose tissue expandability by PAPPA in vitro reflecting in vivo physiology (55, 91, 92), as well as our findings of impaired adipose tissue adaptations and pregnancy-specific insulin resistance in Pappa-deficient mice.
Despite displaying 86% amino acid conservation between human and mouse and sharing catalytic features, Pappa is not highly produced in the placenta during pregnancy in mice (93). Therefore, pregnancy-specific regulation in this species may occur through its expression and function in peripheral tissues, including adipose tissue itself. The effects of Pappa on murine adipose tissue can be mediated through cleavage of Igfbp2, as its expression was specifically up-regulated by pregnancy and it is a known substrate of Pappa (94, 95). The direct role of Pappa on adipose tissue is also supported by the finding that depots displaying lesser impairments exhibit a larger compensatory overexpression of Igf-2, consistent with observations that increased Igf-2 mitigates the effects of Pappa deficiency (96). In human adipose tissue, the largest pregnancy-induced change for a single gene is the enhanced expression of IGFBP5, accompanied by >10-fold elevation in circulating PAPPA net proteolytic activity (50). Thus, although the specific IGF binding proteins and the source of Pappa/PAPPA may differ between mice and humans, the mechanism whereby their regulatory axis enables adipose tissue adaptation to pregnancy is conserved between the two species.
The pregnancy-specific adaptations we observed in mice include a trend toward decreasing total fat pad mass and the appearance of small adipocytes, consistent with increased fat mobilization and adipose tissue hyperplasia. The molecular mechanisms that remodel adipose tissue during pregnancy appear to be variations on mechanisms already known to play a major role in adipose tissue development. In nonpregnant humans, the IGF signaling pathway is known to play a major role in the regulation of adipose tissue expandability (52, 54, 55, 97–99). In mice, expression of IGFBP-4 is regulated by age and obesity, and it can directly modulate adipose tissue expandability in vitro (55).
Adipose tissue expandability is increasingly recognized to play a central role in the regulation of insulin sensitivity through secretion of cytokines and preventing lipotoxicity (100). Failure of adipose tissue to suppress lipolysis or insufficient adipose tissue storage capacity leads to increased lipid delivery to the liver and enhanced gluconeogenesis and hepatosteatosis (29, 101). In our studies, Pappa KO mice displayed greater insulin resistance as evidenced by insulin tolerance tests and fatty liver, consistent with impaired adipose tissue function. Paradoxically, we found enhanced glucose tolerance in both nonpregnant and pregnant conditions in Pappa KO mice. A possible explanation for enhanced glucose tolerance in the face of insulin resistance would be the enhanced activity of insulin-independent glucose consumption mechanisms, such as those associated with exercise. Increased muscle oxidative metabolism has been previously reported in Pappa KO mice (71). Consistent with this possibility, the higher glucose infusion rates during hyperinsulinemic insulin clamps seen in Pappa KO mice were accompanied by enhanced skeletal muscle glucose uptake and increased whole body glycolysis. Nevertheless, there was no decrease in hepatic glucose production, which would be expected if the enhanced glucose turnover was due to increased insulin action. This unusual metabolic state of enhanced peripheral glucose uptake in the presence of insulin resistance is consistent with the similarly unexpected trend toward better glucose tolerance that is seen in Pappa KO mice subjected to high fat diet (102).
Our study carries limitations. We cannot be certain that circulating PAPPA derived from the placenta is mediating subcutaneous human adipose tissue IGFBP5 cleavage. Although the vast majority of circulating PAPPA in human pregnancy is derived from the placenta (103), other tissue also expresses a substantial amount (104), which could therefore be mediating IGF signaling. Further longitudinal studies measuring adipose tissue architecture, PAPPA gene expression in adipose tissue, circulating concentrations of total and active PAPPA, and glucose tolerance over the course of normal human pregnancy may help answer this question. Another limitation is our use of a whole body Pappa KO mouse, in which the consequences of Pappa depletion are not restricted to adipose tissue. It is possible that effects on other tissues, including muscle and pancreas, might contribute to the observed effects on glucose homeostasis during pregnancy. Future studies using tissue-specific Pappa ablation will help determine the extent to which each tissue contributes to metabolic adaptations to pregnancy and understand the respective contributions of circulating versus tissue-restricted Pappa. Despite these limitations, our current findings identify an important, Pappa-dependent, effect of pregnancy on adipose tissue remodeling and a physiologically meaningful role of Pappa on insulin sensitivity during pregnancy.
Gestational diabetes is a growing medical concern, with increasingly recognized long-term health consequences for mothers and their offspring (1, 7). Our finding of a correlation between PAPPA insufficiency and presence of GDM provides impetus for further studies on cause-effect relationships and mechanisms of this association. If these studies prove a direct causative role for PAPPA on incidence or severity of GDM through its effects on human adipose tissue during pregnancy, they would open the possibility for therapeutic use of this protease. Through gathering a better understanding of PAPPA/IGF signaling, initiating a targeted investigation of PAPPA expression regulation, and directly studying the action of PAPPA as a biologic, we will determine its potential as a therapeutic option in mitigating GDM and its transgenerational pathologies.
MATERIALS AND METHODS
Study design
The objective of this study was to determine potential roles of adipose tissue in mediating metabolic adaptations to pregnancy. We performed transcriptomic and histological analysis of adipose tissue using samples from nonpregnant and pregnant women matched for age and BMI. The role of the IGF signaling pathway on adipose tissue expansion was demonstrated in vitro by measuring the outgrowth of cells from adipose tissue explants in the presence or absence of IGFBP5, PAPPA, and the IGF-1 receptor inhibitor NVP-AEW541. The role of PAPPA in vivo was determined by analyzing adipose tissue depot architecture and gene expression in pregnant and nonpregnant PappA KO mice and their WT littermates using histochemistry and reverse transcription polymerase chain reaction, and systemic glucose metabolism was analyzed in nonpregnant PappA KO mice and their WT littermates using hyperinsulinemic-euglycemic glucose clamps. Numbers of animals and experimental replicates are indicated in figure legends. To determine the relationship between circulating PAPPA and glucose homeostasis in human pregnancy, we conducted a retrospective study of 6361 pregnant women for which first trimester PAPPA values and third trimester serum glucose values were available. Blinding was used in all experiments requiring image analysis and in the performance of hyperinsulinemic/euglucemic clamps.
Human subjects
The Institutional Review Board from University of Massachusetts Medical School (UMMS) approved the studies, and all participants were provided and signed informed consent. For Affymetrix analysis, omental and subcutaneous adipose tissues from women delivering at term by Cesaerian delivery and from nonpregnant women undergoing bariatric surgery were obtained as previously described (56). For analysis of adipose tissue architecture, all pregnant women with singleton gestations scheduled for Cesarean section and nonpregnant normoglycemic women without hypertension presenting to University of Massachusetts Memorial Health Care (UMMHC) between June 2015 and November 2016 were considered for enrollment. Diabetes status in the pregnant cohort was classified according to the Carpenter-Coustan criteria. The exclusion criteria included type 1 and type 2 diabetes mellitus, underweight BMI (<18.5 kg/m2, pregestational BMI in pregnant cohort), use of illicit substances including replacement products, HIV/AIDS, hepatitis B or C, autoimmune disease, chronic steroid use, age <18 and >45 years, and plans to move out of the area during study period. Exclusions specific to pregnant cohort included multiple gestations, initiated prenatal care after 13 completed gestational weeks, alcohol use, and previous diagnosis of T2DM.
To determine the relationship between serum PAPPA and categorical diabetes status, we performed retrospective analyses of the electronic health records containing PAPPA values of all pregnant women with singleton gestations who delivered at UMMHC from June 2009 to March 2015. We classified women according to the Carpenter-Coustan criteria, in which normal glucose tolerance is defined as gravidas without pregestational diabetes and with passing value on routine 50-g glucola screening (≤140 mg/dl) or ≤ 1 abnormal value (fasting ≥ 95, 1 hour ≥ 180, 2 hour ≥ 155, 3 hour ≥ 140 mg/dl) on 100-g 3-hour glucose tolerance test (GTT); abnormal glucose tolerance included gravidas without pregestational diabetes and with failed value on routine 50-g glucola screening (>140 mg/dl), independent of the value of 100-g 3-hour GTT; GDM included gravidas without pregestational diabetes and with failed value on routine 50-g glucola screening (>140 mg/dl) and ≥2 abnormal values (fasting ≥ 95, 1 hour ≥ 180, 2 hour ≥ 155, 3 hour ≥ 140 mg/dl) on 100-g 3-hour GTT. For analysis of the relationship between PAPPA and serum glucose, all records containing PAPPA values from routine 10- to 13-week aneuploidy and fasting, 1-, 2-, and 3-hour serum glucose values derived from 50-g glucola screening or 3-hour GTT were used. Data from medical records was extracted using Epic EHR v. 2018.
Human tissue collection
For the pregnant cohorts, specimen collection was done at the time of Cesarean delivery after delivery of the baby. Before skin closure, two samples (1 cm by 1 cm) of subcutaneous adipose tissue were obtained from within the surgical incision usually placed about 2 cm above the pubic bone (Pfannenstiel incision). In the case of repeat Cesarean delivery, subcutaneous adipose tissue biopsies were taken from deep within the incision to decrease scar tissue sampling. For the nonpregnant cohort, specimen collection was done at the Clinical Trials Unit of UMMS during a scheduled site visit. Subcutaneous adipose tissue needle biopsies were obtained from anterior abdominal wall just lateral and inferior to the umbilicus, both away from blood vessels or scars. For acquiring the AT specimens, a 60-ml BD syringe with 14-gauge needle filled with 30 ml of normal saline was used. About 1 g of AT was collected.
Animal husbandry and pregnancy
All procedures were approved by UMMS Institutional Animal Care and Use Committee. The experimental animals used in this study were homozygous PappA (+/+) (WT) and PappA (−/−) (KO) littermates obtained from crosses of heterozygous PappA (+/−) mice (68). All mice were fed normal chow diet (22% protein and 5% fat, Isopro 3000) ad libitum and housed under controlled temperature and 12-hour light/12-hour dark cycle conditions. For all experiments, 10- to 12-week-old mice were used. For the pregnancy experiments, homozygous WT or KO animal trios consisting of one male and two females were housed together. The presence of a vaginal plug was considered as day 1 of gestation. After plug visualization, females were separated from males. All pregnancy experiments were carried out at day 16 of gestation.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v8. Statistical tests and P values are described in figure legends. A P value < 0.05 was considered significant. Data were tested for normality before use of parametric tests, and when normality could not be verified, non-parametric tests were used as described in figure legends. Difference of histograms was calculated using the Wilcoxon matched pairs test, and P values for differences at each size range calculated using multiplicity-adjusted (Sidak) Student’s t tests. Statistical significance between groups was estimated using ordinary one-way ANOVA corrected for multiple comparisons using the Sidak test. For contingency analyses, statistical significance between comparisons to the highest quartile was done using Fisher’s exact test. Statistical significance of the difference between control and treated explants was calculated using paired Student’s t tests. The difference between doses at each time point was assessed using repeated measures two-way ANOVA, and differences between fat depot, genotype, and pregnant state for each gene were assessed using three-way ANOVA corrected for multiple comparisons using Tukey’s test.
Supplementary Material
Fig. S1. Depot-specific pregnancy-induced changes in adipocyte size at early and late stages of pregnancy.
Data file S1. Primary data.
Data file S2. Genes differentially expressed between adipose tissue of nonpregnant and pregnant women.
Data file S3. Pathway analysis of genes affected by pregnancy in both subcutaneous and omental depots.
Acknowledgments:
We acknowledge the use of UMASS Metabolic Phenotyping Core 5U2C-DK093000 and support from cores and programs supported by UMCCTS grant UL1 TR001453.
Funding:
This study was supported by NIH grants DK089101-04 to S.C. and with support from R25GM113686 to R.R.-R.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability:
All data associated with this study are present in the paper or the Supplementary Materials. A uniform biological material transfer agreement between University of Massachusetts and the Mayo Foundation for Medical Education and Research for procurement of Pappa KO mice is available upon request.
REFERENCES AND NOTES
- 1.Zhang C, Olsen SF, Hinkle SN, Gore-Langton RE, Vaag A, Grunnet LG, Yeung EH, Bao W, Bowers K, Liu A, Mills JL, Sherman S, Gaskins AJ, Ley SH, Madsen CM, Chavarro JE, Hu FB; Diabetes & Women’s Health Study team, Diabetes & Women’s Health (DWH) Study: An observational study of long-term health consequences of gestational diabetes, their determinants and underlying mechanisms in the USA and Denmark. BMJ Open 9, e025517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P, Gestational diabetes mellitus. Nat. Rev. Dis. Primers 5, 47 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA, Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes 44, 586–591 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas J-P, Hingorani AD, Williams D, Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 373, 1773–1779 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Kwak SH, Choi SH, Kim K, Jung HS, Cho YM, Lim S, Cho NH, Kim SY, Park KS, Jang HC, Prediction of type 2 diabetes in women with a history of gestational diabetes using a genetic risk score. Diabetologia 56, 2556–2563 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC, Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: A study of discordant sibships. Diabetes 49, 2208–2211 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Farahvar S, Walfisch A, Sheiner E, Gestational diabetes risk factors and long-term consequences for both mother and offspring: A literature review. Expert Rev. Endocrinol. Metab 14, 63–74 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Vargas L, Addison SS, Nistala R, Kurukulasuriya D, Sowers JR, Gestational diabetes and the offspring: Implications in the development of the cardiorenal metabolic syndrome in offspring. Cardiorenal Med. 2, 134–142 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kampmann FB, Thuesen ACB, Hjort L, Olsen SF, Pires SM, Tetens I, Grunnet LG, Exposure to gestational diabetes is a stronger predictor of dysmetabolic traits in children than size at birth. J. Clin. Endocrinol. Metab 104, 1766–1776 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Wicklow BA, Sellers EAC, Sharma AK, Kroeker K, Nickel NC, Philips-Beck W, Shen GX, Association of gestational diabetes and type 2 diabetes exposure in utero with the development of type 2 diabetes in first nations and non-first nations offspring. JAMA Pediatr. 172, 724–731 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson B, Löf M, Olausson H, Forsum E, Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br. J. Nutr 103, 50–57 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC, Fat mass deposition during pregnancy using a four-component model. J. Appl. Physiol (1985) 87, 196–202 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Forsum E, Sadurskis A, Wager J, Estimation of body fat in healthy Swedish women during pregnancy and lactation. Am. J. Clin. Nutr 50, 465–473 (1989). [DOI] [PubMed] [Google Scholar]
- 14.Larciprete G, Valensise H, Vasapollo B, Altomare F, Sorge R, Casalino B, De Lorenzo A, Arduini D, Body composition during normal pregnancy: Reference ranges. Acta Diabetol. 40 (suppl. 1), S225–S232 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS, Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 132, 1639–1647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borel AL, Nazare JA, Smith J, Aschner P, Barter P, Van Gaal L, Eng Tan C, Wittchen HU, Matsuzawa Y, Kadowaki T, Ross R, Brulle-Wohlhueter C, Almeras N, Haffner SM, Balkau B, Després JP, Visceral, subcutaneous abdominal adiposity and liver fat content distribution in normal glucose tolerance, impaired fasting glucose and/or impaired glucose tolerance. Int. J. Obes. (Lond) 39, 495–501 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C, Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci 20, 2358 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasan SK, Osmond C, Canoy D, Christodoulides C, Neville MJ, Di Gravio C, Fall CHD, Karpe F, Comparison of regional fat measurements by dual-energy X-ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int. J. Obes. (Lond) 42, 850–857 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Liu J, Li H, Liu L, Zheng J, Huang Z, Cao X, Xiao H, Li Y, Higher ratio of abdominal subcutaneous to visceral adipose tissue related with preservation of islet β-cell function in healthy individuals. Int. J. Endocrinol 2017, 6180904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS, Abdominal subcutaneous adipose tissue: A protective fat depot? Diabetes Care 32, 1068–1075 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser ME, Kropman E, Kranendonk ME, Koppen A, Hamers N, Stroes ES, Kalkhoven E, Monajemi H, Characterisation of non-obese diabetic patients with marked insulin resistance identifies a novel familial partial lipodystrophy-associated PPARg mutation (Y151C). Diabetologia 54, 1639–1644 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simha V, Garg A, Lipodystrophy: Lessons in lipid and energy metabolism. Curr. Opin. Lipidol 17, 162–169 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CSS, Bottomley W, Vigouroux C, Magré J, Raymond-Barker P, Murgatroyd PR, Chawla A, Skepper JN, Chatterjee VK, Suliman S; LD Screening Consortium, Patch A-M, Agarwal AK, Garg A, Barroso I, Cinti S, Czech MP, Argente J, O’Rahilly S, Savage DB, Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol. Med 1 , 280–287 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hocking SL, Stewart RL, Brandon AE, Suryana E, Stuart E, Baldwin EM, Kolumam GA, Modrusan Z, Junutula JR, Gunton JE, Medynskyj M, Blaber SP, Karsten E, Herbert BR, James DE, Cooney GJ, Swarbrick MM, Subcutaneous fat transplantation alleviates diet-induced glucose intolerance and inflammation in mice. Diabetologia 58, 1587–1600 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Li M, Zou Y, Cao T, Induction of adipocyte hyperplasia in subcutaneous fat depot alleviated type 2 diabetes symptoms in obese mice. Obesity 22, 1623–1631 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Foster MT, Softic S, Caldwell J, Kohli R, de Kloet AD, Seeley RJ, Subcutaneous adipose tissue transplantation in diet-induced obese mice attenuates metabolic dysregulation while removal exacerbates it. Physiol. Rep 1, e00015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa K, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y, Subcutaneous fat modulates insulin sensitivity in mice by regulating TNF-α expression in visceral fat. Horm. Metab. Res 38, 631–638 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, Chen H, Monks BR, Chen J, Rabinowitz JD, Birnbaum MJ, Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 23, 1154–1166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly SM, Ahmadian M, Zamarron BF, Chang L, Uhm M, Poirier B, Peng X, Krause DM, Korytnaya E, Neidert A, Liddle C, Yu RT, Lumeng CN, Oral EA, Downes M, Evans RM, Saltiel AR, A subcutaneous adipose tissue-liver signalling axis controls hepatic gluconeogenesis. Nat. Commun 6, 6047 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortés VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, Smith AR, Ren J, Esser V, Hammer RE, Agarwal AK, Horton JD, Garg A, Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 9, 165–176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ, Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest 115, 1343–1351 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinar S, Berger H, De Souza LR, Ray JG, Difference in visceral adipose tissue in pregnancy and postpartum and related changes in maternal insulin resistance. J. Ultrasound Med 38, 667–673 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Svensson H, Wetterling L, Bosaeus M, Odén B, Odén A, Jennische E, Edén S, Holmäng A, Lönn M, Body fat mass and the proportion of very large adipocytes in pregnant women are associated with gestational insulin resistance. Int. J. Obes. (Lond) 40, 646–653 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas-Rodriguez R, Lifshitz LM, Bellve KD, Min SY, Pires J, Leung K, Boeras C, Sert A, Draper JT, Corvera S, Moore Simas TA, Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia 58, 2106–2114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Resi V, Basu S, Haghiac M, Presley L, Minium J, Kaufman B, Bernard S, Catalano P, Hauguel-de Mouzon S, Molecular inflammation and adipose tissue matrix remodeling precede physiological adaptations to pregnancy. Am. J. Physiol. Endocrinol. Metab 303, E832–E840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts CT Jr., Leroith D, Molecular aspects of insulin-like growth factors, their binding proteins and receptors. Baillieres Clin. Endocrinol. Metab 2, 1069–1085 (1988). [DOI] [PubMed] [Google Scholar]
- 37.Williams KL, Fuller CR, Fagin J, Lund PK, Mesenchymal IGF-I overexpression: Paracrine effects in the intestine, distinct from endocrine actions. Am. J. Physiol. Gastrointest. Liver Physiol 283, G875–G885 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Bikle DD, Chang W, Autocrine and paracrine actions of IGF-I signaling in skeletal development. Bone Res. 1, 249–259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dicarlo M, Bianchi N, Ferretti C, Orciani M, Di Primio R, Mattioli-Belmonte M, Evidence supporting a paracrine effect of IGF-1/VEGF on human mesenchymal stromal cell commitment. Cells Tissues Organs 201, 333–341 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A, Insulin receptor activation by IGF-II in breast cancers: Evidence for a new autocrine/paracrine mechanism. Oncogene 18, 2471–2479 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Minuto F, Barreca A, Del Monte P, Giordano G, Paracrine actions of IGF binding proteins. Acta Endocrinol. 124 (suppl. 2), 63–69 (1991). [PubMed] [Google Scholar]
- 42.Holly J, Physiology of the IGF system. Novartis Found. Symp 262, 19–26 (2004). [PubMed] [Google Scholar]
- 43.Wetterau LA, Moore MG, Lee KW, Shim ML, Cohen P, Novel aspects of the insulin-like growth factor binding proteins. Mol. Genet. Metab 68, 161–181 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X, Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J. Clin. Endocrinol. Metab 86, 847–854 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, Gleich GJ, Sottrup-Jensen L, Conover CA, Oxvig C, Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J. Biol. Chem 275, 31128–31133 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Bischof P, Amandruz M, Weil-Franck C, Baeriswyl JP, Weil A, Hermann WL, Sizonenko PC, The disappearance rate of Pregnancy-Associated Plasma Protein-A (PAPP-A) after the end of normal and abnormal pregnancies. Arch. Gynecol 236, 93–98 (1984). [DOI] [PubMed] [Google Scholar]
- 47.Bischof P, Three pregnancy proteins (PP12, PP14, and PAPP-A): Their biological and clinical relevance. Am. J. Perinatol 6, 110–116 (1989). [DOI] [PubMed] [Google Scholar]
- 48.Brambati B, Macintosh MC, Teisner B, Maguiness S, Shrimanker K, Lanzani A, Bonacchi I, Tului L, Chard T, Grudzinskas JG, Low maternal serum levels of pregnancy associated plasma protein A (PAPP-A) in the first trimester in association with abnormal fetal karyotype. Br. J. Obstet. Gynaecol 100, 324–326 (1993). [DOI] [PubMed] [Google Scholar]
- 49.Wald N, Stone R, Cuckle HS, Grudzinskas JG, Barkai G, Brambati B, Teisner B, Fuhrmann W, First trimester concentrations of pregnancy associated plasma protein A and placental protein 14 in Down’s syndrome. BMJ 305, 28 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gyrup C, Christiansen M, Oxvig C, Quantification of proteolytically active pregnancy-associated plasma protein-A with an assay based on quenched fluorescence. Clin. Chem 53, 947–954 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See LH, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu YC, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, Mishra T, Jain D, Dogan N, Harris RS, Cayting P, Kawli T, Boyle AP, Euskirchen G, Kundaje A, Lin S, Lin Y, Jansen C, Malladi VS, Cline MS, Erickson DT, Kirkup VM, Learned K, Sloan CA, Rosenbloom KR, Lacerda de Sousa B, Beal K, Pignatelli M, Flicek P, Lian J, Kahveci T, Lee D, Kent WJ, Ramalho Santos M, Herrero J, Notredame C, Johnson A, Vong S, Lee K, Bates D, Neri F, Diegel M, Canfield T, Sabo PJ, Wilken MS, Reh TA, Giste E, Shafer A, Kutyavin T, Haugen E, Dunn D, Reynolds AP, Neph S, Humbert R, Hansen RS, De Bruijn M, Selleri L, Rudensky A, Josefowicz S, Samstein R, Eichler EE, Orkin SH, Levasseur D, Papayannopoulou T, Chang KH, Skoultchi A, Gosh S, Disteche C, Treuting P, Wang Y, Weiss MJ, Blobel GA, Cao X, Zhong S, Wang T, Good PJ, Lowdon RF, Adams LB, Zhou XQ, Pazin MJ, Feingold EA, Wold B, Taylor J, Mortazavi A, Weissman SM, Stamatoyannopoulos JA, Snyder MP, Guigo R, Gingeras TR, Gilbert DM, Hardison RC, Beer MA, Ren B; Mouse ENCODE Consortium, A comparative encyclopedia of DNA elements in the mouse genome. Nature 515, 355–364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garten A, Schuster S, Kiess W, The insulin-like growth factors in adipogenesis and obesity. Endocrinol. Metab. Clin. North Am 41, 283–295 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Hoeflich A, Wu M, Mohan S, Fùll J, Wanke R, Froehlich T, Arnold GJ, Lahm H, Kolb HJ, Wolf E, Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology 140, 5488–5496 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Maridas DE, DeMambro VE, Le PT, Mohan S, Rosen CJ, IGFBP4 is required for adipogenesis and influences the distribution of adipose depots. Endocrinology 158, 3488–3500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gealekman O, Gurav K, Chouinard M, Straubhaar J, Thompson M, Malkani S, Hartigan C, Corvera S, Control of adipose tissue expandability in response to high fat diet by the insulin-like growth factor-binding protein-4. J. Biol. Chem 289, 18327–18338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran K-V, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S, Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 123, 186–194 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corvera S, Gealekman O, Adipose tissue angiogenesis: Impact on obesity and type-2 diabetes. Biochim. Biophys. Acta 1842, 463–472 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han J, Lee J-E, Jin J, Lim JS, Oh N, Kim K, Chang S-I, Shibuya M, Kim H, Koh GY, The spatiotemporal development of adipose tissue. Development 138, 5027–5037 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Christiaens V, Lijnen HR, Angiogenesis and development of adipose tissue. Mol. Cell. Endocrinol 318, 2–9 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Murakami T, Felinski EA, Antonetti DA, Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem 284, 21036–21046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J, Comparative evaluation of FGF-2–, VEGF-A–, and VEGF-C–induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res 94, 664–670 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Kim J, Kim YH, Kim J, Park DY, Bae H, Lee D-H, Kim KH, Hong SP, Jang SP, Kubota Y, Kwon Y-G, Lim D-S, Koh GY, YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest 127, 3441–3461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ichikawa-Shindo Y, Sakurai T, Kamiyoshi A, Kawate H, linuma N, Yoshizawa T, Koyama T, Fukuchi J, Iimuro S, Moriyama N, Kawakami H, Murata T, Kangawa K, Nagai R, Shindo T, The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J. Clin. Invest 118, 29–39 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lambert-Messerlian G, Palomaki GE, Canick JA, Examination of the pregnancy-associated plasma protein-A assay on the Beckman Coulter Access(®) platform: Suitability for use in first trimester Down’s syndrome screening. J. Med. Screen 17, 109–113 (2010). [DOI] [PubMed] [Google Scholar]
- 65.García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro H-G, Cozens R, Evans DB, Fabbro D, Furet P, Porta DG, Liebetanz J, Martiny-Baron G, Ruetz S, Hofmann F, In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell 5, 231–239 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, Testa JR, Eisenberg B, von Mehren M, Godwin AK, Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc. Natl. Acad. Sci. U.S.A 105, 8387–8392 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boucher J, Mori MA, Lee KY, Smyth G, Liew CW, Macotela Y, Rourk M, Bluher M, Russell SJ, Kahn CR, Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat. Commun 3, 902 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Füchtbauer E-M, Oxvig C, van Deursen J, Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 131, 1187–1194 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Qiao L, Chu K, Wattez J-S, Lee S, Gao H, Feng G-S, Hay WW Jr., Shao J, High-fat feeding reprograms maternal energy metabolism and induces long-term postpartum obesity in mice. Int. J. Obes. (Lond) 43, 1747–1758 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cinti S, in The ECOG’s eBook on Child and Adolescent Obesity, Frelut ML, Ed. (ebook.ecog-obesity.eu, 2015).
- 71.Conover CA, Bale LK, Nair KS, Comparative gene expression and phenotype analyses of skeletal muscle from aged wild-type and PAPP-A-deficient mice. Exp. Gerontol 80, 36–42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rebrin K, Steil GM, Mittelman SD, Bergman RN, Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J. Clin. Invest 98, 741–749 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perry RJ, Camporez J-PG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang X-M, Ruan H-B, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI, Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith R, Bischof P, Hughes G, Klopper A, Studies on pregnancy-associated plasma protein A in the third trimester of pregnancy. Br. J. Obstet. Gynaecol 86, 882–887 (1979). [DOI] [PubMed] [Google Scholar]
- 75.Bischof P, Purification and characterization of pregnancy associated plasma protein A (PAPP-A). Arch. Gynecol 227, 315–326 (1979). [DOI] [PubMed] [Google Scholar]
- 76.Barnea ER, Bischoff P, Page C, DeCherney AH, Herrmann W, Naftolin F, Placental and circulating pregnancy-associated plasma protein A concentrates in normal and pathological term pregnancies. Obstet. Gynecol 68, 382–386 (1986). [DOI] [PubMed] [Google Scholar]
- 77.Griffin JF, Pregnancy-associated plasma protein levels at term in normal pregnancy, preeclampsia and essential hypertension. Aust. N. Z. J. Obstet. Gynaecol 23, 11–14 (1983). [DOI] [PubMed] [Google Scholar]
- 78.Sutcliffe RG, Kukulska-Langlands BM, Horne CHW, Maclean AB, Jandial V, Sutherland HW, Gibb S, Bowman AW, Studies on the concentration of pregnancy-associated plasma protein-A during normal and complicated pregnancy. Placenta 3, 71–79 (1982). [DOI] [PubMed] [Google Scholar]
- 79.Kristensen T, Oxvig C, Sand O, Moller NPH, Sottrup-Jensen L, Amino acid sequence of human pregnancy-associated plasma protein A derived from cloned cDNA. Biochemistry 33, 1592–1598 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Conover CA, Oxvig C, Overgaard MT, Christiansen M, Giudice LC, Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J. Clin. Endocrinol. Metab 84, 4742–4745 (1999). [DOI] [PubMed] [Google Scholar]
- 81.Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C, Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: Implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 504, 36–40 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Pedersen JF, Sorensen S, Ruge S, Human placental lactogen and pregnancy-associated plasma protein A in first trimester and subsequent fetal growth. Acta Obstet. Gynecol. Scand 74, 505–508 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Baer RJ, Lyell DJ, Norton ME, Currier RJ, Jelliffe-Pawlowski LL, First trimester pregnancy-associated plasma protein-A and birth weight. Eur. J. Obstet. Gynecol. Reprod. Biol 198, 1–6 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Giudice I, Benintende G, Di Nicolò AM, Mangiameli D, Carrara G, Randazzo C, Sapuppo IM, Gulisano A, Correlation of neonatal weight with maternal serum levels of pregnancy-associated plasma protein-A during the first trimester of pregnancy: A retrospective study. J. Perinat. Med 43, 227–232 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Beneventi F, Simonetta M, Lovati E, Albonico G, Tinelli C, Locatelli E, Spinillo A, First trimester pregnancy-associated plasma protein-A in pregnancies complicated by subsequent gestational diabetes. Prenat. Diagn 31 , 523–528 (2011). [DOI] [PubMed] [Google Scholar]
- 86.Lovati E, Beneventi F, Simonetta M, Laneri M, Quarleri L, Scudeller L, Albonico G, Locatelli E, Cavagnoli C, Tinelli C, Spinillo A, Corazza GR, Gestational diabetes mellitus: Including serum pregnancy-associated plasma protein-A testing in the clinical management of primiparous women? A case-control study. Diabetes Res. Clin. Pract 100, 340–347 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Petry CJ, Ong KK, Hughes IA, Acerini CL, Frystyk J, Dunger DB, Early pregnancy-associated plasma protein A concentrations are associated with third trimester insulin sensitivity. J. Clin. Endocrinol. Metab 102, 2000–2008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Husslein H, Lausegger F, Leipold H, Worda C, Association between pregnancy-associated plasma protein-A and gestational diabetes requiring insulin treatment at 11-14 weeks of gestation. J. Matern. Fetal Neonatal Med 25, 2230–2233 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Savvidou MD, Syngelaki A, Muhaisen M, Emelyanenko E, Nicolaides KH, First trimester maternal serum free β-human chorionic gonadotropin and pregnancy-associated plasma protein A in pregnancies complicated by diabetes mellitus. BJOG 119, 410–416 (2012). [DOI] [PubMed] [Google Scholar]
- 90.Wright D, Silva M, Papadopoulos S, Wright A, Nicolaides KH, Serum pregnancy-associated plasma protein-A in the three trimesters of pregnancy: Effects of maternal characteristics and medical history. Ultrasound Obstet. Gynecol 46, 42–50 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Pellegrinelli V, Peirce VJ, Howard L, Virtue S, TQrei D, Senzacqua M, Frontini A, Dalley JW, Horton AR, Bidault G, Severi I, Whittle A, Rahmouni K, Saez-Rodriguez J, Cinti S, Davies AM, Vidal-Puig A, Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat. Commun 9, 4974 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gealekman O, Guseva N, Gurav K, Gusev A, Hartigan C, Thompson M, Malkani S, Corvera S, Effect of rosiglitazone on capillary density and angiogenesis in adipose tissue of normoglycaemic humans in a randomised controlled trial. Diabetologia 55, 2794–2799 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin X, Sexton C, Byun D, Strong DD, Baylink DJ, Mohan S, Differential regulation of pregnancy associated plasma protein (PAPP)-A during pregnancy in human and mouse. Growth Horm. IGF Res 12, 359–366 (2002). [DOI] [PubMed] [Google Scholar]
- 94.Gérard N, Delpuech T, Oxvig C, Overgaard MT, Monget P, Proteolytic degradation of IGF-binding protein (IGFBP)-2 in equine ovarian follicles: Involvement of pregnancy-associated plasma protein-A (PAPP-A) and association with dominant but not subordinated follicles. J. Endocrinol 182, 457–466 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Monget P, Mazerbourg S, Delpuech T, Maurel M-C, Manière S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT, Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: Identification of cleavage site and characterization of IGFBP-2 degradation. Biol. Reprod 68, 77–86 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Bale LK, Conover CA, Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J. Endocrinol 186, 325–331 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Hjortebjerg R, Berryman DE, Comisford R, List EO, Oxvig C, Bjerre M, Frystyk J, Kopchick JJ, Depot-specific and GH-dependent regulation of IGF binding protein-4, pregnancy-associated plasma protein-A, and stanniocalcin-2 in murine adipose tissue. Growth Horm. IGF Res 39, 54–61 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Rajkumar K, Krsek M, Dheen ST, Murphy LJ, Impaired glucose homeostasis in insulin-like growth factor binding protein-1 transgenic mice. J. Clin. Invest 98, 1818–1825 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silha JV, Gui Y, Murphy LJ, Impaired glucose homeostasis in insulin-like growth factor-binding protein-3-transgenic mice. Am. J. Physiol. Endocrinol. Metab 283, E937–E945 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Carobbio S, Pellegrinelli V, Vidal-Puig A, Adipose tissue function and expandability as determinants of lipotoxicity and the metabolic syndrome. Adv. Exp. Med. Biol 960, 161–196 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN, Molecular evidence supporting the portal theory: A causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab 288, E454–E461 (2005). [DOI] [PubMed] [Google Scholar]
- 102.Conover CA, Harstad SL, Tchkonia T, Kirkland JL, Preferential impact of pregnancy-associated plasma protein-A deficiency on visceral fat in mice on high-fat diet. Am. J. Physiol. Endocrinol. Metab 305, E1145–E1153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bersinger NA, Klopper A, Observations on the source of pregnancy-associated plasma protein A (PAPP-A). Br. J. Obstet. Gynaecol 91, 1074–1076 (1984). [DOI] [PubMed] [Google Scholar]
- 104.Gude MF, Hjortebjerg R, Oxvig C, Thyo AA, Magnusson NE, Bjerre M, Pedersen SB, Frystyk J, PAPP-A, IGFBP-4 and IGF-II are secreted by human adipose tissue cultures in a depot-specific manner. Eur. J. Endocrinol 175, 509–519 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Depot-specific pregnancy-induced changes in adipocyte size at early and late stages of pregnancy.
Data file S1. Primary data.
Data file S2. Genes differentially expressed between adipose tissue of nonpregnant and pregnant women.
Data file S3. Pathway analysis of genes affected by pregnancy in both subcutaneous and omental depots.


