Abstract
Severe COVID-19 is characterized by lung and multiorgan inflammation and coagulation in the presence of overactivation of the complement system. Complement is a double edged-sward in SARS-Cov-2 infection. On one hand, it can control the viral infection in milder cases, on the other hand in cases with severe and prolonged infection massive complement activation occurs, which can intensify lung and systemic inflammation and promote a procoagulant and prothrombotic state. Several uncontrolled studies and controlled clinical trials with different complement inhibitors have been performed and others are ongoing. Results are promising in some but negative in others. Further studies are required to elucidate the benefit to risk profile of complement inhibitors in COVID-19 patients at different stages of the disease and to clarify the best targets in the complement cascade.
Keywords: Complement, Complement inhibitory drugs, COVID-19, Endothelial cells, Thrombosis, Inflammation
1. Activation of the complement system in SARS-CoV-2 infection
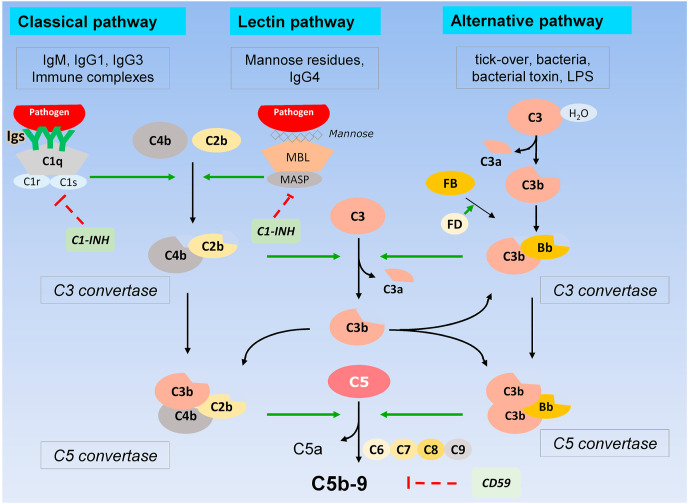
The complement system is an integral component of the innate immune response and represents the host's first immune response to clear pathogens (Noris and Remuzzi, 2013). The complement system is organized in three activation pathways: the classical, the lectin and the alternative pathway (Fig. 1 ) and all are triggered in the presence of pathogens including bacteria and viruses. The three pathways converge on the formation of the classical/lectin and the alternative C3 convertases that cleave C3 into C3b – that binds the surface of pathogens leading to virus aggregation and phagocytosis through interaction with complement receptors present on neutrophils and macrophages - and C3a, a potent anaphylatoxin that attracts and activates inflammatory leukocytes (Noris and Remuzzi, 2013). C3b also contributes to the formation of the C5 convertases, which cleave C5 generating C5a, another anaphylatoxin, and C5b that initiates the formation of the terminal membrane attack complex C5b-9 (Fig. 1) that inserts into the surface of pathogens and of virus-infected cells and causes lysis (Noris and Remuzzi, 2013).
Fig. 1.
Schematic representation of the three complement activation pathways and the common terminal pathway.
Evidence are accumulating that SARS-CoV-2 activates all the three complement pathways (Noris et al., 2020).
SARS-CoV interacts with the lectin pathway recognition molecule mannose binding lectin (MBL), and MBL binding to SARS-CoV-infected cells results in enhanced deposition of C4-activation fragments (Noris et al., 2020; Santiesteban-Lores et al., 2021). SARS-CoV-2 nucleocapsid protein binds the MBL associated serine protease MASP-2, and potentiates MBL interaction and lectin pathway (Santiesteban-Lores et al., 2021).
An in vitro study documented that the SARS-CoV-2 spike protein can directly activate the alternative complement pathway at cell surface level. Indeed, the S1 and S2 subunits of spike protein added to normal human serum increased C5b-9 deposition on a human erythroleukemia cell line. This effect was inhibited by a FD inhibitor that blocks the alternative pathway (Yu et al., 2020).
The classical pathway may be activated in the later phase of infection by virus-specific antibodies and COVID-19-associated autoantibodies (Bosmann, 2021), as documented by staining of COVID-19 patient's lung for C1q along with widely distributed IgG staining (Macor et al., 2021).
Ramlall et al. (2020) by transcriptional profiling of nasopharyngeal swabs of SARS-CoV-2 infected patients, demonstrated strong transcription of genes encoding proteins of the classical/lectin (C1q, C4, C2) and alternative (factor B -FB-, C3) pathways. Finally, in a prospective cohort study, the large majority of COVID-19 hospitalized patients showed higher than normal plasma levels of complement activation products of the classical/lectin (C4d) and alternative pathway (C3bBbP, Fig. 1), as well as of C5a and sC5b-9, indicating systemic activation of the three complement pathways until the terminal common pathway (Holter et al., 2020). Notably, in another study (Ma et al., 2021), plasma sC5b-9 levels were higher in patients with COVID-19 vs. those with influenza and patients with non-COVID-19 respiratory failure, identifying terminal complement activation as a distinctive feature of COVID-19.
Local complement activation and inflammatory response may effectively contribute to the control of SARS-CoV-2 infection in the first phases of the disease. However, unstrained complement activation, as it occurs in the context of intense and prolonged SARS-CoV-2 infections, can be detrimental, contributing to damage of host tissues, and to lung and multi-organ dysfunction.
2. Lung inflammation and injury and complement activation
Immunohistochemical analysis of lungs of patients who died of COVID-19 showed deposits of C1q, C4, C3 and C5b-9 localized in the capillaries of the interalveolar septa, in alveolar epithelial cells as well as in infiltrating leukocytes (Macor et al., 2021; Magro et al., 2020; Carvelli et al., 2020).
Evaluation of complement circulating parameters revealed a strong association of the degree of complement activation – particularly of the terminal pathway-with disease severity and adverse outcome. In a prospective study, plasma levels of sC5b-9 and C4d were significantly higher in COVID-19 patients with respiratory failure than in patients without such complication (Holter et al., 2020), and logistic regression showed an association between sC5b-9 concentrations and odds of respiratory failure (Holter et al., 2020). In another cohort, plasma levels of C3a and sC5b-9 were higher in patients in intensive care unit (ICU) as compared with non-ICU patients (de Nooijer et al., 2021). Also the plasma levels of the other product of the terminal pathway, C5a, are elevated in COVID-19 patients and were found to be proportional to disease severity, reaching maximal values in the group developing acute respiratory distress syndrome (ARDS) (Carvelli et al., 2020).
C5a is a strong chemoattractant that can recruit and activate neutrophils and other myeloid leukocytes at the site of infection, and stimulate the release of reactive oxygen species and neutrophil extracellular traps (NETs) that damage the alveolar epithelium and increase the permeability of alveolar capillaries, causing edema (Santiesteban-Lores et al., 2021). Excessive production of C5a leads to massive release of cytokines from circulating and infiltrating leukocytes, a phenomenon called cytokine storm (Ragab et al., 2020). Blood neutrophils and monocytes of patients with COVID-19-associated pneumonia and ARSD express high levels of the C5a receptor (C5aR1), and in vitro exposure to C5a increased the production of the inflammatory cytokines IL-6, TNF alpha and CCl2 by blood monocytes isolated from COVID-19 patients (Carvelli et al., 2020). Consistently, C5a was detected in the broncoalveolar lavage fluid of COVID-19 patients who developed ARSD along with large numbers of neutrophils and monocytes expressing C5aR1, and inflammatory cytokines and chemokines (Carvelli et al., 2020).
Altogether these data support a role of C5a as a mediator of the exaggerated inflammatory response and the associated lung injury and dysfunction in severe COVID-19.
3. Endothelial dysfunction, coagulopathy, thrombotic microangiopathy, and complement activation
COVID-19 often manifests with a procoagulant state that associates with disease severity and fatal outcome (Noris et al., 2020). It has been estimated that more than 70% of deaths related to COVID-19 are attributable to clotting-associated complications often leading to multiorgan failure (Pujhari et al., 2021).
Patients with COVID-19 associated coagulopathy have strongly elevated levels of plasma D-dimer, a fibrin degradation product present in the blood after activation of fibrinolysis (Spiezia et al., 2020). This was already known from the China experience. In a retrospective study of March 2020 on 191 patients from Wuhan, D-dimer levels over 1 microgr/mL at admission predicted an 18-fold increase in the odds of dying (Zhou et al., 2020).
These findings are consistent with histologic examinations of autoptic tissues from COVID-19 cases that confirmed high incidence of venous thromboembolisms (Lodigiani et al., 2020), and microvascular thrombi with endothelial injury and swelling consistent with a thrombotic microangiopathy, in the lungs but also in the heart, liver, small bowel, kidney and other organs (Goette et al., 2020; Varga et al., 2020; Pellegrini et al., 2021). Such complications have been observed also in pediatric population. In a study on 50 hospitalized pediatric patients with acute SARS-CoV-2 infection, 48% met criteria for thrombotic microangiopathy with hemolytic anemia, thrombocytopenia and organ damage mainly in the liver and the kidney (Diorio et al., 2020).
Evidence is accumulating that complement activation is associated with multi-organ thrombosis in COVID-19 patients. A pattern of microvascular injury and thrombosis with vasculopathy associated with C5b-9 deposition was observed in the lung of 5 patients with severe COVID-19 (Magro et al., 2020). Vascular C5b-9 deposition was also seen in the skin of patients affected by retiform purpura indicating ischemia of the affected tissue (Magro et al., 2020).
Analysis of kidney specimens revealed strong C5b-9 staining in peritubular capillaries, in glomerular afferent and efferent arterioles and traces in the glomerular tuft along with fibrin microthrombi occluding glomerular capillaries (Noris et al., 2020). Other authors described C1q, C4, C3 and C5b-9 deposits on tubules and vessels of kidneys and on hepatic artery and portal vein of the liver (Macor et al., 2021; Pfister et al., 2020). Cardiac microthrombi from COVID-19-positive autopsy cases showed more fibrin and C5b-9 immunostaining compared with intramyocardial thromboemboli from COVID-19 negative patients with ST-elevation myocardial infarction (Pellegrini et al., 2021).
Finally, in a study in 197 hospitalized patients with COVID-19, higher baseline plasma concentrations of C3a and sC5b-9 were observed in patients who developed a thromboembolic event, and sC5b-9 concentrations correlated with D-dimer levels (de Nooijer et al., 2021). Collectively, available data support a link between complement activation and thrombotic complications of SARS-CoV-2 infection.
Among the several effector molecules generated by complement activation cascade, the terminal products C5a and C5b-9 are the most likely candidates for promoting endothelial perturbation and vascular thrombosis, through multiple processes (Noris et al., 2020).
C5a increases tissue factor activity on endothelial cells (Ikeda et al., 1997) and in the circulation (Ritis et al., 2006). C5a and the C5b-9 induce the exocytosis of P-selectin and von Willebrand factor multimers (VWF) from endothelial cells (Hattori et al., 1989) that promote platelet adhesion and aggregation, and the shedding of thrombomodulin from cell surface (Bettoni et al., 2017), which triggers the coagulation cascade. C5a also stimulates the production of NETs which contain antimicrobial peptides important to prevent the spread of infections, but may also trap platelets and promote coagulopathy and endothelial dysfunction (Guo and Ward, 2005).
Of interest, neutrophils isolated from COVID-19 patients spontaneously release NETs expressing tissue factor. In addition, control neutrophils stimulated with platelet rich plasma (PRP) from COVID-19 patients generate NETs that induce tissue factor expression in human aortic endothelial cells in vitro and both effects were attenuated by C5a receptor blockade (Skendros et al., 2020). Finally, plasma from severe COVID-19 patients requiring mechanical ventilation activated control neutrophils to release NETs, an effect that was restrained by an anti-C5a neutralizing antibody (Zhang et al., 2021).
4. The yin and the yang of complement inhibition in COVID-19 patients
Complement inhibition has been proposed as a potential target in limiting tissue inflammation and thrombotic complications associated with COVID-19 (Noris et al., 2020; Risitano et al., 2020), but great care should be taken in choosing between the drugs that are currently available or in advanced clinical development (Noris et al., 2020; Ricklin et al., 2019; Mastellos et al., 2019). Timing is also relevant since shutting down complement activation components that restrict viral propagation may be harmful while preventing uncontrolled activation is desirable (Song and FitzGerald, 2020)
In this context, C3 inhibitors do not appear to be a safe option for COVID-19 patients, since these drugs will prevent the activation of all three complement pathways in response to viral infections. Studies to establish the relationship between the protective role of the lectin complement pathway in virus clearance vs. its potential pathogenetic roles in sustaining inflammatory response and tissue injury will be instrumental in the perspective of clinical studies with specific inhibitors such as anti-MASP2 antibodies (Rambaldi et al., 2020), in COVID-19 patients.
On the other hand, C5 inhibitors could exert a favorable effect by blocking the proinflammatory and prothrombotic actions of the terminal products of the complement cascade (C5a and C5b-9) activated by SARS-CoV-2, while preserving the activity of early complement components that are important for viral clearance and activation of the adaptive immune response (Noris and Remuzzi, 2013; Noris et al., 2020; Stoermer and Morrison, 2011).
Terminal complement inhibition with the anti-C5 monoclonal antibody eculizumab has been shown to be an effective therapeutic tool in thrombotic, hematological and inflammatory diseases (Wijnsma et al., 2019). In particular, eculizumab blocked venous thromboembolic events in paroxysmal nocturnal hemoglobinuria and thrombotic microangiopathy in atypical hemolytic uremic syndrome (Wijnsma et al., 2019). Thus, C5 inhibition could theoretically also protect COVID-19 patients from complement-mediated lung damage and prevent thromboembolic events. In this context, case report and case series showed positive effects on clinical outcomes in patients with severe COVID-19 treated with eculizumab or other C5 blockers (Peffault de Latour et al., 2020), (Laurence et al., 2020), (Diurno et al., 2020), even in combination with the JAK1/2 inhibitor ruxolitinib (Giudice et al., 2020).
In a non-randomized proof-of-concept French study (Annane et al., 2020) in patients with severe COVID-19 (with pneumonia, acute lung injury or ARDS requiring oxygen supplementation) and admitted to ICU, 45 patients were treated with standard care and 35 with standard care plus eculizumab. The initial regimen in the first 10 patients consisted of 900 mg of eculizumab at day 1, 8, 15 and 22 of ICU admission. This dosage resulted in transient and incomplete inhibition of the terminal pathway, which lead to protocol amendment and the subsequent patients received higher and more frequent doses of the drug (1200 mg on days 1, 4 and 8 and 900 mg on days 15 and 22). Survival at day 15 (primary end-point) and at day 28 was higher and survival time was prolonged in eculizumab treated patients compared to control patients (Annane et al., 2020). In addition, C5 inhibition was associated with faster improvement in the ratio of arterial oxygen tension over fraction of inspired oxygen (PaO2/FiO2), with better renal function and steeper decline in cytokine blood levels (Annane et al., 2020). However, total complement activity (CH50) was detectable at day 7 in two third of eculizumab treated patients indicating that the terminal pathway was not completely inhibited by the drug, even with the intensified regimen (Annane et al., 2020). Massive activation of complement and the increased expression of complement components associated with severe and prolonged SARS-CoV-2 infection (Santiesteban-Lores et al., 2021) may have played a role in the insufficient C5 inhibition by eculizumab.
On the other hand, a phase 3 study trial with another anti-C5 antibody, ravulizumab (ClinicalTrials.gov identifier, NCT4369469), in adults with severe COVID-19 requiring mechanical ventilation, has recently halted patient recruitment due to lack of efficacy as revealed by interim analysis (https://ir.alexion.com/news-releases/news-release-details/alexion-provides-update-phase-3-study-ultomirisr-ravulizumab). So, the therapeutic potential of C5 blockade in COVID-19, which patients could benefit the most, along with the suitable dosage, are still unanswered questions. Other trials are investigating the C5 inhibitors zilucoplan (ClinicalTrials.gov Identifiers: NCT04382755,NCT04590586) and eculizumab (ClinicalTrials.gov Identifiers: NCT04346797;NCT04351503) in COVID-19 patients, and FDA approved a program of eculizumab off-label compassionate use for the treatment of non-intubated patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04288713). Finally, a randomised, parallel arm, open-label trial is ongoing with the immunomodulatory agent Baricitinib (Kalil et al., 2021) - a Janus kinase inhibitor- and Ravulizumab in pre-ICU Patients admitted with COVID-19 (ClinicalTrials.gov Identifier: NCT04390464).
Based on the above background, our center activated two compassionate use protocols for an expanded access program to C5 blockade with eculizumab therapy in patients with mild/moderate (in need of high-flow nasal oxygenation) or advanced (in need of continuous positive airway pressure ventilation) COVID-19 pneumonia. Preliminary results in patients with advanced COVID-19 are encouraging showing improved respiratory dysfunction and long-term outcomes.
Blocking C5 activation prevents the formation of both terminal pathway products, C5a and C5b-9, thus exposing the patients to risk of developing infections. In this regard, in the French study, the eculizumab-treated group experienced more secondary bacterial infections versus the control group (Annane et al., 2020).
Selective blocking of C5a/C5aR could have advantages over C5 blockade since it preserves the formation of the C5b-9 membrane attack complex, which is crucial to kill pathogens. In addition, this approach will inhibit the C5a-mediated recruitment of inflammatory leukocytes in lung and other organs (Carvelli et al., 2020), and limit the production of pro-thrombotic factors from immune cells, platelets, and endothelium (Noris et al., 2020; Woodruff and Shukla, 2020; Skendros et al., 2020; Huber-Lang et al., 2001; Riedl et al., 2017).
A preliminary preprint report on two patients who received the anti-C5a antibody BDB-001 showed improvement of clinical condition with normalization of body temperature and increased oxygenation index within a few days (Noris et al., 2020). A multi-center, open-label, randomized parallel controlled phase II/III trial is ongoing to evaluate on the efficacy and safety of BDB-001 injection in the treatment of patients with progressive COVID-19 – with respiratory distress requiring a mask oxygen therapy or high-flow nasal cannula oxygen therapy and pulmonary imaging showing lesion progression >50% within 24–48 hours (ClinicalTrials.gov Identifier: NCT04449588)-.
A proof-of-concept, phase 2 randomized study (first part of the adaptive phase 2/3 PANAMO trial, ClinicalTrials.gov Identifier: NCT04333420) has investigated the effect of the anti-C5a antibody IFX-1 in 15 adults with severe COVID-19 as compared with 15 controls receiving best supportive care only (Vlaar et al., 2020). The relative change in the PaO2/FiO2 in the supine position between baseline and day 5 (primary outcome) did not differ between treatment groups, although secondary outcome results showed a lower incidence of serious pulmonary embolisms in patients receiving IFX-1 than in the control group (Vlaar et al., 2020). However, the study was not powered on this endpoint and results should be confirmed in the ongoing larger Phase 3 of the trial: a double-blind, placebo-controlled, randomized phase comparing standard of care plus IFX-1 versus standard of care plus placebo-to-match in 360 patients (NCT04333420).
Another therapeutic option to inhibit the C5a/C5aR1 signaling may be the blockade of the C5aR1 receptor. The orally active C5aR1 inhibitor avacopan in ANCA-associated vasculitis was effective in replacing high-dose steroids (Jayne et al., 2021). Emerging results of C5aR1 inhibition in COVID-19 are much less encouraging, indeed a phase II clinical trial evaluating the safety and efficacy of the anti-C5aR1 antibody, avdoralimab, in COVID-19 patients with severe pneumonia (ClinicalTrials.gov Identifier: NCT04371367), did not meet its primary endpoints (https://www.thepharmaletter.com/article/avdoralimab-phase-ii-force-trial-in-covid-19-misses-endpoints).
Under clinical investigation are also C1 esterase inhibitors. In the complement system, C1 esterase inhibitor (C1–INH) protein inactivates C1r and C1s of the classical pathway and mannose-binding lectin-associated serine proteases (MASPs) of the lectin pathway (Fig. 1) (Adesanya et al., 2021) and inhibits components of the coagulation cascade, plasmin and kallikrein, reducing complement-driven inflammation and coagulation. High-throughput yeast two-hybrid screening demonstrated that C1–INH interacts with seven different SARS-CoV proteins (Adesanya et al., 2021). In addition, serum levels of C1–INH were found to predict progression to respiratory distress in COVID-19 (Shen et al., 2020) and a single nucleotide polymorphism (SNP) in SERPING1, the gene encoding C1–INH, was one of seven complement-related SNPs most associated with adverse outcome (Ramlall et al., 2020).
In a preliminary study, the human recombinant C1 esterase inhibitor Conestat alpha (Ruconest) was administered to 5 adult patients with severe COVID-19 pneumonia (Urwyler et al., 2020). Treatment was well-tolerated and rapidly reduced fever, levels of inflammatory markers and oxygen supplementation in 4 patients, only one required mechanical ventilation. All five patients recovered (Urwyler et al., 2020). Encouraged by these results, a randomized controlled phase 2 trial has been initiated in the US with Ruconest in the prevention of severe disease in hospitalized patients with COVID-19 (PROTECT-COVID-19-US, ClinicalTrials.gov Identifier: NCT04530136). Inclusion criteria are: evidence of pulmonary involvement on CT scan or X-Ray of the chest; symptom onset within the previous 10 days; and at least one additional risk factor for progression to mechanical ventilation: 1) arterial hypertension, 2) >50 years, 3) obesity (BMI >30.0 kg/m2), 4) history of cardiovascular disease, 5) chronic pulmonary disease, 7) chronic renal disease, 6) C-reactive protein of >35 mg/L, 7) oxygen saturation at rest in ambient air of <94%. A similar trial is ongoing in Switzerland and Brazil (ClinicalTrials.gov Identifier: NCT04414631).
5. Conclusions
Collectively, available evidence clearly demonstrated that alike in bacterial sepsis, severe SARS-Cov-2 infection induces uncontrolled complement activation that involves the classical, lectin and alternative pathway pathways. This process leads to formation of several effectors including the terminal products C5a and C5b-9, which contribute to lung inflammation and injury, endothelial injury and dysfunction with subsequent spreading of the inflammatory response to the circulation and to the other organs. Complement-mediated coagulopathy and systemic microangiopathy result in severe complications, multiorgan failure and eventually death.
Several uncontrolled studies and controlled clinical trials with different complement inhibitors have been performed and others are ongoing. Results are promising in some but negative in others, and further studies are required to define a number of critical points, namely: 1) the optimal time to initiate anti-complement therapy; 2) the best target within the several molecules and activation fragments of the complement system; 3) the suitable primary end-point; and 4) the risk of complication from other infections.
Declaration of competing interest
Marina Noris has received honoraria from Alexion Pharmaceuticals for giving lectures, and for participating in advisory boards and research grants from Omeros, Alnylam and Chemocentryx.
Acknowledgments
This work was partially supported by Fondazione Cav. Lav. CARLO PESENTI.
References
- Adesanya T.M.A., Campbell C.M., Cheng L., Ogbogu P.U., Kahwash R. C1 esterase inhibition: targeting multiple systems in COVID-19. J. Clin. Immunol. 2021;41:729–732. doi: 10.1007/s10875-021-00972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D., Heming N., Grimaldi-Bensouda L., et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine. 2020;28:100590. doi: 10.1016/j.eclinm.2020.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettoni S., Galbusera M., Gastoldi S., et al. Interaction between multimeric von Willebrand factor and complement: a fresh look to the pathophysiology of microvascular thrombosis. J. Immunol. 2017;199:1021–1040. doi: 10.4049/jimmunol.1601121. [DOI] [PubMed] [Google Scholar]
- Bosmann M. Complement control for COVID-19. Sci. Immunol. 2021:6. doi: 10.1126/sciimmunol.abj1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli J., Demaria O., Vely F., et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooijer A.H., Grondman I., Janssen N.A.F., et al. Complement activation in the disease course of coronavirus disease 2019 and its effects on clinical outcomes. J. Infect. Dis. 2021;223:214–224. doi: 10.1093/infdis/jiaa646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio C., McNerney K.O., Lambert M., et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4:6051–6063. doi: 10.1182/bloodadvances.2020003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diurno F., Numis F.G., Porta G., et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- Giudice V., Pagliano P., Vatrella A., et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front. Pharmacol. 2020;11:857. doi: 10.3389/fphar.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goette A., Patscheke M., Henschke F., Hammwohner M. COVID-19-Induced cytokine release syndrome associated with pulmonary vein thromboses, atrial cardiomyopathy, and arterial intima inflammation. TH Open. 2020;4:e271–e279. doi: 10.1055/s-0040-1716717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R.F., Ward P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Hattori R., Hamilton K.K., McEver R.P., Sims P.J. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 1989;264:9053–9060. [PubMed] [Google Scholar]
- Holter J.C., Pischke S.E., de Boer E., et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117:25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Lang M., Sarma V.J., Lu K.T., et al. Role of C5a in multiorgan failure during sepsis. J. Immunol. 2001;166:1193–1199. doi: 10.4049/jimmunol.166.2.1193. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Nagasawa K., Horiuchi T., Tsuru T., Nishizaka H., Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb. Haemostasis. 1997;77:394–398. [PubMed] [Google Scholar]
- Jayne D.R.W., Merkel P.A., Schall T.J., Bekker P. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 2021;384:599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J., Mulvey J.J., Seshadri M., et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin. Immunol. 2020;219:108555. doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Sahu S.K., Cano M., et al. 2021. Increased Complement Activation is a Distinctive Feature of Severe SARS-CoV-2 Infection. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macor P., Durigutto P., Mangogna A., et al. 2021. Multi-organ Complement Deposition in COVID-19 Patients. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos D.C., Ricklin D., Lambris J.D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 2019;18:707–729. doi: 10.1038/s41573-019-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M., Remuzzi G. Overview of complement activation and regulation. Semin. Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peffault de Latour R., Bergeron A., Lengline E., et al. Complement C5 inhibition in patients with COVID-19 - a promising target? Haematologica. 2020;105:2847–2850. doi: 10.3324/haematol.2020.260117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini D., Kawakami R., Guagliumi G., et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- Pfister F., Vonbrunn E., Ries T., et al. Complement activation in kidneys of patients with COVID-19. Front. Immunol. 2020;11:594849. doi: 10.3389/fimmu.2020.594849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujhari S., Paul S., Ahluwalia J., Rasgon J.L. Clotting disorder in severe acute respiratory syndrome coronavirus 2. Rev. Med. Virol. 2021;31:e2177. doi: 10.1002/rmv.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A., Gritti G., Mico M.C., et al. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020;225:152001. doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlall V., Thangaraj P.M., Meydan C., et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020;26:1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Mastellos D.C., Lambris J.D. Therapeutic targeting of the complement system. Nat. Rev. Drug Discov. 2019 doi: 10.1038/s41573-019-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl M., Noone D.G., Khan M.A., et al. Complement activation induces neutrophil adhesion and neutrophil-platelet aggregate formation on vascular endothelial cells. Kidney Int. Rep. 2017;2:66–75. doi: 10.1016/j.ekir.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano A.M., Mastellos D.C., Huber-Lang M., et al. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritis K., Doumas M., Mastellos D., et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- Santiesteban-Lores L.E., Amamura T.A., da Silva T.F., et al. A double edged-sword - the Complement System during SARS-CoV-2 infection. Life Sci. 2021;272:119245. doi: 10.1016/j.lfs.2021.119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Yi X., Sun Y., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72 e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skendros P., Mitsios A., Chrysanthopoulou A., et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.C., FitzGerald G.A. COVID-19, microangiopathy, hemostatic activation, and complement. J. Clin. Invest. 2020;130:3950–3953. doi: 10.1172/JCI140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiezia L., Boscolo A., Poletto F., et al. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemostasis. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoermer K.A., Morrison T.E. Complement and viral pathogenesis. Virology. 2011;411:362–373. doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler P., Moser S., Charitos P., et al. Treatment of COVID-19 with Conestat alfa, a regulator of the complement, contact activation and kallikrein-kinin system. Front. Immunol. 2020;11:2072. doi: 10.3389/fimmu.2020.02072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaar A.P.J., de Bruin S., Busch M., et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020;2:e764–e773. doi: 10.1016/S2665-9913(20)30341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnsma K.L., Ter Heine R., Moes D., et al. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin. Pharmacokinet. 2019;58:859–874. doi: 10.1007/s40262-019-00742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T.M., Shukla A.K. The complement C5a-C5aR1 GPCR Axis in COVID-19 therapeutics. Trends Immunol. 2020;41:965–967. doi: 10.1016/j.it.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Han K., Du C., et al. Carboxypeptidase B blocks ex vivo activation of the anaphylatoxin-neutrophil extracellular trap axis in neutrophils from COVID-19 patients. Crit. Care. 2021;25:51. doi: 10.1186/s13054-021-03482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]