Abstract
Gastrointestinal symptoms (GIS) are commonly reported in children with autism spectrum disorder (ASD). This multi-site study evaluated the prevalence of GIS in preschool-aged children with ASD/(n = 672), with other developmental delays (DD)/(n = 938), and children in the general population (POP)/(n = 851). After adjusting for covariates, children in the ASD group were over 3 times more likely to have parent-reported GIS than the POP group, and almost 2 times more likely than the DD group. Children with GIS from all groups had more behavioral and sleep problems. Within the ASD group, children with developmental regression had more GIS than those without; however, there were no differences in autism severity scores between children with and without GIS. These findings have implications for clinical management.
Keywords: Autism spectrum disorder, Gastrointestinal, Preschool, Developmental delay
Introduction
Children with autism spectrum disorder (ASD) are at increased risk for co-occurring medical conditions (Aldinger et al. 2015). Gastrointestinal symptoms (GIS) have frequently been reported at higher rates in children with ASD and can have an impact on health, behavior, and quality of life (Buie et al. 2010). Therefore, the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee Strategic Plan for Autism Spectrum Disorder Research–2013 Update recommended more research into the etiology of GI issues in individuals with ASD in order to develop adequate interventions. A meta-analysis found that children with ASD have a higher prevalence of GIS than children without developmental delays (DD) (McElhanon et al. 2014). However, there is a lack of studies with diverse, well characterized subjects that include control groups, use objective measures, and are large enough to adjust for potential confounders to guide development of appropriate management guidelines (McElhanon et al. 2014). In addition, identifying clusters of comorbidities in children with ASD may help to elucidate etiologies of ASD (Doshi-Velez et al. 2014).
The primary aim of this study was to determine the prevalence of GIS in a well-characterized and geographically diverse sample of children with ASD compared to children with other DD and children recruited from the general population (POP). The secondary aim was to evaluate associations between GIS and neurodevelopmental phenotypes.
Methods
Participants
The Study to Explore Early Development (SEED) (Schendel et al. 2012) is a multisite, community-based case–control study of children 30–68 months of age designed to evaluate genetic and environmental risk factors for ASD. Children with ASD and other DD were recruited from early intervention and special education programs, and from healthcare providers serving children with disabilities. Children in the POP group were randomly selected from birth certificates at each site. A child was eligible if he or she lived with a caregiver from 6 months of age who was fluent in English (English or Spanish at two sites) and could provide legal consent, and if the child was born and continued to live in the specific catchment areas of the SEED sites located in California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania (Schendel et al. 2012). A detailed description of study methods can be found in Schendel et al. (2012). This study was approved by the institutional review boards at all participating sites. All families provided written consent for participation.
Upon enrollment, all children were screened for ASD with a parent questionnaire, the Social Communication Questionnaire (SCQ) (Rutter et al. 2003), and underwent an in-person developmental evaluation using the Mullen Scales of Early Learning (MSEL) (Mullen 1995). The Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 1999), and the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994) were completed for children with a previous diagnosis of ASD, a SCQ score of 11 or above, or when concerns for ASD were noted during administration of the MSEL. A research classification of ASD was made using an ADOS and ADI-R Algorithm: An ASD classification was used if a child met ADOS criteria and one of three ADI-R relaxed criteria: (1) met the social domain cutoff and was within two points of the communication domain cutoff, or (2) met the communication domain cutoff and was within two points of the social domain cutoff, or (3) met the social domain cutoff and had at least two points on the behavioral domain. As shown in Fig. 1, if a child did not meet our research criteria for ASD they were moved to the DD group or back to the POP group (Wiggins et al. 2015).
Fig. 1.

SEED research classifications. ASD autism spectrum disorder, DD developmental delays, POP children from the general population, SCQ social communication questionnaire, SCQ + (score ≥ 11), SCQ – (score < 11), ADOS autism diagnostic observation schedule, ADI-R autism diagnostic interview-revised. ADOS and ADI-R Algorithm: ASD classification if child met ADOS criteria and one of three ADI-R relaxed criteria: (1) met the social domain cutoff and was within two points of the communication domain cutoff, or (2) met the communication domain cutoff and was within two points of the social domain cutoff, or (3) met the social domain cutoff and had at least two points on the behavioral domain
Procedures
Gastrointestinal Questionnaire (GIQ): A parent-completed survey of GIS, created for SEED, included yes/no questions about GIS present “on a regular basis” (> 2 times per month). Parents were asked about any current GIS and more specific symptoms including vomiting, diarrhea, loose stools, constipation, loose stools alternating with constipation, abdominal pain with meals, abdominal pain relieved by defecation, pain on stooling, gas, and “other” GI problems; treatment for constipation in the previous 30 days; and presence of GIS in the past that were no longer present. This questionnaire has not been validated (Appendix 1).
Stool Diary (SD): Caregivers were also asked to complete a seven-day SD where they recorded each stool and rated its form and consistency based on the Bristol Stool Form Scale (BSS) which characterizes stool into seven types ranked from hard to liquid (Lewis and Heaton 1997). The BSS correlates with intestinal transit time, and has been used in pediatric populations (Russo et al. 2013).
Gastrointestinal Symptoms (GIS): GIS were defined dichotomously in two different ways. The first is based on a parent’s yes/no response to overall GIS and subtypes of GIS from the GIQ (GIS/PR). The second is based on at least one yes from a six-item variable combining some responses from the GIQ with those from the stool diary (GIS/PRSD) including: (1) four or more stools per day; (2) constipation—two or more hard stools per week, less than three stools per week with at least one hard stool, and/or use of a laxative or stool softener; (3) loose stools/diarrhea—more than 1/3 of stools per week are loose or watery; (4) vomiting; (5) abdominal pain with stooling, relieved by stooling, or during meals; or (6) gas. Only “typical weeks” were included. While treatment of constipation was defined only by specific use of a laxative or stool softener in the GIS/PRSD, parent’s answers included the use of any treatment for constipation except food in the GIS/PR. Two developmental pediatricians reviewed the treatments for constipation reported by parents and agreed on a classification of stool softener or laxative.
Demographic and Phenotypic Variables
Information regarding child sex; socio-demographic variables (some race/ethnicity variables were combined to avoid small numbers in some categories); and child’s genetic/neurologic diagnoses (Down Syndrome, Fragile X Syndrome, Rett Syndrome, Tuberous Sclerosis, Cerebral Palsy, and Neurofibromatosis) were collected via a telephone interview with the primary caregiver. In addition, the child’s caregiver was specifically asked if the child was diagnosed with celiac disease, irritable bowel syndrome, Crohn’s disease, and ulcerative colitis. There was also an option for “other.” Behavioral characteristics were obtained from the Child Behavior Checklist (CBCL), a standardized parent/caregiver questionnaire with well-established reliability and validity (Achenbach 1992). The anxious/depressed, attention problems, and aggressive behavior scales were examined (Achenbach 1992). Sleep problems were evaluated using the Children’s Sleep Habits Questionnaire (CSHQ), a 33-item parent-completed questionnaire (Owens et al. 2000a, b) Higher scores indicate more sleep concerns. While a total score of > 41 has been used to define sleep problems clinically (Owens et al. 2000a, b), a more conservative score of > 48 was used for this analysis as previously described (Reynolds et al. 2019). This score was based on the highest quartile total score in the POP group as about 25% of young children with typical development have sleep problems (Teng et al. 2012). Developmental regression was ascertained from the ADI-R (Lord et al. 1994), and severity of ASD symptoms from the ADOS Calibrated Severity Scale (CSS) (Shumway et al. 2012).
Analysis
Multivariable logistic regression generalized estimating equation (GEE) models were used to assess between-group differences in GIS, comparing the ASD group to DD and POP; and among children with ASD, to analyze the association between GIS and developmental regression. Among children with ASD, GEE models were used to compare ADOS CSS least squares mean (LS-mean) scores in children with and without GIS/PR. Among all children, GEE models were used to compared LS-mean scores of anxious/depressed, aggressive behavior, and attention problems subscale scores, and multivariable logistic regression GEE models were used to compare the prevalence of sleep problems (CSHQ total score > 48) between children with and without GIS/PR. All models were adjusted for possible correlation by enrollment site, assuming an independent correlation structure. To account for differences in socioeconomic status (SES) of participants, base models were adjusted for maternal race/ethnicity, education, and age at child’s birth. Models were also adjusted for child sex and MSEL score. Potential confounders were identified a priori and included household income, maternal language, child enrollment age, and presence of a genetic condition. Additional covariates were included in the final model if they were associated with both exposure and outcome at p < 0.20 and changed the estimate of interest by 10% or more. P-values < 0.05 were considered statistically significant in multivariable analyses.
Results
The sample included 672 children with ASD, 938 children with DD, and 851 POP children whose parent completed the GIQ. Of those, 64% also completed a stool diary and 51% completed a stool diary during a typical week as shown in Fig. 2. Children with ASD were more likely to be male and to have a lower MSEL score compared to DD and POP children as shown in Table 1. We have included more details regarding race ethnicity in Supplemental Table 1. Children with and without SD differed in all demographic characteristics except sex as shown in Table 2. In addition, parents of children with ASD treated for constipation were less likely to complete a SD than parents of children in DD and POP groups (44% vs. 58% and 55%). Of the children with a stool diary, children with ASD were less likely to use treatments for constipation that were a laxative or stool softener than DD and POP groups (46% vs. 72 and 71%) as shown in Table 3.
Fig. 2.
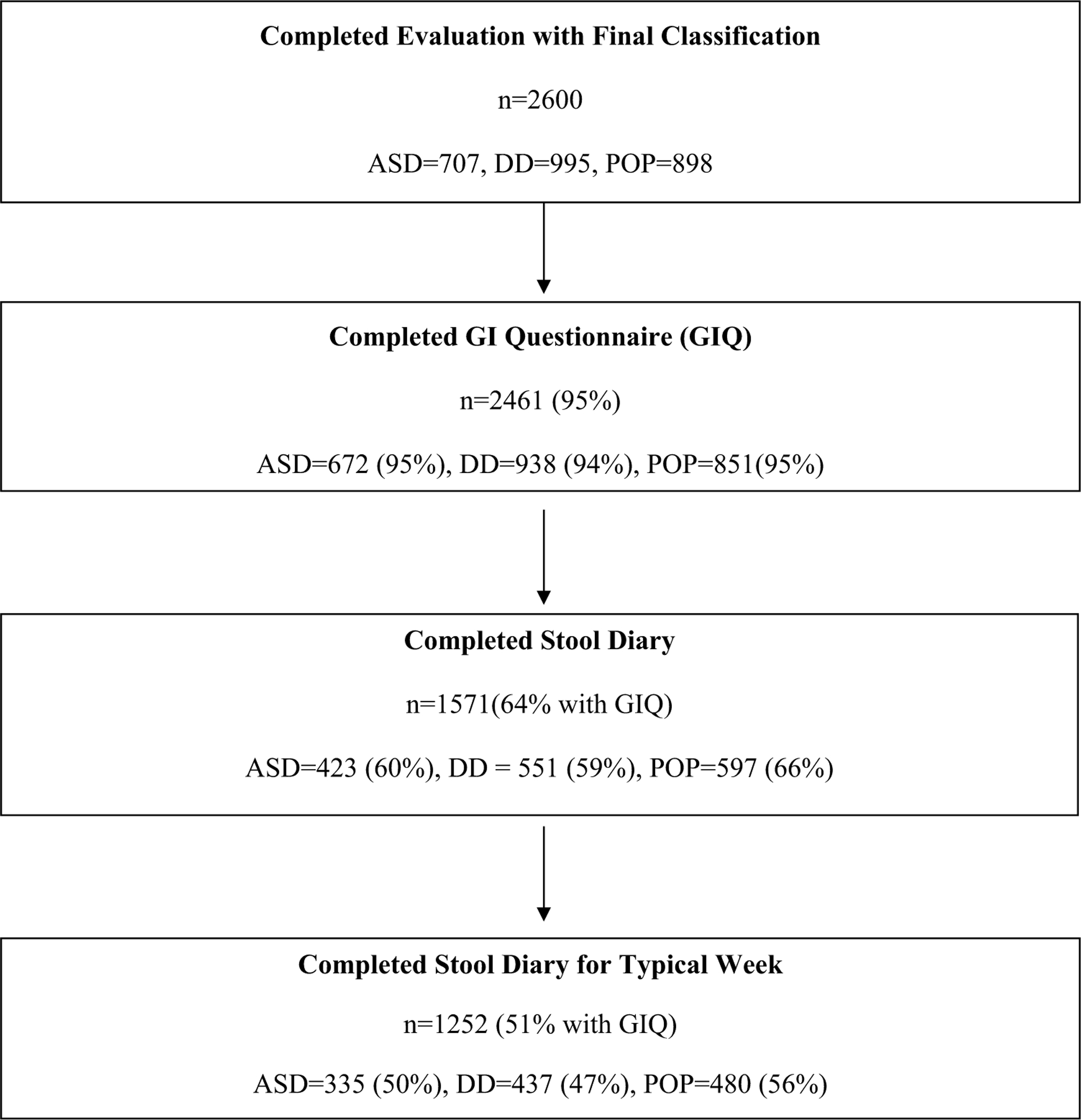
Subject disposition for children with autism spectrum disorder (ASD), developmental delays (DD), and children from the general population (POP)
Table 1.
Demographic characteristics for children whose parent completed the gastrointestinal questionnaire
| Variable | ASD (n = 672) n (%) |
DD (n = 938) n (%) |
POP (n = 51) n (%) |
p-value |
|---|---|---|---|---|
| Child sex | < 0.0001 | |||
| Male | 552 (82.1) | 626 (66.7) | 459 (53.9) | |
| Female | 120 (17.9) | 312 (33.3) | 392 (46.1) | |
| Maternal education | < 0.0001 | |||
| Some college and less | 315 (46.9) | 422 (45.0) | 278 (32.7) | |
| Bachelor’s degree | 207 (30.8) | 283 (30.2) | 309 (36.3) | |
| Graduate school | 142 (21.1) | 227 (24.2) | 255 (30.0) | |
| Missing | 8 (1.2) | 6 (0.6) | 9 (1.1) | |
| Maternal race/ethnicity | < 0.0001 | |||
| White non-hispanic | 366 (54.5) | 553 (59.0) | 596 (70.0) | |
| Other non-hispanic | 214 (31.8) | 245 (26.1) | 166 (19.5) | |
| Hispanic | 83 (12.4) | 130 (13.9) | 75 (8.8) | |
| Missing | 9 (1.3) | 10 (1.1) | 14 (1.6) | |
| Maternal primary language | < 0.0001 | |||
| English | 587 (87.4) | 814 (86.8) | 787 (92.5) | |
| Spanish | 34 (5.1) | 81 (8.6) | 25 (2.9) | |
| Other | 45 (6.7) | 37 (3.9) | 31 (3.6) | |
| Missing | 6 (0.9) | 6 (0.6) | 8 (0.9) | |
| Household income | < 0.0001 | |||
| < $50 Thousand | 252 (37.5) | 342 (36.5) | 212 (24.9) | |
| $50-$90 Thousand | 209 (31.1) | 287 (30.6) | 289 (34.0) | |
| > $90 Thousand | 182 (27.1) | 252 (26.9) | 321 (37.7) | |
| Missing | 29 (4.3) | 57 (6.1) | 29 (3.4) | |
| Enrollment site | 0.0438 | |||
| CA | 110 (16.4) | 144 (15.4) | 144 (16.9) | |
| CO | 133 (19.8) | 166 (17.7) | 176 (20.7) | |
| GA | 132 (19.6) | 213 (22.7) | 158 (18.6) | |
| MD | 96 (14.3) | 101 (10.8) | 116 (13.6) | |
| NC | 103 (15.3) | 192 (20.5) | 151 (17.7) | |
| PA | 98 (14.6) | 122 (13.0) | 106 (12.5) | |
| Genetic/neurological | < 0.0001 | |||
| Yes | 28 (4.2) | 62 (6.6) | 5 (0.6) | |
| No | 628 (93.5) | 866 (92.3) | 832 (97.8) | |
| Missing | 16 (2.4) | 10 (1.1) | 14 (1.6) | |
|
| ||||
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | p-value |
|
| ||||
| Maternal age at child birth | 31.6 (5.6) | 31.6 (5.6) | 32.1 (5.4) | 0.1695 |
| Child enrollment age | 55.6 (6.8) | 55.8 (7.5) | 55.5 (7.7) | 0.6918 |
| Child MSEL scores | 67.0 (20.0) | 86.5 (21.1) | 102.5 (14.7) | < 0.0001 |
ASD autism spectrum disorder, DD other developmental delays, POP children from the general population, MSEL Mullen Scales of Early Learning, Genetic/Neurological associated conditions: down syndrome, fragile X syndrome, Rett syndrome, tuberous sclerosis, cerebral palsy, and neurofibromatosis
Table 2.
Demographic characteristics for all children whose parent completed the gastrointestinal questionnaire and stool diary
| Variable | Complete stool diary (n = 1571) n (%) |
Missing stool diary (n = 890) n (%) |
p-value |
|---|---|---|---|
| Male child | 1045 (66.5) | 592 (66.5) | 1.0 |
| Maternal education | < 0.001 | ||
| Some college and less | 587 (37.4) | 428 (48.1) | |
| Bachelor’s degree | 543 (34.6) | 256 (28.8) | |
| Graduate school | 434 (27.6) | 190 (21.3) | |
| Maternal race/ethnicity | < 0.001 | ||
| White non-hispanic | 1061 (67.5) | 454 (51.0) | |
| Other non-hispanic | 317 (20.2) | 308 (34.6) | |
| Hispanic | 181 (11.5) | 107 (12.0) | |
| Mother’s primary language | < 0.001 | ||
| English | 1400 (89.1) | 788 (88.5) | |
| Spanish | 100 (6.4) | 40 (4.5) | |
| Other | 67 (4.3) | 46 (5.2) | |
| Household income | < 0.001 | ||
| < $50 thousand | 460 (29.3) | 346 (38.9) | |
| $50-$90 thousand | 543 (34.6) | 242 (27.2) | |
| > $90 thousand | 506 (32.2) | 249 (28.0) | |
| Genetic/neurological | |||
| 56 (3.6) | 39 (4.4) | < 0.001 | |
|
| |||
| Variable | Mean (SD) | Mean (SD) | p-value |
|
| |||
| Maternal age at child birth | 32.3 (5.3) | 31 (5.9) | < 0.001 |
| Child enrollment age | 55.2 (7.3) | 56.5 (7.5) | < 0.001 |
| MSEL mean score | 88.6 (23.2) | 83.4 (23.2) | < 0.001 |
MSEL Mullen Scales of Early Learning, Genetic/Neurological Associated conditions: down syndrome, fragile X syndrome, Rett syndrome, tuberous sclerosis, cerebral palsy, and neurofibromatosis
Table 3.
Proportions of subjects using treatments for constipation who completed a stool diary
| ASD | DD | POP | |
|---|---|---|---|
| Parent report of all children within groups receiving a treatment for constipation | 105 (16.0) | 118 (12.9) | 64 (7.6) |
| Parent report of treatment for constipation and completed stool diary | 46 (43.8%) | 68 (57.6%) | 35 (54.6%) |
| Treatment for constipation included use of laxative/stool softener, and completed stool diary | 21 (45.6%) | 49 (72.1%) | 25 (71.4%) |
Autism spectrum disorder (ASD), Developmental delays (DD), and children from the general population (POP)
After adjusting for covariates, the odds of any current overall GIS/PR were higher in children with ASD than children from the DD and POP groups as shown in Table 4. Adjusted odds ratios were also higher in children with ASD compared to DD for all specific GIS/PR except vomiting, abdominal pain with meals or relieved by defecation, other GI problem, and treatment for constipation. Similarly, when SD data were included, children with ASD also had significantly higher odds of having any GIS/PRSD, abdominal pain, and gas than DD controls, however, there was no difference between ASD and DD groups for vomiting or frequency/consistency of stools from SD. When compared to children from the POP group, children with ASD had higher adjusted odds for all GIS/PR except vomiting. Children with ASD had higher odds of having any GIS/PRSD, 4 + stools per day, constipation, or gas compared to POP children. Further, children with ASD were more likely to have past GIS/PR than DD or POP groups. Based on the caregiver interview, very few caregivers (< 1%) endorsed 4 specific GI disorders. There were 4 children with celiac disease in each of the 3 groups (ASD, DD, and POP). Irritable bowel syndrome was reported in 4 children with ASD and 5 with DD. One child in the ASD group was diagnosed with ulcerative colitis, and no child was diagnosed with Crohn’s disease. In the “other” category, gastroesophageal reflux was reported in 1 child with ASD, 3 with DD, and 2 from the POP group. Food allergy was reported in 2 children with DD and 4 from the POP group. There were 3 children with ASD with food intolerance and none from the DD or POP groups. There was 1 child with DD with pyloric stenosis and 1 child with ASD with fecal impaction (Supplementary Table 2).
Table 4.
Comparison of the proportion of children with gastrointestinal symptoms (GIS) based on parent responses on the gastrointestinal questionnaire (GIS/PR) between children with an autism spectrum disorder (ASD), developmental delays (DD), and children from the general population (POP)
| GIS questionnaire only (GIS/PR) | Case | DD | POP | ASD vs DD |
ASD vs POP |
||
|---|---|---|---|---|---|---|---|
| (n = 656) n (%) | (n = 932) n (%) | (n = 837) n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Presence of any current GIS/PR | 227 (34.6) | 202 (22.1) | 101 (12.0) | 1.91 (1.62–2.26)* | 1.85 (1.54–2.22)* | 3.90 (3.07–4.95)* | 3.42 (2.11–5.54)* |
| Vomiting | 30 (4.6) | 29 (3.2) | 8 (0.9) | 1.49 (0.89–2.49) | 1.08 (0.56–2.08) | 5.01 (3.17–7.94)* | 2.04 (0.54–7.64) |
| Diarrhea | 66 (10.1) | 24 (2.6) | 13 (1.5) | 4.23 (2.69–6.66)* | 3.57 (2.40–5.30)* | 7.16 (4.83–10.61)* | 4.74 (2.70–8.31)* |
| Loose stools | 105 (16.0) | 50 (5.5) | 26 (3.1) | 3.36 (2.21–5.12)* | 2.73 (1.77–4.20)* | 6.00 (4.32–8.33)* | 4.04 (2.88–5.68)* |
| Constipation | 154 (23.5) | 149 (16.3) | 72 (8.5) | 1.61 (1.4–1.86)* | 1.53 (1.39–1.67)* | 3.29 (2.40–4.52)* | 2.58 (1.14–5.84)* |
| Loose alternating with Constipation | 94 (14.3) | 40 (4.4) | 19 (2.2) | 3.73 (2.69–5.17)* | 3.09 (2.05–4.65)* | 7.27 (5.54–9.54)* | 5.25 (3.73–7.39)* |
| Abdominal pain with meals | 33 (5.0) | 35 (3.8) | 19 (2.2) | 1.36 (0.88–2.10) | 1.75 (0.88–3.48) | 2.30 (1.61–3.29)* | 5.06 (2.39–10.74)* |
| Abdominal pain relieved by defecation | 50 (7.6) | 69 (7.6) | 37 (4.4) | 1.03 (0.77–1.38) | 1.43 (0.83–2.44) | 1.80 (1.21–2.69)* | 3.40 (1.20–9.60)* |
| Pain on stooling | 81 (12.3) | 77 (8.4) | 35 (4.1) | 1.56 (1.10–2.22)* | 1.86 (1.39–2.48)* | 3.26 (2.28–4.65)* | 4.29 (1.57–11.76)* |
| Gas | 105 (16.0) | 73 (8.0) | 30 (3.6) | 2.24 (1.48–3.40)* | 1.97 (1.42–2.74)* | 5.18 (3.67–7.31)* | 4.86 (2.44–9.67)* |
| Other | 35 (5.3) | 28 (3.1) | 6 (0.7) | 1.82 (1.03–3.21)* | 1.72 (0.78–3.81) | 7.88 (3.03–20.48)* | 5.46 (1.24–24.08)* |
| Current Treatment for Constipation | 105(16.0)a | 118(12.9)a | 64 (7.6)a | 1.30 (0.97–1.73) | 1.19 (0.90–1.57) | 2.29 (1.65–3.18)* | 2.21 (1.10–4.47)* |
| Past GI problems (no longer present) | 180(27.4)b | 177(19.4)b | 113(13.4)b | 1.65 (1.33–2.03)* | 1.76 (1.24–2.50)* | 2.53 (2.01–3.19)* | 3.50 (2.26–5.43)* |
|
GIS based on an algorithm combining gastrointestinal questionnaire and stool diary using the Bristol stool scale (GIS/PRSD) | |||||||
| Gastrointestinal symptom (GIS/PRSD) | Case | DD | POP | ASD vs DD |
ASD vs POP |
||
| (n = 335) n (%) | (n = 437) n (%) | (n = 480) n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|
| |||||||
| Presence of any current GIS below | 169 (50.4) | 186 (42.6) | 147 (30.6) | 1.37 (1.20–1.57)* | 1.29 (1.07–1.56)* | 2.31 (1.99–2.68)* | 2.22 (1.56–3.14)* |
| Four or more stools per day | 5 (1.5) | 5(1.1) | 1 (0.2) | 1.31 (0.43–3.99) | 0.65 (0.29–1.49) | 7.26 (1.90–27.66)* | 11.18(2.37–52.77)* |
| Constipation (GIS/PRSD) | 80 (23.9) | 120 (27.5) | 102 (21.3) | 0.83 (0.69–1.00)* | 0.84 (0.67–1.05) | 1.16 (1.03–1.32)* | 1.48 (1.14–1.92)* |
| Loose stool or diarrhea | 34 (10.1) | 37 (8.5) | 30 (6.3) | 1.22 (0.84–1.77) | 1.03 (0.75–1.43) | 1.69 (1.19–2.41)* | 1.05 (0.48–2.28) |
| Vomiting | 25 (7.5) | 20 (4.6) | 8 (1.7) | 1.68 (1.06–2.67)* | 1.24 (0.80–1.93) | 4.76 (2.20–10.28)* | 2.30 (0.94–5.64) |
| Abdominal pain | 55 (16.4) | 52 (11.9) | 38 (7.9) | 1.45 (1.18–1.79)* | 1.62 (1.11–2.35)* | 2.28 (1.71–3.05)* | 2.48 (0.80–7.68) |
| Gas | 49 (14.6) | 32 (7.3) | 18 (3.8) | 2.17 (1.46–3.23)* | 1.71 (1.10–2.65)* | 4.40 (2.97–6.52)* | 4.01 (1.68–9.57)* |
All models are adjusted for maternal race/ethnicity, education level, age at child’s birth, child sex and Mullen Scales of Early Learning score, and accounted for correlation by enrollment site
ASD autism spectrum disorder, DD other developmental delays, POP children from the general population, constipation (GIS/PRSD): 2 or more hard stools/week, < 3 stools/week and at least one is hard, and/or use of a laxative or stool softener
p-value < 0.05
Percent calculated from total of 667 ASD, 936 DD, 849 POP
Percent calculated from total of 635 ASD, 913 DD, 837 POP
After adjustment for covariates, children with ASD and regression had increased odds of having several GIS/PR compared to children with ASD without regression as shown in Table 5, and had increased odds of having vomiting and diarrhea/loose stools, but slightly decreased odds of constipation when stool diary was included. There was no difference in ASD severity between children with ASD with and without GIS as shown in Table 6. Among all children, sleep problems and CBCL subscale scores for anxious/depressed, aggressive behavior, and attention problems were associated with GIS/PR in unadjusted and adjusted analyses as shown in Table 7.
Table 5.
Comparison of gastrointestinal symptoms (GIS) between children with an autism spectrum disorder with and without regression in speech or social skills
| GIS questionnaire only (GIS/PR) | Regression | No regression | Regression vs. No regression |
|
|---|---|---|---|---|
| (n = 161) n (%) | (n = 472) n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Current GIS(overall) | 69 (42.9) | 150 (31.8) | 1.61 (1.46–1.78)* | 1.53(1.33–1.77)* |
| Vomiting | 15 (9.3) | 15 (3.2) | 3.13 (1.56–6.26)* | 2.67 (1.54–4.61)* |
| Diarrhea | 30 (18.6) | 33 (7.0) | 3.05 (1.68–5.53)* | 2.77 (1.59–4.82)* |
| Loose stools | 36 (22.4) | 63 (13.3) | 1.87 (1.07–3.26)* | 1.85 (1.03–3.36)* |
| Constipation | 46 (28.6) | 104 (22.0) | 1.42 (1.08–1.86)* | 1.43 (1.05–1.96)* |
| Loose alternating with Constipation | 32 (19.9) | 59 (12.5) | 1.74 (1.34–2.26)* | 1.83 (1.35–2.48)* |
| Abdominal pain with meals | 12 (7.5) | 21 (4.4) | 1.73 (0.94–3.19) | 1.85 (0.84–4.08) |
| Abdominal pain relieved by defecation | 18 (11.2) | 32 (6.8) | 1.73 (1.16–2.58)* | 1.86 (1.32–2.63)* |
| Pain on stooling | 23 (14.3) | 57 (12.1) | 1.21 (0.67–2.18) | 1.22 (0.68–2.19) |
| Gas | 33 (20.5) | 70 (14.8) | 1.48 (0.95–2.31) | 1.43 (0.87–2.37) |
| Other | 10 (6.2) | 23 (4.9) | 1.29 (0.48–3.47) | 1.54 (0.70–3.39) |
| Past GI problems (no longer present) | 50 (31.1)a | 124 (26.3)a | 1.25 (0.91–1.73) | 1.33 (0.90–1.96) |
| Treatment for Constipation | 32 (19.9)b | 69 (14.6)b | 1.41 (1.03–1.93)* | 1.35 (0.89–2.03) |
|
GIS based on an algorithm combining gastrointestinal questionnaire and stool diary using the Bristol stool scale (GIS/PRSD) | ||||
| Gastrointestinal symptom (GIS/PRSD) | Regression | No regression | Regression vs. No regression |
|
| (n = 82) n (%) | (n = 241) n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|
| ||||
| GI problems (any of the above) | 41 (50.0) | 123 (51.0) | 0.96 (0.74–1.24) | 0.86 (0.62–1.19) |
| Four or more stools per day | 2(2.4) | 3(1.2) | 1.98(0.22–17.68) | 2.60 (0.23–29.72) |
| Constipation: 2 or more hard stools/week, < 3 stools/week and at least one is hard, use of laxative or stool softener | 17 (20.7) | 61 (25.3) | 0.77 (0.52–1.14) | 0.71 (0.51–0.99)* |
| Loose stool or diarrhea | 13 (15.9) | 18 (7.5) | 2.33 (1.32–4.12)* | 1.88 (1.29–2.76)* |
| Vomiting | 11 (13.4) | 14 (5.8) | 2.51 (1.34–4.70)* | 2.31 (1.13–4.70)* |
| Abdominal pain: pain on stooling, pain relieved by stooling, pain during meals | 13 (15.9) | 42 (17.4) | 0.89 (0.45–1.79) | 0.89 (0.43–1.81) |
| Gas | 13 (15.9) | 35 (14.5) | 1.11 (0.61–2.02) | 1.20 (0.63–2.27) |
All models are adjusted form maternal race/ethnicity, education level, age at child’s birth, child sex, genetic condition, and Mullen Scales of Early Learning score, and accounted for correlation by enrollment site
p-value < 0.05
Percent calculated from total of 166 with regression and 476 without regression
Percent calculated from total of 157 with regression and 457 without regression
Table 6.
Comparison of mean autism severity score in children with autism spectrum disorders with and without gastrointestinal symptoms (GIS)
| GIS questionnaire only (GIS/PR) | Autism severity score mean(se) |
Difference in average Autism severity score |
||
|---|---|---|---|---|
| GIS mean (se) | No GIS mean (se) | Crude difference (95% CI) | Adjusted difference (95% CI)a | |
| Current GI problems (overall) | 7.18 (0.18) | 7.13 (0.12) | 0.05 (− 0.12–0.22) | 0.04 (− 0.13–0.20) |
| Vomiting | 7.2 (0.26) | 7.14 (0.14) | 0.06 (− 0.45–0.56) | − 0.05 (− 0.49–0.40) |
| Diarrhea | 7.26 (0.27) | 7.13 (0.13) | 0.12 (− 0.24–0.49) | 0.04 (− 0.36–0.45) |
| Loose stools | 7.43 (0.26) | 7.09 (0.12) | 0.33 (− 0.02–0.69) | 0.26 (− 0.05–0.58) |
| Constipation | 7.2 (0.21) | 7.13 (0.12) | 0.07 (− 0.18–0.33) | 0.05 (− 0.25–0.35) |
| Loose alternating with Constipation | 7.2 (0.15) | 7.14 (0.14) | 0.07 (− 0.11–0.24) | 0.05 (− 0.13–0.23) |
| Abdominal pain with meals | 6.85 (0.36) | 7.16 (0.14) | − 0.31 (− 1.11–0.49) | − 0.15 (− 0.90–0.61) |
| Abdominal pain relieved by defecation | 6.9 (0.19) | 7.17 (0.13) | − 0.27 (− 0.54–0.01) | − 0.04 (− 0.25–0.16) |
| Pain on stooling | 6.85 (0.2) | 7.19 (0.14) | − 0.34 (− 0.70–0.03) | − 0.17 (− 0.46–0.11) |
| Gas | 7.17 (0.27) | 7.14 (0.12) | 0.03 (− 0.35–0.41) | 0.06 (− 0.31–0.44) |
| Other | 6.86 (0.37) | 7.16 (0.13) | − 0.30 (− 0.96–0.35) | − 0.27 (− 1.03–0.48) |
| Past GI problems (no longer present) | 7.26 (0.12) | 7.11 (0.15) | 0.16 (0.06–0.25)* | 0.16 (0.00–0.32) |
| Treatment for Constipation | 7.12 (0.21) | 7.16 (0.14) | − 0.03 (− 0.30–0.24) | − 0.07 (− 0.33–0.20) |
|
GIS based on an algorithm combining parent report and stool diary using the Bristol stool scale (GIS/PRSD) | ||||
| GI problems (any of the above) | 7.14 (1.58) | 6.94 (1.60) | 0.20 (− 0.26–0.65) | 0.22 (− 0.29–0.72) |
| Four or more stools per day | 6.80 (1.10) | 7.04 (1.60) | − 0.24 (− 1.33–0.84) | − 0.34 (− 1.29–0.6) |
| Constipation: 2 or more hard stools/week, < 3 stools/week and at least one is hard, use of laxative or stool softener | 7.11 (1.76) | 7.02 (1.54) | 0.10 (− 0.53–0.72) | 0.15 (− 0.45–0.76) |
| Loose stool or diarrhea | 7.44 (1.65) | 6.99 (1.58) | 0.45 (0.00–0.90) | 0.28 (− 0.04–0.60) |
| Vomiting | 7.28 (1.37) | 7.02 (1.61) | 0.26 (− 0.25–0.77) | 0.16 (− 0.49–0.81) |
| Abdominal pain: pain on stooling, pain relieved by stooling, pain during meals | 6.91 (1.54) | 7.06 (1.60) | − 0.16 (− 0.76–0.45) | 0.00 (− 0.43–0.42) |
| Gas | 7.12 (1.70) | 7.02 (1.58) | 0.10 (− 0.62–0.82) | 0.22 (− 0.52–0.96) |
All models are adjusted form maternal race/ethnicity, education level, age at child’s birth, child sex, genetic condition, and Mullen Scales of Early Learning score, and accounted for correlation by enrollment site
p-value < 0.05
Additional adjustment for maternal place of birth (GIS/PR only)
Table 7.
Association between mean Child Behavior Checklist (CBCL) subscale scores and Children’s Sleep Habits Questionnaire (CSHQ) total score > 48 in all children (autism spectrum disorder, developmental delays and from the general population) with and without gastrointestinal symptoms (GIS)
| CBCL subscales | Mean CBCL score |
|
||
|---|---|---|---|---|
| GI symptoms | No GI symptoms | Crude difference (95% CI) | Adjusted difference (95% CI) | |
| Anxious Depressed | 3.26 | 2.31 | 0.95 (0.75–1.15)* | 0.87 (0.66–1.08)* |
| Aggressive Behavior | 13.70 | 9.56 | 4.14 (3.55–4.72)* | 3.29 (2.55–4.04)* |
| Attention Problems | 4.17 | 2.93 | 1.24 (1.00–1.48)* | 0.86 (0.76–0.96)* |
|
| ||||
| CSHQ total score | GIS n (%) | No GIS n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)a |
|
| ||||
| CSHQ Score > 48 | 228 (46.8) | 544 (30.5) | 2.01 (1.77–2.28)* | 2.01 (1.70–2.37)* |
p-value < 0.05
Adjusted for maternal race/ethnicity, education level, age at child’s birth, child sex, and Mullen Scales of Early Learning score, and accounted for correlation by enrollment site
Discussion
In SEED, preschool-aged children with ASD had more current and past GIS than children from the DD and POP groups based on parental report (GIS/PR) and after including data from stool diaries (GIS/PRSD). This is consistent with previous studies (McElhanon et al. 2014). This study adds very well characterized ASD and control groups and is large enough to adjust for covariates. The etiology of GIS in children with ASD is likely heterogeneous. Gastrointestinal issues are complex and may result from multiple etiologies simultaneously. The following characteristics seen in children with ASD may be related to GIS: (1) Developmental differences: stool frequency and consistency may vary with age and toilet training status; (2) Behavioral differences: stool withholding contributing to constipation is common in children with ASD; (3) Dietary: children with ASD are likely to have restrictive diets with poor fiber intake (Hyman et al. 2012); (4) Neurologic/Autonomic/Anxiety: children with ASD have been found to have differences in autonomic function, high rates of anxiety, and differences in muscle tone, which may contribute to changes in motility (Ferguson et al. 2017); (5) Immunologic: food allergy and intolerance including disorders such as eosinophilic esophagitis have been reported in children with ASD (Buie et al. 2010; Kushak et al. 2016); (6) Microbiome: children with ASD have been reported to have differences in the microbiome, which can affect gut function (Alam et al. 2017; Fung et al. 2017); and (7) Genetic: genetic differences associated with GIS have been found in children with ASD (Campbell et al. 2009). This study was able to adjust for developmental level and some neurological/genetic differences. The study also examined associations between anxiety, autism severity, and developmental regression. Next steps will include evaluation of genetic, immunological, perinatal risk factors, and dietary associations with GIS, and to use GIS as a phenotypic subtype in children with ASD.
Several significant differences were found in specific GIS between children with ASD and children with other DD with notable exceptions of treatment for constipation and stool frequency and consistency on the SD. Marked differences were found between children with ASD and POP children in all specific GIS/PR except vomiting, with some attenuation of effects after incorporating SD data. The significant differences in the prevalence of GIS in children with ASD in comparison to children from the DD and POP groups are consistent with findings in some but not all studies (Chaidez et al. 2014; McElhanon et al. 2014). Prevalence of constipation was higher in the ASD group when using only parent report; however, when combined with SD data, the proportions in all study groups were more similar. This may be explained by measurement differences. A diagnosis of constipation was not equivalent between measures. Children treated for constipation may not demonstrate constipation on a SD if the treatment has the desired effect; therefore, we added treatment with a stool softener or laxative to the criteria for constipation on GIS/PRSD. Children with ASD who were being treated for constipation were less likely to have a SD and more likely to use a treatment for constipation that was not a stool softener or laxative such as fiber products or suppositories.
Children with ASD and regression were more likely to have GIS/PR than children with ASD without regression. No difference was found in autism severity scores between children with ASD with and without GIS. The findings of higher GIS/PR in children with ASD with regression compared to those without regression are consistent with other studies (Ferguson et al. 2017; Valicenti-McDermott et al. 2008). However, when SD data were included, higher levels of GIS were only significant for vomiting and loose stools/diarrhea. The finding that autism severity was not associated with GIS is consistent with a study that found no association between GIS and autism severity (autism vs. pervasive developmental disorder/Asperger) except for diarrhea (Chaidez et al. 2014). In contrast, another study found that GIS were more common in children with more severe ASD (Wang et al. 2011). These differences between studies may be related to how autism severity is defined. We used ADOS CSS, which should reflect more about symptoms of autism than overall level of functioning.
Despite adjusting for developmental level and SES, children from all three groups with GIS had more aggression, anxiety, attention, and sleep concerns than children without GIS. Chaidez et al. found that children with ASD and GIS had higher scores on the Aberrant Behavior Checklist on irritability, social withdrawal, stereotypy and hyperactivity than children with ASD without GIS (Chaidez et al. 2014). Children with ASD or other DD often have difficulty communicating pain or discomfort. Irritability or aggression may be the only identified symptoms when a child with ASD has pain (Buie et al. 2010). Anxiety and stress have been associated with GIS in the general population and in children with ASD (Ferguson et al. 2017; Mazurek et al. 2013). This may be mediated by the autonomic nervous system, which responds to anxiety and stress and can directly impact gut motility. Children with ASD have been found to have high rates of anxiety, hyperarousal/difficulty with arousal modulation, and autonomic dysfunction (Ferguson et al. 2017; Mazurek et al. 2013). An association between GIS and sleep problems in children with ASD has been described in three large cohorts of children with ASD (Aldinger et al. 2015; Mazurek et al. 2013). These cohorts had no comparison groups or siblings were used for comparison. In SEED, similar associations between sleep problems and GIS were found in children in the DD and POP groups, which would imply that this is not specific to children with ASD. Discomfort from GIS may disrupt sleep; conversely, anxiety and hyperarousal may contribute to both GIS and sleep problems.
This study has several strengths. SEED is the largest, geographically diverse, well-characterized sample designed to assess GIS among children with ASD that includes two different comparison groups, enabling assessment of the prevalence of GIS in children with ASD compared to children with other DD and children from the general population and exploration of the specificity of various symptoms to children with ASD. The large sample size and comprehensive data collection allowed adjustment for many potential confounders, including developmental level. Children with ASD were also recruited from multiple sources rather than from a clinic-based sample, which reduces selection bias. Case classification was based on rigorous research-reliable methods rather than relying on past clinical diagnoses. Finally, this study is also one of the first to use a stool diary as a more objective measure of stool frequency and consistency.
This study has limitations. Clinical diagnoses of gastrointestinal dysfunction were not available; however, a study of children with ASD found that parent report of GIS was concordant with a clinical diagnosis by a gastroenterologist (Gorrindo et al. 2012). The GI questionnaire developed for SEED has not been validated. A questionnaire based on Rome III criteria was created to define functional GIS but was not available when the study was designed or implemented. Therefore, Rome III criteria for functional constipation could not be applied to SEED data as information regarding large stools, stool retention, history of large fecal mass, or soiling were not collected. SEED did use a priori definitions for constipation and diarrhea/loose stools based on stool consistency and frequency collected in the stool diary. Parent report of GIS in children with ASD may have been impacted by recall bias due to general concern that GIS is common in children with ASD. However, a large prospective cohort in Norway, which would mitigate concerns of recall bias, found that mothers reported a higher prevalence of constipation, food allergy/intolerance, and diarrhea in 6- to 36-month-old children who were later diagnosed with ASD compared to children with typical development (Bresnahan et al. 2015). While the stool diary added more objective data, only 51% of caregivers completed it during a typical week. In addition, children with stool diaries were also more likely to have higher cognitive scores, higher household income, and to have a mother with more education and who was white non-Hispanic. Parents of children with ASD who were receiving treatment for constipation were less likely to complete a stool diary when compared to DD and POP groups. Only use of a laxative or stool softener were used in the definition of constipation in the GIS/PRSD data; children with ASD were less likely to use a laxative or stool softener to treat constipation, and therefore less likely to meet criteria for constipation on the GIS/PRSD.
Conclusion
In a large, geographically diverse and well-characterized sample of children with ASD, a higher prevalence of GIS was found in children with ASD compared to children from the DD and POP groups. The presence of two control groups helps to identify symptoms that are specific to ASD vs. those associated with other developmental concerns or with young children in general. Children with ASD and regression were found to have greater GIS/PR than children with ASD without regression, and all children, irrespective of group, with GIS/PR had more behavioral and sleep concerns. Our findings suggest that clinicians who provide care to children with ASD and DD consider screening these children for GIS, so that adequate management of GIS is provided. These results also suggest that clinicians who care for young children be aware that GIS may be associated with sleep issues, irritability, aggression, and/or anxiety in young children. Researchers may use these data to inform future studies to better understand the etiologies of GIS in children with ASD and DD. Understanding etiology may lead to better treatments and to a better understanding of the association of GIS with anxiety, behavior and sleep. These findings may also be informative for understanding phenotypic subtypes in ASD in future analyses of risk factors for ASD.
Supplementary Material
Acknowledgments
Special thanks to SEED family participants, investigators, and staff at the SEED sites. We thank the SEED Data Coordinating Center team at the Clinical and Translational Sciences Institute of Michigan State University for their support throughout this study. This project was supported by Centers for Disease Control and Prevention (CDC) Cooperative Agreements announced under the following RFA’s: Grant Nos. 01086, 02199, DD11–002, DD06–003, DD04–001, and DD09–002 (https://www.cdc.gov/ncbddd/autism/seed.html). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. This work was also supported by NIH/NCATS Colorado CTSA Grant No. UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Funding
The authors have no financial relationships relevant to this article to disclose.
Footnotes
Conflict of Interest The authors have no conflicts of interest relevant to this article to disclose. Dr. Reynolds is participating in clinical trials with Roche and is providing consultation to Ovid Therapeutics regarding evaluation of sleep severity and improvement in clinical trials.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10803-020-04786-9) contains supplementary material, which is available to authorized users.
References
- Achenbach T. (1992). Child behavior checklist. Burlington: Achenbach System of Empirically based Assessment. [Google Scholar]
- Alam R, Abdolmaleky HM, & Zhou JR (2017). Microbiome, inflammation, epigenetic alterations, and mental diseases. American Journal of the Medical Genetics Part B Neuropsychiatric Genetics. 10.1002/ajmg.b.32567 [DOI] [PMC free article] [PubMed]
- Aldinger KA, Lane CJ, Veenstra-VanderWeele J, & Levitt P. (2015). Patterns of risk for multiple co-occurring medical conditions replicate across distinct cohorts of children with autism spectrum disorder. Autism Res, 8(6), 771–781. 10.1002/aur.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan M, Hornig M, Schultz AF, Gunnes N, Hirtz D, Lie KK, … Lipkin WI (2015). Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry, 72(5), 466–474. doi: 10.1001/jamapsychiatry.2014.3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ 3rd, Furuta GT, Levy J, Vandewater J, … Winter H. (2010). Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics, 125(Suppl 1), S1–18. doi: 10.1542/peds.2009-1878C [DOI] [PubMed] [Google Scholar]
- Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, Perrin JM, & Levitt P. (2009). Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics, 123(3), 1018–1024. 10.1542/peds.2008-0819 [DOI] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, & Hertz-Picciotto I. (2014). Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders, 44(5), 1117–1127. 10.1007/s10803-013-1973-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi-Velez F, Ge Y, & Kohane I. (2014). Comorbidity clusters in autism spectrum disorders: An electronic health record time-series analysis. Pediatrics, 133(1), e54–63. 10.1542/peds.2013-0819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Marler S, Altstein LL, Lee EB, Akers J, Sohl K, … Beversdorf DQ (2017). Psychophysiological Associations with Gastrointestinal Symptomatology in Autism Spectrum Disorder. Autism Res, 10(2), 276–288. doi: 10.1002/aur.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TC, Olson CA, & Hsiao EY (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nature Neuroscience, 20(2), 145–155. 10.1038/nn.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, & Levitt P. (2012). Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Res, 5(2), 101–108. 10.1002/aur.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SL, Stewart PA, Schmidt B, Cain U, Lemcke N, Foley JT, … Ng PK (2012). Nutrient intake from food in children with autism. Pediatrics, 130(Suppl 2), S145–153. doi: 10.1542/peds.2012-0900L [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushak RI, Buie TM, Murray KF, Newburg DS, Chen C, Nestoridi E, & Winter HS (2016). Evaluation of intestinal function in children with autism and gastrointestinal symptoms. Journal of Pediatric Gastroenterology and Nutrition, 62(5), 687–691. 10.1097/MPG.0000000000001174 [DOI] [PubMed] [Google Scholar]
- Lewis SJ, & Heaton KW (1997). Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology, 32(9), 920–924. 10.3109/00365529709011203 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A. (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, & Risi S. (1999). Autism diagnostic observation schedule. Los Angeles: Western Psychological Services. [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, … Lowery LA (2013). Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnorm Child Psychol, 41(1), 165–176. doi: 10.1007/s10802-012-9668-x [DOI] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, & Sharp WG (2014). Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics, 133(5), 872–883. 10.1542/peds.2013-3995 [DOI] [PubMed] [Google Scholar]
- Mullen E. (1995). Mullen scales of early learning. San Antonio: Pearson. [Google Scholar]
- Owens JA, Maxim R, Nobile C, McGuinn M, & Msall M. (2000). Parental and self-report of sleep in children with attention-deficit/hyperactivity disorder. Archives of Pediatrics and Adolescent Medicine, 154(6), 549–555. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, & McGuinn M. (2000). The children’s sleep habits questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23(8), 1043–1051. [PubMed] [Google Scholar]
- Reynolds AM, Soke GN, Sabourin KR, Hepburn S, Katz T, Wiggins LD, … Levy SE (2019). Sleep problems in 2- to 5-year-olds with autism spectrum disorder and other developmental delays. Pediatrics, 143(3). doi: 10.1542/peds.2018-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M, Martinelli M, Sciorio E, Botta C, Miele E, Vallone G, & Staiano A. (2013). Stool consistency, but not frequency, correlates with total gastrointestinal transit time in children. Journal of Pediatrics, 162(6), 1188–1192. 10.1016/j.jpeds.2012.11.082 [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C. (2003). SCQ: Social communication questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Schendel DE, Diguiseppi C, Croen LA, Fallin MD, Reed PL, Schieve LA, … Yeargin-Allsopp M. (2012). The Study to Explore Early Development (SEED): A multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) network. J Autism Dev Disord, 42(10), 2121–2140. doi: 10.1007/s10803-012-1461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway S, Farmer C, Thurm A, Joseph L, Black D, & Golden C. (2012). The ADOS calibrated severity score: Relationship to phenotypic variables and stability over time. Autism Res, 5(4), 267–276. 10.1002/aur.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng A, Bartle A, Sadeh A, & Mindell J. (2012). Infant and toddler sleep in Australia and New Zealand. Journal of Paediatrics and Child Health, 48(3), 268–273. 10.1111/j.1440-1754.2011.02251.x [DOI] [PubMed] [Google Scholar]
- Valicenti-McDermott MD, McVicar K, Cohen HJ, Wershil BK, & Shinnar S. (2008). Gastrointestinal symptoms in children with an autism spectrum disorder and language regression. Pediatric Neurology, 39(6), 392–398. 10.1016/j.pediatrneurol.2008.07.019 [DOI] [PubMed] [Google Scholar]
- Wang LW, Tancredi DJ, & Thomas DW (2011). The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. Journal of Developmental and Behavioral Pediatrics, 32(5), 351–360. 10.1097/DBP.0b013e31821bd06a [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Reynolds A, Rice CE, Moody EJ, Bernal P, Blaskey L, … Levy SE (2015). Using standardized diagnostic instruments to classify children with autism in the study to explore early development. J Autism Dev Disord, 45(5), 1271–1280. doi: 10.1007/s10803-014-2287-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


