Abstract
Introduction:
To determine drug delivery/toxicity, and pathological/surgical outcomes of muscle-invasive bladder cancer (MIBC) patients receiving neoadjuvant gemcitabine-cisplatin (GC) plus radical cystectomy-pelvic lymph node dissection (RC-PLND).
Patients and Methods:
Chemotherapy and surgical/pathologic outcomes were retrospectively analyzed with 5-year survival follow-up at a referral center. Post-neoadjuvant chemotherapy (NAC) pathologic endpoints included complete response (pT0N0), residual non-MIBC (pTa/Tis/T1N0) and ≥MIBC (≥pT2 and/or N+). Associations of pathologic/surgical findings with overall survival (OS), disease-free survival (DFS), and surgical management with RC-PLND were analyzed (Cox regression).
Results:
Clinical T2a-T4aN0M0 MIBC patients (154) from 1/2000–10/2012 received GC plus RC-PLND. Patients (117, 76%) received GCx4 and 136 (88%) GCx3. Five-year OS was 61% (95% CI 53–71). Median number of resected lymph nodes (LN) was 19. Down-staging was observed as follows: pT0N0: 21%; pTa/Tis/T1N0: 25%, with similar 5-yr OS (85% and 89%, respectively). Five-year OS for <pT2 vs. ≥pT2 residual disease was 87% (95% CI, 78%−98%) vs. 38% (95% CI, 27%−53%); p<0.001. Post-NAC stage ≥pT2 (HR 6.79; 95% CI 2.63–17.53; p<0.001), positive LN (HR 3.64; 95% CI 1.84–7.19; p<0.001) and positive margins (HR 4.15; 95% CI 1.68–10.25; p=0.002) were associated with increased risk of all-cause death (multivariable analysis). A hazard ratio of 0.97 (95% CI: 0.94–1.00) was observed for each additional node removed, but this effect was not statistically significant (p=0.056).
Conclusions:
Neoadjuvant GC achieves meaningful pathologic responses. Patients with ≥pT2 residual disease, positive margins, or positive LN post-chemotherapy have inferior survival.
Keywords: Neoadjuvant chemotherapy, radical cystectomy, urothelial carcinoma
Micro-Abstract
We sought to define the efficacy and tolerability of the commonly used regimen of neoadjuvant gemcitabine and cisplatin (GC) followed by surgery at a single center. Retrospective analysis of 154 patients who received neoadjuvant GC revealed a pathologic downstaging rate of 46% and a 5-year overall survival of 87% with any degree of downstaging from muscle invasion. No significant delay to surgery or surgical complication rates were observed following GC administration, and no difference in response rates were observed when cisplatin was split over days 1 and 8. These data support the use of GC as an effective, tolerable regimen in the management of muscle-invasive bladder cancer.
1. INTRODUCTION
Primary surgical management of MIBC with RC-PLND results in cure of ~50% of patients.(1) Neoadjuvant chemotherapy (NAC) incorporation before RC-PLND is recommended based on Level 1 evidence demonstrating further survival improvements. In the phase III SWOG 8710 trial, addition of neoadjuvant methotrexate, vinblastine, doxorubicin and cisplatin (M-VAC) demonstrated an improved OS compared to RC-PLND alone.(2) A meta-analysis from the Advanced Bladder Cancer group analyzing 3,005 patients with MIBC reported a 5% improved OS with the incorporation of any neoadjuvant cisplatin-based regimen.(3) Although M-VAC and cisplatin, methotrexate, and vinblastine (CMV) (4) are the only NAC regimens supported by Level 1 evidence, significant toxicities have spurred exploration of alternative neoadjuvant combinations with improved tolerability and similar clinical activity.
Gemcitabine and cisplatin (GC) has been adopted as an alternative regimen to M-VAC despite the lack of randomized data supporting its use.(5) A phase III clinical trial comparing GC to MVAC in the metastatic setting found comparable efficacy but significantly less toxicity for GC.(6) Evidence for neoadjuvant GC is sparse, derived from small phase II trials and retrospective analyses.(7–11) In one retrospective review, 42 patients treated at Memorial Sloan Kettering Cancer Center (MSKCC) receiving neoadjuvant GC from 11/2000–11/2006 showed comparable rates of downstaging to pT0 and <pT2 compared to MVAC.(12) Although GC is a National Comprehensive Cancer Network (NCCN)-recommended neoadjuvant regimen(13), the clinical, surgical and survival outcomes utilizing GC in multi-modality treatment have been incompletely characterized.
Retrospective studies support the concept that the extent and quality of PLND impacts OS in bladder cancer. Post-hoc analysis from SWOG 8710 demonstrated that patients with <10 resected LN had an inferior OS compared to patients with ≥10 LN, regardless of metastatic involvement.(14) While a number of analyses have demonstrated a similar survival benefit for a minimum number of resected nodes (15–17), other studies have shown that when node status is correlated with cancer-specific outcome, as opposed to OS, a discrete cut point for minimum number of retrieved nodes could not be defined. In one analysis, a significant association was noted between improved cancer-specific survival and every additional node removed.(18) Finally, the impact of PLND surgical factors on OS has never been formally evaluated in patients receiving neoadjuvant GC.
We sought to characterize the efficacy of neoadjuvant GC and RC-PLND in a retrospective analysis of patients treated at MSKCC with a focus on the contribution of both NAC and PLND.
2. PATIENTS AND METHODS
2.1. Patients
Following IRB approval, clinical and pathologic data were reviewed from MIBC patients treated at MSKCC with neoadjuvant GC and RC-PLND from 1/2000–10/2012. MIBC was diagnosed by transurethral resection of the bladder tumor (TURBT) and underwent independent MSKCC pathology review. Eligible histo-pathologic subtypes were urothelial carcinoma (UC) and UC with secondary features (mixed), as long as UC was the predominant histology. All patients underwent pre-NAC imaging. Repeat examination under anesthesia (EUA) and TURBT were performed at the urologist’s discretion and maximal TURBT was not required pre-chemotherapy. Final pre-NAC clinical staging was determined using pathology, imaging and EUA. Hydronephrosis or perivesical fat infiltration on imaging or palpable bladder mass on EUA was considered clinical T3 disease. Only patients with clinical stage T2-T4aN0M0 UC of the bladder were included.
Patients received up to 4 cycles of GC with expected treatment duration of either 84 days with a 21-day/cycle schedule or 112 days using a 28-day/cycle schedule. GC schedules included gemcitabine 1000mg/m2 day 1 (d1) and d8 and cisplatin 70mg/m2 d1 (21-day cycle), split-dose with cisplatin 35mg/m2 and gemcitabine 1000 mg/m2 on d1 and d8 (21-day cycle), or gemcitabine 1000mg/m2 d1, d8, d15 and cisplatin 70mg/m2 d1 (28-day cycle). Total planned gemcitabine dose goal was 8,000mg/m2 for the 21-day cycle regimens or 12,000 mg/m2 for the 28-day cycle regimen. All regimens had a total cisplatin dose goal of 280 mg/m2. Select chemotherapy-associated hematologic [white blood cell (WBC), hemoglobin, platelet number] and renal (creatinine) toxicities were collected and graded using Common Terminology Criteria for Adverse Events Version 4.0. Vascular thromboembolic events (VTE) were recorded, including deep venous thrombosis, pulmonary embolism, cerebrovascular attacks and myocardial infarction, for patients not on baseline anticoagulation. Cycle-limiting toxicities were qualitatively captured. Toxicity events were attributed to chemotherapy if occurring between the start of chemotherapy and 30 days after last treatment.
Time from chemotherapy completion to RC-PLND was collected. The extent of PLND was based on surgeon preference and not standardized among treating urologists. Extended PLND included at a minimum the bilateral hypogastric, external iliac, obturator, common iliac and presacral nodes. The lower para-aortic and para-caval nodes up to the base of the inferior mesenteric artery represented the highest extent removed. Lesser dissections were performed based on patient or tumor factors and surgeon preference. Patients undergoing bladder-sparing approaches (partial cystectomy, post-chemotherapy intravesical therapy) or post-RC-PLND adjuvant protocols were excluded. Pathologic responses were defined as: complete pathologic response (pT0), residual non-MIBC (pTa/pTis/pT1N0), all non-MIBC (pT0/pTa/pTis/pT1N0)(<pT2), residual MIBC or greater (≥pT2), and residual node positive (≥N1).
2.2. Statistical Methods
Patient and disease characteristics were summarized using the median (range) when continuous or the number (%) when categorical. Linear regression was used to test for association between patient and disease characteristics and number of nodes removed. Overall survival (OS) was defined as death from any cause, and was calculated from date of RC to date of death or last follow-up. Disease-free survival (DFS) was defined as recurrence or death from disease, and was calculated from date of RC to date of recurrence or death from disease. Patients who did not recur or died from other causes were censored for DFS at the date of last follow-up or death. Date of RC was used as the start time for both OS and DFS to obtain a consistent interpretation of results. The Kaplan-Meier method was used to estimate survival times and the log-rank test, stratified by surgeon, was used for univariable between-group comparisons. Multivariable Cox regression models were adjusted for potential confounders that were determined a priori and were stratified by surgeon. The functional form of the association between number of nodes removed and OS and DFS was assessed using Martingale residual plots, and a linear form was chosen based on these results and for generalizability and interpretability.
A p-value < 0.05 was considered statistically significant. All analyses were conducted using R software version 3.2.2 (R Core Development Team, Vienna, Austria) including the ‘survival’ package.
3. RESULTS
Initial review of all patients receiving NAC followed by RC-PLND yielded 167 patients potentially eligible for analysis. Thirteen patients were excluded: 1 with pure micropapillary histology; 9 who underwent a bladder-sparing approach; 1 with GC-related cardiac complications delaying RC-PLND; and 2 who underwent investigational adjuvant treatment. Of the remaining 154 patients, the median age was 65 years with a predominance of current or former smokers (81%), Caucasians (88%), and males (68%) (Table 1). Over 70% of patients received the 21-day split-dose regimen. A median of 4 cycles (range, 0.5–4) was delivered over a median of 86 days (range, 21–121). One hundred seventeen patients (76%) received all 4 cycles. The achieved dose intensity for cisplatin was 90%, with a median weekly cisplatin dose of 21.5 mg/m2/week (range 10.1–26.1). The achieved dose intensity for gemcitabine was 89%, resulting in a median dose of 615.4 mg/m2/week (range 289.3–746.7) (Table 1).
Table 1.
Patient characteristics. Summary measures presented are N (%) when categorical and median (min, max) when continuous.
| Variable | Summary measure |
|---|---|
| Age at diagnosis | 65 years (39.1, 82.9) |
| Sex | |
| Male | 105 (68.2) |
| Female | 49 (31.8) |
| Race | |
| White | 135 (87.7) |
| Black | 6 (3.9) |
| Asian | 2 (1.3) |
| Other/unknown | 11 (7.1) |
| Smoking status | |
| Never | 30 (19.5) |
| Former | 83 (53.9) |
| Current | 41 (26.6) |
| cT stage | |
| cT2 | 69 (44.8) |
| cT3 | 74 (48.1) |
| cT4a | 11 (7.1) |
| Histology | |
| UC | 105 (68.2) |
| UC, mixed | 49 (31.8) |
| Palpable mass on EUA | |
| Yes | 37 (24.0) |
| No | 63 (40.9) |
| N/A | 54 (35.1) |
| Mobile bladder on EUA | |
| Yes | 95 (61.7) |
| No | 5 (3.2) |
| N/A | 54 (35.1) |
| Hydronephrosis | |
| Yes | 41 (26.6) |
| No | 113 (73.4) |
| KPS | |
| 70 | 2 (1.3) |
| 80 | 36 (23.4) |
| 90 | 116 (75.3) |
| Chemotherapy regimen | |
| Split-dose | 112 (72.7) |
| Standard | 40 (26.0) |
| Gemcitabine weekly | 2 (1.3) |
| Chemotherapy treatment time (days) | 86.0 (21.0, 121.0) |
| Days | |
| Days from chemotherapy end to RC-PLND * | 33.5 (0.0, 106.0) |
| Cycles completed | 4.0 (0.5, 4.0) |
| Gemcitabine dose intensity | 615.4 mg/m2/week (289.3, 746.7) |
| Cisplatin dose intensity | 21.5 mg/m2/week (10.1, 26.1) |
| Gemcitabine total dose | 8000 mg/m2 (1000, 12000) |
| Cisplatin total dose | 280.0 mg/m2 (25.0, 295.0) |
| pT stage: patients (%) | |
| pT0 | 32 (21) |
| pTa | 2 (1) |
| pTis | 25 (16) |
| pT1 | 12 (8) |
| pT2 | 22 (14) |
| pT3 | 55 (36) |
| pT4 | 6 (4) |
| pTa + pTcis + pT1 | 39 (25) |
| pT0 + pTa + pTcis + pT1 (<pT2) | 71 (46) |
| ≥pT2 | 83 (54) |
| Median total lymph nodes | 19.0 (0.0, 61.0) |
| Positive lymph nodes | |
| Yes | 32 (21) |
| No | 122 (79) |
| Median Number positive Lymph Nodes (32) | 3.0 (1.0, 12.0) |
| Positive margin (bladder or urethra) | |
| Yes | 11 (7.1) |
| No | 143 (92.9) |
Imputed end date of chemotherapy was used. In one patient, chemotherapy was terminated mid-cycle and RC was performed 3 weeks after the last dose, with a 0-day interval between the imputed cycle end date and the date of RC.
Grade 3/4 hematologic toxicities included anemia (10%), leukopenia (12%), and thrombocytopenia (8%). Grades 3/4 acute renal toxicity occurred in <1% of patients. The incidence of VTE was 20% (30 of 149, with 5 patients excluded); 17% had a venous event and 3% had an arterial event. Only 6 events (4%) resulted in cycle attenuation. The most common cycle-limiting toxicities were hematologic, drug tolerance, infection, and renal insufficiency.
Median number of days from chemotherapy completion to RC-PLND was 34 (range 0–106) with 70% and 89% of patients undergoing surgery within 6 and 9 weeks, respectively. One death occurred within 30 days and 6 deaths total (4%) within 90 days of RC-PLND. Four deaths were related to disease progression and 2 to post-surgical complications.
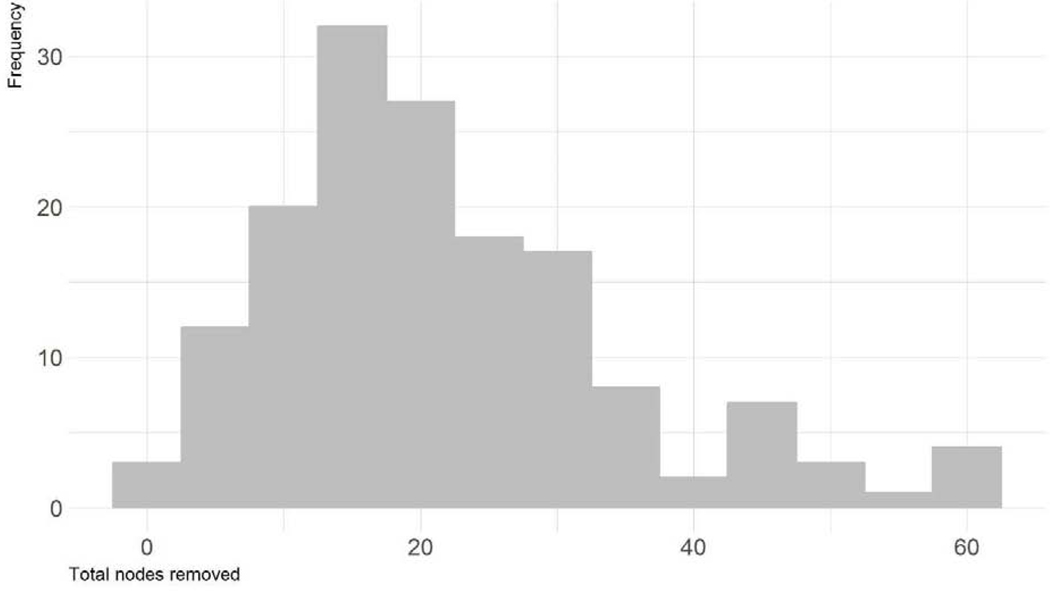
At RC, 46% of patients had <pT2 disease: 21% had pT0 and 25% had residual NMIBC (Table 1). The median number of nodes retrieved was 19 (range 0–61) (Figure 1), and 21% of patients had node involvement, with a median of 3 positive LN (range 1–12) among node positive patients. Eleven patients (7%) had positive invasive surgical margins. When excluding patients with urethral UC, the positive margin rate was 5%.
Figure 1.
Distribution of number of lymph nodes removed
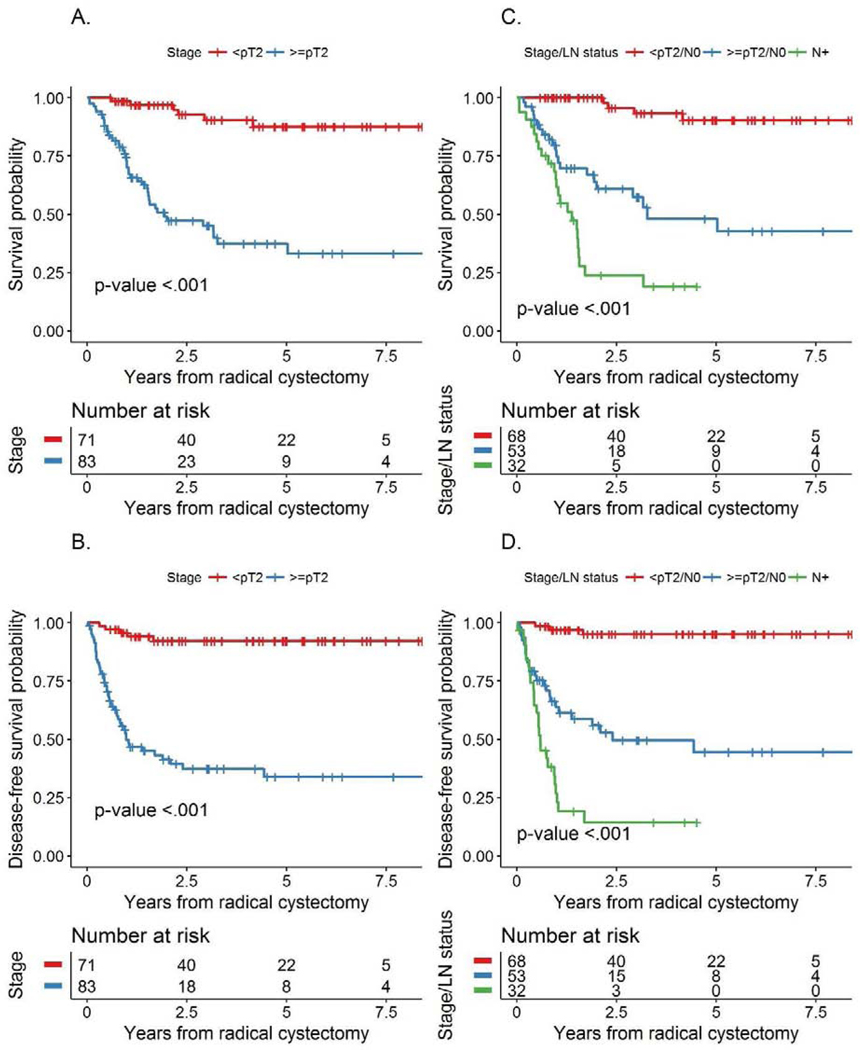
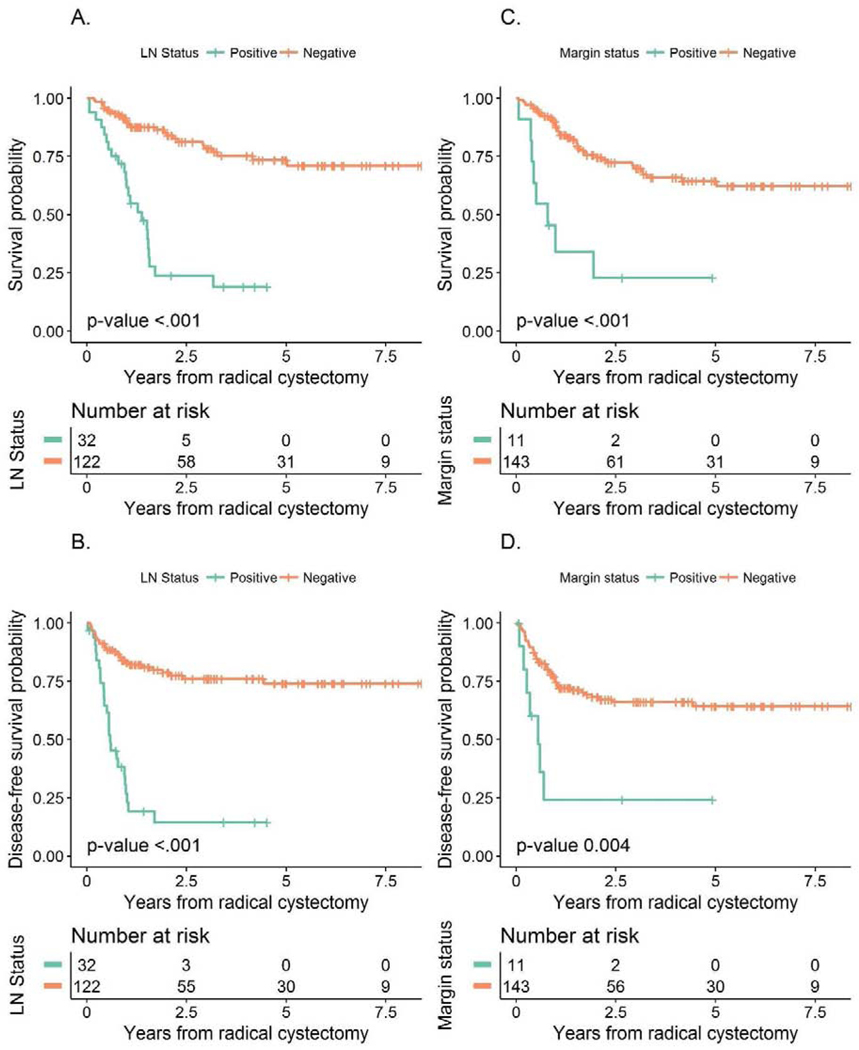
Median follow-up among survivors was 3 years (range: 0.4 – 9.2). During this time, 48 patients died from any cause while 52 had a recurrence or died from disease. Overall survival was 72% (95% CI 64–80%) and 66% (95% CI 59–75%) and DFS was 66% (95% CI 58–74%) and 64% (95% CI 56–72%) at 2 years and 3 years, respectively. Since pT0 and residual non-MIBC responses did not differ with regard to OS (p=0.868) or DFS (p = 0.247), a combined pathologic outcome of patients with <pT2 versus ≥pT2 disease was used. Patients down-staged to <pT2 had a significantly better OS compared to residual ≥pT2 disease (p<0.001, Figure 2A) with 3-year OS of 90% (95% CI 82–98%) versus 45% (95% CI 26–53%). Similarly, DFS was better in <pT2 responders as compared to non-responders (≥pT2) (p<0.001; Figure 2B), with 3-year DFS of 92% (95% CI 86–99%) versus 37% (95% CI 27–51%). Patients with node positive disease had a significantly worse OS and DFS than patients with any residual bladder disease (Figures 2C and D) and those without node involvement (both p<0.001; Figures 3A and 3B). No node positive patient survived 5 years and only 4 node positive patients survived 3 years, with a maximal survival time of 3.17 years. Both OS (p<0.001) and DFS (p=0.004) were similarly poor in patients with positive surgical margins compared with negative or non-invasive margins (Figures 3C and 3D).
Figures 2A-B:
Association of pathological stage with A) overall survival and B) disease-free survival; figures 2C-D: Association of LN positivity with C) overall survival and D) disease-free survival
Figure 3 –
Association between LN status and A) Overall Survival and B) Disease-Free Survival and the association between margin status and C) Overall Survival and D) Disease-Free Survival
On univariable analysis, clinical stage (cT2 vs. cT3/T4), hydronephrosis, number of chemotherapy cycles administered (<4 vs. 4), gemcitabine and cisplatin dose intensities and total doses, pathologic stage, LN status, and margin status were all significantly associated with OS (all p<0.05). Clinical stage, hydronephrosis, pathologic stage, LN status and margin status were significantly associated with DFS (all p<0.05). The number of LN retrieved was not associated with the presence of positive LN (p=0.647), OS (p=0.275), DFS (p=0.511), clinical stage (p=0.062), or pathologic stage (p=0.060). There was a strong association between initial clinical stage and final pathologic stage (p<0.001).
On multivariable analysis, the presence of residual post-chemotherapy ≥pT2 disease (HR 6.79; 95% CI 2.63–17.53; p<0.001), positive LN (HR 3.64; 95% CI 1.84–7.19; p<0.001) and positive surgical margins (HR 4.15; 95% CI 1.68–10.25; p=0.002) (Table 2) were significantly associated with an increased risk of death. The hazard ratio for number of nodes retrieved was <1, but this association did not reach statistical significance (HR 0.97; 95% CI 0.94–1.00; p=0.056). Moreover, residual post-chemotherapy ≥pT2 disease and positive node status were significantly associated with an increased risk of recurrence or death from disease (both p<0.001).
Table 2 –
Multivariable Cox regression results
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| <pT2 vs ≥pT2 | 6.79 (2.63 – 17.53) | <0.001 | 9.72 (3.65 – 25.9) | <0.001 |
| Positive Margin | 4.15 (1.68 – 10.25) | 0.002 | 1.54 (0.62 – 3.85) | 0.355 |
| Positive LN | 3.64 (1.84 – 7.19) | <0.001 | 4.16 (2.11 – 8.22) | <0.001 |
| Number of nodes removed | 0.97 (0.94 – 1) | 0.056 | 0.98 (0.95 – 1.01) | 0.241 |
4. CONCLUSIONS
The hallmark of an efficacious NAC regimen is the safe delivery of anti-neoplastic agents at doses sufficient to result in tumor cell death without compromising post-chemotherapy management. Several neoadjuvant cisplatin-based chemotherapy regimens have shown a survival benefit in MIBC. While the optimal regimen remains undefined, this retrospective analysis validates the GC doublet as a tolerable neoadjuvant regimen that results in significant pathologic downstaging of MIBC and good long-term survival when integrated with appropriate surgical management, supporting its inclusion as a neoadjuvant regimen that should be recommended for routine clinical use. Several historical series of RC alone have shown a ~50% 5-year survival rate that is improved with the incorporation of NAC. Our data showing a 61% 5-year OS recapitulate these findings and underscore the efficacy of GC as an effective neoadjuvant regimen.(1) Limitations to this study include its retrospective nature and potential biases inherent to a single, tertiary care referral center; however, an integrated medical and surgical team performed the analysis. Moreover, this study includes 5-year survival follow-up of all patients, representing a very mature OS analysis.
Complete disease eradication within the bladder following chemotherapy (pT0) has traditionally been considered the sole surrogate for long-term recurrence-free and overall survival, based on the SWOG 8710 study in which pT0 responders had an 85% 5-year overall survival. However, any degree of pathologic down-staging from muscle invasion (<pT2) has also been reported to be associated with improved survival in patients receiving cisplatin-based NAC.(19) While the pT0 rate observed with GC was lower than reported in SWOG 8710 (21% vs. 38%, respectively), the rate of down-staging to < pT2 was 46%, similar to the 44% rate in the SWOG study. The 5-year OS for patients achieving < pT2 responses was nearly identical to that of pT0 responders (89% vs. 85%, respectively). These data support the use of <pT2 response as an efficacy endpoint for clinical trials of NAC in MIBC.
Cisplatin-based chemotherapy has been associated with a 14–18% incidence of venous and arterial thromboembolic events.(20, 21) A retrospective study found a 15% rate of VTEs in patients receiving GC for metastatic or locally unresectable urothelial carcinoma.(22) Of 30 patients who developed VTEs in this study, 6 (4%) required early cessation of NAC, and most of these events were incidentally identified, underscoring the overall tolerability of preoperative GC.
The GC regimen did not result in significant delays to RC-PLND, minimizing concerns from reports demonstrating inferior disease-specific and overall survival with a delay of >12 weeks in time to RC.(23–25) In our cohort, 96% of patients underwent surgery within 12 weeks. Post-surgical mortality at 30 days (0.6%) was not impacted by chemotherapy administration or toxicities.
Splitting the cisplatin dose over days 1 and 8 is a frequent permutation of the GC regimen used to reduce the risk of platinum-induced renal toxicity in an older patient population. Few groups have examined the pathologic down-staging rates and survival outcomes with split-dose therapy.(26, 27) In this analysis, the majority of patients (112) received split-dose cisplatin and usage did not impact pathologic response.
On multivariable analysis, ≥pT2 disease, node involvement, and positive surgical margins all increased the risk of death. These results are concordant with previously reported prognostic factors.(28, 29) This study also observed an improved survival from an increasing number of resected LN, similar to retrospective studies reporting that the absolute number of nodes retrieved is a weak surrogate for quality and/or extent of PLND, and that there are an extensive number of clinical, surgical and pathological variables that impact these analyses beyond a single node count. Surgical factors affecting survival outcomes are being addressed in the SWOG 1011 study, comparing extended versus standard PLND.
An underlying concern regarding NAC is that patients who do not achieve pathologic down-staging have missed the window for cure with upfront RC-PLND. This concern has fueled the search for biomarkers that effectively discriminate between responders and non-responders. SWOG1314, a randomized trial of 2 cisplatin-based NAC regimens, is incorporating a multi-platform analysis of pre-treatment TUR specimens to identify genomic biomarkers of response. Additional studies have shown an association between defective DNA damage repair processes and sensitivity to cisplatin-based chemotherapy.(30, 31) Efforts to prospectively validate these findings are ongoing and should serve as the basis for further studies of NAC in pre-selected patients.
Clinical Practice Points.
Gemcitabine and cisplatin (GC) is a commonly used neoadjuvant chemotherapy regimen for the management of muscle-invasive bladder cancer. While one prospective trial (SWOG 1314) contains outcomes for patients who received neoadjuvant GC, long-term survival and toxicity data are lacking. Our analysis of 154 patients who received neoadjuvant GC followed by radical cystectomy and pelvic lymph node dissection showed a pathologic down-staging rate of 46%, similar to that observed in prospective trials of the alternative 4-drug regimen of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC). The GC doublet was well tolerated without significant delays to surgery or a higher post-surgical complication rate. Notably, any degree of pathologic down-staging was associated with improved survival, and no significant difference in survival was observed between complete responders and those with down-staging to non-muscle-invasive disease. The presence of involved nodes and positive surgical margins were associated with poor survival. Importantly, split dose cisplatin, commonly used to ameliorate the toxicities of platinum chemotherapy, did not impact pathologic response rates. These data support the use of <pT2 as a surrogate endpoint for long-term disease-free survival in patients receiving neoadjuvant chemotherapy prior to cystectomy.
Key Points.
Neoadjuvant gemcitabine and cisplatin therapy is associated with significant pathologic down-staging to <pT2
Down-staging from muscle-invasive disease at the time of cystectomy is associated with equivalent overall survival compared to complete pathologic response following neoadjuvant gemcitabine and cisplatin
Gemcitabine and cisplatin did not result in significant delays to radical cystectomy and pelvic lymph node dissection
Acknowledgements
Funding: NIH Training Grant T-32CA09207 and The Zena and Michael A. Wiener Research and Therapeutic Program in Bladder Cancer, and the Core Grant (P30 CA008748).
Footnotes
Disclosures:
No relevant conflicts of interest exist for the authors of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–75. [DOI] [PubMed] [Google Scholar]
- 2.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. [DOI] [PubMed] [Google Scholar]
- 3.Advanced Bladder Cancer Meta-analysis C. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–5; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 4.International Collaboration of T, Medical Research Council Advanced Bladder Cancer Working P, European Organisation for R, Treatment of Cancer Genito-Urinary Tract Cancer G, Australian Bladder Cancer Study G, National Cancer Institute of Canada Clinical Trials G, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zargar H, Espiritu PN, Fairey AS, Mertens LS, Dinney CP, Mir MC, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67(2):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. [DOI] [PubMed] [Google Scholar]
- 7.Scosyrev E, Messing EM, van Wijngaarden E, Peterson DR, Sahasrabudhe D, Golijanin D, et al. Neoadjuvant gemcitabine and cisplatin chemotherapy for locally advanced urothelial cancer of the bladder. Cancer. 2012;118(1):72–81. [DOI] [PubMed] [Google Scholar]
- 8.Yeshchina O, Badalato GM, Wosnitzer MS, Hruby G, RoyChoudhury A, Benson MC, et al. Relative efficacy of perioperative gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. 2012;79(2):384–90. [DOI] [PubMed] [Google Scholar]
- 9.Hussain SA, Palmer DH, Lloyd B, Collins SI, Barton D, Ansari J, et al. A study of split-dose cisplatin-based neo-adjuvant chemotherapy in muscle-invasive bladder cancer. Oncol Lett. 2012;3(4):855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairey AS, Daneshmand S, Quinn D, Dorff T, Dorin R, Lieskovsky G, et al. Neoadjuvant chemotherapy with gemcitabine/cisplatin vs. methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive urothelial carcinoma of the bladder: a retrospective analysis from the University of Southern California. Urol Oncol. 2013;31(8):1737–43. [DOI] [PubMed] [Google Scholar]
- 11.Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121(15):2586–93. [DOI] [PubMed] [Google Scholar]
- 12.Dash A, Pettus JAt, Herr HW, Bochner BH, Dalbagni G, Donat SM, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113(9):2471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11(4):446–75. [DOI] [PubMed] [Google Scholar]
- 14.Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere White R, Sarosdy MF, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22(14):2781–9. [DOI] [PubMed] [Google Scholar]
- 15.Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. The Journal of urology. 2002;167(3):1295–8. [PubMed] [Google Scholar]
- 16.Konety BR, Joslyn SA, O’Donnell MA. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer: analysis of data from the Surveillance, Epidemiology and End Results Program data base. The Journal of urology. 2003;169(3):946–50. [DOI] [PubMed] [Google Scholar]
- 17.Herr HW. Extent of surgery and pathology evaluation has an impact on bladder cancer outcomes after radical cystectomy. Urology. 2003;61(1):105–8. [DOI] [PubMed] [Google Scholar]
- 18.Koppie TM, Vickers AJ, Vora K, Dalbagni G, Bochner BH. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer. 2006;107(10):2368–74. [DOI] [PubMed] [Google Scholar]
- 19.Splinter TA, Scher HI, Denis L, Bukowski R, Simon S, Klimberg I, et al. The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. European Organization for Research on Treatment of Cancer--Genitourinary Group. The Journal of urology. 1992;147(3):606–8. [DOI] [PubMed] [Google Scholar]
- 20.Duivenvoorden WC, Daneshmand S, Canter D, Lotan Y, Black PC, Abdi H, et al. Incidence, Characteristics and Implications of Thromboembolic Events in Patients with Muscle Invasive Urothelial Carcinoma of the Bladder Undergoing Neoadjuvant Chemotherapy. J Urol. 2016;196(6):1627–33. [DOI] [PubMed] [Google Scholar]
- 21.Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29(25):3466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tully CM, Apolo AB, Zabor EC, Regazzi AM, Ostrovnaya I, Furberg HF, et al. The high incidence of vascular thromboembolic events in patients with metastatic or unresectable urothelial cancer treated with platinum chemotherapy agents. Cancer. 2016;122(5):712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CT, Madii R, Daignault S, Dunn RL, Zhang Y, Montie JE, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. The Journal of urology. 2006;175(4):1262–7; discussion 7. [DOI] [PubMed] [Google Scholar]
- 24.Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS, Urologic Diseases in America P. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2009;115(5):988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen ME, Palapattu GS, Karakiewicz PI, Lotan Y, Bastian PJ, Lerner SP, et al. A delay in radical cystectomy of >3 months is not associated with a worse clinical outcome. BJU international. 2007;100(5):1015–20. [DOI] [PubMed] [Google Scholar]
- 26.Kim YR, Lee JL, You D, Jeong IG, Song C, Hong B, et al. Gemcitabine plus split-dose cisplatin could be a promising alternative to gemcitabine plus carboplatin for cisplatin-unfit patients with advanced urothelial carcinoma. Cancer chemotherapy and pharmacology. 2015;76(1):141–53. [DOI] [PubMed] [Google Scholar]
- 27.Maughan BL, Agarwal N, Hussain SA, Boucher KM, Von Der Maase H, Kaufman DS, et al. Pooled analysis of phase II trials evaluating weekly or conventional cisplatin as first-line therapy for advanced urothelial carcinoma. Clinical genitourinary cancer. 2013;11(3):316–20. [DOI] [PubMed] [Google Scholar]
- 28.Henningsohn L, Wijkstrom H, Dickman PW, Bergmark K, Steineck G. Distressful symptoms after radical cystectomy with urinary diversion for urinary bladder cancer: a Swedish population-based study. Eur Urol. 2001;40(2):151–62. [DOI] [PubMed] [Google Scholar]
- 29.Novara G, Svatek RS, Karakiewicz PI, Skinner E, Ficarra V, Fradet Y, et al. Soft tissue surgical margin status is a powerful predictor of outcomes after radical cystectomy: a multicenter study of more than 4,400 patients. The Journal of urology. 2010;183(6):2165–70. [DOI] [PubMed] [Google Scholar]
- 30.Van Allen EM, Mouw KW, Kim P, Iyer G, Wagle N, Al-Ahmadie H, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer discovery. 2014;4(10):1140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plimack ER, Dunbrack RL, Brennan TA, Andrake MD, Zhou Y, Serebriiskii IG, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol. 2015;68(6):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]