Abstract
Background
The aim of this study was to compare the outcomes following anterior cervical discectomy and fusion with zero-profile anchored spacer-ROI-C-fixation (ROI-C) vs combined intervertebral cage and anterior cervical discectomy and fusion (ACDF).
Material/Methods
We retrospectively analyzed 87 patients who underwent operations between January 2015 and January 2019, including 42 patients that underwent ROI-C treatment (group A) and 45 that were treated by the ACDF approach (group B). Operative duration, blood loss, dysphagia, Neck Disability Index scores (NDI), Japanese Orthopaedic Association scores (JOA), and other complications were compared between these groups. In addition, implant settlement, fusion, and cervical Cobb angle were assessed via imaging analyses.
Results
Patients in group A and group B were followed for 22.6±3.3 months and 27.1±3.5 months, respectively (range: 13–30 months). Relative to preoperative values, JOA scores were increased and NDI scores were reduced in both groups following treatment (P<0.05), with comparable outcomes between groups (P>0.05). However, operative duration, intraoperative blood loss, and postoperative complications did differ significantly between these groups (P<0.05). Specifically, rates of short-term dysphagia were lower and recovery time was faster in group A relative to group B (P<0.05).
Conclusions
The findings from this study showed that ROI-C fixation achieved satisfactory outcomes, improved cervical curvature, restored intervertebral height, and was associated with shorter operative duration, reduced blood loss, and less dysphagia.
Keywords: Cervical Vertebrae, Neck Pain, Spondylosis
Background
Cervical spondylosis can have a severe adverse impact on quality of life [1,2]. In cases where conservative treatment alone is ineffective, surgery is generally required to alleviate symptoms and to improve patient well-being [3]. Recent technological and surgical advances have improved the treatment of cervical spondylosis [4]. Anterior cervical surgery is an increasingly common treatment strategy for these patients, as it is associated with relatively minimal trauma and is sufficient to achieve high rates of precise curative outcomes [5]. Titanium cage bone grafting combined with titanium plate fixation and fusion is often used to treat cervical spondylosis. While very effective, this treatment is associated with common complications, including plate displacement, loosening of surgical screws, esophageal damage, and dysphagia [6–8]. Efforts to reduce the rates of these complications led to the successful development and clinical implementation of ROI-C. Therefore, the present study aimed to compare the clinical outcomes following anterior cervical discectomy and fusion with ROI-C vs ACDF.
Material and Methods
General Information
This retrospective study was supported by the Institutional Committee of Danyang People’s Hospital for Clinical Studies. Between January 2015 and January 2019, a total of 87 cervical spondylosis patients were treated in our hospital. Of these patients, 42 (23 females, 19 males; average age: 62.59±8.21 years; range: 50–73 years) underwent ROI-C (group A), while 45 (25 females, 20 males; average age: 61.15±7.52; range: 51–72 years) underwent treatment using ACDF (group B). Patients included in the present study met the following criteria: (1) Preoperative X-ray, preoperative computed tomography (CT), and preoperative magnetic resonance imaging (MRI) scans of the patient revealed single-level spinal cord or nerve root compression and osteophyte formation; (2) patients were experiencing symptoms including numbness, limb weakness, gait instability, or neck and shoulder pain that were not improved following 6 or more weeks of conservative treatment; and (3) follow-up data pertaining to > 12 months was available. Patients excluded in the present study met the following criteria: (1) showed posterior longitudinal ligament stenosis and ossification; (2) exhibited severe cervical instability or trauma; or (3) had any history of tumors, serious general disease, or prior cervical surgery.
Surgical Procedures
All patients were placed in supine position, with the head tilted back using a cervical cushion. Patients were administered general anesthesia, and a right transverse incision was made to expose the vertebral body. For patients in group A, following vertebral segment exposure, the hyperplastic tissue was removed to prevent compression of the spinal cord and nerve roots. Appropriate cage size was determined using the test model. The local osteophyte that had been cut was placed in the center of the cage, which was subsequently implanted in the intervertebral space. The cage position was then assessed in the lateral and positive position. Following cage implantation, 2 self-locking cervical fixation clips were installed to ensure stability. For patients in group B, the corresponding vertebral body was exposed, after which the corresponding intervertebral disc, the posterior longitudinal ligament, and the bone hyperplasia behind the vertebral body were removed to alleviate dura mater and nerve root compression. The removed bone was placed into the intervertebral cage, and an appropriate length of steel plate was placed in front of the cervical vertebra, after which the plate was fixed using 4 screws. A cervical bracket was used for 4 weeks to support appropriate fixation [9].
Clinical Evaluation
For each patient, data pertaining to age, sex, operative duration, intraoperative blood loss, complication rates, dysphagia incidence, symptom duration, and symptom relief were recorded. JOA scores [10] and NDI scores [11] were used to compare postoperative outcomes to preoperative findings in each patient. Odom’s criteria were used to assess the efficacy of surgery [12] as follows: excellent – symptoms were completely alleviated following surgery; good – normal functionality was restored and symptoms were relieved; satisfactory – partial symptom improvement was achieved, but functionality remained abnormal; poor – symptoms were largely unchanged postoperatively. Bazaz score was used to evaluate the degree of dysphagia into 4 grades as follows: severe, moderate, mild, or none [13].
Radiographic Imaging
Collected radiographic images included preoperative X-ray, CT, and cervical MRI scans, and postoperative X-ray scans. Cobb angle values were measured with the cervical spine in the sagittal position by assessing the vertical line formed by the inferior endplate of C2 and C7. Radiologic fusion was considered to have been achieved when the following criteria were met: a) adjacent vertebral displacement was <2° in flexion with neck extension; b) target cervical intervertebral space height remained unchanged; c) no transparent lines were detected between the grafted bone and the upper and lower vertebral body endplates; d) it is judged as subsidence if the height of intervertebral space is more than 3 mm. Two experienced surgeons independently assessed all radiographic data to reduce the mean error associated with these analyses.
Statistical Analysis
SPSS 19.0 (SPSS, Inc, IL, USA) was used for all statistical testing. Clinical and radiologic outcome data were compared via t tests. Categorical variables including complication and fusion rates were compared via chi-squared tests. P < 0.05 was the significance threshold for this study.
Results
Clinical Evaluation
Operations for all patients were completed successfully without any incidence of hoarseness, cerebrospinal fluid leakage, or esophageal fistulae. Representative cases in group A are shown in Figures 1 and 2. Representative cases in group B are shown in Figures 3 and 4. The average operative duration for group A (84±23 min) was lower than that for group B (98±27 min) (P<0.05, Table 1). Similarly, intraoperative blood loss was lower for group A (139±22 mL) relative to group B (154±33 mL) (P<0.05, Table 1). No significant differences were observed between groups with respect to JOA or NDI scores, which were increased and decreased, respectively, in both groups postoperatively (P>0.05, Table 2). Clinical symptoms were significantly improved in all patients following treatment.
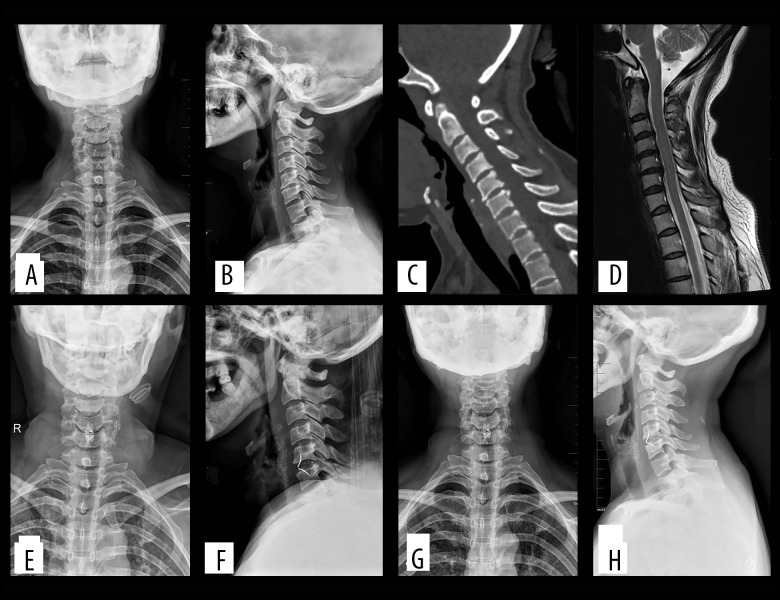
Figure 1.
(A–D) Preoperatively, cervical degeneration, hyperosteogeny, C5–6 cervical disc compresses the spinal cord. (E, F) Two days after surgery, X-ray images revealed appropriate C5–6 cervical discectomy and proper positioning of the ROI-C interbody fusion cage. (G, H) One-year after surgery, X-ray imaging revealed appropriate C5–6 cervical fusion and proper positioning of the ROI-C interbody fusion cage. (C – cervical vertebra).
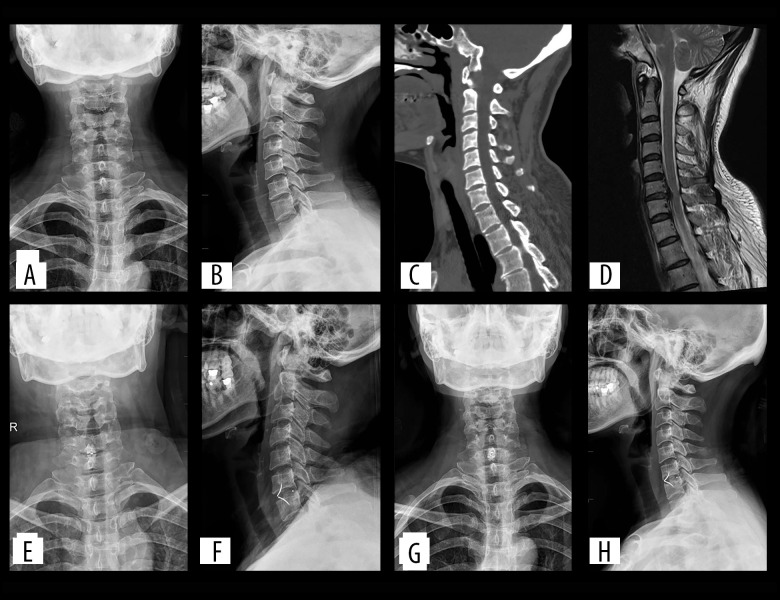
Figure 2.
(A–D) Preoperatively, cervical degeneration, hyperosteogeny, C6–7 cervical disc compresses the spinal cord. (E, F) Two days after surgery, X-ray images revealed appropriate C6–7 cervical discectomy and proper positioning of the ROI-C interbody fusion cage. (G, H) One-year after surgery, X-ray imaging revealed appropriate C6–7 cervical fusion and proper positioning of the ROI-C interbody fusion cage.
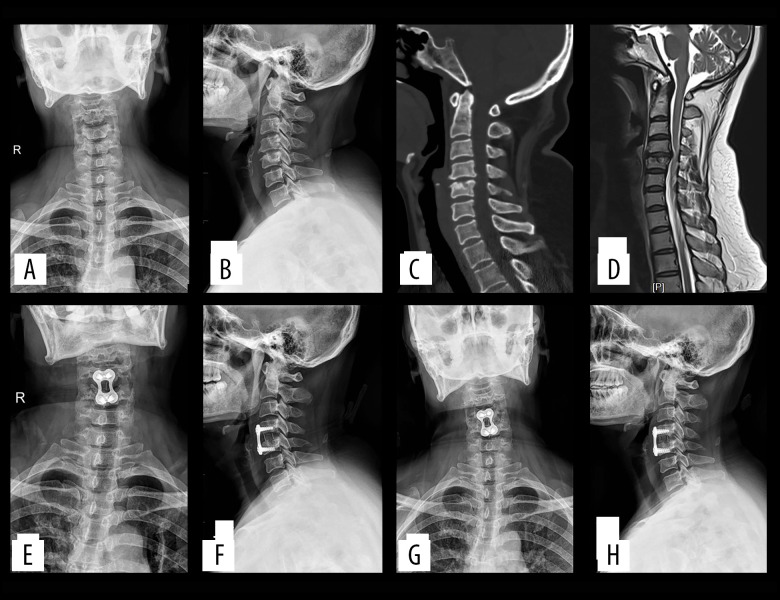
Figure 3.
(A–D) Preoperatively, cervical degeneration, hyperosteogeny, C4–5 cervical disc compresses the spinal cord. (E, F) Two days after surgery, X-ray imaging revealed appropriate C4–5 cervical discectomy and proper positioning of the titanium mesh and plate; (G, H) One-year after surgery, X-ray imaging revealed appropriate C4–5 cervical fusion and proper positioning of the titanium mesh and plate.
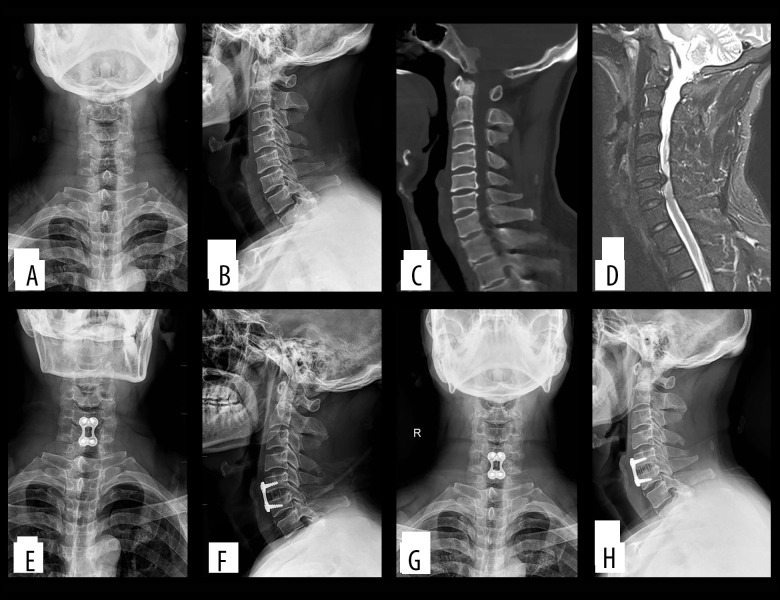
Figure 4.
(A–D) Preoperatively, cervical degeneration, hyperosteogeny, C5–6 c cervical disc compresses the spinal cord. (E, F) Two days after surgery, X-ray imaging revealed appropriate C5–6 cervical discectomy and proper positioning of the titanium mesh and plate; (G, H) One-year after surgery, X-ray imaging revealed appropriate C5–6 cervical fusion and proper positioning of the titanium mesh and plate.
Table 1.
General information.
| Group A | Group B | P value | |
|---|---|---|---|
| Number | 42 | 45 | – |
| Sex | |||
| Male | 19 | 20 | – |
| Female | 23 | 25 | – |
| Age (year) | 62.59±8.21 | 61.15±7.52 | 0.396 |
| Follow-up (months) | 26.6±3.3 | 27.1±3.5 | 0.496 |
| Operation time (minutes) | 84±23 | 98±27 | 0.011 |
| Blood loss (ml) | 139±22 | 154±33 | 0.015 |
P value is given for comparison between both groups. P<0.05, statistically significant.
Table 2.
JOA score and NDI score results (mean±SD).
| Group A | Group B | P value | |
|---|---|---|---|
| JOA scores | |||
| Preoperative | 9.9±1.6* | 9.7±1.9* | 0.598 |
| Postoperative 1 month | 13.2±2.1** | 13.8±1.7** | 0.626 |
| Postoperative 3 month | 14.9±1.7** | 15.4±1.9** | 0.201 |
| Last follow-up | 14.1±1.5** | 14.4±1.8** | 0.403 |
| NDI scores | |||
| Preoperative | 34.1±9.5* | 33.5±8.9* | 0.736 |
| Postoperative 1 month | 16.9±4.4** | 15.5±4.8** | 0.161 |
| Postoperative 3 month | 15.4±4.2** | 15.6±4.3** | 0.827 |
| Last follow-up | 14.7±4.6** | 15.1±4.4** | 0.679 |
JOA – Japanese Orthopedic Association, NDI – Neck Disability Index.
P value is given for comparison between both groups;
P<0.05 compared with preoperative value.
Radioactivity Evaluation
The postoperative Cobb angle of group A (17.5±9.1°) was significantly better than the preoperative value in these same patients (12.7±8.4°) (P<0.05, Table 3). Similarly, the postoperative cervical lordosis angle in group B (19.8±9.3°) was significantly improved relative to preoperative values in these same patients (11.9±9.1°) (P<0.05, Table 3). There were no differences in cervical lordosis angle observed between groups postoperatively (P>0.05). Three months postoperatively, fusion rates in groups A and B were 85.7% and 82.2%, respectively, with no significant difference between these groups (P>0.05, Table 3). All patients were fused at the final follow-up. Six cases of subsidence were observed in group A and 9 cases in group B, with overall subsidence rates being comparable between groups (P>0.05, Table 3).
Table 3.
The mean outcomes of radiological parameters measured before operation and during follow-up (mean±SD).
| Group A | Group B | P value | |
|---|---|---|---|
| Cervical lordosis | |||
| Preoperative | 12.7±8.4* | 11.9±9.1* | 0.672 |
| Postoperative 1 month | 17.5±9.1** | 19.8±9.3** | 0.247 |
| Postoperative 3 month | 17.1±10.3** | 17.9±9.1** | 0.702 |
| Last follow-up | 16.4±9.5** | 17.2±9.8** | 0.701 |
| Fusion rate | |||
| Postoperative 3 month | 85.7% (36/42) | 82.2% (37/45) | 0.658 |
| Final fusion | 100% | 100% | |
| Subsidence | 14.2% (6/42) | 20.0% (9/45) | 0.481 |
P value is given for comparison between both groups;
P<0.05 compared with preoperative value.
Complications
No patients reported preoperative dysphagia. At 1 month after surgery, 9 patients reported dysphagia at 1 month after surgery, with 5 of these patients reporting mild dysphagia and 4 reporting moderate dysphagia. At 3 months after surgery, 2 patients in group B still reported mild dysphagia, but it had resolved by the last follow-up. In group B, 19 patients reported dysphagia at 1 month after surgery, with 12 of these patients reporting mild dysphagia and 7 reporting moderate dysphagia. At 3 months after surgery, 3 patients in group B still reported moderate dysphagia, while 6 reported mild dysphagia. At the last follow-up, 3 patients in group B still reported mild dysphagia. At 1 and 3 months following surgery, rates of dysphagia were significantly different between these groups, whereas they did not differ significantly at the last follow-up (P>0.05, Table 4).
Table 4.
Incidence of dysphagia.
| Groups | Group A | Group B | P value |
|---|---|---|---|
| Dysphagia | |||
| One month postoperatively | 21.4% (9/42) | 42.2% (19/45) | 0.038 |
| Three months postoperatively | 4.7% (2/42) | 20.0% (9/45) | 0.033 |
| Final follow-up | 0.0% (0/42) | 6.67% (3/45) | 0.089 |
P value is given for comparison between both groups. P<0.05, statistically significant.
Discussion
The JOA and NDI scores improved significantly in the 2 groups in the present study, with significant cervical lordosis improvement and good surgical efficacy. As such, both of these tested surgical approaches can effectively alleviate spinal cord and nerve root compression. ACDF is the surgical approach that is most frequently employed to treat cervical disc disease [14,15], and it is associated with a number of advantages, including a high fusion rate and good cervical spine stability. However, ACDF is also linked with many potential complications, including dysphagia, screw or plate displacement, and adjacent segment degeneration linked to plate implantation in the anterior spine [16,17]. Clinically, a novel ROI-C has been developed and implemented to minimize these surgery-associated risks [18,19]. This ROI-C is composed of 2 self-locking clips and a PEEK box. By entering the vertebral body via the lamina, the locking plate can prevent cage movement, serving as an independent anchoring gasket and thereby providing independent biomechanical stability. Indeed, from a stability perspective, ROI-C exhibits efficacy similar to that of a fusion cage with a titanium plate [9].
ROI-C implantation completely within the intervertebral space was successfully achieved without any esophageal compression in the present study. The incidence of early dysphagia in group A was significantly lower than that in group B, but the final follow-up results showed no statistical significance. ROI-C may thus be able to decrease the incidence of early dysphagia and to reduce esophageal recovery time in patients who are affected by this complication. The mechanistic basis for dysphagia in treated patients has not been clearly elucidated, but may be linked to 2 primary factors. First, surgical instruments can induce potential esophageal damage. During anesthetization, tracheal intubation can injure the pharyngeal mucosa and cause postoperative dysphagia. Second, a retractor must be used to gently move the esophagus to clearly expose the position of the target vertebrae, thereby causing direct mechanical compression of the esophagus, which can cause damage, and prolonged traction can result in postoperative dysphagia. In this study cohort, the operative time of group B was longer than that of group A, and the incidence of dysphagia in group B was higher than that in group A. Decreasing the operative duration can thus also lower rates of postoperative dysphagia. In addition, anterior vertebral plate utilization can increase the incidence of dysphagia [20,21]. Many clinical studies have shown that following ACDF fixation with a steel plate, this plate can protrude from the vertebral body surface, resulting in slight esophageal compression. In agreement with this, the use of thinner steel plates has been reported to reduce rates of dysphagia and attachment [16]. Some studies have found that steel plate adhesion and attachment to the esophagus can cause dysphagia to develop in treated patients [22], and not using steel plates has also been linked to a lower risk of dysphagia [23].
In the present study, there were 6 cases of subsidence in group A and 9 cases of subsidence in group B, with no significant differences in subsidence rates between these groups. Intervertebral cage subsidence can induce a range of complications, including intervertebral foramen and intervertebral disc height loss and segmental spinal instability. Endplate preparation can reduce vertebral stiffness and strength, making it important to preserve as much of the cortical bone endplate as possible to decrease the risk of intervertebral cage collapse. The risk of subsidence is higher for a single interbody fusion cage, resulting in the loss of kyphosis and adjacent segment degeneration [24]. Cage sinking has previously been found to occur in roughly 44% of cases [25]. In their study, Gercek et al found that the anterior or posterior height loss of 5 intervertebral discs in 9 fusion segments was >3 mm, with 1 patient exhibiting recurrent radiculopathy at 6-month follow-up [26]. In summary, both of these approaches can decrease the risk of subsidence and displacement, consistent with the ability of the surgeon to appropriately preserve the bone endplates and to select accurately sized cages.
Our study also has the following limitations. Firstly, a longer follow-up period would be needed to investigate the vertebral subsidence and long-term cervical stabilization. Secondly, the sample size was small, and the use of a single center may have introduced bias. We expect to perform a randomized prospective study with more patients included in the future.
Conclusions
The findings from this study showed that ROI-C fixation achieved satisfactory outcomes, improved cervical curvature, restored intervertebral height, and was associated with shorter operative duration, reduced intraoperative bleeding, and a reduced incidence of postoperative dysphagia.
Acknowledgment
Thanks to all patients and doctors involved in this study.
Footnotes
Financial support: Departmental sources
References
- 1.Boogaarts HD, Bartels RH. Prevalence of cervical spondylotic myelopathy. Eur Spine J. 2015;24(Suppl 2):139–41. doi: 10.1007/s00586-013-2781-x. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Yan C, Shen Y, Wu Z. Relation between neck pain and modic changes in cervical spondylotic myelopathy. Med Sci Monit. 2020;26:e923908. doi: 10.12659/MSM.923908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witwer BP, Trost GR. Cervical spondylosis: ventral or dorsal surgery. Neurosurgery. 2007;60(Supp 1):S130–36. doi: 10.1227/01.NEU.0000215351.32372.CE. [DOI] [PubMed] [Google Scholar]
- 4.Matsunaga S, Komiya S, Toyama Y. Risk factors for development of myelopathy in patients with cervical spondylotic cord compression. Eur Spine J. 2015;24(Suppl 2):142–49. doi: 10.1007/s00586-013-2839-9. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Chen D, Wang X, et al. Zero-profile integrated plate and spacer device reduces rate of adjacent-level ossification development and dysphagia compared to ACDF with plating and cage system. Arch Orthop Trauma Surg. 2015;135(6):781–87. doi: 10.1007/s00402-015-2212-z. [DOI] [PubMed] [Google Scholar]
- 6.Guo X, Lu M, Xie N, et al. Multilevel anterior cervical discectomy and fusion with plate fixation for juvenile unilateral muscular atrophy of the distal upper extremity accompanied by cervical kyphosis. J Spinal Disord Tech. 2014;27(7):E241–46. doi: 10.1097/BSD.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen C, Sanchez K, Roren A, et al. Anatomical specificities of the degenerated cervical spine: a narrative review of clinical implications, with special focus on targeted spinal injections. Ann Phys Rehabil Med. 2016;59(4):276–81. doi: 10.1016/j.rehab.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Riley LH, 3rd, Skolasky RL, Albert TJ, et al. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine. 2005;30(22):2564–69. doi: 10.1097/01.brs.0000186317.86379.02. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wang H, Li X, et al. Comparison of a zero-profile anchored spacer (ROI-C) and the polyetheretherketone (PEEK) cages with an anterior plate in anterior cervical discectomy and fusion for multilevel cervical spondylotic myelopathy. Eur Spine J. 2016;25(6):1881–90. doi: 10.1007/s00586-016-4500-x. [DOI] [PubMed] [Google Scholar]
- 10.Yonenobu K, Abumi K, Nagata K, et al. Interobserver and intraobserver reliability of the Japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine. 2001;26(17):1890–94. doi: 10.1097/00007632-200109010-00014. [DOI] [PubMed] [Google Scholar]
- 11.Vernon H, Mior S. The Neck Disability Index: A study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–15. [PubMed] [Google Scholar]
- 12.Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc. 1958;166(1):23–28. doi: 10.1001/jama.1958.02990010025006. [DOI] [PubMed] [Google Scholar]
- 13.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: A prospective study. Spine. 2002;27(22):2453–58. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Epstein NE, Hollingsworth R. Diagnosis and management of traumatic cervical central spinal cord injury: A review. Surg Neurol Int. 2015;6(Suppl 4):S140–53. doi: 10.4103/2152-7806.156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofstetter CP, Kesavabhotla K, Boockvar JA. Zero-profile anchored spacer reduces rate of dysphagia compared with ACDF with anterior plating. J Spinal Disord Tech. 2015;28(5):E284–90. doi: 10.1097/BSD.0b013e31828873ed. [DOI] [PubMed] [Google Scholar]
- 16.Cho HJ, Hur JW, Lee JB, et al. Cervical stand-alone polyethere-therketone cage versus Zero-Profile anchored spacer in single-level anterior cervical discectomy and fusion: Minimum 2-year assessment of radiographic and clinical outcome. J Korean Neurosurg Soc. 2015;58(2):119–24. doi: 10.3340/jkns.2015.58.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alimi M, Njoku I, Hofstetter CP, et al. Anterior cervical discectomy and fusion (ACDF): Comparison between Zero Profile implants and anterior cervical plate and spacer. Cureus. 2016;8(4):e573–80. doi: 10.7759/cureus.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Li Y, Jiang W. A comparison of zero-profile anchored spacer (ROI-C) and plate fixation in 2-level noncontiguous anterior cervical discectomy and fusion – a retrospective study. BMC Musculoskelet Disord. 2018;19(1):119. doi: 10.1186/s12891-018-2033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucci MN, Oh D, Cowan RS, et al. The ROI-C zero-profile anchored spacer for anterior cervical discectomy and fusion: Biomechanical profile and clinical outcomes. Med Devices. 2017;10:61–69. doi: 10.2147/MDER.S127133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Bao W, Wang Z, et al. Comparison of the clinical effects of zero-profile anchored spacer (ROI-C) and conventional cage-plate construct for the treatment of noncontiguous bilevel of cervical degenerative disc disease (CDDD): A minimum 2-year follow-up. Medicine. 2018;97(5):e9808. doi: 10.1097/MD.0000000000009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan Y, Yang Y, Wang Y, et al. Comparison of anterior cervical discectomy and fusion with the zero-profile device versus plate and cage in treating cervical degenerative disc disease: A meta-analysis. J Clin Neurosci. 2016;33:11–18. doi: 10.1016/j.jocn.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Tong M-J, Xiang G-H, He Z-L, et al. Zero-profile spacer versus cage-plate construct in anterior cervical diskectomy and fusion for multilevel cervical spondylotic myelopathy: Systematic review and meta-analysis. World Neurosurg. 2017;104:545–53. doi: 10.1016/j.wneu.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Zhu R, Yang H, et al. Zero-profile implant (Zero-p) versus plate cage benezech implant (PCB) in the treatment of single-level cervical spondylotic myelopathy. BMC Musculoskelet Disord. 2015;16:290. doi: 10.1186/s12891-015-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YS, Kim YB, Park SW. Risk factors for postoperative subsidence of single-level anterior cervical discectomy and fusion: The significance of the preoperative cervical alignment. Spine (Phila Pa 1976) 2014;39:1280–87. doi: 10.1097/BRS.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 25.Fujibayashi S, Neo M, Nakamura T. Stand-alone interbody cage versus anterior cervical plate for treatment of cervical disc herniation: Sequential changes in cage subsidence. J Clin Neurosci. 2008;15:1017–22. doi: 10.1016/j.jocn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Gercek E, Arlet V, Delisle J, et al. Subsidence of stand-alone cervical cages in anterior interbody fusion: Warning. Eur Spine J. 2003;12:513–16. doi: 10.1007/s00586-003-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]