Abstract
Objectives:
Although there have been associations between diabetes and mortality in COVID-19 patients, it is unclear whether this is driven by the disease itself or whether it can be attributed to an inability to exhibit effective glucose control.
Methods:
We conducted a retrospective cohort study of 292 patients admitted to a tertiary referral center to assess the association of mortality and glycemic control among COVID-19-positive patients. We used a logistic regression model to determine whether average fasting glycemic levels were associated with in-hospital mortality.
Results:
Among the diabetic and non-diabetic patients, there were no differences between mortality or length of stay. Mean glucose levels in the first 10 days of admission were higher on average among those who died (150–185 mg/dL) compared with those who survived (125–165 mg/dL). When controlling for multiple variables, there was a significant association between mean fasting glucose and mortality (odds ratio = 1.014, p < 0.001). The associations between glucose and mortality remained when controlled for comorbidities and glucocorticoid use.
Conclusion:
The results of this retrospective study show an association between mortality and inpatient glucose levels, suggesting that there may be some benefit to tighter glucose control in patients diagnosed with COVID-19.
Keywords: COVID, blood glucose, diabetes, mortality
Introduction
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the cause of the global pandemic with more than 179 million cases and 3.9 million confirmed deaths as of 26 June 2021.1 SARS-CoV-2 has been shown to affect nearly every organ but primarily causes tissue damage in the upper respiratory tract and lungs, resulting in pneumonia.2 Severe cases of COVID-19 can rapidly progress to acute respiratory distress syndrome, shock, and acute kidney injury necessitating mechanical ventilation and vasopressor support.3 Elderly individuals, along with those with pre-existing conditions, such as hypertension and diabetes mellitus, have demonstrated a higher risk for developing severe cases of COVID-19 and a higher risk of mortality.4 Patients with diabetes mellitus who develop COVID-19 infections have a higher risk of intensive care unit (ICU) admissions and a higher mortality risk.5 The increase in mortality was seen in both type 1 and type 2 diabetes mellitus; however, international studies have shown conflicting findings on whether diabetes mellitus is an independent predictor of mortality among COVID-19 cases.6,7 A recent meta-analysis has shown that patients with diabetes mellitus are likely to encounter severe COVID-19 infections;8 however, it is still unknown whether an increase in mortality can be attributed to diabetes mellitus or the inability to exhibit effective glycemic control in these patients. COVID-19 patients with uncontrolled hyperglycemia have been shown to have more extended hospital stays and increased mortality compared with patients who did not exhibit hyperglycemia.9 Blood glucose levels have been identified as a prognostic factor in both disease progression and fatality.10 Hyperglycemia has been shown to be an independent risk factor for morbidity and mortality in previous SARS viruses.11 These associations between diabetes mellitus and worse outcomes in viral infections are not surprising, as hyperglycemia and diabetes have been associated with increased inflammatory markers;12 previous studies in ICU patients who developed hyperglycemia have shown worse outcomes.13
Despite a growing collection of evidence of pre-existing diabetes mellitus influencing patients’ outcomes with COVID-19, there is insufficient data on the importance of inpatient glycemic control. Recently, several studies have suggested fasting glucose levels on admission to be a significant contributor to disease severity.14,15 A key challenge for clinicians is to improve outcomes in the face of a new and complex disease, and one potential avenue may be glycemic management. The correlation of glycemic control and plasma glucose levels with clinical outcomes, including mortality in diabetic patients with COVID-19, has been demonstrated recently in the literature, but not well established. Therefore, in this study, we examined the association between glycemic control and mortality among patients with COVID-19.
Methods
We conducted a retrospective study of COVID-19 patients admitted to a tertiary referral center to ascertain whether glycemic control among diabetic and non-diabetic patients was associated with in-hospital mortality. This study was approved by the institutional review board of Hackensack Meridian Health, and consent and HIPAA waiver was granted due to the observational nature of the study (Approval No. Pro2020-0697).
All enrolled patients were confirmed COVID-19 cases with RT-PCR (reverse transcription polymerase chain reaction) and admitted to Jersey Shore University Medical Center, New Jersey, USA, from 10 March 2020 to 14 September 2020. To detect a difference in plasma glucose of 20 mg/dL between groups with 80% power and an alpha of 0.05, assuming 17% mortality in hospitalized COVID-19 patients, a sample of 253 patients was required. A real-time RT-PCR assay for SARS-CoV-2 was performed based on the Centers for Disease Control and Prevention (CDC) recommendation. All patients included in this study had clinical outcomes and laboratory parameters recorded. All patients received standard of care treatment at the time of hospitalization, including respiratory support, antiviral therapy, antimicrobial therapy, and symptomatic and supportive therapies, as appropriate.
Epidemiological and clinical characteristics, laboratory parameters, clinical status, and outcomes were obtained from electronic medical record (EMR). Data were entered and cross-reviewed by at least two independent team members. The information recorded included demographic data and past medical history, including diabetes mellitus, hypertension, coronary artery disease, chronic lung disease, congestive heart failure, dyslipidemia, and obesity. Home diabetic medications, including oral and injectable antihyperglycemic agents and insulin, were recorded. Treatment measures and medications were obtained via EMR. Complications such as ICU admission and diagnosis of acute respiratory distress syndrome were considered. The following information was collected for the study: the laboratory findings, including random blood glucose on admission, daily metabolic panel, cellular immunity, metabolic enzymes, and other biochemical parameters and inflammatory markers.
Due to the retrospective nature of the study, glycosylated hemoglobin was not always available, but it was recorded in 149 of 292 patients either at the time of admission or within the previous 6-month period available in the EMR. Patients with an established diagnosis of type 1 or type 2 diabetes mellitus or who were currently using antidiabetic medications on hospital admission were considered to have diabetes mellitus. Morning glucose was obtained from daily metabolic panels. Outcome measures included in-hospital mortality.
Descriptive statistics were used for patient baseline data. Categorical variables were described as frequency and percentages (%), and continuous variables were described with median and standard deviation values. A logistic regression model was used to analyze the association between inpatient fasting glucose with the outcome variable as in-hospital fatality. Variables reported in previous literature were included in the final analysis. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 22.0 (IBM Software, Armonk, NY, USA) and GraphPad Prism 8 (GraphPad, San Diego, CA, USA).
Results
From 10 March 2020 to 14 September 2020, there were 292 patients diagnosed with COVID-19 included in our study. Baseline data for continuous and categorical variables of our study population are included in Table 1. The median age of our population was 64.7 years. Our patient population was mostly male (63.0%) and primarily Caucasian (63.4%). We had 130 patients with type 2 diabetes mellitus, 4 patients with new-onset diabetes mellitus, and 5 patients with type 1 diabetes mellitus. Most patients were admitted to telemetry floors (81.7%). Of note, many of the telemetry beds were converted to acute care beds due to the nature of the pandemic and the hospital being a referral center, which diminishes the distinction between telemetry and ICU. Most of the patients with type 2 diabetes mellitus were taking oral medications. Metformin was the most common medication used in type 2 diabetes mellitus with 70 of 139 diabetic patients (50.4%) and 24.0% of the total study population. Of 139 diabetic patients, 55 were on home insulin before admission (39.6%) and 18.8% of the total study population.
Table 1.
Demographics and characteristics of patients with COVID-19, by survival status.
| Total (n = 292) | Survivors (n = 229) | Death (n = 63) | p-value | |
|---|---|---|---|---|
| Age, years (SD) | 64.7 ± 16.7 | 63.2 ± 17.3 | 70.1 ± 12.8 | <0.001 |
| Male (%) | 184 (63.0) | 137 (59.8) | 47 (74.6) | 0.039 |
| White (%) | 185 (63.4) | 144 (62.9) | 41 (65.1) | 0.770 |
| BMI (%) | 29.9 ± 6.76 | 30.2 ± 7.1 | 29.1 ± 5.2 | 0.244 |
| Diabetes mellitus (%) | 139 (47.6) | 107 (46.7) | 32 (50.8) | 0.573 |
| Hypertension (%) | 188 (64.4) | 147 (64.2) | 41 (65.1) | 1.00 |
| Hyperlipidemia (%) | 119 (40.8) | 98 (42.8) | 21 (33.3) | 0.195 |
| Obesity (%) | 50 (17.1) | 42 (18.3) | 8 (12.7) | 0.348 |
| CAD (%) | 49 (16.8) | 36 (15.7) | 13 (20.6) | 0.347 |
| Insulin (%) | 55 (18.8) | 41 (17.9) | 14 (22.2) | 0.063 |
| HbA1c (SD) | 7.44 ± 2.6 | 7.3 ± 2.7 | 7.8 ± 2.5 | 0.295 |
| Glucocorticoid (%) | 99 (33.9) | 61 (26.6) | 38 (60.3) | <0.001 |
| ICU admission | 119 (40.8) | 65 (28.4) | 54 (85.7) | <0.001 |
| Mechanical ventilation (%) | 117 (40.1) | 59 (25.8) | 58 (92.1) | <0.001 |
| ARDS (%) | 95 (32.5) | 43 (18.8) | 52 (82.5) | <0.001 |
| Average FPG (SD) | 146.6 ± 53.9 | 139.2 ± 54.5 | 172.8 ± 42.7 | <0.001 |
SD: standard deviation; BMI: body mass index; CAD: coronary artery disease; ICU: intensive care unit; ARDS: acute respiratory distress syndrome; FPG: fasting plasma glucose.
On admission, most of our patients were on nasal cannula as their highest supplemental oxygen (46.9%). The recommended treatment regimen for COVID-19 changed throughout this study. Many patients included in the study were treated with azithromycin (63.0%), hydroxychloroquine (70.9%), zinc (63.0%), and vitamin C (54.8%) and glucocorticoids (33.9%). Although we had a 43% discharge to home rate, 21.6% of our patients died in the hospital. This study took place during the beginning of the COVID-19 pandemic and the facility became a referral center for multiple hospitals in the area for higher acuity COVID-19 patients. Based on meta-analysis, this mortality rate is consistent with COVID-19 inpatients in general, which was 17% as we had a high percentage of critically ill patients admitted to the ICU (40.8%), where the mortality rate was found to be much higher at 40.5%.16
Patients with higher oxygen requirements on admission were associated with higher mortality (p < 0.001). There were no differences in admission inflammatory markers, mortality, or length of stay among diabetic and non-diabetic patients. Glycosylated hemoglobin levels obtained at admission, nor most recent levels were correlated with mortality or any outcome difference within the limited sample of 149 patients.
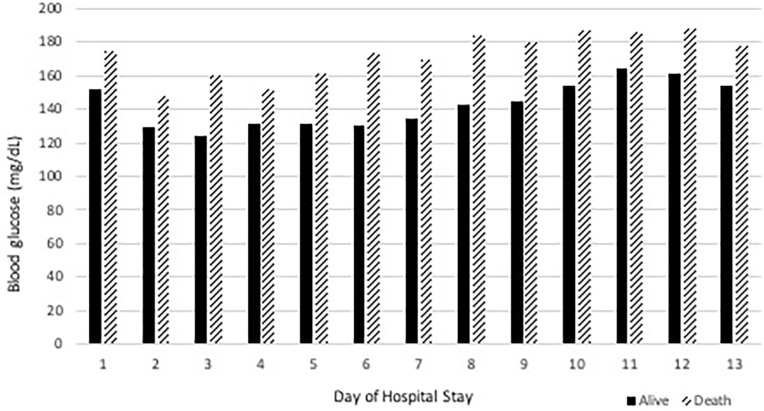
In addition, there were no documented episodes of hypoglycemia in the study population. The mean glucose levels on days 1 through 10 in patients who died in hospital ranged from 150 to 185 mg/dL. The mean glucose levels on days 1 through 10 in patients who survived ranged from 125 to 165 mg/dL, as shown in Figure 1. These results persisted in both diabetic and non-diabetic patients (p < 0.001). This association between fasting glucose and mortality persisted both in patients treated with steroids (p < 0.001) and in patients not treated with steroids (p < 0.001).
Figure 1.
Morning metabolic panel glucose levels and survival status.
The results of the logistic regression model with the dichotomous outcome of survival versus mortality is shown in Table 2. In the 292 patients, daily fasting glucose (mg/dL) averaged over the duration of the hospital stay was significantly associated with in-hospital mortality (odds ratio (OR) = 1.014, p < 0.001). Other factors that were significantly associated with the odds of inpatient mortality were age in years (OR = 1.06, p < 0.001), male sex (OR = 3.36, p = 0.002), and glucocorticoid use (OR = 3.720, p < 0.001). Interestingly, when included in the model, there was a trend toward association between diabetes and mortality, but when adjusted for daily fasting glucose, the association was no longer significant.
Table 2.
Multivariate logistic regression for mortality among COVID-19 patients.
| Disease fatality | ||
|---|---|---|
| OR | p-value | |
| Age, years (SD) | 1.063 | <0.001 |
| Male (%) | 3.362 | 0.002 |
| White (%) | 0.748 | 0.458 |
| BMI (%) | 0.967 | 0.277 |
| Diabetes mellitus (%) | 2.132 | 0.097 |
| Hypertension (%) | 0.760 | 0.511 |
| Hyperlipidemia (%) | 0.627 | 0.247 |
| Obesity (%) | 1.009 | 0.986 |
| CAD (%) | 1.425 | 0.445 |
| Insulin (%) | 1.205 | 0.712 |
| Glucocorticoid (%) | 3.720 | <0.001 |
| Average FPG (SD) | 1.014 | <0.001 |
OR: odds ratio; SD: standard deviation; BMI: body mass index; CAD: coronary artery disease; FPG: fasting plasma glucose.
Discussion
The purpose of this retrospective study was to determine the associations between glycemic control and mortality among patients with COVID-19. In contrast to other studies, diabetes was not a significant predictor of mortality in our population of COVID-19 patients. In addition, there was no association with hemoglobin A1C or fasting blood glucose on admission. As expected, older patients were associated with higher mortality. Similarly, those who presented with lower oxygen saturation suffered higher mortality. There was a significant difference in mean blood glucose from fasting metabolic panels between survivors and patients who died. This difference persisted when controlled for multiple variables, including in both diabetes and glucocorticoid use.
Previous studies have shown that glycemic control is an essential factor in critically ill patients. High glycemic variability has been associated with higher mortality among critical patients in an ICU setting.17 Stress-induced hyperglycemia has been shown to increase mortality compared with hyperglycemia due to diabetes in critically ill patients.18 While hyperglycemia has been implicated as a harmful state for critically ill patients,19 it remains a correctable one. Not only is hyperglycemia known to be dangerous, but episodes of hypoglycemia and wide glycemic variability have all been shown to be independent risk factors for mortality.20
Multiple landmark trials have investigated glycemic control in the intensive care setting. The Leuven Surgical trial in 2001 found a benefit for intensive glycemic control (80–110 mg/dL) with a reduction in mortality among patients who required more than 5 days in intensive care.21 The Leuven Medical trial in 2006 found that patients under intensive therapy had higher mortality with an ICU stay for less than 3 days, but those with stays of 3 or more days had a reduction in morbidity and mortality with intensive therapy (80–110 mg/dL).22 The Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial in 2009 countered the previous findings by showing that intensive treatment was associated with an increase in mortality in its population, which was driven mostly by episodes of hypoglycemia.23 In our study sample, there were no episodes of hypoglycemia on morning metabolic panels. Patients who were successfully discharged had significantly lower morning glucose on metabolic panels, while patients who died in hospital had higher glucose on average. Although tight glucose control increases the risk of hypoglycemia and has the potential for worse outcomes, COVID-19 patients who have prolonged hospital courses may benefit from more strict glucose control.
Barriers to appropriate glucose control in our hospital and many others during this pandemic will likely remain. High-dose steroids have become standard of care, resulting in hyperglycemia and impaired insulin sensitivity. Due to isolation and limited supplies of personal protective equipment, entrance into COVID-19 rooms was strategically limited, especially early during the pandemic. This resulted in bundled care limiting staff exposure, but it may also limit the number of point-of-care glucose checks and insulin administrations throughout the day. On-time meal delivery became difficult. As the rates of COVID-19 hospitalizations climb once again, attempts to limit staff exposure will likely hinder the ability to demonstrate tighter control.
This study has several limitations. First, this was a retrospective study. Second, not all patients included in the study had hemoglobin A1C drawn at the time of admission, which may have resulted in undiagnosed diabetes in the study population. Third, we were unable to gather records for every COVID-19 patient during the study period, and patients were skewed toward the early pandemic, which could lead to some selection bias. Fourth, while there were no hypoglycemia episodes in our population, these measures were taken from morning metabolic panels, which may not capture all the glycemic excursions. Finally, the prevalence of diabetes in our study population was 47.6%, higher than the reported ranges of 20% due to selective recruiting; however, we do not think this is a potential confounder because the glycemic association with mortality persisted when adjusted for diabetes.
Conclusion
In conclusion, mean glucose levels were associated with in-hospital mortality. Patients who did not survive hospitalization had significantly higher daily fasting glucose levels than patients who were successfully discharged. We feel that tighter glycemic control may be beneficial in COVID-19 patients, especially those with extended hospital courses, regardless of diabetic status. During this pandemic, treating hyperglycemia more aggressively could potentially improve patient outcomes, which needs further study on a larger scale.
Acknowledgments
The authors are grateful to the Institution and its Library staff for providing easy and prompt access to the published literature.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved the by institutional review board of Hackensack Meridian Health, Study No. Pro2020-0697.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Patient consent was waived by institutional review board (IRB) due to observational nature of the study.
ORCID iD: Christopher Lesniak  https://orcid.org/0000-0002-2614-9431
https://orcid.org/0000-0002-2614-9431
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard, https://covid19.who.int (accessed 26 June 2021).
- 2.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020; 77(2): 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan E, Song J, Deane AM, et al. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta-analysis. Chest 2021; 159: 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395(10239): 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roncon L, Zuin M, Rigatelli G, et al. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol 2020; 127: 104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Li J, Huang J, et al. Association between diabetes and COVID-19: a retrospective observational study with a large sample of 1,880 cases in Leishenshan Hospital, Wuhan. Front Endocrinol 2020; 11: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020; 8(10): 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta Y, Goyal A, Kubihal S, et al. A guidance on diagnosis and management of hyperglycemia at COVID care facilities in India. Diabetes Metab Syndr 2021; 15(1): 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol 2020; 14(4): 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 2006; 23(6): 623–628. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Shen M, Tao Y, et al. Elevated glucose level leads to rapid COVID-19 progression and high fatality. BMC Pulm Med 2021; 21(1): 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soliman AT, Prabhakaran Nair A, Al Masalamani MS, et al. Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus versus nondiabetic symptomatic patients with COVID-19: a comparative study. Acta Biomed 2020; 91(3): e2020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87(3): 978–982. [DOI] [PubMed] [Google Scholar]
- 14.Mazori AY, Bass IR, Chan L, et al. Hyperglycemia is associated with increased mortality in critically ill patients with COVID-19. Endocr Pract 2021; 27(2): 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar B, Mittal M, Gopalakrishnan M, et al. Effect of plasma glucose at admission on COVID-19 mortality: experience from a tertiary hospital. Endocr Connect 2021; 10(6): 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macedo A, Gonçalves N, Febra C.COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann Epidemiol 2021; 57: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh M, Upreti V, Singh Y, et al. Effect of glycemic variability on mortality in ICU settings: a prospective observational study. Indian J Endocrinol Metab 2018; 22(5): 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan KH, Thimmareddygari D, Ramahi A, et al. Clinical characteristics and outcome in patients with combined diabetic ketoacidosis and hyperosmolar hyperglycemic state associated with COVID-19: a retrospective, hospital-based observational case series. Diabetes Res Clin Pract 2020; 166: 108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falciglia M, Freyberg RW, Almenoff PL, et al. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009; 37(12): 3001–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krinsley JS, Egi M, Kiss A, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care 2013; 17(2): R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345(19): 1359–1367. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354(5): 449–461. [DOI] [PubMed] [Google Scholar]
- 23.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360(13): 1283–1297. [DOI] [PubMed] [Google Scholar]