Abstract
The genus Calonectria includes many important plant pathogens with a wide global distribution. In order to better understand the reproductive biology of these fungi, we characterised the structure of the mating type locus and flanking genes using the genome sequences for seven Calonectria species. Primers to amplify the mating type genes in other species were also developed. PCR amplification of the mating type genes and multi-gene phylogenetic analyses were used to investigate the mating strategies and evolution of mating type in a collection of 70 Calonectria species residing in 10 Calonectria species complexes. Results showed that the organisation of the MAT locus and flanking genes is conserved. In heterothallic species, a novel MAT gene, MAT1-2-12 was identified in the MAT1-2 idiomorph; the MAT1-1 idiomorph, in most cases, contained the MAT1-1-3 gene. Neither MAT1-1-3 nor MAT1-2-12 was found in homothallic Calonectria (Ca.) hongkongensis, Ca. lateralis, Ca. pseudoturangicola and Ca. turangicola. Four different homothallic MAT locus gene arrangements were observed. Ancestral state reconstruction analysis provided evidence that the homothallic state was basal in Calonectria and this evolved from a heterothallic ancestor.
Keywords: Cylindrocladium, fungal biology, fungal pathogens, MAT locus, mating type, phylogeny, sexual reproduction
INTRODUCTION
Calonectria is an Ascomycete genus that accommodates many important plant pathogens having a broad global distribution (Crous 2002, Lombard et al. 2010c). Approximately 335 plant species residing in 100 plant families are hosts to these fungi (Crous 2002, Lombard et al. 2010c). Calonectria species reside in two main phylogenetic groups. These are known as the Prolate Group and the Sphaero-Naviculate Group, and they are differentiated based on the shape of the vesicles in their conidiogenous apparatuses (Lombard et al. 2010b, Pham et al. 2019).
Ten species complexes are defined in Calonectria. Eight of these are in the Prolate Group, which includes the Ca. brassicae, Ca. candelabrum, Ca. colhounii, Ca. cylindrospora, Ca. mexi-cana, Ca. pteridis, Ca. reteaudii and Ca. spathiphylli species complexes. The remaining two species complexes reside in the Sphaero-Naviculate Group and they include the Ca. kyotensis and the Ca. naviculata species complexes (Lombard et al. 2010b, 2016). To date, 172 Calonectria species have been identified based on comparisons of DNA sequence data. Of these, approximately 99 were isolated from diseased tissues and about 73 from soil samples (Lombard et al. 2010b, 2016, Marin-Felix et al. 2017, Crous et al. 2019, Pham et al. 2019).
Both homothallic and heterothallic mating systems have been reported in Calonectria spp., but their sexual morphs are rarely seen in nature or in laboratory culture (Crous 2002, Lombard et al. 2010a). This is not unusual given that sexual reproduction is a complex process that is commonly species-specific, and strongly influenced by the environment and the compatibility of isolates (Goodenough & Heitman 2014). Consequently, the absence of sexual structures in Calonectria does not preclude the fact that species may be capable of sexual outcrossing (Billiard et al. 2012). This is an important consideration given that sexual reproduction is the dominant mechanism generating genetic diversity, eliminating deleterious mutations, ensuring survival of species and their overall population health (Crow 1994, Gordo & Campos 2008, Lumley et al. 2015).
Ascomycetes have a bipolar mating system that is controlled by mating type (MAT) genes at a single MAT locus (MAT1) with two non-allelic forms referred to as the MAT1-1 and MAT1-2 idiomorphs (Turgeon & Yoder 2000). The MAT1-1 idiomorph is characterised by a MAT1-1-1 gene, which encodes an alpha box motif protein homologous to MATa1 of Saccharomyces cerevisiae (Turgeon & Yoder 2000). The MAT1-2 idiomorph contains a MAT1-2-1 gene that encodes a protein with a high mobility group (HMG) domain (Wilson et al. 2015a). Eight additional genes (MAT1-1-2 to MAT1-1-9) have been identified in the MAT1-1 idiomorph and 10 genes (MAT1-2-2 to MAT1-2-11) in the MAT1-2 idiomorph (Wilken et al. 2017). These have been named sequentially in the order of their discovery (Wilken et al. 2017). The expression of these genes is most often related to the sexual life cycle of the fungi in which they occur (Ferreira et al. 1998, Kim et al. 2012, Zheng et al. 2013).
In heterothallic Ascomycetes, the two opposite mating type idiomorphs exist in different isolates. These individuals are self-sterile and require a compatible partner to mate and produce sexual spores. In contrast, homothallic species are self-fertile, where a single individual possesses both mating type idiomorphs, and can therefore complete the sexual cycle on its own (Ni et al. 2011, Wilson et al. 2015b). Transitions between homothallism and heterothallism are well-known in genera of the Ascomycetes (Labarere & Noel 1992, Lin & Heitman 2007, Ni et al. 2011).
Mating strategy and the ratio of mating type genes are commonly used in population genetics and epidemiology studies of plant pathogens (McDonald & Linde 2002, Alby et al. 2009, Adamson et al. 2018). The MAT gene sequences have also been used to track the evolutionary direction of mating systems based on thallism and molecular phylogenies (James et al. 2006, Fraser et al. 2007, Nagel et al. 2018). These genes can be used as molecular markers to establish species boundaries and to delimitate cryptic species (O’Donnell et al. 2004, Lopes et al. 2017). Mating strategies have consequently served as important criteria in the taxonomy of Calonectria (Schoch et al. 1999, Lombard et al. 2010a). Similarly, using genome sequences and PCR amplification of MAT genes, populations of Calonectria species have been defined based on their mating type (Malapi-Wight et al. 2014, 2019). For example, Malapi-Wight et al. (2019) showed in a collection from four continents, that all isolates of Ca. henricotiae were MAT1-1 whereas all isolates of Ca. pseudonaviculata were MAT1-2.
Some studies have considered the mating types of Calonectria spp., however, sexual reproduction is still not well understood in this genus. For example, it is not known which MAT genes occur at the MAT loci of homothallic Calonectria species, how they are arranged, or whether there is significant conservation of MAT genes or gene sequences at these loci. Universal mating type markers for MAT1-1 idiomorph are not available to enable easy detection of the thallism in Calonectria species, although MAT1-2-1 gene markers were designed for Calonectria by Schoch et al. (2000). In addition, nothing is known regarding the evolution of the mating systems in Calonectria and the probable ancestral state (homothallism or heterothallism) has not been determined.
An important basis to control the spread and prevalence of plant pathogens is to understand their life cycles and modes of reproduction. In order to further understand the possible role of sexual reproduction in Calonectria, we identified and characterised the MAT loci and flanking genes of seven species of Calonectria using whole genome sequences. Mating type primers were then designed to consider the mating strategies of 65 Calonectria species from 10 Calonectria species complexes. The data were also used to consider the evolutionary history of mating in the genus.
MATERIALS AND METHODS
Isolates, DNA extraction and identification
A total of 123 isolates, representing 65 Calonectria species residing in 10 Calonectria species complexes (Lombard et al. 2010b, 2016) were utilised in this study (Table 1). Two isolates were acquired from the culture collection of the China Eucalypt Research Centre (CERC), Chinese Academy of Forestry (CAF); 32 from the culture collection (CBS) of the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands and 89 from the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa. Cultures were incubated and maintained on 2 % malt extract agar (MEA) at room temperature.
Table 1.
Species of Calonectria used in this study.
| Species | Isolate number1 | Host | Origin | Thallism2 | Mating type | GenBank accession No.3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAT1-1-1 | MAT1-1-3 | MAT1-2-1 | MAT1-2-12 | tub2 | cmdA | his3 | tef1 | ||||||
| Ca. acaciicola | CBS 1435574,5; CMW 47173 | Soil in Acacia auriculiformis plantation | Nghe An, Vietnam | P_HE | MAT1-1 | MN959486 | No6 | No | No | MH119285 | MH119252 | MH119186 | MH119219 |
| CBS 143558; CMW 47174 | Soil in A. auriculiformis plantation | Nghe An, Vietnam | P_HE | MAT1-1 | MN959487 | No | No | No | MH119286 | MH119253 | MH119187 | MH119220 | |
| Ca. aciculata | CBS 1428835; CMW 47645; CERC 5342 | Eucalyptus urophylla × E. grandis leaf | YunNan, China | HO | homothallic | MN959488 | MN959560 | MN959612 | MN959697 | MF442989 | MF442874 | MF442759 | MF442644 |
| Ca. aeknauliensis | CBS 1435595; CMW 48253 | Soil in Eucalyptus plantation | North Sumatra, Indonesia | P_HE | MAT1-2 | No | No | MN959613 | No | –7 | MH119259 | MH119193 | MH119226 |
| CBS 143560; CMW 48254 | Soil in Eucalyptus plantation | North Sumatra, Indonesia | P_HE | MAT1-2 | No | No | MN959614 | No | – | MH119260 | MH119194 | MH119227 | |
| Ca. amazonica | CBS 115486; CMW 51223; CPC 3894 | E. tereticornis | Brazil | HE | MAT1-2 | No | No | MN959615 | No | KX784611 | KX784554 | – | KX784681 |
| CBS 1162505; CMW 51234; CPC 3534 | E. tereticornis | Brazil | HE | MAT1-1 | MN959489 | MN959561 | No | No | KX784612 | KX784555 | – | KX784682 | |
| Ca. arbusta | CBS 1360795; CMW 31370; CERC 1705 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959490 | MN959562 | MN959616 | No | KJ462904 | KJ463018 | KJ463135 | KJ462787 |
| CBS 136098; CMW 37981; CERC 1944; CPC 23519 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959491 | MN959563 | MN959617 | No | – | KJ463019 | KJ463136 | KJ462788 | |
| Ca. auriculiformis | CBS 1435615; CMW 47178 | Soil in A. auriculiformis plantation | Thanh Hoa, Vietnam | P_HE | MAT1-2 | No | No | MN959618 | MN959698 | MH119287 | MH119254 | MH119188 | MH119221 |
| CBS 143562; CMW 47179 | Soil in A. auriculiformis plantation | Thanh Hoa, Vietnam | P_HE | MAT1-2 | No | No | MN959619 | MN959699 | MH119288 | MH119255 | MH119189 | MH119222 | |
| Ca. baviensis | CBS 1435635; CMW 47410 | E. urophylla leaf | Hanoi, Vietnam | P_HE | MAT1-1 | MN959492 | No | No | No | MH119289 | MH119256 | MH119190 | MH119223 |
| CBS 143564; CMW 47433 | E. pellita leaf | Hanoi, Vietnam | P_HE | MAT1-1 | MN959493 | No | No | No | MH119290 | MH119257 | MH119191 | MH119224 | |
| Ca. blephiliae | CBS 1364255; CMW 51321; CPC 21859 | Blephilia ciliata stem | North Carolina, USA | P_HE | MAT1-1 | MN959494 | No | No | No | KF777246 | – | – | KF777243 |
| Ca. brachiatica | CBS 1237005; CMW 25298 | Pinus maximinoi | Buga, Colombia | P_HE | MAT1-2 | No | No | MN959620 | MN959700 | FJ696388 | GQ267366 | FJ696396 | GQ267296 |
| CMW 25302 | P. tecunumanii | Buga, Colombia | P_HE | MAT1-2 | No | No | MN959621 | MN959701 | FJ716708 | GQ267365 | FJ716712 | GQ267295 | |
| CMW 25307 | P. tecunumanii | Buga, Colombia | P_HE | MAT1-2 | No | No | MN959622 | MN959702 | FJ716709 | GQ267366 | FJ716713 | GQ267296 | |
| Ca. brasiliana | CBS 1114845; CMW 51187; CPC 1924 | Soil | Brazil | P_HE | MAT1-2 | No | No | MN959623 | MN959703 | KX784616 | KX784559 | – | KX784686 |
| CBS 111485; CMW 51188; CPC 1929 | Soil | Brazil | P_HE | MAT1-2 | No | No | MN959624 | MN959704 | KX784617 | KX784560 | – | KX784687 | |
| Ca. brasiliensis | CBS 230.515; CMW 23670; CPC 2390; CMW 51160 | Eucalyptus sp. | Brazil | P_HE | MAT1-1 | MN959495 | MN959564 | No | No | GQ267241 | GQ267421 | GQ267259 | GQ267328 |
| Ca. brevistipitata | CBS 110837; CMW 51163; CPC 913 | Soil | Mexico | HE | MAT1-2 | No | No | MN959625 | MN959705 | KX784621 | KX784563 | – | KX784691 |
| CBS 110928; CMW 51170; CPC 951 | Soil | Mexico | HE | MAT1-1 | MN959496 | MN959565 | No | No | KX784622 | KX784564 | – | KX784692 | |
| CBS 1156715; CMW 51226; CPC 949 | Soil | Mexico | HE | MAT1-1 | MN959497 | MN959566 | No | No | KX784623 | KX784565 | – | KX784693 | |
| Ca. bumicola | CBS 1435755; CMW 48257 | Soil in Eucalyptus plantation | North Sumatra, Indonesia | HO | homothallic | MN959498 | MN959567 | MN959626 | No | – | MH119271 | MH119205 | MH119238 |
| Ca. candelabra | CMW 310005; CPC 1675 | Eucalyptus sp. | Brazil | HE | MAT1-1 | MN959499 | MN959568 | No | No | FJ972426 | GQ267367 | FJ972476 | FJ972525 |
| CMW 31001; CPC 1679 | Eucalyptus sp. | Brazil | HE | MAT1-2 | No | No | MN959627 | MN959706 | GQ421779 | GQ267368 | GQ267246 | GQ267298 | |
| Ca. clavata | CBS 1145575; CMW 23690; CPC 2536 | Callistemon viminalis | USA | HE | MAT1-1 | MN959500 | MN959569 | No | No | AF333396 | GQ267377 | DQ190623 | GQ267305 |
| CBS 114666; CMW 30994; CPC 2537 | Root debris in peat | USA | HE | MAT1-2 | No | No | MN959628 | MN959707 | DQ190549 | GQ267378 | DQ190624 | GQ267306 | |
| Ca. colombiana | CBS 1156385; CMW 30766; CPC 1161 | Soil | Colombia | P_HE | MAT1-1 | MN959501 | MN959570 | No | No | FJ972422 | GQ267456 | FJ972441 | FJ972491 |
| Ca. colombiensis | CBS 1122215; CMW 30985; CPC 724 | E. grandis | Colombia | HO | homothallic | MN959502 | MN959571 | MN959629 | No | AY725620 | AY725749 | AY725663 | AY725712 |
| Ca. crousiana | CBS 1271995; CMW 27253 | E. grandis | FuJian, China | HO | homothallic | MN959503 | MN959572 | MN959630 | MN959708 | HQ285795 | MF527085 | HQ285809 | HQ285823 |
| Ca. curvispora | CBS 1161595; CMW 23693; CPC 765 | Soil | Tamatave, Madagascar | P_HE | MAT1-1 | MN959504 | MN959573 | No | No | AF333395 | GQ267374 | AY725664 | GQ267302 |
| Ca. densa | CBS 1252615; CMW 31182 | Soil | Pichincha, Ecuador | P_HE | MAT1-1 | MN959505 | MN959574 | No | No | GQ267232 | GQ267444 | GQ267281 | GQ267352 |
| Ca. ericae | CBS 114456; CMW 51209; CPC 1984 | Erica capensis | California, USA | P_HE | MAT1-2 | No | No | MN959631 | MN959709 | KX784627 | KX784569 | – | KX784697 |
| CBS 114457; CMW 51210; CPC 1985 | Erica capensis | California, USA | P_HE | MAT1-2 | No | No | MN959632 | MN959710 | KX784628 | KX784570 | – | KX784698 | |
| CBS 1144585; CMW 51211; CPC 2019 | Erica capensis | California, USA | P_HE | MAT1-2 | No | No | MN959633 | MN959711 | KX784629 | KX784571 | – | KX784699 | |
| Ca. eucalypti | CBS 1252755; CMW18444 | E. grandis leaf | Sumatra Utara, Indonesia | HO | homothallic | MN959506 | MN959575 | MN959634 | MN959712 | GQ267218 | GQ267430 | GQ267267 | GQ267338 |
| CBS 125276; CMW 18445 | E. grandis leaf | Sumatra Utara, Indonesia | HO | homothallic | MN959507 | MN959576 | MN959635 | MN959713 | GQ267219 | GQ267431 | GQ267268 | GQ267339 | |
| Ca. expansa | CBS 1362475; CMW 31392; CERC 1727 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959508 | MN959577 | MN959636 | No | KJ462914 | KJ463029 | KJ463146 | KJ462798 |
| Ca. foliicola | CBS 1366415; CMW 31393; CERC 1728 | E. urophylla × E. grandis leaf | Guangxi, China | P_HE | MAT1-2 | No | No | MN959637 | MN959714 | KJ462916 | KJ463031 | KJ463148 | KJ462800 |
| Ca. fujianensis | CBS 127200; CMW 27254 | E. grandis leaf in plantation | FuJian, China | HO | homothallic | MN959509 | MN959578 | MN959638 | MN959715 | HQ285791 | MF527088 | HQ285805 | HQ285819 |
| CBS 1272015; CMW 27257 | E. grandis leaf in plantation | FuJian, China | HO | homothallic | MN959510 | MN959579 | MN959639 | MN959716 | HQ285792 | MF527089 | HQ285806 | HQ285820 | |
| Ca. gracilis | CBS 111284; CMW 51175 | Soil | Brazil | HO | homothallic | MN959511 | No | MN959640 | MN959717 | DQ190567 | GQ267408 | DQ190647 | GQ267324 |
| CBS 1118075; CMW 51189 | Manilkara zapota | Brazil | HO | homothallic | MN959512 | No | MN959641 | MN959718 | AF232858 | GQ267407 | DQ190646 | GQ267323 | |
| Ca. guangxiensis | CBS 1360925; CMW 35409; CERC 1900; CPC 23506 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959513 | MN959580 | MN959642 | No | KJ462919 | KJ463034 | KJ463151 | KJ462803 |
| CBS 136094; CMW 35411; CERC 1902; CPC 23507 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959514 | MN959581 | MN959643 | No | KJ462920 | KJ463035 | – | KJ462804 | |
| Ca. henricotiae* -1 | CBS 138102 5,8 | Buxus sempervirens | Lokeren, East Flanders, Belgium | HE | MAT1-1 | JX535308 | KF815157 | KF815185 | – | ||||
| Ca. heveicola | CBS 1435715; CMW 49928 | Soil | Binh Phuoc, Vietnam | P_HE | MAT1-2 | No | No | MN959644 | No | MH119296 | MH119267 | MH119201 | MH119234 |
| CBS 143572; CMW 49935 | Soil | Binh Phuoc, Vietnam | P_HE | MAT1-2 | No | No | MN959645 | No | MH119297 | MH119268 | MH119202 | MH119235 | |
| Ca. honghensis | CBS 142884; CMW 47668; CERC 5571 | Soil in Eucalyptus plantation | YunNan, China | HO | homothallic | MN959515 | MN959582 | MN959646 | MN959719 | MF442996 | MF442894 | MF442779 | MF442664 |
| CBS 1428855; CMW 47669; CERC 5572 | Soil in Eucalyptus plantation | YunNan, China | HO | homothallic | MN959516 | MN959583 | MN959647 | MN959720 | MF442997 | MF442895 | MF442780 | MF442665 | |
| Ca. hongkongensis | CBS 1148285; CMW 51217; CPC 4670 | Soil | Hong Kong | HO | homothallic | MN959517 | No | MN959648 | No | AY725622 | AY725755 | AY725667 | AY725717 |
| Ca. hongkongensis* -2 | CMW 47271; CERC 3570 | Soil in Eucalyptus plantation | GuangXi, China | HO | homothallic | MN959518 | No | MN959649 | No | MF443001 | MF442899 | MF442784 | MF442669 |
| CMW 47499; CERC 7132 | Soil | FuJian, China | HO | homothallic | MN959519 | No | MN959650 | No | MF443004 | MF442902 | MF442787 | MF442672 | |
| Ca. indonesiae | CBS 1128235; CMW 23683; CPC 4508 | Soil | Warambunga, Indonesia | P_HE | MAT1-2 | No | No | MN959651 | No | AY725623 | AY725756 | AY725668 | AY725718 |
| Ca. lantauensis | CBS 142887; CMW 47251; CERC 3301 | Soil | Hong Kong, China | P_HE | MAT1-2 | No | No | MN959652 | No | – | MF442906 | MF442791 | MF442676 |
| CBS 1428885; CMW 47252; CERC 3302 | Soil | Hong Kong, China | P_HE | MAT1-2 | No | No | MN959653 | No | – | MF442907 | MF442792 | MF442677 | |
| Ca. lateralis | CBS 1366295; CMW 31412; CERC 1747 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959520 | No | MN959654 | No | KJ462955 | KJ463070 | KJ463186 | KJ462840 |
| Ca. lauri | CBS 749.705; CMW 23682 | Llex aquifolium | Netherlands | P_HE | MAT1-1 | MN959521 | No | No | No | GQ267210 | GQ267388 | GQ267250 | GQ267312 |
| Ca. leucothoes* -3 | CBS 1091665,8; CMW 30977 | Leucothoe axillaris leaf | Florida, USA | HE | MAT1-2 | FJ918508 | GQ267392 | FJ918523 | FJ918553 | ||||
| Ca. lichi | CERC 88665; CGMCC3.18733 | Soil | HeNan, China | HO | homothallic | MN959522 | MN959584 | MN959655 | MN959721 | MF527097 | MF527071 | MF527055 | MF527039 |
| CERC 8890; CGMCC3.18734 | Soil | HeNan, China | HO | homothallic | MN959523 | MN959585 | MN959656 | MN959722 | MF527099 | MF527073 | MF527057 | MF527041 | |
| Ca. malesiana | CBS 112710; CMW 51199; CPC 3899 | Leaf litter | Thailand | P_HE | MAT1-1 | MN959524 | MN959586 | No | No | AY725626 | AY725759 | AY725671 | AY725721 |
| CBS 1127525; CMW 23687; CPC 4223 | Soil | Indonesia | P_HE | MAT1-1 | MN959525 | MN959587 | No | No | AY725627 | AY725760 | AY725672 | AY725722 | |
| Ca. mossambicensis | CBS 1372435; CMW 36327 | E. grandis × E. camaldulensis cutting | Manica, Mozambmbique | P_HE | MAT1-2 | No | No | MN959657 | MN959723 | – | JX570722 | JX570726 | JX570718 |
| CMW 36329 | E. grandis and E. urophylla cutting | Zambézia, Mozambmbique | P_HE | MAT1-2 | No | No | MN959658 | MN959724 | – | JX570721 | JX570725 | JX570717 | |
| Ca. naviculata* -4 | CBS 1011215,8; CMW 30974 | Leaf litter | Joao Pessoa, Brazil | HE | MAT1-1 | GQ267211 | GQ267399 | GQ267252 | GQ267317 | ||||
| Ca. orientalis | CBS 125259; CMW 20273 | Soil | Teso East, Indonesia | P_HE | MAT1-1 | MN959526 | MN959588 | No | No | GQ267237 | GQ267449 | GQ267286 | GQ267357 |
| CBS 1252605; CMW 20291 | Soil | Lagan, Indonesia | P_HE | MAT1-1 | MN959527 | MN959589 | No | No | GQ267236 | GQ267448 | GQ267285 | GQ267356 | |
| Ca. ovata | CBS 1112995; CMW 16724 | E. tereticornis | Tucuruí, Para, Brazil | HE | MAT1-2 | No | No | MN959659 | No | GQ267212 | GQ267400 | GQ267253 | GQ267318 |
| CBS 111307; CMW 30979 | E. tereticornis | Tucuruí, Para, Brazil | HE | MAT1-1 | MN959528 | No | No | No | AF210868 | GQ267401 | GQ267254 | GQ267319 | |
| Ca. papillata | CBS 136096; CMW 37972; CERC 1935; CPC 23515 | Soil in Eucalyptus plantation | Guangdong, China | P_HE | MAT1-1 | MN959529 | No | No | No | KJ462963 | KJ463078 | KJ463194 | KJ462848 |
| CBS 1360975; CMW 37976; CERC 1939; CPC 23517 | Soil in Eucalyptus plantation | Guangdong, China | P_HE | MAT1-1 | MN959530 | No | No | No | KJ462964 | KJ463079 | KJ463195 | KJ462849 | |
| Ca. parakyotensis | CBS 1360855; CMW 35169; CERC 1845 | Soil in Eucalyptus plantation | Guangdong, China | HO | homothallic | MN959531 | MN959590 | MN959660 | No | – | KJ463081 | KJ463197 | KJ462851 |
| Ca. pauciramosa* -5 | CBS 1388245; CMW 5683; CPC 971 | E. grandis | South Africa | HE | MAT1-2 | No | No | MN959661 | MN959725 | FJ918514 | GQ267405 | FJ918531 | FJ918565 |
| CMW 2151 | E. nitens | South Africa | HE | MAT1-2 | No | No | MN959662 | MN959726 | FJ972400 | – | FJ972468 | FJ972517 | |
| Ca. pauciramosa* -6 | CMW 7592 | E. grandis | Uruguay | HE | MAT1-1 | MN959532 | MN959591 | No | No | FJ972380 | – | FJ972447 | FJ972497 |
| CMW 9151 | A. mearnsii | South Africa | HE | MAT1-2 | No | No | MN959663 | MN959727 | FJ972384 | – | FJ972451 | FJ972501 | |
| CMW 30823; CPC 416 | E. grandis | South Africa | HE | MAT1-1 | MN959533 | MN959592 | No | No | FJ918515 | GQ267404 | FJ918532 | FJ918566 | |
| CMW 30875; CPC 415 | Eucalyptus sp. | South Africa | HE | MAT1-1 | MN959534 | MN959593 | No | No | FJ972390 | – | FJ972457 | FJ972507 | |
| Ca. pentaseptata | CBS 1333495; CMW 51318 | Eucalyptus hybrid | Bavi, Hanoi, Vietnam | P_HE | MAT1-1 | MN959535 | MN959594 | No | No | JX855942 | – | JX855946 | JX855958 |
| CBS 133351; CMW 51319 | Macadamia sp. | Bavi, Hanoi, Vietnam | P_HE | MAT1-1 | MN959536 | MN959595 | No | No | JX855944 | – | JX855948 | JX855960 | |
| Ca. plurilateralis | CBS 1114015; CMW 51178; CPC 1637 | Soil | Ecuador | P_HE | MAT1-2 | No | No | MN959664 | MN959728 | KX784648 | KX784586 | – | KX784719 |
| Ca. polizzii | CBS 1234025; CMW 51312 | Arbutus unedo | Sicily, Italy | HE | MAT1-1 | MN959537 | MN959596 | No | No | FJ972419 | – | FJ972438 | FJ972488 |
| CBS 125270; CMW 7804; CPC 2681 | Callistemon citrinus | Sicily, Italy | HE | MAT1-1 | MN959538 | MN959597 | No | No | FJ972417 | GQ267461 | FJ972436 | FJ972486 | |
| CBS 125271; CMW 10151; CPC 2771 | Arbutus unedo | Sicily, Italy | HE | MAT1-2 | No | No | MN959665 | MN959729 | FJ972418 | GQ267462 | FJ972437 | FJ972487 | |
| Ca. pseudocolhounii | CBS 1271955; CMW 27209 | E. dunnii leaf in plantation | FuJian, China | HO | homothallic | MN959539 | MN959598 | MN959666 | MN959730 | HQ285788 | MF527091 | HQ285802 | HQ285816 |
| CBS 127196; CMW 27213 | E. dunnii leaf in plantation | FuJian, China | HO | homothallic | MN959540 | MN959599 | MN959667 | MN959731 | HQ285789 | MF527092 | HQ285803 | HQ285817 | |
| Ca. pseudoecuadoriae | CBS 1114125; CMW 51180; CPC 1648 | Soil | Ecuador | P_HE | MAT1-2 | No | No | MN959668 | MN959732 | DQ190601 | KX784590 | – | KX784724 |
| Ca. pseudomexicana | CBS 1303545; CMW 51313 | Callistemon sp. (rouge) | Carthage, Tunis, Tunisia | P_HE | MAT1-2 | No | No | MN959669 | MN959733 | JN607281 | – | JN607266 | JN607296 |
| CBS 130355; CMW 51314 | Callistemon sp. (rouge) | Carthage, Tunis, Tunisia | P_HE | MAT1-2 | No | No | MN959670 | MN959734 | JN607282 | – | JN607267 | JN607297 | |
| Ca. pseudonaviculata* -7 | CBS 139394 5,8 | Sarcococca hookeriana | Maryland, USA | HE | MAT1-2 | KR011242 | – | – | – | ||||
| Ca. pseudopteridis | CBS 163.285; CMW 51159 | Washingtonia robusta | USA | P_HE | MAT1-1 | MN959541 | MN959600 | No | No | – | KM396076 | – | KM395902 |
| Ca. pseudoreteaudii* -8 | YA51 5,8 | Eucalyptus sp. | Fujian, China | HE | MAT1-2 | – | – | – | – | ||||
| Ca. pseudoscoparia | CBS 125255; CMW 15215 | E. grandis | Pichincha, Ecuador | P_HE | MAT1-2 | No | No | MN959671 | MN959735 | GQ267227 | GQ267439 | GQ267276 | GQ267347 |
| CBS 1252575; CMW 15218 | E. grandis | Pichincha, Ecuador | P_HE | MAT1-2 | No | No | MN959672 | MN959736 | GQ267229 | GQ267441 | GQ267278 | GQ267349 | |
| Ca. pseudoturangicola | CBS 1428905; CMW 47496; CERC 7126 | Soil | FuJian, China | HO | homothallic | MN959542 | No | MN959673 | No | MF443080 | MF442980 | MF442865 | MF442750 |
| CBS 142891; CMW 47497; CERC 7127 | Soil | FuJian, China | HO | homothallic | MN959543 | No | MN959674 | No | MF443081 | MF442981 | MF442866 | MF442751 | |
| Ca. pseudouxmalensis | CBS 110923; CMW 51165; CPC 941 | Soil | Mexico | P_HE | MAT1-2 | No | No | MN959675 | MN959737 | KX784653 | – | – | KX784725 |
| CBS 1109245; CMW 51166; CPC 942 | Soil | Mexico | P_HE | MAT1-2 | No | No | MN959676 | MN959738 | KX784654 | – | – | KX784726 | |
| CBS 115677; CMW 51228; CPC 943 | Soil | Mexico | P_HE | MAT1-2 | No | No | MN959677 | MN959739 | KX784655 | – | – | KX784727 | |
| Ca. pseudoyunnanensis | CBS 1428925; CMW 47655; CERC 5376 | Soil in Eucalyptus plantation | YunNan, China | HO | homothallic | MN959544 | MN959601 | MN959678 | No | MF443083 | MF442983 | MF442868 | MF442753 |
| CBS 142893; CMW 47656; CERC 5377 | Soil in Eucalyptus plantation | YunNan, China | HO | homothallic | MN959545 | MN959602 | MN959679 | No | MF443084 | MF442984 | MF442869 | MF442754 | |
| CBS 142894; CMW 47657; CERC 5378 | Soil in Eucalyptus plantation | YunNan, China | HO | homothallic | MN959546 | MN959603 | MN959680 | No | MF443085 | MF442985 | MF442870 | MF442755 | |
| Ca. putriramosa | CBS 1114495; CMW 51181; CPC 1951 | Eucalyptus cutting | Brazil | P_HE | MAT1-2 | No | No | MN959681 | MN959740 | KX784656 | KX784591 | – | KX784728 |
| CBS 111470; CMW 51182; CPC 1940 | Soil | Brazil | P_HE | MAT1-2 | No | No | MN959682 | MN959741 | KX784657 | KX784592 | – | KX784729 | |
| CBS 111477; CMW; 51183; CPC 1928 | Soil | Brazil | P_HE | MAT1-2 | No | No | MN959683 | MN959742 | KX784658 | KX784593 | – | KX784730 | |
| CBS 116076; CMW 51230; CPC 604 | Eucalyptus cutting | Brazil | P_HE | MAT1-2 | No | No | MN959684 | MN959743 | – | – | – | KX784731 | |
| Ca. seminaria | CBS 1366325; CMW 31450; CERC 1785; CPC 23488 | E. urophylla × E. grandis seedling leaf | Guangdong, China | P_HE | MAT1-2 | No | No | MN959685 | MN959744 | KJ462998 | KJ463115 | KJ463231 | KJ462885 |
| CBS 136639; CMW 31489; CERC 1824 | E. urophylla × E. grandis seedling leaf | Guangdong, China | P_HE | MAT1-2 | No | No | MN959686 | MN959745 | KJ462999 | KJ463116 | KJ463232 | KJ462886 | |
| Ca. sphaeropedunculata | CBS 1360815; CMW 31390; CERC 1725 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959547 | MN959604 | MN959687 | No | KJ463003 | KJ463120 | KJ463236 | KJ462890 |
| Ca. sulawesiensis | CBS 125253; CMW 14879 | Eucalyptus sp. | Sulawesi, Indonesia | P_HE | MAT1-1 | MN959548 | No | No | No | GQ267222 | GQ267434 | GQ267271 | GQ267342 |
| CBS 1252775; CMW 14878 | Eucalyptus sp. | Sulawesi, Indonesia | P_HE | MAT1-1 | MN959549 | No | No | No | GQ267220 | GQ267432 | GQ267269 | GQ267340 | |
| Ca. sumatrensis | CBS 1128295; CMW 23698; CPC4518 | Soil | Indonesia | P_HE | MAT1-1 | MN959550 | MN959605 | No | No | AY725649 | AY725771 | AY725696 | AY725733 |
| CBS 112934; CMW 30987; CPC 4516 | Soil | Indonesia | P_HE | MAT1-1 | MN959551 | MN959606 | No | No | AY725651 | AY725773 | AY725698 | AY725735 | |
| Ca. terrestris | CBS 1366425; CMW 35180; CERC 1856 | Soil in Eucalyptus plantation | Guangdong, China | P_HE | MAT1-2 | No | No | MN959688 | MN959746 | KJ463004 | KJ463121 | KJ463237 | KJ462891 |
| CBS 136645; CMW 35178; CERC 1854 | Soil in Eucalyptus plantation | Guangdong, China | P_HE | MAT1-2 | No | No | MN959689 | MN959747 | KJ463007 | KJ463124 | KJ463240 | KJ462894 | |
| Ca. tetraramosa | CBS 1366355; CMW 31474; CERC 1809; CPC 23489 | E. urophylla × E. grandis seedling leaf | Guangdong, China | P_HE | MAT1-2 | No | No | MN959690 | MN959748 | KJ463011 | KJ463128 | KJ463244 | KJ462898 |
| CBS 136637; CMW 31476; CERC 1811 | E. urophylla × E. grandis seedling leaf | Guangdong, China | P_HE | MAT1-2 | No | No | MN959691 | MN959749 | KJ463012 | KJ463129 | KJ463245 | KJ462899 | |
| Ca. tonkinensis | CBS 1435765; CWM 47430 | Soil in Eucalyptus plantation | Hanoi, Vietnam | P_HE | MAT1-1 | MN959552 | No | No | No | MH119291 | MH119258 | MH119192 | MH119225 |
| Ca. turangicola | CBS 1360775; CMW 31411; CERC 1746; CPC 23479 | Soil in Eucalyptus plantation | Guangxi,China | HO | homothallic | MN959553 | No | MN959692 | No | KJ463013 | – | KJ463246 | KJ462900 |
| CBS 136093; CMW 35410; CERC 1901 | Soil in Eucalyptus plantation | Guangxi, China | HO | homothallic | MN959554 | No | MN959693 | No | KJ463014 | KJ463130 | KJ463247 | KJ462901 | |
| Ca. vegrandis | CBS 1435655; CMW 48245 | Soil in Eucalyptus plantation | North Sumatra, Indonesia | P_HE | MAT1-1 | MN959555 | MN959607 | No | No | – | MH119261 | MH119195 | MH119228 |
| CBS 143566; CMW 48246 | Soil in Eucalyptus plantation | North Sumatra, Indonesia | P_HE | MAT1-1 | MN959556 | MN959608 | No | No | – | MH119262 | MH119196 | MH119229 | |
| Ca. yunnanensis | CBS 142895; CMW 47642; CERC 5337 | Soil in Eucalyptus plantation | YunNan, China | HO | homothallic | MN959557 | MN959609 | MN959694 | No | MF443086 | MF442986 | MF442871 | MF442756 |
| CBS 1428975;CMW 47644; CERC 5339 | Soil in Eucalyptus plantation | YunNan, China | HO | homothallic | MN959558 | MN959610 | MN959695 | No | MF443088 | MF442988 | MF442873 | MF442758 | |
| Ca. zuluensis | CBS 1252685; CMW 9188 | E. grandis | Kwa-Zulu Natal, South Africa | HE | MAT1-2 | No | No | MN959696 | MN959750 | FJ972414 | GQ267459 | FJ972433 | FJ972483 |
| CBS 125272; CMW 9896 | E. grandis × E. urophylla cutting | Pietermarizburg, South Africa | HE | MAT1-1 | MN959559 | MN959611 | No | No | FJ972415 | GQ267460 | FJ972434 | FJ972484 | |
1 CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CERC: China Eucalypt Research Centre, Chinese Academy of Forestry, Zhanjiang, GuangDong Province, China; CMW: culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; CPC: Pedro Crous working collection housed at CBS; CGMCC: Microbiological Culture Collection Center, Beijing, China; YA: Quanzhu Chen working culture collection number (Ye et al. 2017).
2HE = Heterothallic; HO = Homothallic; P_HE = Putative heterothallic.
3tub2 = β-tubulin; cmdA = calmodulin; his3 = histone H3; tef1 = translation elongation factor 1-alpha.
4Isolates representing ex-type cultures are indicated in bold.
5Isolate sequences were used in phylogenetic analyses.
6‘No’ represents the relative MAT locus was not amplified successfully by the primers designed in the current study.
7‘–’ represents sequences that are not available.
8Genome sequences of the isolate were from public genomic databases and for which no cultures were available in this study.
9The genome sequences were generated in this study.
Genome Ca. henricotiae*−1 = PGWR000000008; Ca. hongkongensis*−2 = JAACJA0000000009; Ca. leucothoes*−3 = NAJI000000008; Ca. naviculata*−4 = NAGG000000008; Ca. pauciramosa*−5 = JAACIZ0000000009; Ca. pauciramosa*−6 = JAACIY0000000009; Ca. pseudonaviculata*−7 = JYJY000000008; Ca. pseudoreteaudii*−8 = MOCD000000008.
All cultures were purified using single hyphal tip transfers to ensure that they represented a single genotype. After three to five days of growth on MEA, the mycelium was harvested and genomic DNA was extracted using Prepman™ Ultra Sample Preparation Reagent (Thermo Fisher Scientific, Waltham, MA, USA) following a protocol described by Duong et al. (2012). DNA concentrations were determined using a NanoDrop ND-2000 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) and diluted to 25–50 ng/μL using sterile distilled water.
The translation elongation factor 1-alpha (tef1) gene region was amplified for all 123 Calonectria isolates using the primers and protocols described by Lombard et al. (2016). Amplification reactions were conducted in 25 μL reaction volumes consisting of 12.5 μL 2 × TopTaq™ Master Mix (Qiagen Inc., Hilden, Germany), 1 μL of each of the two primers (10 mM), 2 μL genomic DNA and 8.5 μL sterile distilled water. The PCR products were visualized under UV light after 2 % agarose gel electrophoresis with 3 % SYBR Safe DNA gel stain (Thermo Fisher Scientific Inc., USA). Amplicons were sequenced in both directions using the same primers used for PCR amplification by the Beijing Genomics Institution, Guangzhou, China. The sequences were edited and assembled using Geneious v. 7.0 (Kearse et al. 2012). The tef1 sequences were used to confirm the identification of isolates based on a pairwise similarity comparison with sequences published on NCBI (https://guides.lib.berkeley.edu/ncbi/blast).
Analysis of the MAT loci in seven Calonectria species and primer design
Genome sequences
The genome sequences of seven Calonectria species (eight isolates) were used to analyse the MAT locus. Three of the genomes were sequenced in this study. This included one isolate of Ca. hongkongensis (CMW 47271) that is self-fertile and resides in the Sphaero-Naviculate Group of Calonectria (Crous et al. 2004, Lombard et al. 2010b, Li et al. 2017) and two isolates of Ca. pauciramosa (CMW 5683 and CMW 7592) known to be self-sterile, of opposite mating type, and which reside in the Prolate Group of Calonectria (Lombard et al. 2010a, b). Genomic DNA was extracted using the phenol/chloroform method described by Goodwin et al. (1992). Pair-end libraries (350 bp average insert size) and mate pair libraries (5 000 bp average insert size) for CMW 47271 and CMW 5683, as well as pair-end libraries (350 bp average insert size) for CMW 7592, were prepared and sequenced using the Illumina HiSeq 2500 platform. Quality control procedures on the raw sequencing reads, and the removal of adapters, were done using Trimmomatic v. 0.36 (Bolger et al. 2014). Genome assembly, assembly of contigs into scaffolds and gap filling were conducted as described by Duong et al. (in Wingfield et al. 2016) for the genome assembly of CMW 2644 (Grosmannia penicillata). The completeness of assembly was evaluated with BUSCO v. 3 (https://busco.ezlab.org/) using the Sordariomycetes odb9 dataset (Simão et al. 2015). All three genomic sequences were deposited in GenBank.
Sequences for the other five species, including Ca. henricotiae (CBS 138102), Ca. leucothoes (CBS 109166), Ca. naviculata (CBS 101121), Ca. pseudonaviculata (CBS 139394) and Ca. pseudoreteaudii (YA51), were obtained from public genomic databases at NCBI with accession numbers PGWR00000000, NAJI00000000, NAGG00000000, JYJY00000000 and MOCD00000000, respectively (Malapi-Wight et al. 2016a, b, Ye et al. 2017). All additional available genome sequences for Calonectria spp. published to date (Malapi-Wight et al. 2016a, b, 2019, Ye et al. 2017, LeBlanc et al. 2019) were also screened for inclusion in this study of the mating type locus. These included three genome sequences of Ca. henricotiae (CB077, NL009 and NL017) with NCBI accession numbers PGSE00000000, PGSF00000000 and PHMY00000000, respectively, and seven genome sequences of Ca. pseudonaviculata (CB002, CBS 114417, CBS 139395, CT13, ICMP 14368, NC-BB1 and ODA1) with NCBI accession numbers RQSK00000000, PHMX00000000, PGGA00000000, PGWW00000000, PHNA00000000, PHMZ00000000 and PHNB00000000, respectively. All three genome sequences of Ca. henricotiae harboured the same MAT1-1 idiomorph as the ex-type isolate of this species (CBS 138102) and all seven genome sequences of Ca. pseudonaviculata contained the same MAT1-2 idiomorph as CBS 139394. The genome sequences of CBS 114417, which is the ex-type culture for Ca. pseudonaviculata, harboured only partial MAT gene sequences while CBS 139394 contained the full MAT gene sequences. Consequently, isolates CBS 138102 (Ca. henricotiae) and CBS 139394 (Ca. pseudonaviculata) were chosen to describe their MAT loci.
Determination of the MAT locus structures
The MAT genes in each of the available eight Calonectria genome sequences were characterised using a tBLASTx search on the CLC Main Workbench v. 7.9.1 using the MAT genes (MAT1-2-1, MAT1-1-3, MAT1-1-2 and MAT1-1-1) reported in Fusarium anguioides NRRL 25385 (heterothallic, NCBI accession number MH742713; Jacobs-Venter et al. 2018) and F. graminearum 3639 (homothallic, NCBI accession number AF318048; Yun et al. 2000). These Fusarium spp., for which data are available regarding the MAT genes, are close relatives of Calonectria in the Nectriaceae. The contigs that produced hits with an E-value ≤ 10−2 were used to predict MAT genes and flanking regions using the online AUGUSTUS tool (http://bioinf.uni-greifswald.de/augustus/; Stanke et al. 2004). The MAT genes and their flanking regions were identified by BLASTp (NCBI), and further confirmed by comparison of homologs published on NCBI. The functional domains of the MAT genes were determined using the Conserved Domain search on NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Comparison of MAT loci
A comparison of the MAT loci mined from genome sequences of the eight Calonectria isolates was generated using BLASTn with a maximum E-value cut off of 0.0001, and visualized using Easyfig v. 2.2.2 (Sullivan et al. 2011). Easyfig is a Python application used to create linear comparative figures of multiple genomic loci with an easy-to-use graphical user interface. Pairwise similarity comparisons (BLASTn, tBLASTx) between multiple genomic regions were generated using the Easyfig interface (Sullivan et al. 2011).
Primer design for MAT genes
MAT1-1-1 and MAT1-2-1 primers were designed to determine the mode of sexual reproduction in a collection of 65 Calonectria species residing in 10 Calonectria species complexes. In addition, the available genome sequences were used to design primers for MAT1-1-3 or MAT1-2-12, which were present in the heterothallic Calonectria isolates but absent in the one homothallic species (Ca. hongkongensis, CMW 47271).
The sequences of the MAT1-1-1 and MAT1-1-3 genes extracted from the genomes of Ca. henricotiae (CBS 138102), Ca. hongkongensis (CMW 47271, only for MAT1-1-1 due to absence of MAT1-1-3), Ca. naviculata (CBS 101121) and Ca. pauciramosa (CMW 7592) were aligned. This alignment was used to design primers using the primer design function in CLC Main Workbench v. 7.9.1. following the software instructions. The alpha box domain in the MAT1-1-1 gene and the HMG box domain in the MAT1-1-3 gene were specifically targeted for primer design because these regions had the greatest similarity across all species.
The MAT1-2-1 primers designed previously by Schoch et al. (2000) were based on the partial HMG box domain and produced fragments of approximately 170 bp. The whole MAT1-2-1 gene region was used to design MAT1-2-1 primers again in this study and aimed to obtain a longer MAT1-2-1 fragment. The target areas for primer design for the MAT1-2-1 and MAT1-2-12 genes were based on the aligned sequences of the MAT1-2-1 or MAT1-2-12 gene found in the genomes of Ca. hongkongensis (CMW 47271, only for MAT1-2-1 due to absence of MAT1-2-12), Ca. leucothoes (CBS 109166), Ca. pauciramosa (CMW 5683), Ca. pseudonaviculata (CBS 139394) and Ca. pseudoreteaudii (YA51) using CLC Main Workbench v. 7.9.1. The MAT1-2-1 primers were designed in HMG box domain and overlapped with those designed by Schoch et al. (2000); MAT1-2-12 primers were designed in the conserved areas.
MAT gene amplification and mating type assignment
All 123 isolates representing 65 Calonectria species were screened for four MAT genes (MAT1-1-1, MAT1-1-3, MAT1-2-1 and MAT1-2-12). PCR amplification reaction conditions for these MAT genes were as follows: initial denaturation at 95 °C for 3 min, followed by 30 cycles of 95 °C denaturation for 30 s, 53 °C (MAT1-1-1) or 58 °C (MAT1-2-1) or 48 °C (MAT1-1-3 or MAT1-2-12) annealing for 30 s, and 72 °C extension for 1 min, followed by a final extension at 72 °C for 10 min. PCR amplification mixtures, verification of PCR products, amplicon sequencing and sequence editing, assembly tools for MAT gene amplification and analyses were the same as those used to obtain the tef1 gene regions described above. The sequences were aligned using the online version of MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/; Katoh & Standley 2013). Alignments of four MAT gene sequences were deposited in TreeBASE (http://treebase.org).
The conserved domains for each MAT gene sequence in all 123 Calonectria isolates were determined by the Pfam domain search on CLC Main Workbench v. 7.9.1. All of these sequences were deposited in GenBank (Table 1). Species having both MAT1-1-1 and MAT1-2-1 genes in a single isolate were designated as homothallic. Heterothallic species were identified by the presence of either MAT1-1-1 or MAT1-2-1 in different isolates. Species were considered to be putatively heterothallic when only the MAT1-1-1 or MAT1-2-1 gene was detected in all the isolates of a particular species (Duong et al. 2016).
Phylogenetic analysis and ancestral state reconstruction
To investigate the evolutionary history of sexual reproduction in Calonectria, a multi-gene phylogenetic tree based on Maximum Likelihood (ML) analysis for the combined dataset of the tef1, histone H3 (his3), calmodulin (cmdA) and partial β-tubulin (tub2) gene regions was generated using PhyML v. 3.1 (Guindon & Gascuel 2003). A single isolate representing each of 70 Calonectria species (Table 1) was selected for the phylogenetic analyses. These included the five species for which the genome sequences are publicly available and for which cultures were not used in this study (Table 1). All sequences used to construct the phylogenetic tree were either downloaded directly from NCBI (http://www.ncbi.nlm.nih.gov) or extracted from the genome sequences. Confidence levels for the nodes were determined with 1 000 bootstrap replicates. Curvicladiella cignea (CBS 109167) was used as the outgroup taxon in the analyses (Lombard et al. 2016). Alignment of sequence combination of four gene regions was deposited in TreeBASE (http://treebase.org).
The homothallic or heterothallic mode of reproduction in each of the 70 Calonectria species was mapped onto the backbone of the multi-gene phylogenetic tree. Ancestral state reconstruction based on the ML approach was performed using an unordered parsimony model in Mesquite v. 3.5 (Maddison & Maddison 2018).
RESULTS
Isolates and identification
The DNA for all 123 isolates representing 65 Calonectria spp. was successfully extracted. Confirmation of these previously identified and published isolates was achieved based on a comparison of tef1 sequences generated in this study and published on NCBI (Table 1).
Genome sequencing
For CMW 47271 (Ca. hongkongensis), CMW 5683 (Ca. pauciramosa) and CMW 7592 (Ca. pauciramosa), the estimated genome sizes were 61.7 Mb, 62.4 Mb and 62.3 Mb, respectively. The average coverage of all three assembled genomes were higher than 736×. The assembled genome of CMW 47271 (Ca. hongkongensis) had 76 scaffolds larger than 500 bp, a N50 contig size of 1.7 Mb and a mean GC content of 49.0 %. The genomes for CMW 5683 and CMW 7592 (Ca. pauciramosa) contained 83 scaffolds (> 500 bp) with N50 of 3.1 Mb, and 104 scaffolds (> 500 bp) with N50 of 1.4 Mb, respectively. These two genomes had a similar GC content of 49.3 %. The BUSCO analysis indicated a high level of completeness for all three assemblies based on the Sordariomycetes dataset and less than 1.2 % BUSCO orthologs were missing. GenBank accession numbers of these three genome sequences were JAACJA000000000, JAACIZ000000000 and JAACIY000000000, respectively (Table 1).
MAT locus structure and MAT genes in the eight Calonectria genomes
The MAT idiomorphs in each of the eight selected Calonectria isolates for which genome sequences were available were detected in a single contig (scaffold) based on a tBLASTx search on the CLC Main Workbench. Contigs from Ca. leucothoes (CBS 109166), Ca. pauciramosa (CMW 5683), Ca. pseudonaviculata (CBS 139394) and Ca. pseudoreteaudii (YA51) contained sequences very similar to those of the MAT1-2-1 gene sequences in F. graminearum 3639 (E-value: 2.31E-8 to 4.14E-5). None of the contigs had similarity to the gene sequences of the MAT1-1 idiomorph. These isolates were considered to contain only a MAT1-2 idiomorph. Calonectria henricotiae (CBS 138102), Ca. naviculata (CBS 101121) and Ca. pauciramosa (CMW 7592) were designated as containing the MAT1-1 idiomorph based on the presence of a MAT1-1-1 gene and the absence of a MAT1-2-1 gene in the MAT locus of each isolate. In addition, Ca. hongkongensis (CMW 47271) was found to have both MAT1-1-1 and MAT1-2-1 in a single scaffold and was confirmed as homothallic.
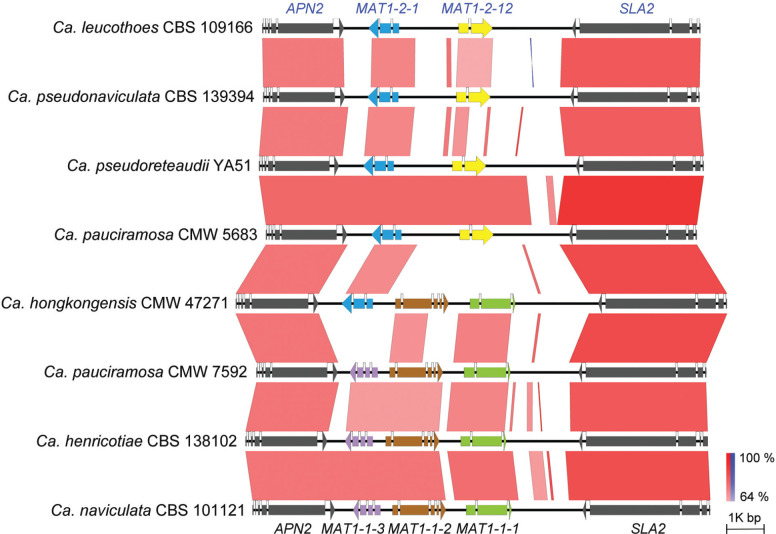
The length of the MAT idiomorph of Ca. hongkongensis (CMW 47271) was 4.66 kb. The MAT1-1 idiomorph of Ca. henricotiae (CBS 138102), Ca. naviculata (CBS 101121) and Ca. pauciramosa (CMW 7592) were approximately 4.3 kb long, and the length of the MAT1-2 idiomorph in Ca. leucothoes (CBS 109166), Ca. pauciramosa (CMW 5683), Ca. pseudonaviculata (CBS 139394) and Ca. pseudoreteaudii (YA51) was approximately 3.3 kb. The structural arrangement of the MAT locus and flanking genes was conserved in all isolates (Fig. 1). The MAT locus was flanked by the genes APN2 (DNA lyase) and SLA2 (cytoskeleton assembly control protein) gene.
Fig. 1.
Pairwise MAT loci comparison among eight Calonectria isolates representing seven species. Black horizontal lines represent genomic sequences. Colour coded arrows represent annotated genes. Red or blue boxes between genomic sequences indicates pairwise similarity based on BLASTn; red suggest that both regions are in the same orientation and blue are in opposite directions. Calonectria hongkongensis CMW 47271 represents the only homothallic individual containing both MAT1-1 and MAT1-2 idiomorph.
The MAT1-1 and MAT1-2 idiomorphs in the genomes of the six heterothallic Calonectria species were identical in order and orientation (Fig. 1). The MAT1-1 idiomorph in Ca. henricotiae (CBS 138102), Ca. naviculata (CBS 101121) and Ca. pauciramosa (CMW 7592) possessed the MAT1-1-1, MAT1-1-2 and MAT1-1-3 genes. A MAT1-2-1 gene as well as an open reading frame (ORF) of unknown function were observed in the MAT1-2 idiomorph of Ca. leucothoes (CBS 109166), Ca. pauciramosa (CMW 5683), Ca. pseudonaviculata (CBS 139394) and Ca. pseudoreteaudii (YA51). The MAT1-1-3 gene and the ORF of unknown function, found respectively in the MAT1-1 and MAT1-2 locus of the heterothallic species, were absent in the MAT locus of homothallic Ca. hongkongensis (CMW 47271), which contained the MAT1-1-1, MAT1-1-2 and MAT1-2-1 genes. The ORF found in the MAT1-2 locus of heterothallic Calonectria species was different to all other genes previously observed at a MAT locus. This was consequently recognised as a new mating type gene and is designated here as MAT1-2-12. This gene was previously designated as MAT1-2-2 by Malapi-Wight et al. (2019).
The predicted MAT1-1-1 (1.2 kb) gene in the eight Calonectria genomes contain two introns, and encode a 372 to 383 amino acid (aa) protein with a conserved MATalpha_HMGbox domain (GenBank: pfam04769) that spans a 49 bp intron. Both the MAT1-1-3 (737 bp to 751 bp) and MAT1-2-1 gene (809 bp to 837 bp) encode an HMG box domain (GenBank: cd01389), which is interrupted by an intron (about 50 bp). The predicted MAT1-1-3 gene has a CDS approximately 600 bp in size and contains three introns. The putative MAT1-2-1 gene has a CDS of approximately 720 bp and contains two introns. A conserved putative protein 1-1-2 domain (GenBank: pfam17043) was found in all MAT1-1-2 (1.4 kb) genes. Although four introns were present in the MAT1-1-2 gene, the conserved putative protein 1-1-2 domain was not interrupted by any of them. The novel mating type gene defined in this study as MAT1-2-12 was approximately 910 bp long, has a predicted 60 bp intron and encodes for a putative protein around 285 aa with unknown domains.
A comparison of nucleotide and amino acid sequences of mating type genes among the eight isolates for which whole genome sequences were available, showed that non-coding intronic regions were more variable than the coding regions. This was with the exception of MAT1-1-2 and MAT1-2-12 (Table 2). The full nucleotide sequence (around 49 %) of the MAT1-2-12 gene was more conserved than amino acid sequences (about 40 %), and both sequences had very similar variation in MAT1-1-2 genes. The sequences of APN2 were more variable than MAT1-1-1 and MAT1-1-3 in the eight Calonectria isolates (Table 2) used in this study and for which whole genome sequences were available.
Table 2.
Nucleotide and amino acid conservation of mating type and flanking genes in the genomes of eight Calonectria isolates.
| Isolates | Nucleotide conservation (%) |
||||||
|---|---|---|---|---|---|---|---|
| SLA2 | MAT1-1-1 | MAT1-1-2 | MAT1-1-3 | MAT1-2-1 | MAT1-2-12 | APN2 | |
| Ca. henricotiae CBS 138102 | 66.37 (2 463/3 711)1 | 60.82 (742/1 220) | 45.63 (657/1 440) | 66.93 (500/747) | 54.20 (1 188/2 192) | ||
| Ca. naviculata CBS 101121 | 71.95 (2 463/3 423) | 60.77 (742/1 221) | 45.72 (657/1 437) | 67.84 (500/737) | 53.71 (1 188/2 212) | ||
| Ca. pauciramosa CMW 7592 | 71.89 (2 463/3 426) | 59.50 (742/1 247) | 45.94 (657/1 430) | 66.58 (500/751) | 54.57 (1 188/2 177) | ||
| Ca. hongkongensis CMW 47271 | 71.31 (2 463/3 454) | 60.92 (742/1 218) | 45.98 (657/1 429) | 56.99 (477/837) | 53.71 (1 188/2 212) | ||
| Ca. leucothoes CBS 109166 | 71.62 (2 463/3 439) | 58.24 (477/819) | 49.34 (452/916) | 54.22 (1 188/2 191) | |||
| Ca. pauciramosa CMW 5683 | 71.87 (2 463/3 427) | 58.96 (477/809) | 49.83 (452/907) | 54.57 (1 188/2 177) | |||
| Ca. pseudonaviculata CBS 139394 | 71.08 (2 463/3 465) | 57.26 (477/833) | 49.24 (452/918) | 54.20 (1 188/2 192) | |||
| Ca. pseudoreteaudii YA51 | 71.81 (2 463/3 430) | 58.10 (477/821) | 49.83 (452/907) | 55.38 (1 188/2 145) | |||
| Isolates | Amino acid conservation (%) |
||||||
|---|---|---|---|---|---|---|---|
| SLA2 | MAT1-1-1 | MAT1-1-2 | MAT1-1-3 | MAT1-2-1 | MAT1-2-12 | APN2 | |
| Ca. henricotiae CBS 138102 | 83.48 (945/1 132)2 | 68.10 (254/373) | 45.61 (187/410) | 75.00 (150/200) | 67.75 (416/614) | ||
| Ca. naviculata CBS 101121 | 89.83 (945/1 052) | 68.10 (254/373) | 45.61 (187/410) | 76.53 (150/196) | 66.99 (416/621) | ||
| Ca. pauciramosa CMW 7592 | 89.83 (945/1 052) | 66.32 (254/383) | 45.95 (187/407) | 75.00 (150/200) | 68.53 (416/607) | ||
| Ca. hongkongensis CMW 47271 | 89.83 (945/1 052) | 68.28 (254/372) | 45.95 (187/407) | 62.30 (152/244) | 66.99 (416/621) | ||
| Ca. leucothoes CBS 109166 | 89.83 (945/1 052) | 62.81 (152/242) | 39.65 (113/285) | 68.42 (416/608) | |||
| Ca. pauciramosa CMW 5683 | 89.83 (945/1 052) | 63.87 (152/238) | 40.07 (113/282) | 68.53 (416/607) | |||
| Ca. pseudonaviculata CBS 139394 | 89.83 (945/1 052) | 62.04 (152/245) | 39.51 (113/286) | 67.75 (416/614) | |||
| Ca. pseudoreteaudii YA51 | 89.83 (945/1 052) | 62.81 (152/242) | 40.07 (113/282) | 68.99 (416/603) | |||
1The percentage of conserved nucleotides including exon and intron (length of conserved nucleotides/full-length of nucleotides).
2The percentage of conserved amino acid (length of conserved amino acid/full-length of amino acid).
MAT loci amplification and mating type assignment
Mating type markers designed in this study (Table 3) were used in PCRs to amplify portions of the MAT1-1-1 (primers Cal_MAT111_F and Cal_MAT111_R), MAT1-1-3 (primers Cal_MAT113_F and Cal_MAT113_R), MAT1-2-1 (primers Cal_MAT121_F and Cal_MAT121_R) and MAT1-2-12 (primers Cal_MAT1212_F and Cal_MAT1212_R) genes in the 123 Calonectria isolates representing 10 Calonectria species complexes. These resulted in PCR products of approximately 330 bp, 430 bp, 240 bp and 670 bp, respectively. The MAT1-1-1 DNA sequences produced by PCR amplification all encoded a putative 110 amino acid sequence that included an alpha box domain. The MAT1-1-3 encoded a sequence of 104 amino acids and MAT1-2-1 encoded a sequence of 61 amino acids; the former having two predicted introns of about 50 bp and the latter an intron of 55 bp. Both sequences had an HMG domain that was interrupted by a single intron (Table 3). The alignments of each of the datasets of four MAT genes were deposited in TreeBASE (TreeBASE no 25663; http://treebase.org). An alignment analysis of the MAT1-1-1, MAT1-1-3, MAT1-2-1 and MAT1-2-12 sequences revealed little or no sequence variation in the genes within species but a high level of variation in the genes between species.
Table 3.
Primers for amplification of mating type gene fragments.
| Target gene | Primer name | Primer sequence (5’ to 3’) | Tm (°C) | Fragment size (bp) | Target area |
|---|---|---|---|---|---|
| MAT1-1-1 | Cal_MAT111_F | ATGCTTCCTCAGTCTTTGCT | 53 | 330 |
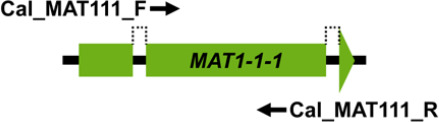
|
| Cal_MAT111_R | CTTGAAYRGGGTTGGTGG | ||||
| MAT1-1-3 | Cal_MAT113_F | CCTCCAGAAGTACCGACT | 48 | 430 |
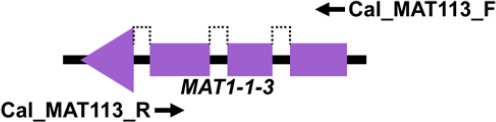
|
| Cal_MAT113_R | GCTGTCGTTCTTCTTCCT | ||||
| MAT1-2-1 | Cal_MAT121_F | GCAAGGAYCGCCACCRAAT | 58 | 240 |
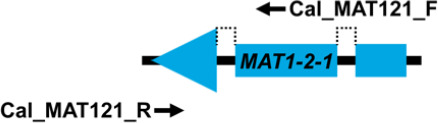
|
| Cal_MAT121_R | GACACCTCKGCGTTTCTTCTCAG | ||||
| MAT1-2-12 | Cal_MAT1212_F | TCATCAGTTTCGCCCATT | 48 | 670 |
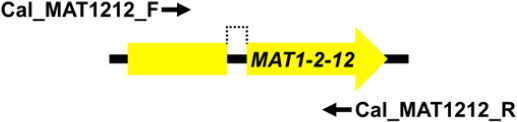
|
| Cal_MAT1212_R | CGTCGTACTTCTTCTTCCG |
Based on the MAT gene amplification profile, 21 species (36 isolates) were identified as homothallic and 22 isolates representing eight species were heterothallic (Table 1). The remaining 36 species (65 isolates) were tentatively designated as heterothallic because only a MAT1-1-1 or a MAT1-2-1 gene was detected in isolates of these species. For the 21 homothallic species, 17 were first described from China, two (Ca. eucalypti CBS 125275 and Ca. bumicola CBS 143575) from Indonesia, Ca. colombiensis CBS 112221 from Colombia and Ca. gracilis CBS 111807 was from Brazil (Table 1).
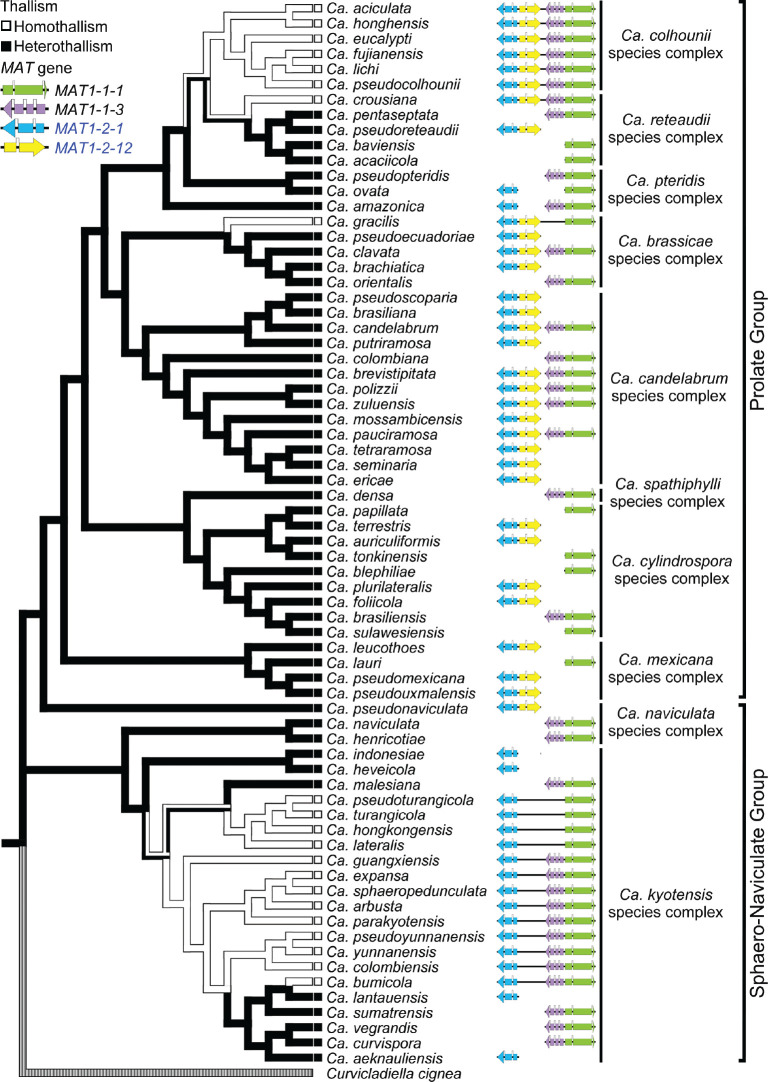
The PCR amplification results revealed four different homothallic MAT loci in Calonectria (Fig. 2). In the Prolate Group, the MAT locus of most homothallic species contained the MAT1-1-1, MAT1-1-3, MAT1-2-1 and MAT1-2-12 genes. This was with the exception of Ca. gracilis in which the MAT1-1-3 gene was not detected. In the Sphaero-Naviculate Group, the MAT1-2-12 gene was absent in all homothallic species. In the clade represented by Ca. lateralis, the MAT1-1-3 gene was absent in all of these species.
Ancestral state reconstruction of sexual thallism
The alignment of sequence combination of tef1, his3, cmdA and tub2 genes was deposited in TreeBASE (TreeBASE no 25663; http://treebase.org). The ancestral state reconstruction analysis suggested that heterothallism is the ancestral state in Calonectria. This emerged from tracing the history of mating type characters onto the multi-gene phylogenetic species tree (Fig. 2). Three independent transitions from heterothallism to homothallism appear to have occurred across the phylogeny. One transition from homothallism to heterothallism was observed in the Ca. kyotensis species complex. Either a homothallic or a heterothallic lifestyle has occurred across Calonectria species in both the Prolate and Sphaero-Naviculate Groups. In most of the cases, the species with the same thallism grouped together in the phylogeny. Heterothallism was the most common state across the genus but homothallism was dominant for species in the Sphaero-Naviculate Group.
Fig. 2.
Ancestral state reconstruction of sexual thallism of 70 Calonectria species. Homothallic species are marked with an open line, heterothallic species are marked with a solid line. Green, purple, blue and yellow coded arrows represent the MAT1-1-1, MAT1-1-3, MAT1-2-1 and MAT1-2-12 gene, respectively.
DISCUSSION
Analyses of genome sequences enabled the characterisation of the MAT loci in eight isolates representing seven species of Calonectria. In addition, the mating strategies of 65 Calonectria species were revealed using primers developed for four MAT genes. The MAT locus and flanking region was shown to have a conserved APN2-MAT1-SLA2 structure, with differences observed in the genes of the MAT locus. From these results, and using ancestral state reconstruction, heterothallism was found to represent the ancestral reproductive state in Calonectria.
MAT loci and mating type genes
Species residing in the Hypocreales have commonly been found to harbour the MAT1-1-1, MAT1-1-2 and MAT1-1-3 genes in the MAT1-1 idiomorph (Bushley et al. 2013). This is consistent with the results of the present study for heterothallic Calonectria species. In the MAT1-2 idiomorph, in addition to the MAT1-2-1 gene that was always present, the MAT1-2-12 gene was described in this study. The discovery of this MAT gene in Calonectria represents a third gene to be discovered in this idiomorph in the Hypocreales. The other two genes include the MAT1-2-8 in Ustilaginoidea (Yu et al. 2015, Wilken et al. 2017) and MAT1-2-9 in Fusarium (Martin et al. 2011, Wilken et al. 2017). These three genes have not been detected in any fungi outside the Hypocreales, suggesting that they are probably restricted to this order. Gene deletions showed the MAT1-2-9 (previously named MAT1-2-3, Wilken et al. 2017) have a similar expression pattern to the MAT1-1-1 and MAT1-2-1 in F. graminearum and F. asiaticum (Kim et al. 2012). The function of MAT1-2-8 and MAT1-2-12 in sexual reproduction has yet to be determined (Wilken et al. 2017, Malapi-Wight et al. 2019).
Neither the MAT1-1-3 nor MAT1-2-12 genes were observed in the MAT locus of the homothallic Ca. hongkongensis, Ca. lateralis, Ca. pseudoturangicola and Ca. turangicola. The MAT1-1-3 gene has been reported as absent in the MAT1-1 idiomorph of other Hypocreales fungi (Yokoyama et al. 2006, Bushley et al. 2013). Interestingly the MAT1-1-3 gene was present in the various closely related species including Ca. arbusta, Ca. bumicola, Ca. colombiensis, Ca. expansa, Ca. guangxiensis, Ca. parakyotensis, Ca. pseudoyunnanensis, Ca. sphaeropedunculata and Ca. yunnanensis. This could reflect two different branches of evolution for the MAT locus in Calonectria spp. Mutation analyses of MAT1-1-2 and MAT1-1-3 have shown that these two genes have similar expression profiles and may possess overlapping functions in sexual development (Ferreira et al. 1998, Zheng et al. 2013). In addition, species maintaining the MAT1-1-3 gene in the Hypocreales are also located at a more ancestral position in the mating type tree than species lacking the MAT1-1-3 gene (Yokoyama et al. 2006). We consequently hypothesize that the MAT locus lacking the MAT1-1-3 gene in Calonectria may have evolved from an ancestral locus containing all three genes (MAT1-1-1, MAT1-1-2 and MAT1-1-3).
Distribution of mating types
Previous studies have shown that most species in Calonectria are heterothallic with a biallelic mating system (Crous et al. 1998, Crous 2002, Lombard et al. 2010a–c). This was supported in the results of the present study, where 44 of 65 Calonectria species were found to be heterothallic. These results also suggest that heterothallism is the ancestral state in Calonectria. The 21 homothallic species reside primarily in the Ca. colhounii and Ca. kyotensis species complexes. But in both these complexes, heterothallism is basal. This suggests that these species had a common homothallic ancestor, which has evolved from a heterothallic state.
The MAT genes observed in Ca. bumicola, Ca. crousiana and Ca. gracilis suggest that these species are homothallic while their closest neighbours in the same clade/group are all heterothallic. This is unusual and in contrast to views in a previous study (Duong et al. 2016) where species residing in the same complex consistently shared the same mode of sexual reproduction. The fact that only the MAT1-1-1 or MAT1-2-1 genes amplified in a number of isolates of Calonectria, provides a level of confidence in our results. It is, however, possible that the primers designed for the MAT1-1-3 and MAT1-2-12 failed to allow the detection of these genes and whole genome sequences would be needed to confirm this result.
Evolution of mating type
The results of this study indicated that heterothallism represents the ancestral reproductive state in Calonectria. Furthermore, that one independent transition from homothallism back to heterothallism has occurred in the Ca. kyotensis species complex. Evolution of homothallism from heterothallism has apparently occurred due to unequal crossing over and translocation of the MAT idiomorphs in various Ascomycete fungi, including Bipolaris = Cochliobolus (Yun et al. 1999), Stemphylium = Pleospora (Inderbitzin et al. 2005), Crivellia = Alternaria (Inderbitzin et al. 2006), Neurospora (Nygren et al. 2011, Gioti et al. 2012) and Eutiarosporella (Thynne et al. 2017). In contrast, fewer studies have shown heterothallic fungi have been derived from homothallic ancestors via gene loss. In this way, partial gene sequences of the genes residing in the MAT1-2 idiomorph have been incorporated into the MAT1-1 idiomorph or vice versa, such as Aspergillus fumigatus (Paoletti et al. 2005), Botrytis cinerea (Amselem et al. 2011) and Cordyceps takaomontana (Yokoyama et al. 2003). Although it is possible that the transition between homothallism and heterothallism in Ascomycetes could occur in either direction, a switch from one state should logically reflect an evolutionary advantage. In this regard, heterothallism would offer the advantage of enhanced genetic diversity and adaption to the environment (Lumley et al. 2015). In contrast, homothallism offers the benefits of sexual recombination without needing isolates of the opposite mating type (Wilson et al. 2015b).
A proposed evolution model for mating type
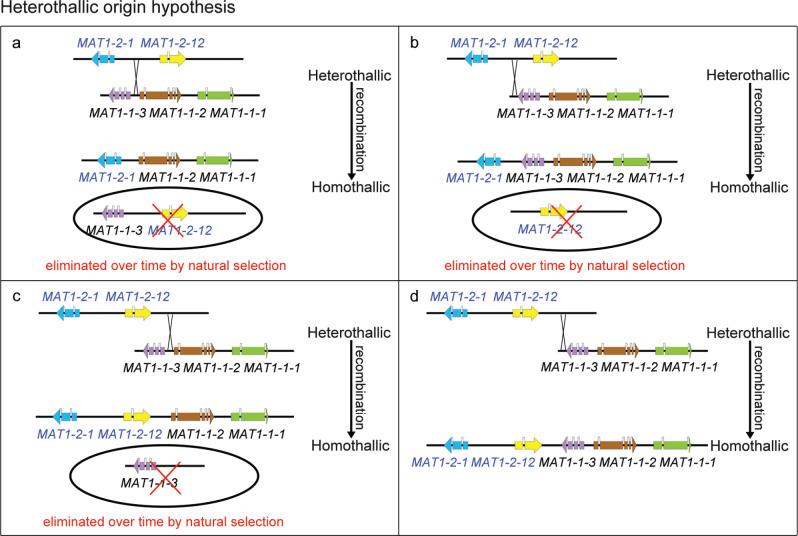
The structure of mating type loci in Calonectria species revealed in this study makes it possible to explain the evolution of the mating types following two possible hypotheses (Fig. 3, 4). In one case, which we consider as the recombination hypothesis, there has been an ancestral shift from heterothallism to homothallism in four independent unequal recombination events (Fig. 3a–d). These would have resulted in the mating type idiomorphs observed in the present study.
Fig. 3.
Evolution models of mating type in Calonectria spp.: Heterothallic origin hypothesis. a–d. Four scenarios under which the mating type loci of heterothallic ancestors undergo an independent recombination event (unequal crossing over), resulting in the present homothallic mating type locus.
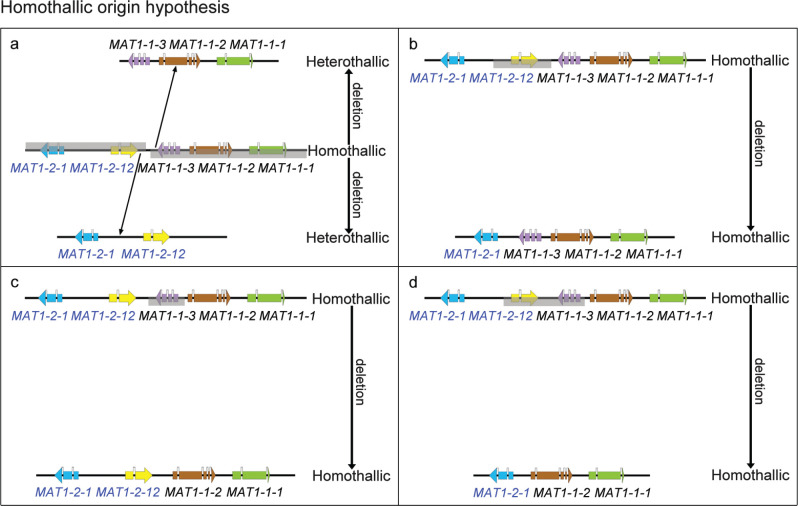
Fig. 4.
Evolution models of mating type in Calonectria spp.: Homothallic origin hypothesis. a. Primary homothallic ancestor mating type locus undergoes two deletions events (gene loss) and this results in the mating type locus of two heterothallic offspring; b–d. primary homothallic ancestor mating type locus undergoes an independent deletion event which results in the present homothallic mating type locus.
An alternative hypothesis would involve a shift from a homothallic ancestor containing all the MAT genes (MAT1-1-1, MAT1-1-2, MAT1-1-3, MAT1-2-12 and MAT1-2-1) to a heterothallic state via at least two deletion events (Fig. 4a–d). In this case, the homothallic ancestor would have also undergone three independent deletion events to arrive at the currently identified homothallic species. This hypothesis is less parsimonious than the recombination hypothesis. Based on parsimony (Rasmussen & Ghahramani 2001), a heterothallic origin hypothesis is more probable than the homothallic origin hypothesis. However, it is not possible to rule out the possibility that the original ancestor of the heterothallic species was in fact not homothallic and that species in this genus have evolved from homothallism to heterothallism and then some have switched back to homothallism.
Reproductive modes and pathogenicity
Results of this study have made it possible to easily characterise the mating type of important Calonectria spp. This will enhance the value of population genetic studies on these fungi where the presence or absence of sexual reproduction can be considered. The results will also support quarantine regulations that should seek to prevent the introduction of opposite mating type strains in heterothallic Calonectria spp., where only one of these is known to be present in a country. This can preclude the generation of new genotypes of such pathogens and a breakdown of resistance developed in the host (McDonald & Linde 2002, Lombard et al. 2010a, Malapi-Wight et al. 2014).
Acknowledgements
This study was supported financially by the special fund for basic scientific research of State Key Laboratory of Tree Genetics and Breeding (SKLTGB) of China (project no. TGB2017001), the National Natural Science Foundation of China (NSFC) (project no. 31622019), the National Key R&D Program of China (project no. 2017YFD0600103), the National Ten-thousand Talents Program (project No. W03070115), the GuangDong Top Young Talents Program (project No. 20171172), the Genomics Research Institute and members of the Tree Protection and Cooperation Programme (TPCP), South Africa. We thank Dr. Seonju Marincowitz for assistance in sourcing cultures and Mr. Jan Nagel, Dr. Tuan Duong, Dr. Markus Wilken and Ms. Katrin Fitza for advice.
REFERENCES
- Adamson K, Mullett MS, Solheim H, et al. 2018. Looking for relationships between the populations of Dothistroma septosporum in northern Europe and Asia. Fungal Genetics and Biology 110: 15–25. [DOI] [PubMed] [Google Scholar]
- Alby K, Schaefer D, Bennett RJ. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amselem J, Cuomo CA, Van Kan JA, et al. 2011. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genetics 7: e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard S, López-Villavicencio M, Hood ME, et al. 2012. Sex, outcrossing and mating types: unsolved questions in fungi and beyond. Journal of Evolutionary Biology 25: 1020–1038. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushley KE, Li Y, Wang WJ, et al. 2013. Isolation of the MAT1-1 mating type idiomorph and evidence for selfing in the Chinese medicinal fungus Ophiocordyceps sinensis. Fungal Biology 117: 599–610. [DOI] [PubMed] [Google Scholar]
- Crous PW. 2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, USA. [Google Scholar]
- Crous PW, Alfenas AC, Junghans TG. 1998. Variability within Calonectria ovata and its anamorph Cylindrocladium ovatum from Brazil. Sydowia 50: 1–3. [Google Scholar]
- Crous PW, Carnegie AJ, Wingfield MJ, et al. 2019. Fungal Planet description sheets: 868–950. Persoonia 42: 291–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Risède JM, et al. 2004. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JF. 1994. Advantages of sexual reproduction. Developmental Genetics 15: 205–213. [DOI] [PubMed] [Google Scholar]
- Duong TA, De Beer ZW, Wingfield BD, et al. 2012. Phylogeny and taxonomy of species in the Grosmannia serpens complex. Mycologia 104: 715–732. [DOI] [PubMed] [Google Scholar]
- Duong TA, De Beer ZW, Wingfield BD, et al. 2016. Mating type markers reveal high levels of heterothallism in Leptographium sensu lato. Fungal Biology 120: 538–546. [DOI] [PubMed] [Google Scholar]
- Ferreira AV, An Z, Metzenberg RL, et al. 1998. Characterization of mat A-2, mat A-3 and ΔmatA mating-type mutants of Neurospora crassa. Genetics 148: 1069–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JA, Stajich JE, Tarcha EJ, et al. 2007. Evolution of the mating type locus: insights gained from the dimorphic primary fungal pathogens Histoplasma capsulatum, Coccidioides immitis, and Coccidioides posadasii. Eukaryotic Cell 6: 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioti A, Mushegian AA, Strandberg R, et al. 2012. Unidirectional evolutionary transitions in fungal mating systems and the role of transposable elements. Molecular Biology and Evolution 29: 3215–3226. [DOI] [PubMed] [Google Scholar]
- Goodenough U, Heitman J. 2014. Origins of eukaryotic sexual reproduction. Cold Spring Harbor Perspectives in Biology 6: a016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin SB, Drenth A, Fry WE. 1992. Cloning and genetic analyses of two highly polymorphic, moderately repetitive nuclear DNAs from Phytophthora infestans. Current Genetics 22: 107–115. [DOI] [PubMed] [Google Scholar]
- Gordo I, Campos PR. 2008. Sex and deleterious mutations. Genetics 179: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Inderbitzin P, Harkness J, Turgeon BG, et al. 2005. Lateral transfer of mating system in Stemphylium. Proceedings of the National Academy of Sciences 102: 11390–11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderbitzin P, Shoemaker RA, O’Neill NR, et al. 2006. Systematics and mating systems of two fungal pathogens of opium poppy: the heterothallic Crivellia papaveracea with a Brachycladium penicillatum asexual state and a homothallic species with a Brachycladium papaveris asexual state. Botany 84: 1304–1326. [Google Scholar]
- Jacobs-Venter A, Laraba I, Geiser DM, et al. 2018. Molecular systematics of two sister clades, the Fusarium concolor and F. babinda species complexes, and the discovery of a novel microcycle macroconidium-producing species from South Africa. Mycologia 110: 1189–1204. [DOI] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch CL, et al. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443: 818–822. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Cho EJ, Lee S, et al. 2012. Functional analyses of individual mating-type transcripts at MAT loci in Fusarium graminearum and Fusarium asiaticum. FEMS Microbiology Letters 337: 89–96. [DOI] [PubMed] [Google Scholar]
- Labarere J, Noel T. 1992. Mating type switching in the tetrapolar basidiomycete Agrocybe aegerita. Genetics 131: 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc N, Gehesquière B, Salgado-Salazar C, et al. 2019. Limited genetic diversity across pathogen populations responsible for the global emergence of boxwood blight identified using SSRs. Plant Pathology 68: 861–868. [Google Scholar]
- Li J, Wingfield MJ, Liu Q, et al. 2017. Calonectria species isolated from Eucalyptus plantations and nurseries in South China. IMA Fungus 8: 259–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Heitman J. 2007. Mechanisms of homothallism in fungi and transitions between heterothallism and homothallism. In: Heitman J, Kronstad JW, Taylor JW, et al. (eds), Sex in fungi: molecular determination and evolutionary implications: 35–57. ASM Press, Washington DC. [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, et al. 2010a. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology 66: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, et al. 2010b. Phylogeny and systematics of the genus Calonectria. Studies in Mycology 66: 31–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, et al. 2010c. Species concepts in Calonectria (Cylindrocladium). Studies in Mycology 66: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Wingfield MJ, Alfenas AC, et al. 2016. The forgotten Calonectria collection: pouring old wine into new bags. Studies in Mycology 85: 159–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes A, Phillips AJ, Alves A. 2017. Mating type genes in the genus Neofusicoccum: mating strategies and usefulness in species delimitation. Fungal Biology 121: 394–404. [DOI] [PubMed] [Google Scholar]
- Lumley AJ, Michalczyk Ł, Kitson JJ, et al. 2015. Sexual selection protects against extinction. Nature 522: 470–473. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2018. Mesquite: a modular system for evolutionary analysis. Version 3.5. http://www.mesquiteproject.org [accessed 18 June 2018].
- Malapi-Wight M, Demers JE, Veltri D, et al. 2016a. LAMP detection assays for boxwood blight pathogens: a comparative genomics approach. Scientific Reports 6: 26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapi-Wight M, Hébert JB, Rivera Y, et al. 2014. Comparative genomics in the boxwood blight system: Insights into the global diversity of the mating-type locus. (Abstr.) Phytopathology 104: S74. [Google Scholar]
- Malapi-Wight M, Salgado-Salazar C, Demers JE, et al. 2016b. Sarcococca blight: use of whole-genome sequencing for fungal plant disease diagnosis. Plant Disease 100: 1093–1100. [DOI] [PubMed] [Google Scholar]
- Malapi-Wight M, Veltri D, Gehesquière B, et al. 2019. Global distribution of mating types shows limited opportunities for mating across populations of fungi causing boxwood blight disease. Fungal Genetics and Biology 131: 103246. [DOI] [PubMed] [Google Scholar]
- Marin-Felix Y, Groenewald JZ, Cai L, et al. 2017. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology 86: 99–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SH, Wingfield BD, Wingfield MJ, et al. 2011. Structure and evolution of the Fusarium mating type locus: new insights from the Gibberella fujikuroi complex. Fungal Genetics and Biology 48: 731–740. [DOI] [PubMed] [Google Scholar]
- McDonald BA, Linde C. 2002. Pathogen population genetics, evolutionary potential, and durable resistance. Annual Review of Phytopathology 40: 349–379. [DOI] [PubMed] [Google Scholar]
- Nagel JH, Wingfield MJ, Slippers B. 2018. Evolution of the mating types and mating strategies in prominent genera in the Botryosphaeriaceae. Fungal Genetics and Biology 114: 24–33. [DOI] [PubMed] [Google Scholar]
- Ni M, Feretzaki M, Sun S, et al. 2011. Sex in fungi. Annual Review of Genetics 45: 405–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren K, Strandberg R, Wallberg A, et al. 2011. A comprehensive phylogeny of Neurospora reveals a link between reproductive mode and molecular evolution in fungi. Molecular Phylogenetics and Evolution 59: 649–663. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Ward TJ, Geiser DM, et al. 2004. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genetics and Biology 41: 600–623. [DOI] [PubMed] [Google Scholar]
- Paoletti M, Rydholm C, Schwier EU, et al. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Current Biology 15: 1242–1248. [DOI] [PubMed] [Google Scholar]
- Pham NQ, Barnes I, Chen SF, et al. 2019. Ten new species of Calonectria from Indonesia and Vietnam. Mycologia 111: 78–102. [DOI] [PubMed] [Google Scholar]
- Rasmussen CE, Ghahramani Z. 2001. Occam’s razor. In: Leen TK, Dietterich TG, Tresp V. (eds), Advances in Neural Information Processing Systems 13: 294–300. MIT Press, Cambridge MA. [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, et al. 1999. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia 91: 286–298. [Google Scholar]
- Schoch CL, Crous PW, Witthuhn RC, et al. 2000. Recombination in Calonectria morganii and phylogeny with other heterothallic small-spored Calonectria species. Mycologia 92: 665–673. [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, et al. 2015. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31: 3210–3212. [DOI] [PubMed] [Google Scholar]
- Stanke M, Steinkamp R, Waack S, et al. 2004. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Research 32: W309–W312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27: 1009–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thynne E, McDonald MC, Solomon PS. 2017. Transition from heterothallism to homothallism is hypothesised to have facilitated speciation among emerging Botryosphaeriaceae wheat-pathogens. Fungal Genetics and Biology 109: 36–45. [DOI] [PubMed] [Google Scholar]
- Turgeon BG, Yoder OC. 2000. Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genetics and Biology 31: 1–5. [DOI] [PubMed] [Google Scholar]
- Wilken PM, Steenkamp ET, Wingfield MJ, et al. 2017. Which MAT gene? Pezizomycotina (Ascomycota) mating-type gene nomenclature reconsidered. Fungal Biology Reviews 31: 199–211. [Google Scholar]
- Wilson AM, Godlonton T, Van der Nest MA, et al. 2015a. Unisexual reproduction in Huntiella moniliformis. Fungal Genetics and Biology 8: 1–9. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Wilken PM, Van der Nest MA, et al. 2015b. Homothallism: an umbrella term for describing diverse sexual behaviours. IMA Fungus 6: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield BD, Duong TA, Hammerbacher A, et al. 2016. Draft genome sequences for Ceratocystis fagacearum, C. harringtonii, Grosmannia penicillata, and Huntiella bhutanensis. IMA Fungus 7: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Liu H, Jin Y, et al. 2017. Transcriptomic analysis of Calonectria pseudoreteaudii during various stages of Eucalyptus infection. PLoS One 12: e0169598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama E, Arakawa M, Yamagishi K, et al. 2006. Phylogenetic and structural analyses of the mating-type loci in Clavicipitaceae. FEMS Microbiology Letters 264: 182–191. [DOI] [PubMed] [Google Scholar]
- Yokoyama E, Yamagishi K, Hara A. 2003. Structures of the mating-type loci of Cordyceps takaomontana. Applied and Environmental Microbiology 69: 5019–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Sun WX, Yu MN, et al. 2015. Characterization of mating-type loci in rice false smut fungus Villosiclava virens. FEMS Microbiology Letters 362: fnv014. [DOI] [PubMed] [Google Scholar]
- Yun SH, Arie T, Kaneko I, et al. 2000. Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genetics and Biology 31: 7–20. [DOI] [PubMed] [Google Scholar]
- Yun SH, Berbee ML, Yoder OC, et al. 1999. Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proceedings of the National Academy of Sciences 96: 5592–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Hou R, Ma J, et al. 2013. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS One 8: e66980. [DOI] [PMC free article] [PubMed] [Google Scholar]