Abstract
To investigate the sociological, environmental, and economic impact of hormonally active contraceptives, a series of comprehensive literature surveys were employed. Sociological effects are discussed including abortion, exploitation of women, a weakening of marriage, and an increase in divorce with deleterious effects on children such as child poverty, poorer health, lower educational achievement, suicide risks, drug and alcohol abuse, criminality, and incarceration, among others. The environmental impact is discussed briefly and includes the feminization and trans-gendering of male fish downstream from the effluent of city wastewater treatment plants with declining fish populations. The potential economic impact of most of these side effects is estimated based on epidemiologic data and published estimates of costs of caring for the diseases which are linked to the use of hormonally active contraceptives. Hormonally active contraceptives appear to have a deleterious impact on multiple aspects of women’s health as well as negative economic and environmental impacts. These risks can be avoided through the use of nonhormonal methods and need to be more clearly conveyed to the public.
Summary:
Hormonal contraceptives have wide-ranging effects. The potential economic impact of the medical side effects is estimated. Sociological effects are discussed including abortion, exploitation of women, a weakening of marriage and an increase in divorce with negative effects on children such as child poverty, poorer health, lower educational achievement, suicide risks, drug and alcohol abuse, criminality and incarceration among others. The environmental impact includes hormonal effects on fish with declining fish populations. Women seeking birth control have a right to know about how to avoid these risks by using effective hormone-free methods like Fertility Awareness Methods.
Keywords: Autoimmunity, Cancer, Cardiovascular disease, Contraception, Depression, Economic impact, Epidemiology, Human interaction with the environment, Osteoporosis, Sociology
In Part II, we review the sociological, economic, and environmental impact of the widespread use of hormonal contraceptives. The sociological impacts are derived from the published literature. Similarly, the environmental impacts have been documented in a number of published studies. The economic impacts are calculated from the medical consequences of the use of contraceptives as noted previously in Part I. We used disease incidence and prevalence, and published economic costs for care of these diseases, to calculate the economic impact.
Sociological Impact
The National Health and Social Life Survey (NHSLS; Laumann et al. 1994)1 studied the first thirty years of the contraceptive revolution. The NHSLS studied adults who were aged eighteen to fifty-nine in 1992 in America, hence those who turned eighteen beginning in 1951. Given that the oral contraceptive pill began to be prescribed in 1960 in the United States, the data allowed comparison between adults who turned eighteen during the decade before the advent of the pill and those who came of age during the first thirty-some years of the contraceptive revolution. The NHLS is considered the most advanced study on sexual behavior since Kinsey (Kinsey, Pomeroy, and Martin 1948; Kinsey et al. 1953) and an improvement over his work, because it used widely accepted research methods (including a nationally representative sample) whereas Kinsey did not. It is the most comprehensive study of sexuality ever conducted in America.
The NHLS found a steady decline in the age of first intercourse and an increase in the number of sexual partners and risky sexual behaviors during high school. The percentage of pre-coitarche individuals at the age of nineteen in the 1950s and 1960s was 45 percent of women and 25 percent of men, but by the 1970s and 1980s, it was only 17 percent of women and 15 percent of men (Laumann et al. 1994, 326). From another perspective, among women who came of age in the 1950s and 1960s, and whoever engaged in vaginal intercourse, 45 percent had first intercourse in marriage. That metric was only 5 percent by the 1980s (Laumann et al. 1994, 329).
In contrast, in the first thirty years of the sexual revolution (1960s–1990s), young people were less likely to have sex before the age of eighteen if they had more education, lived with both parents, and were religious (Laumann et al. 1994, 324). After the sexual revolution, fewer children lived with both parents and perhaps fewer were religious, opening the door to potential harms. From about 1960 on, there was general concern about issues of morality, pregnancy, and sexually transmitted disease, and through the 1980s and mid-1990s, the rates of unwed births (Ventura et al. 2003, 1–16), teen pregnancies, and sexually transmitted diseases (Mascola et al. 1983, 25–30; Centers for Disease Control and Prevention [CDC] 1995, 81–4, 1996, 121–25; Nicholle 2005; Aral, Fenton, and Holmes 2007, 257–66) all increased.
Exploitation and violence toward young women are also important aspects. The NHSLS found that when young men first had intercourse, half did so out of curiosity and only a quarter out of affection. These numbers were the reverse for young women (Laumann et al. 1994, 329). (Among those who wanted their first experience of intercourse, other reasons for having first intercourse were physical pleasure—12.2 percent for men and 6.6 percent for women; wedding night—6.9 percent for men and 21.1 percent for women; peer pressure—4.2 percent for men and 3.3 percent for women; under the influence of alcohol or drugs—0.7 percent for men and 0.3 percent for women; and pregnancy—0.5 percent for men and 0.6 percent for women.) Young women often did not want their first intercourse experience. Many went along against their wishes because of affection for their partner or peer pressure, and one in twenty reported being forced (Laumann et al. 1994, 328, 347). Women who ever had coerced sexual experiences had more active and passive oral, anal, and group sex and more sexual partners in their lifetime (Laumann et al. 1994, 339), findings similar to those for women who had ever been sexually coerced as children (Laumann et al. 1994, 344). For males, 8 percent also did not want to have their first intercourse (Laumann et al. 1994, 328) but went along because of peer pressure (Laumann et al. 1994, 330). Rates of child abuse rose in parallel with the rise in the rates of contraception, unwed births, abortion, divorce (Bennett 1993; Orr 1999), and the rates of children not living with two parents (Michael 2005, table 5.2; US Census Bureau 2020, see figure CH-1).
Table 5.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Increased Prevalence of Cervical Cancer.
| Prevalent cases of breast cancer | Ever use of COCs | Cervical cancer ever users | ||
|---|---|---|---|---|
| 3,418,124 | 79.3 percent | 257,524 | ||
| Adjusted estimate of cases if no use of COCs | 204,217 | 1.6 (1.1–2.3) | Relative risk (95 percent confidence interval ) | |
| Excess cases | 76,581 | |||
| Annual cost per patient with cervical cancer | US$13,749 | |||
| Estimated total costs | US$1,052,914,912 | |||
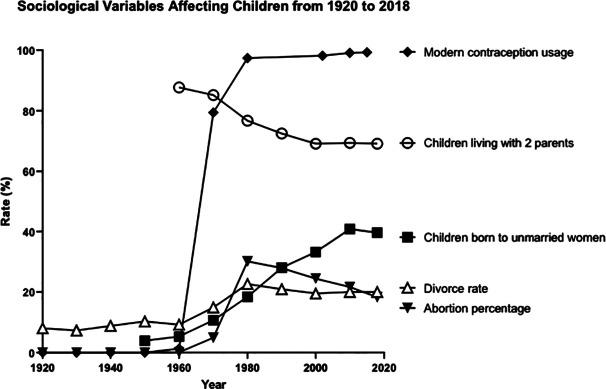
Research also indicates that contraception has increased the rate of abortion (Figure 1). From 1935 to 1965, the percentage of pregnancies ending in induced abortions was essentially zero. Since 1975, the percentage of all pregnancies ending in induced abortion has ranged from 16 to 30 percent (Johnston 2014). The abortion rate was 0.01 percent in 1960–1963 and 0.02 percent in 1964–1965. Even in 1969, it was still below 1 percent. It jumped to 22 percent by 1974, 25 percent in 1975, peaking at 30 percent from 1979 to 1984, then declining to 22 percent in 2005 and 20 percent in 2013. One author (Michael 2005, 94) reported a rate of 22 percent from 1975 to 2000; the current rate is 16 percent. The link between increases in contraception and abortion also appears to exist internationally as noted in reports of Spain (Dueñas et al. 2011, 82–87), Belarus, Russia, and Ukraine (Denisov, Sakevich, and Jasilioniene 2012, e49986). One hypothesis is that abortions are frequently used to deal with failures of contraception. These data appear to support that contention.
Figure 1.
Sociological variables affecting children from 1920 to 2018. The sources for the data are as follows: Children living with two parents: 1960–2000 from Michael (2005, table 5.2) and 1960–2018 from US Census Bureau (2020, CH-1); divorce rate: 1920–1970 from Michael (1988, table A-1), 1950–2000 from Michael (2005, table 5.1), and 2010 and following estimate from Feldhahn (2014); children born to unmarried women: 1950–2000 from Michael (2005, table 5.2) and Martin et al. (2019); abortion rates: all years from Johnston (2014); modern contraception usage: 1920–1974 from Michael (1988, table A-1); and Key Statistics from the National Survey of Family Growth—C Listing available at https://www.cdc.gov/nchs/nsfg/key_statistics/c.htm#everused.
A meta-analysis was performed on the research literature published internationally from 1995 to 2009 evaluating the mental health impact of abortion on women (Coleman 2011, 180–86). The results revealed that “…women who had undergone an abortion experienced an 81% increased risk of mental health problems, and nearly 10% of the incidence of mental health problems was shown to be attributable to abortion.” The author stated,
This review offers the largest quantitative estimate of mental health risks associated with abortion available in the world literature. Calling into question the conclusions from traditional reviews, the results revealed a moderate to highly increased risk of mental health problems after abortion. Consistent with the tenets of evidence-based medicine, this information should inform the delivery of abortion services. (Coleman 2011, 180–86)
Women’s reproductive health has also become compromised. Assisted reproductive technology, along with sexually transmitted diseases and induced abortion, have contributed to the significant national rise in multifetal gestations, preterm birth, lower than the replacement-level birth rate, infertility, miscarriages, ectopic pregnancies, stillbirths, and neonatal deaths (Hilgers 2010, 18–22).
In the 1970s, contraception and abortion led, almost immediately, to the weakening of marriage. With contraception came a historic increase in the divorce rate (Figure 1; Abma et al. 1997, 1–114; W. Mosher and Pratt 1990; Peterson 1995; W. Mosher and Bachrach 1988; Ford 1979; Michael 1988, 2005). Robert Michael, who was one of the researchers in the NHSLS (Laumann et al. 1994), stated, “This contraception variable is strongly positively related to the divorce rate” (Michael 1988, 384). He attributed 50 percent of the rise in the divorce rate directly to the rise of modern contraception (Michael 1988, 385; Michael, 2005, 110).
Michael noted that with the barrier methods before the pill, a woman could expect to have zero to five conceptions over twenty years. The marriage contract necessarily was about procreation and educating children, and men and women entered marriage with that expectation. Using the pill and IUD, however, a woman could expect to have zero to one conception over twenty years, and with the option of abortion, a woman could attain a birth rate of zero. The marriage contract changed midstream for marriages navigating through the contraceptive revolution and modified the potential understanding of marriage thereafter. The meaning of marriage became whatever adults thought would fulfill them. Michael observed that individuals could have affairs or find a new partner who they felt would be more suited to satisfy them under this new contract (Michael 2005, 110–11). Suggesting another way, hormonal contraceptives may have contributed to the rise in divorce, researchers found an effect of contraceptive steroid hormones on mate selection (Wedekind and Penn 2000, 1269–71; Klaus and Cortes 2015, 283–300). The researchers noted that steroid contraceptives skewed the selection of sexual partners from men with differing HLA complex alleles to men with similar HLA complex alleles. This could in turn result in later incompatibility between the partners once the steroid contraceptives had been discontinued.
As the divorce rate climbed, psychology journals published research minimizing the impact of divorce and concluding that children do not need their fathers. For example, see Hertzog and Sudia (1973), reviewed with larger discussion (Popenoe 2009, 60f; Byrd 2010, 102–23). Regnerus (2012) revealed worse child outcomes in all alternative families—usually families minus biological fathers in the home (pp. 252–70). Similar findings are noted in reviews of studies of alternative families (Marks 2012, 735–51; Smock and Greenland 2010, 576–93). Researchers argued that we should not be trying so hard to save marriages. Instead, we should be implementing policies to have more community support for working single mothers. A new family model was evolving that demanded that government, not fathers, take care of mothers and children. This model requires the government to grow to meet their needs and creates more dependents on the state.
After contraception was legalized and divorce laws were reformed in the United States, the long-term results of increased divorce have been revealed. The number of children in poverty increased. One study concluded, “Overall, child poverty would be nearly a third lower today if the traditional two-parent family had not deteriorated between 1960 and 2000” (Rector, Johnson, and Fagan 2002). The authors calculated, however, that if single parents of children living in poverty “were married to spouses with matching demographic characteristics, only 6.7 percent of these children would remain poor” (Rector, Johnson, and Fagan 2002).
Research shows that children do best when raised by their biological parents who remain in a stable marriage (Marks 2012, 735–51; Regnerus 2012, 252–70), not just until the children leave home but until death parts them (Regnerus 2012, 252–70). Compared to the children of single parents, cohabiting biological parents, or stepparents, children raised by their married biological parents do better in many areas such as health, length of life, “suicide risks, drug and alcohol abuse, criminality and incarceration, intergenerational poverty, education and/or labor force contribution, early sexual activity and early childbearing, and divorce rates as adults” (Marks 2012, 735–51). Alternate family configurations continue to grow—for example, families of donor or surrogate parents and same-sex parents. It appears unlikely that these new alternate family types will meet the needs of children as well as the natural family. Additional examples of alternative family structures include families who utilized sperm or egg donors (Marquardt, Glenn, and Clark 2010; Center for Bioethics and Culture Network n.d.), surrogate parents (Lahl et al. 2019; Global Surrogacy 2021; Center for Bioethics and Culture Network n.d.), same-sex parents (American Psychological Association 2015; Schumm 2018; Sutton and Cretella 2019, 101–7), and parents in consensual nonmonogamy relationships characterized as polyamorous, open, swinging, or relationship anarchy (American Psychological Association, Division 44 Consensual Nonmonogamy Task Force n.d.; Kegu and Silverstein 2019).
Modern contraception separated procreation from sex and from relationships. Increasingly, procreation has been separated from marriage (Smock and Greenland 2010, 576). Fewer children are born, and fewer have the dignity, security, and benefits of being born into the stable marriage of their mother and father.
Of the forty-eight states, thirty had laws prohibiting the prescribing and marketing of contraceptives, mostly enacted by Protestant legislators beginning in the nineteenth century. The practice of contraception was still a core aspect of theology that both Protestants (e.g., Lambeth Conference Resolution [1930] 13) and Catholics universally condemned. This condemnation of contraception, as well as abortion and infanticide, was based on continuous Church teachings from the first century forward and on Scripture (Calvin n.d.; Wesley n.d.; Provan 1989; Harrison 1996; Hardon 1981). The first Protestant exception was from the Lambeth Conference 1930 Resolution 15, a watershed for contraception (Public Broadcasting System n.d.; Anglican Consultative Council 2005). The Supreme Court created a constitutional right to privacy and legalized the prescribing and marketing of contraceptives for married couples in 1965 and for the unmarried in 1967 (Public Broadcasting System n.d.), the same doctrine is used in 1973 to legalize abortion.
By 1973, a mere eight years from when the US Supreme Court legalized contraception in 1965, the use of modern contraception (the pill and IUD) became nearly universal. In the same year (see Figure 1), abortion was legalized (abortion rose from essentially 0–25 percent), child abuse and sexual exploitation of adolescent girls increased, there was an increase in out-of-wedlock births, teen pregnancies, and sexually transmitted diseases, the divorce rate nearly doubled, reproduction fell below the replacement rate levels (risking future population demographics), homosexuality was removed from the Diagnostic and Statistical Manual, and the traditional definition of marriage as pair-bonding for the procreation and education of children faded. These were the results of separating sex from its procreative meaning. Marriage, family, and the value of human life have fragmented. Dissenters from these new paradigms are pressured to conform to this new world, including Catholics and other Christians who agree with the Church’s teaching and members of other religions who hold similar beliefs on sex and marriage.
Although abortion rates have declined, currently the induced abortion rate is 16 percent of all pregnancies.2 Of children who are born, about 40 percent are born out of wedlock (Martin et al. 2019)—some to cohabiting parents, and these relationships are significantly less stable than marriages. See research reviews (Marks 2012, 735–51; Smock and Greenland 2010, 576–93; Regnerus 2012, 252–70) showing poorer child outcomes on average for children of cohabiting versus married parents (Marks 2012, 735; Marquardt, Glenn, and Clark 2010) regarding children of married parents versus alternate family structures: “(a) health, mortality, suicide risks, (b) drug and alcohol abuse, (c) criminality and incarceration, (d) intergenerational poverty, (e) education and/or labor force contribution, (f) early sexual activity and early childbearing, and (g) divorce rates as adults.” Also note the percentage of children who experience a separation of their parents: 15 percent by age 1, half by age 5, and two-thirds by age 10 of cohabiting parents versus 4 percent, 15 percent, and 28 percent, respectively, of married parents (Smock and Greenland 2010, 584).
The remaining children are born to their married parents, but about a quarter of first marriages end in divorce. It is difficult to know how many children experience the divorce of their parents before they reach eighteen years of age, as the US government no longer collects divorce statistics and divorce estimates vary (Feldhahn 2014). One optimistic estimate put the divorce rate for first marriages at 20–25 percent and for all marriages at 31 percent (Feldhahn 2014), hence a marriage persistence rate of 69–80 percent. Feldhahn argued that the 50 percent divorce rate has been erroneously repeated based on one researcher’s prediction during the divorce revolution that future divorce rates would rise to 50 percent, but the divorce rate has never actually been 50 percent. This has a negative impact on the children. Thus, the proportion of conceived children who are born, born to their married parents, and raised by their parents continuously to maturity is a minority. Reconnecting procreation to sex, marriage, and family is a matter of justice for children.
Environmental Impact
One area that is infrequently discussed is the environmental impact of the widespread use of hormonal contraceptives. Based on data from the Guttmacher Institute, 11 million women aged fifteen to forty-four in the United States are currently using oral or injectable hormonal contraceptives (Guttmacher Institute 2020). A 2015 study reports that 21 percent of reproductive-age women are using a hormonal contraceptive or about 13 million women (Daniels et al. 2015, 1–14). This has resulted in a significant increase in the release of synthetic progestogens (such as levonorgestrel) and synthetic estrogens (such as ethinyl estradiol [EE2]) into the aquatic environment via treated wastewater effluent (Besse and Garric 2009, 3485–94; King et al. 2016, 1378–85). EE2 undergoes first-pass metabolism in the liver, but ∼6 percent of the administered dose is excreted as nonmodified EE2 in the urine and ∼9 percent in the feces (Stanczyk, Archer, and Bhavnani 2013, 706–27). Even at low concentrations (King et al. 2016, 1378–85), these compounds can act as potent endocrine disruptors, affecting the growth, development, and reproduction of exposed aquatic organisms (Tyler, Jobling, and Sumpter 1998, 319–61; Larsson et al. 1999, 91–97). EE2 is one of the most studied synthetic hormones in aquatic environments, for which assessments of environmental concentrations and the quantification of endocrine-related effects have been documented in a range of aquatic species (Purdom et al. 1994, 275–85; Jobling et al. 1998, 2498–506; Kirby et al. 2004, 748–58; Jobling et al. 2006, 32–39). Numerous studies on aquatic organisms have led to the derivation of a predicted no-effect concentration of 0.1 ng/L for EE2 (Caldwell et al. 2012, 1396–406).
In 1993, the first publication to bring attention to the issue of synthetic chemicals mimicking natural estrogen in the environment appeared (Sharpe and Skakkebaek 1993, 1392–95). The study pointed to environmental pollutants, which were having a deleterious effect on male fetuses in utero—via endocrine disruption. These included polychlorinated biphenyls, detergents, dioxins, and hormonal contraceptives. In 1995, another study (Sumpter 1995, 737–42) noted that male fish in twenty-eight rivers across Britain were being “feminized” by pollutants. In 2002, a study was published that focused specifically on the effects of endocrine-disrupting chemicals in the environment (Jobling et al. 2002, 515–24). They demonstrated reduced fertility in fish populations in areas downstream of effluent from sewage plants located along tributaries of the River Thames. In 2007, the results of a seven-year Canadian lake study were published which examined the effects of EE2 (Kidd et al. 2007, 8897–901). The researchers released a quantity of EE2 equivalent to what would come into the waterways via sewage from a city of 200,000 people. They witnessed an immediate feminization and transgendering of male fish, which resulted in the “near extinction” of the fathead minnow population (Kidd et al. 2007, 8897–901). Although the minnow populations neared extinction, they rebounded as soon as the researchers stopped adding EE2 to the lake. A 2006 study from the US Geological Survey on smallmouth bass in the Shenandoah and Monocacy Rivers found that more than 80 percent of all the male bass living in these waterways were growing eggs in their testes (US Fish and Wildlife Service n.d.).
A study was carried out on fish populations relative to the sewage treatment plants of three major Colorado cities: Denver, Boulder, and Colorado Springs (Woodling et al. 2006, 10–15). At each municipality, they set up a location immediately upstream from where the effluent was released, and another immediately downstream. The upstream fish enjoyed a balanced 1:1 female-to-male sex ratio. Downstream, there were five female fish for every male, and 20 percent of the reduced male population demonstrated intersex characteristics, such as eggs in their testes and the presence of vitellogenin, an egg yolk protein normally found only in fertile females. The consequences also appeared to ascend the food chain in a measurable way, specifically with the feminization of trout, mink frogs, and green frogs (Park and Kidd 2009, 2027–36). Both the predicted and the measured concentrations of EE2 in the United States, including effluent of wastewater treatment plants, surface water, or groundwater, exceed the predicted no-effect concentrations on fish populations (Kostich, Flick, and Martinson 2013, 271–77).
Environmental factors have been implicated in declining fertility rates (Skakkebaek et al. 2016, 55–97). A 2017 study found that sperm counts in human men have dropped by more than 50 percent since 1973 (Levine et al. 2017, 646–59). While it has been noted that environmental exposure to individual steroidal estrogens, as well as their mixtures, is unlikely to dramatically affect endocrine signaling in humans, it is not clear whether more subtle effects are possible (Kostich, Flick, and Martinson 2013, 271–77). More recently, the environmental effects of levonorgestrel have been postulated (King et al. 2016, 1378–85).
There is a clear effect of environmental EE2 on fish populations as well as on higher phylum species such as frogs. An effect on humans is also possible but has not been established. However, a clear economic impact can be deduced.
Patients Affected and Economic Impact
As noted in Part I, the use of hormonal contraceptives is linked to an increased incidence and prevalence of numerous diseases. These disease burdens carry a cost of care that can be calculated. In some cases, we have calculated the estimated number of excess cases and consequent economic impact by taking into account those who are currently using combined oral contraceptives (COCs) or progestin-only contraceptives (POCs), and in other cases those who have ever used COCs or POCs. According to the CDC, 15.9 percent of women (9,699,000) aged fifteen to forty-four in the United States currently take ‘the pill’ (CDC 2020a; Daniels et al. 2015, 1–14). According to the National Survey of Family Growth (CDC, National Survey for Family Growth 2019), 79.3 percent of women, or 94,599,191 women over eighteen (Howden and Meyer 2011), in the United States have ever used the pill. For POCs, the National Survey of Family Growth (CDC, National Survey for Family Growth 2019) notes that between 16.8 percent and 25.4 percent of women aged fifteen to forty-four in 2002–2015 have ever used “three-month injectable (Depo-Provera™).” For a conservative estimate, we use the lowest of these numbers (16.8 percent or 20,041,191 women). This would not include POCs administered by other routes.
Calculation Methods
In some cases, we have calculated the estimated number of excess cases and consequent economic impact by taking into account those who are currently using COCs and those who have ever used COCs. According to the CDC, 15.9 percent of women aged fifteen to forty-four in the United States currently take “the pill” (CDC: Contraceptive Use, 2020a). There are 61 million US women of reproductive age (fifteen to forty-four; Daniels et al. 2015). This yields 9,699,000 women in the United States currently on COCs. Note that this is a low estimate as it does not include women using intravaginal and transdermal formulations and is lower than the estimate by Daniels et al. (2015, 1–14).
According to the National Survey of Family Growth (CDC, National Survey for Family Growth 2019), 79.3 percent of women surveyed from 2011 to 2015 have ever used “the pill,” slightly lower than the 2006–2010 survey, and 82.3 percent in the 2002 survey. The number for “ever use” of 79.3 percent is used in subsequent calculations. According to the 2010 census (Howden and Meyer 2011), there were 156,964,212 women in the United States, of whom 24 percent were under eighteen years of age. Thus, there were 119,292,801 women aged eighteen years or older. This implies that 94,599,191 women in the United States have ever used the pill. As noted above, this does not include women using intravaginal and transdermal formulations. This will be a low estimate as there are women younger than eighteen who have used COCs or POCs.
The numbers 9,699,000 for current use and 94,599,191 for “ever use” of COCs were utilized in some of these calculations. In other calculations, the census data for specific age groups were used if they were the groups most likely to be impacted by the current or recent use of COCs.
For POCs, the National Survey of Family Growth (CDC, National Survey for Family Growth 2019) notes that 25.4 percent of women aged fifteen to forty-four in 2011–2015 have ever used “three-month injectable (Depo-Provera™).” This is up from 23.2 percent in 2006–2010 and 16.8 percent in 2002. For a conservative estimate, we will use the lowest of these numbers (16.8 percent or 20,041,191 women). This would not include POCs administered by other routes.
For some of these diseases and conditions, data were available regarding incidence (number of new cases in a year) and prevalence (number of women with these diseases/conditions in the population). When possible, both incident cases and costs (excess cases and costs incurred by new COC-/POC-related cases each year) and prevalent costs (excess cases and costs incurred by all COC-/POC-related cases in the population) were calculated. The number of incident cases per year is shown in Figure 2A, while the number of prevalent cases in the population is shown in Figure 2B for selected diseases where these could be calculated.
Figure 2.
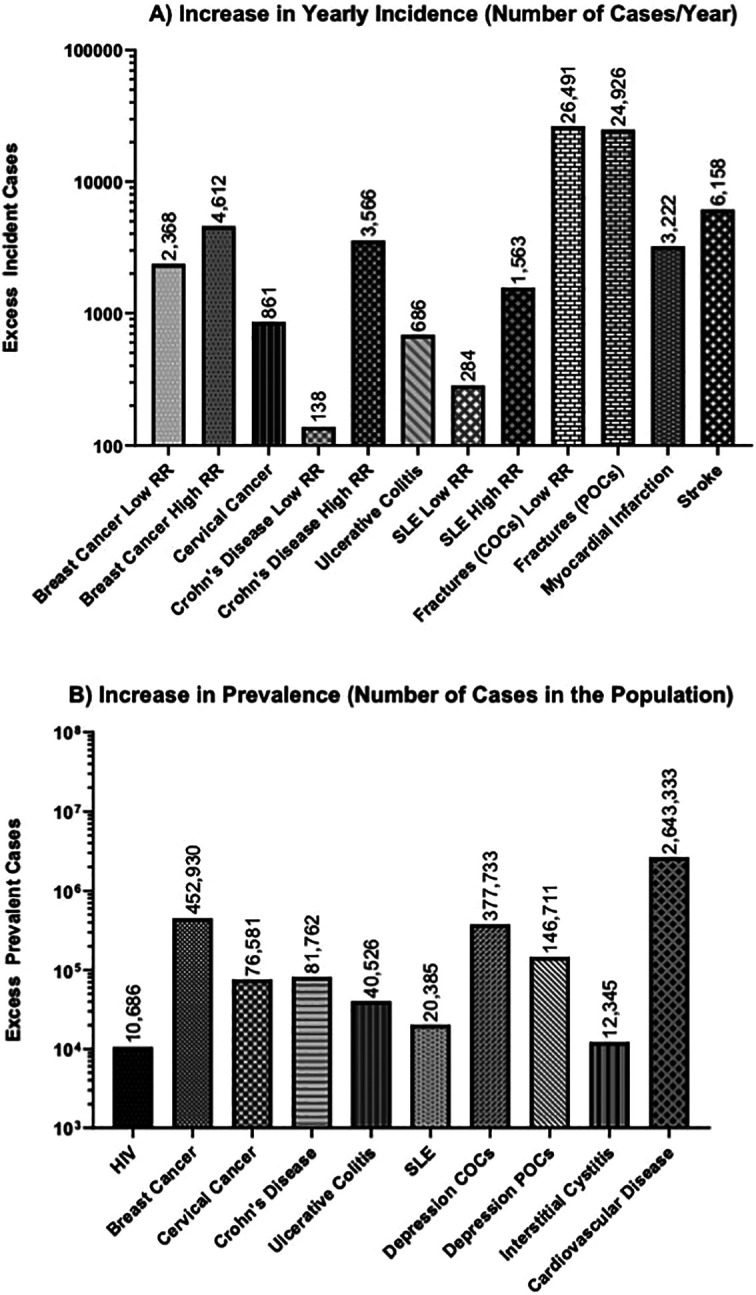
Estimated increases in the yearly incidence (A) and prevalence (B) due to contraceptive use in the diseases noted. The data are derived from Tables 1–19. Where different estimates were noted, the lowest relative risk (RR) from the lowest reported disease incidence (low RR) and the highest RR from the highest reported disease incidence (high RR) are shown. Note that the y-axis for both graphs is on the log scale. Estimated numbers are shown above each bar.
HIV
According to the CDC (2020c), an estimated 255,900 women were living with HIV at the end of 2014. Of these, it is estimated 87 percent were via sexual contact (this proportion was relatively stable from 2014 to 2018; CDC 2020b, Table 1). Annual medical cost estimates for HIV-infected persons, adjusted for age, sex, race/ethnicity, and transmission risk group were from the HIV Research Network (range US$1,854–US$4,545/month) and for HIV-uninfected persons were from the Medical Expenditure Panel Survey (range US$73–US$628/month; Schackman et al. 2015, 293–301). Using this information along with the prevalence of depot medroxyprogesterone acetate (DMPA) use of 16.8 percent suggests an annual cost of treatment for HIV infection due to DMPA use of ∼US$157–US$573 million (Table 1).
Table 1.
Estimated Economic Impact of DMPA due to Increased Prevalence of HIV Infection.
| Women with HIV | 255,900 |
| Sexual transmission | 87 percent |
| Cases due to sexual transmission | 222,633 |
| Ever use of DMPA | 16.80 percent |
| Women with HIV with DMPA use | 37,402 |
| Relative risk (95 percent confidence interval) of HIV with DMPA use | 1.4 (1.16–1.69) |
| Adjusted estimate | 26,716 |
| Excess cases | 10,686 |
| Highest estimated individual annual costs | US$53,664 |
| Lowest estimated individual annual costs | US$14,712 |
| Highest estimated total annual costs | US$573,474,111 |
| Lowest estimated total annual costs | US$157,218,081 |
Breast Cancer
A recent study in the United States (Blumen, Fitch, and Polkus 2016, 23–32) notes that the average costs per patient allowed by the insurance company in the year after diagnosis were between US$60,637 and US$134,682 for disease stage 0, I/II, III, and IV, while the average costs allowed per patient in the twenty-four months after the index diagnosis similarly ranged from US$71,909 to US$182,655. For all patients, they note that the average cost for the first twelve months following diagnosis is US$85,772, and for the second twelve months is US$22,127 with a total of US$103,735 for the twenty-four months following diagnosis. For these calculations, we use the first-year costs to estimate costs for incident (new) cases among current users of COCs and the second-year cost to approximate the average annual cost of care for all patients diagnosed with breast cancer. According to the National Institutes of Health (NIH) Surveillance, Epidemiology, and End Results Program statistics (SEER n.d.b), the incidence of breast cancer is 128.5 per 100,000 women per year. Approximately 12.9 percent of women will be diagnosed with female breast cancer at some point during their lifetime. According to epidemiology studies (Mørch et al. 2017, 2228–39; Heikkinen et al. 2016, 249–58; Lund et al. 2007, 645–48), and a meta-analysis (Kahlenborn et al. 2006, 1290–302), the relative risk (RR) of ever use of COCs for the development of breast cancer is 1.19–1.37. Based on this information, the estimated increase in cost from the use of COCs due to incident (new) cases of breast cancer is between US$203 million and US$395 million each year (Table 2).
Table 2.
Estimated Economic Impact of Combined Oral Contraceptives due to Increased Incidence of Breast Cancer.
| Women of reproductive age | Number on the pill | Incidence | ||
|---|---|---|---|---|
| 61,000,000 | 9,699,000 | 0.001285 | ||
| Estimated women on the pill at risk | 12,463 | |||
| Adjusted estimate of cases | 14,831 | 1.19 (1.14–1.26) | Low RR (95 percent CI) | |
| Adjusted estimate of cases | 17,075 | 1.37 (1.12–1.68) | High RR (95 percent CI) | |
| Excess cases | 2,368 | Low RR | ||
| Excess cases | 4,612 | High RR | ||
| Annual cost per patient with breast cancer | US$85,772 | |||
| Estimated annual costs | US$203,108,096 | Low RR | ||
| Estimated annual costs | US$395,580,464 | High RR | ||
Note: CI = confidence interval; RR = relative risk.
To evaluate the impact of “ever use” of COCs on prevalent breast cancer, we noted that the best meta-analysis (Kahlenborn et al. 2006, 1290–302) showed a 1.19 odds ratio (OR) of breast cancer with COCs. According to the SEER statistics, there are currently 3,577,264 prevalent cases of breast cancer in the United States. The estimated increase in cost from the treatment of excess cases of breast cancer is estimated to be US$10 billion annually (Table 3).
Table 3.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Increased Prevalence of Breast Cancer.
| Prevalent cases of breast cancer | Ever use of COCs | Breast cancer ever users | ||
|---|---|---|---|---|
| 3,577,264 | 79.3 percent | 2,836,770 | ||
| Adjusted estimate of cases if no use of COCs | 2,383,841 | 1.19 (1.14–1.26) | Relative risk (95 percent confidence interval) | |
| Excess cases | 452,930 | |||
| Annual cost per patient with breast cancer | US$22,127 | |||
| Estimated total costs | US$10,021,975,916 | |||
Cervical Cancer
A recent study (Pendrith et al. 2016, e109–15) on the cost of invasive cervical cancer treatment noted that the mean overall medical care cost was US$39,187 in the first year after diagnosis. At five years after diagnosis, the mean overall unadjusted cost was US$68,745. For these calculations, we will assume a cost of US$39,187 annually for incident cases and US$13,749 (= US$68,745/5) annually for prevalent cases of invasive cervical cancer. According to the NIH SEER statistics (SEER n.d.a), the incidence of invasive cervical cancer is 7.4 per 100,000 person-years. According to the American Cancer Society: Cervical Cancer (n.d.), it is estimated that 13,170 women will be diagnosed with invasive cervical cancer in the United States in 2019. In 2015, there were an estimated 257,524 women living with invasive cervical cancer in the United States. According to one epidemiology study (Roura et al. 2016, e0147029), the RR of ever use of COCs for the development of invasive cervical cancer is 1.6 (95 percent CI 1.1–2.3) and the RR for current use is 2.2 (95 percent CI 1.3–4.0). Based on this information, the estimated increase in cost from the use of COCs due to incident (new) cases of cervical cancer is ∼US$33 million each year (Table 4).
Table 4.
Estimated Economic Impact of Combined Oral Contraceptives due to Increased Incidence of Cervical Cancer.
| Women of reproductive age | Number on the pill | Incidence | ||
|---|---|---|---|---|
| 61,000,000 | 9,699,000 | 0.000074 | ||
| Estimated women on the pill at risk | 718 | |||
| Adjusted estimate of cases | 1,579 | 2.2 (1.3–4.0) | Relative risk (95 percent confidence interval) | |
| Excess cases | 861 | |||
| Annual cost per patient with cervical cancer | US$39,187 | |||
| Estimated annual costs | US$33,750,635 | |||
To evaluate the impact of “ever use” of COCs on prevalent cervical cancer, we noted that the best study (Roura et al. 2016, e0147029) showed a RR of 1.6 for cervical cancer with COCs. According to the NIH SEER statistics, there are currently 257,524 cases of cervical cancer prevalent in the United States. The estimated increase in cost from the treatment of the excess cases of cervical cancer is estimated to be ∼US$1 billion annually (Table 5).
Crohn’s Disease (CD)
A US study (Rao et al. 2017, 107–15) estimated the five-year cost of the treatment of CD as US$116,838 per patient (annual cost US$23,368). This was higher with worsening disease activity. The incidence of CD is 3.1–20.2 cases per 100,000 person-years (Gajendran et al. 2018, 20–57). According to epidemiology studies (Khalili et al. 2013, 1153–59; García Rodríguez et al. 2005, 309–15), and meta-analysis (Cornish et al. 2008, 2394–400), the RR of current COC use is 1.46–2.82 for the development of CD. Based on this information, the estimated increase in cost just from treatment of the new (incident) excess cases of CD, only looking at current use and not past use of COCs, is between US$3 million and US$83 million annually (Table 6).
Table 6.
Estimated Economic Impact of Combined Oral Contraceptives due to Increased Incidence of Crohn’s Disease.
| Women of reproductive age | Number on the pill | Low incidence | High incidence | ||
|---|---|---|---|---|---|
| 61,000,000 | 9,699,000 | 0.000031 | 0.000202 | ||
| Estimated women on the pill at risk | 301 | 1,959 | |||
| Adjusted estimate | 439 | 2,860 | 1.46 (1.26–1.70) | Low RR (95 percent CI) | |
| Adjusted estimate | 848 | 5,525 | 2.82 (1.65–4.82) | High RR (95 percent CI) | |
| Excess cases | 138 | 901 | Low RR | ||
| Excess cases | 547 | 3,566 | High RR | ||
| Annual cost per patient with Crohn’s disease | US$23,368 | ||||
| Estimated annual costs | US$3,231,920 | US$21,059,968 | Low RR | ||
| Estimated annual costs | US$12,787,162 | US$83,324,221 | High RR | ||
Note: CI = confidence interval; RR = relative risk.
To evaluate the impact of “ever use” of COCs, we noted that the cohort study (Khalili et al. 2013, 1153–59) and meta-analysis (Cornish et al. 2008, 2394–400) showed a 1.43 and 1.44 RR of CD, respectively. The prevalence of CD in adults is 201 cases per 100,000 person-years (Dahlhamer et al. 2016, 1166–69). Taking the lower number of 1.43, the estimated increase in cost from the treatment of the excess cases of CD due to COC use is approximately US$1.9 billion annually (Table 7).
Table 7.
Estimated Economic Impact of COCs due to Increased Prevalence of Crohn’s Disease.
| Women ≥18 in 2010 Census | Ever use of COCs | Prevalence | ||
|---|---|---|---|---|
| 119,292,801 | 94,599,191 | 0.000201 | ||
| Estimated women on the pill at risk | 190,144 | |||
| Adjusted estimate | 271,906 | 1.44 (1.12–1.86) | RR (95 percent CI) | |
| Excess cases | 81,762 | 1.44 (1.12–1.86) | RR (95 percent CI) | |
| Annual cost per patient with Crohn’s disease | US$23,368 | |||
| Estimated total costs | US$1,910,583,605 | 1.44 (1.12–1.86) | RR (95 percent CI) | |
Note: COCs = combined oral contraceptives; CI = confidence interval; RR = relative risk.
Ulcerative Colitis (UC)
A recent study in the United States (Cohen et al. 2015, 447–56) noted that compared with controls, patients with UC had higher adjusted total direct (US$15,548 vs. US$4,812) and indirect costs (US$4,125 vs. US$1,961) annually. This implies a total annual increase in the cost of ∼US$12,900 for UC. The incidence of UC is estimated as 12.2 cases per 100,000 person-years (Shivashankar et al. 2017, 857–63). According to epidemiology studies noted (Khalili et al. 2013, 1153–59; García Rodríguez et al. 2005, 309–15), and meta-analysis (Cornish et al. 2008, 2394–400), the RR of current COC use is 1.22–1.58 for the development of UC. Based on this information, the estimated increase in cost just from the treatment of excess incident cases of UC, only looking at current use and not past use of COCs, is between US$3.3 and US$8.9 million per year (Table 8).
Table 8.
Estimated Economic Impact of Combined Oral Contraceptives due to Increased Incidence of Ulcerative Colitis.
| Women of reproductive age | Number on the pill | Incidence | ||
|---|---|---|---|---|
| 61,000,000 | 9,699,000 | 0.000122 | ||
| Estimated women on the pill at risk | 1,183 | |||
| Adjusted estimate | 1,444 | 1.22 (0.74–2.07) | Low RR (95 percent CI) | |
| Adjusted estimate | 1,870 | 1.58 (0.71–3.52) | High RR (95 percent CI) | |
| Excess cases | 260 | Low RR | ||
| Excess cases | 686 | High RR | ||
| Annual cost per patient with ulcerative colitis | US$12,900 | |||
| Estimated annual costs | US$3,358,143 | Low RR | ||
| Estimated annual costs | US$8,853,286 | High RR | ||
Note: CI = confidence interval; RR = relative risk.
To evaluate the impact of “ever use” of COCs, we noted that the cohort study (Khalili et al. 2013, 1153–59) showed a 1.18 RR of UC. The prevalence of UC in adults in the United States in one large study was estimated as 238 per 100,000 population (Kappelman et al. 2007, 1424–29). The estimated increase in the cost of the excess cases of UC due to the use of COCs is US$522 million annually (Table 9).
Table 9.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Increased Prevalence of Ulcerative Colitis.
| Women ≥18 in 2010 census | Ever use of COCs | Prevalence | ||
|---|---|---|---|---|
| 119,292,801 | 94,599,191 | 0.000238 | ||
| Estimated women on the pill at risk | 225,146 | |||
| Adjusted estimate | 265,672 | 1.18 (0.92–1.52) | RR (95 percent CI) | |
| Excess cases | 40,526 | 1.18 (0.92–1.52) | RR (95 percent CI) | |
| Annual cost per patient with ulcerative colitis | US$12,900 | |||
| Estimated total costs | US$522,789,187 | 1.18 (0.92–1.52) | RR (95 percent CI) | |
Note: CI = confidence interval; RR = relative risk.
Systemic Lupus Erythematosus (SLE)
A recent study in the United States (S. Y. Chen et al. 2015, 180–86) noted that mean total healthcare costs were US$21,535 among all SLE patients over the one-year study period. According to the CDC, the incidence of SLE is 6.5–10.6 cases per 100,000 women-years (CDC 2018). In terms of prevalence, “A conservative estimate suggests a prevalence of 161,000 with definite SLE and 322,000 with definite or probable SLE.” According to the epidemiology studies that evaluated the current use of COCs (Bernier et al. 2009, 476–81), the RR of current COC use is 1.45–2.52 for the development of SLE. Based on this information, the estimated increase in cost just from treatment of the new (incident) excess cases of SLE, only looking at current use and not past use of COCs, is US$6.1 million to US$33.6 million annually (Table 10).
Table 10.
Estimated Economic Impact of Combined Oral Contraceptives due to Increased Incidence of Systemic Lupus Erythematosus.
| Women of reproductive age | Number on the pill | Low incidence | High incidence | ||
|---|---|---|---|---|---|
| 61,000,000 | 9,699,000 | 0.000065 | 0.0001065 | ||
| Estimated women on the pill at risk | 630 | 1,028 | |||
| Adjusted estimate | 914 | 1,491 | 1.45 | Low RR | |
| Adjusted estimate | 1,589 | 2,591 | 2.52 | High RR | |
| Excess cases | 284 | 463 | Low RR | ||
| Excess cases | 958 | 1,563 | High RR | ||
| Annual cost per patient with systemic lupus erythematosus | US$21,535 | ||||
| Estimated annual costs | US$6,109,388 | US$9,963,002 | Low RR | ||
| Estimated annual costs | US$20,636,155 | US$33,652,807 | High RR | ||
Note: RR = relative risk.
To evaluate the impact of “ever use” of COCs, we noted that the cohort studies (Costenbader et al. 2007, 1251–62; Bernier et al. 2009, 476–81) showed a 1.19–2.3 RR of SLE. The estimated increase in the cost of the excess cases of SLE due to the use of COCs is US$439 million–US$1.55 billion annually (Table 11).
Table 11.
Estimated Economic Impact of COCs due to Increased Prevalence of Systemic Lupus Erythematosus.
| Women ≥18 in 2010 census | Ever use of COCs | Prevalence | ||
|---|---|---|---|---|
| 119,292,801 | 94,599,191 | 161,000 | ||
| Estimated women on the pill at risk | 127,673 | |||
| Adjusted estimate | 107,288 | 1.19 (0.98–1.45) | Low RR (95 percent CI) | |
| Adjusted estimate | 55,510 | 2.3 (1.0–5.0) | High RR (95 percent CI) | |
| Excess cases | 20,385 | 1.19 (0.98–1.45) | Low RR (95 percent CI) | |
| Excess cases | 72,163 | 2.3 (1.0–5.0) | High RR (95 percent CI) | |
| Annual cost per patient with systemic lupus erythematosus | US$21,535 | |||
| Estimated total costs | US$438,985,908 | 1.19 (0.98–1.45) | Low RR (95 percent CI) | |
| Estimated total costs | US$1,554,030,205 | 2.3 (1.0–5.0) | High RR (95 percent CI) | |
Note: COCs = combined oral contraceptives; CI = confidence interval; RR = relative risk.
Multiple Sclerosis (MS)
As the most rigorous cohort studies did not show an increase in the risk of developing MS, a rigorous cost analysis was not performed. However, using the information from a case-control study (Hellwig et al. 2016, e0149094), an increased OR of 1.51 was noted. If this is accurate, this can be used along with a study of total MS costs from 1997 to 2013 (A. Y. Chen, Chonghasawat, and Leadholm 2017, 180–86). They noted that, “The total charges on managing MS range from US$161 million in 1997 to US$755 million in 2013.” Conservatively assuming constant costs since 2013, noting a female to male ratio of 3:1 in MS (National Multiple Sclerosis Society n.d.), we can calculate that 79.3 percent of the costs in women were incurred by “ever users” of COCs. This yields US$449 million. If these women had not used COCs, there would have been a proportionate reduction in costs of US$151 million annually.
Interstitial Cystitis (IC)
According to one recent paper (Tung et al. 2017, 474–82), having IC was associated with an average US$7,223 higher total healthcare costs annually compared with not having IC. The prevalence of IC has been estimated at 2.7 percent using a high specificity definition (McLennan 2014, 385–95), while another study in a managed care population (Clemens et al. 2005, 98–102) indicated a prevalence between 45 percent and 197 percent per 100,000 women, depending on the definition of IC used. Using the most conservative estimate (Champaneria, D’Andrea, and Latthe 2016, 709–22), “ever use” of COCs is associated with an OR of 2.31 for IC. Assuming 61 million women of reproductive age, with a 79.3 percent of exposure to COCs, this suggests ∼11,500 excess cases to ∼50,500. This yields an annual cost of US$83–US$365 million (Table 12) for excess cases of IC for COCs.
Table 12.
Estimated Annual Economic Impact of Combined Oral Contraceptives due to Increased Prevalence of Interstitial Cystitis.
| Low prevalence of interstitial cystitis | 0.00045 |
| High prevalence of interstitial cystitis | 0.00197 |
| Women of reproductive age | 61,000,000 |
| Number with ever use of the pill | 48,373,000 |
| Number of women with interstitial cystitis—low prevalence | 21,768 |
| Number of women with interstitial cystitis—high prevalence | 95,295 |
| Odds ratio (95 percent confidence interval) | 2.31 (1.03–5.16) |
| Excess cases of interstitial cystitis—low prevalence | 12,345 |
| Excess cases of interstitial cystitis—high prevalence | 54,042 |
| Annual cost | US$7,223 |
| Annual cost of interstitial cystitis—low prevalence | US$89,165,215 |
| Annual cost of interstitial cystitis—high prevalence | US$390,343,584 |
Osteoporotic Bone Fracture Risk
According to a recent review (Ballane et al. 2017, 1531–42), in North America, the incidence of osteoporotic vertebral fractures, on average, is 960 per 100,000 per year. The annual excess cost of care for women with osteoporotic vertebral fractures was estimated to be US$11,655 per patient (Kilgore et al. 2009, 2050–55). Using the most relevant RR of 1.07 (Barad et al. 2005, 374–83), this implies an annual cost of ∼US$308 million in the United States from COC use (Table 13).
Table 13.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Increased Annual Incidence of Vertebral Fractures.
| Women ≥50 in 2010 census | Ever use of COCs | Incidence of osteoporotic vertebral fractures | ||
|---|---|---|---|---|
| 53,151,456 | 42,149,105 | 0.0096 | ||
| Estimated women on the pill with Fractures | 404,631 | |||
| Adjusted estimate | 378,160 | 1.07 (1.01–1.15) | Relative risk (95 percent confidence interval) | |
| Excess cases | 26,471 | |||
| Annual cost per patient with osteoporotic vertebral fractures | US$11,655 | |||
| Estimated total annual costs | US$308,521,992 | |||
The best cohort study on fracture risk with POCs showed a RR of 1.51 for ever use of DMPA (Lanza et al. 2013, 593–600), the most widely used POC. Assuming 16.8 percent of women have used POCs, this yields an annual cost of ∼US$290 million in the United States from POC use (Table 14).
Table 14.
Estimated Economic Impact of Progesterone-Only Contraceptives (POCs) due to Increased Annual Incidence of Vertebral Fractures.
| Women ≥50 in 2010 census | Ever use of POCs | Incidence of osteoporotic vertebral fractures | ||
|---|---|---|---|---|
| 53,151,456 | 8,929,445 | 0.0096 | ||
| Estimated women on the pill with Fractures | 85,723 | |||
| Adjusted estimate | 60,796 | 1.41 (1.35–1.47) | Relative risk (95 percent confidence interval) | |
| Excess cases | 24,926 | |||
| Annual cost per patient with osteoporotic vertebral fractures | US$11,655 | |||
| Estimated total annual costs | US$290,517,770 | |||
Venous Thromboembolism, Atherosclerosis, and Cardiovascular Disease
About one in every four female deaths is due to heart disease; it is the leading cause of death for women in the United States (CDC 2020e). A review of recent population studies revealed that the overall prevalence of peripheral arterial disease for women is 15.6 percent (compared to 13.4 percent for men; https://www.medscape.org/viewarticle/711179_2). In 2008, coronary heart disease was prevalent in 7.5 million women (https://www.healthline.com/health/heart-disease/women-statistics-facts#1). The total mean direct medical costs for cardiovascular disease is US$18,953 annually (Nichols et al. 2010, e86–93). Using the median RR of the most popular birth control brands, the RR is 1.8 (Table 15) which yields an increased annual cost of over US$50 billion.
Table 15.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Increased Incidence of Cardiovascular Disease.
| Coronary heart disease | Ever use of COCs | Coronary Heart Disease in ever users | ||
|---|---|---|---|---|
| 7,500,000 | 79.3 percent | 5,947,500 | ||
| Adjusted estimate of cases if no use of COCs | 3,304,167 | 1.8a | Relative risk | |
| Excess cases | 2,643,333 | |||
| Annual cost per patient for cardiovascular disease | US$18,953 | |||
| Estimated total costs | US$50,099,090,349 | |||
a This is the median of the most popular birth control brands.
A more conservative estimate would assume that the increased risk for cardiovascular disease is limited to women aged fifteen to forty-nine years, which was the group studied by Lidegaard et al. (2012, e2990). According to the US Census in 2010, the population is broken down by age-group (Howden and Meyer 2011). The rate of cardiovascular events is similarly broken down by Lidegaard et al. (2012, e2990). Thus, the number of cases by age-group is shown in Table 16.
Table 16.
Cardiovascular Events in Women by Age-Group.
| Census Data | Events per 100,000 Person-Years (Lidegaard et al. 2012) | Events per Year | |||
|---|---|---|---|---|---|
| Age-Group | Number of Women | Myocardial Infarction | Stroke | Myocardial Infarction # | Stroke # |
| Fifteen to nineteen years | 10,736,677 | 0.4 | 3.4 | 43 | 365 |
| Twenty to twenty-four years | 10,571,823 | 0.7 | 5.6 | 74 | 592 |
| Twenty-five to twenty-nine years | 10,466,258 | 2.2 | 10.5 | 230 | 1,099 |
| Thirty to thirty-four years | 9,965,599 | 5 | 15.4 | 498 | 1,535 |
| Thirty-five to thirty-nine years | 10,137,620 | 12.2 | 23.3 | 1,237 | 2,362 |
| Forty to forty-four years | 10,496,987 | 25.4 | 39.2 | 2,666 | 4,115 |
| Forty-five to forty-nine years | 11,499,506 | 38.2 | 64.4 | 4,393 | 7,406 |
| Total number of events per year | 9,141 | 17,473 | |||
Using these estimates, with the annual cost of care for cardiovascular disease and the RR noted above, this calculates to ∼US$61 million in excess costs for myocardial infarctions and ∼US$117 million in excess costs for strokes (Table 17).
Table 17.
Cost of Cardiovascular Events in Women Attributable to Combined Oral Contraceptive (COC) Use.
| Myocardial Infarction | Stroke | |||
|---|---|---|---|---|
| Total events per year | 9,141 | 17,473 | ||
| Ever use of the pill | 79.30 percent | |||
| Number of events on COCs | 7,249 | 13,856 | 1.8a | Relative risk ever use |
| Adjusted estimate | 4,027 | 7,697.96 | ||
| Excess cases | 3,222 | 6,158 | ||
| Estimated annual costs | US$18,953 | US$18,953 | ||
| Estimated excess annual costs | US$61,062,935 | US$116,719,504 | ||
a This is the median of the most popular birth control brands.
Depression
A study by Skovlund et al. (2016, 1154–62) indicated a 1.1 RR for depression with COCs and a 1.2 RR with POCs. This study evaluated women aged fifteen to thirty-four and then followed them for a mean of five years. According to Brody, Pratt, and Hughes (2018, 1–8), the prevalence of depression in women aged twenty to thirty-nine is 10.1 percent. An analysis of medical claims conducted by the insurer Blue Cross Blue Shield (2018) found that “in 2016, Blue Cross plans spent US$10,673 on those diagnosed with ‘major depression’ compared to US$4,283 on those without a depression diagnosis.” With this information, noting from the US Census data (Howden and Meyer 2011) that there are 52 million women aged fifteen to thirty-nine, we calculate that the excess annual cost of depression attributable to COCs is US$2.4 billion (Table 18) and from POCs is US$937 million (Table 19).
Table 18.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Increased Prevalence of Depression.
| Women aged fifteen to thirty-nine | 51,877,977 |
| Percent with depression | 10.1 percent |
| Women aged fifteen to thirty-nine with depression | 5,239,675.68 |
| Ever use of COCs | 79.30 percent |
| Fifteen to thirty-nine years old COC users with depression | 4,155,063 |
| Relative risk (95 percent confidence interval) of depression with COC use | 1.1 (1.08–1.14) |
| Adjusted estimate | 3,777,330 |
| Excess cases | 377,733 |
| Estimated individual annual costs | US$6,390 |
| Estimated total annual costs | US$2,413,713,761 |
Table 19.
Estimated Economic Impact of Progesterone-Only Contraceptives (POCs) due to Increased Prevalence of Depression.
| Women aged fifteen to thirty-nine | 51,877,977 |
| Percent with depression | 10.1 percent |
| Women aged fifteen to thirty-nine with depression | 5,239,675.68 |
| Ever use of POCs | 16.80 percent |
| Fifteen to thirty-nine years old COC users with depression | 880,266 |
| Relative risk (95 percent confidence interval) of depression with POC use | 1.2 (1.08–1.14) |
| Adjusted estimate | 733,555 |
| Excess cases | 146,711 |
| Estimated individual annual costs | US$6,390 |
| Estimated total annual costs | US$937,482,772 |
Diseases with Decreased Prevalence on COCs
Hyperthyroidism
COCs have been associated with a decreased prevalence of hyperthyroidism but not hypothyroidism (Williams 2017, 275–95). Three studies evaluated the RR for the development of hyperthyroidism in women taking COCs (Vestergaard et al. 2002, 69–75; Strieder et al. 2003, 396–401; Frank and Kay 1978). Of these, one cohort study did not report statistical information (Frank and Kay 1978, 1531) but noted a RR of 0.71 for current use and 0.9 for “ever use” of COCs. One study included both COCs and hormone replacement therapy and did not separate these out (Strieder et al. 2003, 396–401). One case–control study (Vestergaard et al. 2002, 69–75) reported an OR of 0.68 (95 percent CI 0.49–0.93) for ever use of COCs. Treatment for hyperthyroidism includes radioiodine therapy, with an estimated cost of €1,856 or US$2,264 (Kahaly and Dietlein 2002, 909–14). Since this is typically a one-time therapy, the costs are typically incurred at the time of diagnosis (incident cases). The incidence of hyperthyroidism has been estimated as 4.6 per 1,000 women over twelve years or an incidence of 0.383 per 1,000 women per year. As noted in Table 20, this results in a decrease in 1,748 cases per year and a cost savings of −US$3,956,680 in the United States.
Table 20.
Estimated Economic Impact of Combined Oral Contraceptives due to Decreased Incidence of Hyperthyroidism.
| Women of reproductive age | Number on the pill | Incidence |
|---|---|---|
| 61,000,000 | 9,699,000 | 0.000383 |
| Estimated women on the pill at risk | 3715 | |
| Relative risk (95 percent confidence interval) | 0.68 (0.49–0.93) | |
| Adjusted estimate | 5463 | |
| Decrease in cases | −1,748 | |
| Annual cost per patient | US$2,264 | |
| Estimated annual cost reduction | −US$3,956,680 | |
Uterine cancer
The RR and OR for uterine cancer have been estimated to decrease on COCs (Williams et al. 2018) with an overall OR of 0.76 over five years of use (Hüsing et al. 2016, 51–60) or 0.95 per year of use (Collaborative Group on Epidemiological Studies on Endometrial Cancer 2015, 1061–70). The incidence of uterine cancer is estimated as 27.8 cases per 100,000 women per year with an estimated 793,846 women living with uterine cancer in the United States (estimated prevalence of 6.655 per 1,000 women; SEER n.d.d.). The costs associated with uterine cancer (all stages) have been estimated as £9,197 for the first two years after diagnosis (US$12,416) or US$6,208 per year (Pennington et al. 2016, e0165539). The total costs at five years were estimated as a mean of £11,705 (US$15,801.75) for annual costs of US$3,160.35. The overall decrease in incident costs is estimated as −US$5,285,295, while the decrease in prevalent costs is estimated as −US$628,302,197 (Tables 21 and 22).
Table 21.
Estimated Economic Impact of Combined Oral Contraceptives due to Decreased Incidence of Uterine Cancer.
| Women of reproductive age | Number on the pill | Incidence |
|---|---|---|
| 61,000,000 | 9,699,000 | 0.000278 |
| Estimated women on the pill at risk | 2,696 | |
| Relative risk per year of use (95 percent confidence interval) | 0.95 (0.93–0.96) | |
| Adjusted estimate | 2,838 | |
| Decrease in cases | −142 | |
| Annual cost per patient | US$6,208 | |
| Estimated annual cost reduction | −US$5,285,295 | |
Table 22.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Decreased Prevalence of Uterine Cancer.
| Women ≥18 in 2010 census | Ever use of COCs | Prevalence | ||
|---|---|---|---|---|
| 119,292,801 | 94,599,191 | 0.006655 | ||
| Estimated women on the pill at risk | 629,558 | |||
| Adjusted estimate | 828,366 | 0.76 (0.73–0.78) | Relative risk | |
| Decrease in cases | −198,808 | |||
| Annual cost per patient with uterine cancer | US$3,160.35 | |||
| Estimated total costs | −US$628,302,197 | |||
Ovarian cancer
The cost of care for ovarian cancer in the initial year after diagnosis is estimated at US$93,632 (Bercow et al. 2017, 1269–75). The average annual cost of care for ovarian cancer can be calculated from the total cost in the United States (National Cancer Institute 2020), which in 2018 was US$5,862,600,000, and the total number of cases in the United States estimated as 225,000 (SEER n.d.c), yielding US$26,056 annually per patient. Based on the most reliable studies, the RR for “ever use” of COCs for ovarian cancer is 0.84–0.86 (Tsilidis et al. 2011, 1436–42; Fortner et al. 2015, 1196–208). This indicates a reduction in 31,487 cases and an annual savings of −US$820,419,141 (Table 23).
Table 23.
Estimated Economic Impact of Combined Oral Contraceptives (COCs) due to Decreased Prevalence of Ovarian Cancer.
| Ovarian cancer | Ever use of COCs | Ovarian cancer in ever users | ||
|---|---|---|---|---|
| 225,000 | 79.3 percent | 178,425 | ||
| Adjusted estimate of cases if no use of COCs | 209,912 | 0.85 | Relative risk | |
| Decrease in cases | −31,487 | |||
| Annual cost per patient for ovarian cancer | US$26,056 | |||
| Estimated total costs | −US$820,419,141 | |||
Other Conditions
There are numerous other conditions that can be decreased in incidence and/or severity by COCs and POCs, such as ovarian cysts, anemia, primary dysmenorrhea, and benign breast disease (Osborn 2018). Similarly, there are numerous other conditions that can be increased in incidence and/or severity by COCs and POCs, such as premenstrual syndrome, headache/migraine, breast pain/tenderness/discomfort, nausea/vomiting, abdominal pain/tenderness/discomfort, and mood changes (Bayer HealthCare Pharmaceuticals 2001). These were not subjected to cost analysis in this article as they are typically are of lesser severity than the adverse events described in more detail here.
Overall Costs
Based on the information above, using the most conservative estimates, over 1.04 million women have developed diseases or disorders linked to the use of hormonal contraceptives (Figure 2), with costs to society of over US$16 billion annually (Table 24). These estimates do not include cardiovascular disease as noted in Table 17, using the more conservative estimates from Tables 18 and 19.
Table 24.
Estimated Total Burden of Disease and Economic Costs.
| Disease | Estimated Excess Cases | Estimated Excess Costs |
|---|---|---|
| HIV | 10,686 | US$157,218,081 |
| Breast cancer | 452,930 | US$10,021,975,916 |
| Cervical cancer | 76,581 | US$1,052,914,912 |
| Crohn’s disease | 81,762 | US$1,910,583,605 |
| Ulcerative colitis | 40,526 | US$522,789,187 |
| Systemic lupus erythematosus | 20,385 | US$438,985,908 |
| Depression combined oral contraceptives (COCs) | 377,733 | US$2,413,713,761 |
| Depression progesterone-only contraceptives (POCs) | 146,711 | US$937,482,772 |
| Interstitial cystitis | 12,345 | US$89,165,215 |
| Fractures COCs | 26,471 | US$308,521,992 |
| Fractures POCs | 24,926 | US$290,517,770 |
| Myocardial Infarction | 3,222 | US$61,062,935 |
| Cerebrovascular Accident | 6,158 | US$116,719,504 |
| Hyperthyroidism | −1,748 | −US$3,956,680 |
| Uterine cancer | −198,808 | −US$628,302,197 |
| Ovarian cancer | −31,487 | −US$820,419,141 |
| Totals | 1,048,393 | US$16,868,973,540 |
One cost not calculated as part of this analysis is the cost of prenatal care and childbirth. These costs were not considered relevant as there are effective alternatives to COCs and POCs that do not incur and an increased risk of disease. These can be termed risk avoidance.
Risk Avoidance
The abovementioned sociological and environmental impact, as well as economic costs, can be avoided by using nonhormonal methods of birth control, for example, modern fertility awareness methods (FAMs). The efficacy of avoiding pregnancy for modern FAMs like the billings ovulation method, the sympto-thermal method, and Creighton model system is similar to the COC pill (Turner 2016, 375–76; Hilgers 2002, 185–96). Women seeking fertility control have a right to be informed and educated about effective nonhormonal methods like FAMs that do not have the risks of potent steroid hormones, such as COCs and POCs.
Conclusion
In addition to their impact on women’s health, hormonal contraceptive agents have a variety of deleterious effects sociologically. The data reviewed above show negative effects on relationships, with the exploitation of women, a weakening of marriage, and an increase in divorce rates. These effects are far-reaching as divorce also has negative effects on children such as child poverty, worse health, lower educational achievement, suicide risks, drug and alcohol abuse, criminality, and incarceration, among others. The widespread use of contraception is also linked to an increase in abortion, with the many negative effects of abortion on women which have been well-documented elsewhere (Fehring, Bouchard, and Meyers 2018, 167–77).
The widespread use of contraception has also led to a large number of medical illnesses with a huge economic impact. As per Figure 2, this includes millions of cases of cardiovascular disease, over half a million cases of cancer, over half a million cases of depression, well over a hundred thousand cases of autoimmune disease, and tens of thousands of osteoporotic fractures each year. Note that these analyses are limited to the United States only, so the worldwide impact is much higher. The cost of treating all of these diseases is conservatively estimated as over US$17 billion (Table 20). This does not take into account the potential contribution of the increases in cases of cardiovascular disease (Table 17) which may affect over 2.5 million women with a cost of over US$50 billion. This is a large cost for a society to bear in exchange for chemically sterilizing sexual intercourse.
Less widely understood is the environmental impact. Research in this area is at an early stage, but the feminization effects on fish populations are well-documented. Although the concentration of contraceptive steroids in the water supply appears too low to exert an effect on humans, the effect on other species is not negligible.
All of these effects need to be taken into consideration for our society. With the advent of effective fertility awareness-based methods, we as a society need to rethink the use of hormonally active contraceptives. Women of childbearing age should also be made aware of these issues.
Supplemental Material
Supplemental Material, sj-pdf-1-lqr-10.1177_00243639211005121 for Hormonally Active Contraceptives, Part II: Sociological, Environmental, and Economic Impact by William V. Williams, Joel Brind, Laura Haynes, Michael D. Manhart, Hanna Klaus, Angela Lanfranchi, Gerard Migeon, Michael Gaskins, Elvis I. Šeman, Lester Ruppersberger and Kathleen M. Raviele in The Linacre Quarterly
Biographical Note
William V. Williams, MD, FACP, is the President and CEO of BriaCell Therapeutics Corporation and an adjunct professor of medicine at the University of Pennsylvania. He has participated in drug development for over twenty years. He is also a permanent deacon in the Archdiocese of Philadelphia. He and his wife Lorraine have three children and four grandchildren.
Joel Brind, PhD, earned his PhD from New York University in basic medical sciences, just retired after thirty-four years as a professor of biology and endocrinology at Baruch College, City University of New York. He specializes in research into sex steroid hormone synthesis and metabolism and their connections to human disease. He cofounded the Breast Cancer Prevention Institute in 1999, and from 2003 to 2006, he served on the federal CDC Advisory Committee on the early detection and control of breast and cervical cancer. He has published extensively on the connections between abortion and breast cancer and between contraceptive steroids and HIV transmission.
Laura Haynes, PhD, MA, is a PhD holder in counseling psychology from Biola University, La Mirada, CA, and has done MA in experimental–general psychology from Southern Methodist University. She reviews research, writes and speaks internationally on sexuality and gender. She has testified before legislative hearing committees in several states in the United States, trained therapists from twenty-six nations, and presented to UN diplomats and high-level government officials. She retired from clinical psychology practice in 2018 after more than forty years of experience.
Michael D. Manhart, PhD, is currently serves as a senior scientific consultant for the Couple to Couple League (CCL) and is a member of the FACTS Advisory Council. After receiving his doctorate in microbiology from the University of Cincinnati College of Medicine, he embarked on a career in Research and Development (R&D) with Procter & Gamble. While there, he held numerous management roles of increasing responsibility in various global healthcare and pharmaceutical organizations; he retired in 2008 as a director of R&D. He also served on the CCL Board of Directors from 2007 to 2009. In July 2009, he was appointed CCL Executive Director and led the organization until 2016.
Hanna Klaus, MD, FACOG, is a Medical Mission Sister who has served in Pakistan and Bangladesh and on the faculties of Washington, St. Louis, and George Washington University medical schools. She is the cofounder of the international Teen STAR (Sexuality Teaching in the context of Adult Responsibility) program.
Angela Lanfranchi, MD, FACS, is a clinical assistant professor of surgery at Rutgers Robert Wood Johnson Medical School and the president of the Breast Cancer Prevention Institute. She practiced breast surgery in New Jersey for thirty-three years.
Gerard Migeon, ESCP is the cofounder and CEO of Natural Womanhood and naturalwomanhood.org, an organization dedicated to transforming reproductive health and relationships by empowering women and couples to embrace fertility awareness, natural family planning, and comprehensive women’s health.
Michael Gaskins, BA is a journalist and author of the book, In the Name of the Pill, and has researched and written extensively about the history of birth control and the risks associated with various contraceptives. His email address is mgaskins@gmail.com.
Elvis I. Šeman, MBBS, FRANZCOG, EUCOGE, FRCOG, NFPMC, PhD, trained in obstetrics and gynecology in Australia and the United Kingdom. He is currently a medical lead in urogynecology at Flinders Medical Centre and senior lecturer at Flinders University, South Australia. He is actively involved in MaterCare and the Catholic Medical Association of South Australia. He also has an interest in restorative reproductive medicine and gained a PhD in laparoscopic pelvic floor repair. He is married to Marija. They have two sons and a delightful nineteen-month-old granddaughter.
Lester Ruppersberger, DO, is a retired board-certified NFP-only OB/GYN with thirty-seven years of clinical experience. He is a past president of the Catholic Medical Association and current president of the Philadelphia Guild of the CMA. He was a host with his wife of fifty years, Betty, on Catholic radio for fifteen years discussing NFP. He is the medical director for two crisis pregnancy centers in the Philadelphia area. He serves on the Board of the Couple to Couple League. He has authored several articles and a self-published book for parents called Wonderfully Made Babies. He is a frequent speaker for CMA sponsored programs. He has two grown children and ten grandchildren.
Kathleen M. Raviele, MD, is a fellow in the American College of Obstetricians and Gynecologists and is a past president of the Catholic Medical Association. She practiced gynecology in the Atlanta area for thirty-nine years. Her email address is ravielek@gmail.com.
Notes
The National Health and Social Life Survey reported in Laumann et al. (1994), Chapter 9: Formative Sexual Experiences, especially conclusions on page 347.
This was calculated from 186 abortions per 1,000 live births. The abortion percentage is defined as number of abortions per 1,000/(number of abortions per 1,000 live births + 1,000 live births) or 186/(186+1,000) = 15.7 percent, rounded to 16 percent (see Centers for Disease Control and Prevention 2020d).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: William V. Williams, MD, FACP  https://orcid.org/0000-0003-3611-9284
https://orcid.org/0000-0003-3611-9284
Michael D. Manhart, PhD  https://orcid.org/0000-0003-1727-1846
https://orcid.org/0000-0003-1727-1846
Supplemental Material: The supplemental material for this article is available online.
References
- Abma J., Chandra A., Mosher W., Peterson L., Piccinino L.. 1997. “Fertility, Family Planning, and Women’s Health: New Data from the 1995 National Survey of Family Growth. National Center for Health Statistics.” Vital Health Statistics 23, no. 19: 1–114. [PubMed] [Google Scholar]
- American Cancer Society: Cervical Cancer. n.d. “Key Statistics for Cervical Cancer.” https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html.
- American Psychological Association. 2015. “Children of Same-Sex Parents Face Challenges, but Will Be OK.” https://www.apa.org/news/press/releases/2015/07/same-sex-parents.
- American Psychological Association, Division 44 Consensual Non-Monogamy Task Force. n.d. “Committee on Consensual Non-Monogamy.” https://www.div44cnm.org.
- Anglican Consultative Council. 2005. “The Lambeth Conference. Resolutions Archive from 1930.” Anglican Communion Office. https://www.anglicancommunion.org/media/127734/1930.pdf.
- Aral S. O., Fenton K. A., Holmes K. K.. 2007. “Sexually Transmitted Diseases in the USA: Temporal Trends.” Sexually Transmitted Infections 83, no. 4: 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballane G., Cauley J. A., Luckey M. M., El-Hajj Fuleihan G.2017. “Worldwide Prevalence and Incidence of Osteoporotic Vertebral Fractures.” Osteoporosis International 28, no. 5: 1531–42. [DOI] [PubMed] [Google Scholar]
- Barad D., Kooperberg C., Wactawski-Wende J., Liu J., Hendrix S. L., Watts N. B.. 2005. “Prior Oral Contraception and Postmenopausal Fracture: A Women’s Health Initiative Observational Cohort Study.” Fertility and Sterility 84, no. 2: 374–83. [DOI] [PubMed] [Google Scholar]
- Bayer HealthCare Pharmaceuticals. 2012. “YASMIN Highlights of Prescribing Information.” https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021098s022lbl.pdf.
- Bennett W.1993. The Index of Leading Cultural Indicators. Washington, DC: The Heritage Foundation and Empower America. [Google Scholar]
- Bercow A. S., Chen L., Chatterjee S., Tergas A. I., Hou J. Y., Burke W. M., Ananth C. V., Neugut A. I., Hershman D. L., Wright J. D.. 2017. “Cost of Care for the Initial Management of Ovarian Cancer.” Obstetrics & Gynecology 130:1269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M. O., Mikaeloff Y., Hudson M., Suissa S.. 2009. “Combined Oral Contraceptive Use and the Risk of Systemic Lupus Erythematosus.” Arthritis and Rheumatism 61, no. 24: 476–81. [DOI] [PubMed] [Google Scholar]
- Besse J.-P., Garric J.. 2009. “Progestagens for Human Use, Exposure and Hazard Assessment for the Aquatic Environment.” Environmental Pollution 157, no. 12: 3485–94. [DOI] [PubMed] [Google Scholar]
- Blue Cross Blue Shield. 2018. “Major Depression: The Impact on Overall Health.” Blue Cross Blue Shield, The Health of America Report®. https://www.bcbs.com/sites/default/files/file-attachments/health-of-america-report/HoA_Major_Depression_Report.pdf.
- Blumen H., Fitch K., Polkus V.. 2016. “Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service.” American Health and Drug Benefits 9, no. 1: 23–32. [PMC free article] [PubMed] [Google Scholar]
- Brody D. J., Pratt L. A., Hughes J. P.. 2018. “Prevalence of Depression among Adults Aged 20 and Over: United States, 2013-2016.” National Center for Health Statistics Data Brief 303, no. 8: 1–8. [PubMed] [Google Scholar]
- Byrd D.2010. “Dual-Gender Parenting for Optimal Child Development.” Journal of Human Sexuality 2:102–23. [Google Scholar]
- Caldwell D. J., Mastrocco F., Anderson P. D., Lange R., Sumpter J. P.. 2012. “Predicted-No-Effect Concentrations for the Steroid Estrogens Estrone, 17b-Estradiol, Estriol, and 17a-Ethinylestradiol.” Environmental Toxicology and Chemistry 31, no. 6: 1396–406. [DOI] [PubMed] [Google Scholar]
- Calvin J.n.d. “Commentary on Genesis, Volume 2, Gen. 38:8 and 10.” https://www.christianity.com/bible/commentary.php?com=clvn&b=1&c=38.
- CDC (Centers for Disease Control and Prevention). 1995. “Update: AIDS Among Women—United States, 1994.” MMWR Morbidity and Mortality Weekly Report 44, no. 7: 81–84. http://www.cdc.gov/mmwr//preview/mmwrhtml/00035899.htm. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 1996. “Mortality Attributable to HIV Infection among Persons Aged 25-44 Years—United States 1994.” MMWR Morbidity and Mortality Weekly Report 45, no. 6: 121–25. http://www.cdc.gov/mmwr/preview/mmwrhtml/00040227.htm. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2018. “Systemic Lupus Erythematosus (SLE).” https://www.cdc.gov/lupus/facts/detailed.html.
- CDC (Centers for Disease Control and Prevention). 2020. a. “Contraceptive Use.” National Center for Health Statistics. https://www.cdc.gov/nchs/fastats/contraceptive.htm. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2020. b. “Estimated HIV Incidence and Prevalence in the United States, 2014–2018.” HIV Surveillance Supplemental Report 25, no. 1. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-1.pdf. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2020. c. “HIV and Women.” Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. https://www.cdc.gov/hiv/group/gender/women/index.html.
- CDC (Centers for Disease Control and Prevention). 2020. d. “Reproductive Health. Data and Statistics.” https://www.cdc.gov/reproductivehealth/data_stats/index.htm.
- CDC (Centers for Disease Control and Prevention). 2020. e. “Women and Heart Disease.” https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_women_heart.htm.
- CDC (Centers for Disease Control and Prevention), National Survey for Family Growth. 2019. “Key Statistics from the National Survey of Family Growth—C Listing.” https://www.cdc.gov/nchs/nsfg/key_statistics/c.htm#everused.
- Center for Bioethics and Culture Network. n.d. “Making Life.” http://www.cbc-network.org/issues/making-life/.
- Champaneria R., D’Andrea R. M., Latthe P. M.. 2016. “Hormonal Contraception and Pelvic Floor Dysfunction: A Systematic Review.” International Urogynecology Journal 27, no. 5: 709–22. [DOI] [PubMed] [Google Scholar]
- Chen A. Y., Chonghasawat A. O., Leadholm K. L.. 2017. “Multiple Sclerosis: Frequency, Cost, and Economic Burden in the United States.” Journal of Clinical Neuroscience 45, no. November: 180–86. [DOI] [PubMed] [Google Scholar]
- Chen S. Y., Choi C. B., Li Q., Yeh W. S., Lee Y. C., Kao A. H., Liang M. H.. 2015. “Glucocorticoid Use in Patients with Systemic Lupus Erythematosus: Association between Dose and Health Care Utilization and Costs.” Arthritis Care and Research (Hoboken) 67, no. 8: 1086–94. [DOI] [PubMed] [Google Scholar]
- Clemens J. Q., Meenan R. T., Rosetti M. C., Gao S. Y., Calhoun E. A.. 2005. “Prevalence and Incidence of Interstitial Cystitis in a Managed Care Population.” Journal of Urology 173, no. 1: 98–102; discussion 102. [DOI] [PubMed] [Google Scholar]
- Cohen R., Skup M., Ozbay A. B., Rizzo J., Yang M., Diener M., Chao J.. 2015. “Direct and Indirect Healthcare Resource Utilization and Costs Associated with Ulcerative Colitis in a Privately-Insured Employed Population in the US.” Journal of Medical Economics 18, no. 6: 447–56. [DOI] [PubMed] [Google Scholar]
- Coleman P.2011. “Abortion and Mental Health: Quantitative Synthesis and Analysis of Research Published 1995-2009.” Journal of British Psychiatry 199, no. 3: 180–86. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. 2015. “Endometrial Cancer and Oral Contraceptives: An Individual Participant Meta-Analysis of 27 276 Women with Endometrial Cancer from 36 Epidemiological Studies.” Lancet Oncology 16, no. 9: 1061–70. [DOI] [PubMed] [Google Scholar]
- Cornish J. A., Tan E., Simillis C., Clark S. K., Teare J., Tekkis P. P.. 2008. “The Risk of Oral Contraceptives in the Etiology of Inflammatory Bowel Disease: A Meta-Analysis.” American Journal of Gastroenterology 103, no. 9: 2394–400. [DOI] [PubMed] [Google Scholar]
- Costenbader K. H., Feskanich D., Stampfer M. J., Karlson E. W.. 2007. “Reproductive and Menopausal Factors and Risk of Systemic Lupus Erythematosus in Women.” Arthritis and Rheumatism 56, no. 4: 1251–62. [DOI] [PubMed] [Google Scholar]
- Dahlhamer J. M., Zammitti E. P., Ward B. W., Wheaton A. G., Croft J. B.. 2016. “Prevalence of Inflammatory Bowel Disease among Adults Aged ≥18 Years—United States, 2015.” MMWR Morbidity and Mortality Weekly Report 65, no. 42: 1166–69. [DOI] [PubMed] [Google Scholar]
- Daniels K., Daugherty J., Jones J., Mosher W.. 2015, November10. “Current Contraceptive Use and Variation by Selected Characteristics among Women Aged 15–44: United States, 2011–2013.” National Health Statistics Report 86:1–14. [PubMed] [Google Scholar]
- Denisov B. P., Sakevich V. I., Jasilioniene A.. 2012. “Divergent Trends in Abortion and Birth Control Practices in Belarus, Russia and Ukraine.” PLoS One 7, no. 11: e49986. 10.1371/journal.pone.0049986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueñas J. L., Lete I., Bermejo R., Arbat A., Pérez-Campos E., Martínez-Salmeán J., Serrano I., Doval J. L., Coll C.. 2011. “Trends in the Use of Contraceptive Methods and Voluntary Interruption of Pregnancy in the Spanish Population during 1997-2007.” Contraception 83, no. 1: 82–87. [DOI] [PubMed] [Google Scholar]
- Fehring R. J., Bouchard T., Meyers M.. 2018. “Influence of Contraception Use on the Reproductive Health of Adolescents and Young Adults.” The Linacre Quarterly 85, no. 2: 167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhahn S.2014. The Good News about Marriage; Debunking Discouraging Myths about Marriage and Divorce. Colorado Springs, CO: Multnomah Books. [Google Scholar]
- Ford K.1979, September. “Contraceptive Utilization, United States.” Vital and Health Statistics, Series 23, No. 2. http://www.cdc.gov/nchs/data/series/sr_23/sr23_002.pdf. [PubMed]
- Fortner R. T., Ose J., Merritt M. A., Schock H., Tjønneland A., Hansen L., Overvad K., et al. 2015. “Reproductive and Hormone-Related Risk Factors for Epithelial Ovarian Cancer by Histologic Pathways, Invasiveness and Histologic Subtypes: Results from the EPIC Cohort.” International Journal of Cancer 137, no. 5: 1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank P., Kay C. R.. 1978. “Incidence of Thyroid Disease Associated with Oral Contraceptives.” British Medical Journal 2:1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajendran M., Loganathan P., Catinella A. P., Hashash J. G.. 2018. “A Comprehensive Review and Update on Crohn’s Disease.” Disease Monthly 64, no. 2: 20–57. [DOI] [PubMed] [Google Scholar]
- García Rodríguez L. A., González-Pérez A., Johansson S., Wallander M. A.. 2005. “Risk Factors for Inflammatory Bowel Disease in the General Population.” Alimentary Pharmacology & Therapeutics 22, no. 4: 309–15. [DOI] [PubMed] [Google Scholar]
- Global Surrogacy. 2021. “Find a Surrogate with Global Surrogacy Inc.”https://www.globalsurrogacyinc.com/parents/.
- Guttmacher Institute. 2020, April. “Contraceptive Use in the United States.” https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states
- Hardon J.1981. The Catholic Catechism: A Contemporary Catechism of the Teachings of the Catholic Church. New York: Image Books. [Google Scholar]
- Harrison B.1996. “The Sin of Onan Revisited.” Living Tradition—Journal of the Roman Theological Forum 67. November 1996. http://www.rtforum.org/lt/lt67.html. [Google Scholar]
- Heikkinen S., Koskenvuo M., Malila N., Sarkeala T., Pukkala E., Pitkäniemi J.2016. “Use of Exogenous Hormones and the Risk of Breast Cancer: Results from Self-Reported Survey Data with Validity Assessment.” Cancer Causes Control 27, no. 2: 249–58. [DOI] [PubMed] [Google Scholar]
- Hellwig K., Chen L. H., Stancyzk F. Z., Langer-Gould A. M.2016. “Oral Contraceptives and Multiple Sclerosis/Clinically Isolated Syndrome Susceptibility.” PLoS One 11, no. 3: e0149094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog E., Sudia C.. 1973. “Children in Fatherless Families.” In Review of Child Development Research, Chapter 3, edited by B. Caldwell and H. Ricciuti, 214, 220–21. Chicago: The University of Chicago Press. [Google Scholar]
- Hilgers T. W.2002. “The Statistical Evaluation of Contraceptive Methods.” In Reproductive Anatomy and Physiology, edited by Hilgers T. W., 185–95. Omaha, NE: Pope Paul VI Institute Press. [Google Scholar]
- Hilgers T. W.2010. The NaProTECHNOLOGY Revolution: Unleashing the Power in a Woman’s Cycle. New York: Beaufort Books. [Google Scholar]
- Howden L. M., Meyer J. A.. 2011. Age and Sex Composition. 2010 Census Briefs. US Department of Commerce Economics and Statistics Administration, US Census Bureau. https://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. [Google Scholar]
- Hüsing A., Dossus L., Ferrari P., Tjønneland A., Hansen L., Fagherazzi G., Baglietto L., et al. 2016. “An Epidemiological Model for Prediction of Endometrial Cancer Risk in Europe.” European Journal of Epidemiology 31:51–60. [DOI] [PubMed] [Google Scholar]
- Jobling S., Coey S., Whitmore J. G., Kime D. E., Van Look K. J., McAllister B. G., Beresford N., et al. 2002. “Wild Intersex Roach (Rutilus rutilus) Have Reduced Fertility.” Biology of Reproduction 67, no. 2: 515–24. [DOI] [PubMed] [Google Scholar]
- Jobling S., Nolan M., Tyler C. R., Brighty G., Sumpter J. P.. 1998. “Widespread Sexual Disruption in Wild Fish.” Environmental Science and Technology 32, no. 17: 2498–506. [Google Scholar]
- Jobling S., Williams R., Johnson A., Taylor A., Gross-Sorokin M., Nolan M., Tyler C. R., van Aerle R., Santos E., Brighty G.. 2006. “Predicted Exposures to Steroid Estrogens in U.K. Rivers Correlate with Widespread Sexual Disruption in Wild Fish Populations.” Environmental Health Perspectives 114, no. Suppl. 1: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston W. R.2014. “United States Abortion Rates, 1960-2013.” http://www.johnstonsarchive.net/policy/abortion/graphusabrate.html: Calculated from the 2016 abortion ratio of 186 abortions per 1,000 live births.
- Kahaly G. J., Dietlein M.. 2002. “Cost Estimation of Thyroid Disorders in Germany.” Thyroid 12, no. 10: 909–14. [DOI] [PubMed] [Google Scholar]
- Kahlenborn C., Modugno F., Potter D. M., Severs W. B.. 2006. “Oral Contraceptive Use as a Risk Factor for Premenopausal Breast Cancer: A Meta-Analysis.” Mayo Clinic Proceedings 81, no. 10:1290–302. [DOI] [PubMed] [Google Scholar]
- Kappelman M. D., Rifas-Shiman S. L., Kleinman K., Ollendorf D., Bousvaros A., Grand R. J., Finkelstein J. A.. 2007. “The Prevalence and Geographic Distribution of Crohn’s Disease and Ulcerative Colitis in the United States.” Clinical Gastroenterology and Hepatology 5, no. 12: 1424–29. [DOI] [PubMed] [Google Scholar]
- Kegu J., Silverstein J.. 2019. “Not Just ‘One Big Orgy’: Fighting the Stigma of Consensual Non-Monogamy.” https://www.cbsnews.com/news/polyamory-relationships-not-just-one-big-orgy-stigma-of-consensual-non-monogamy-cbsn-originals/.
- Khalili H., Higuchi L. M., Ananthakrishnan A. N., Richter J. M., Feskanich D., Fuchs C. S., Chan A. T.. 2013. “Oral Contraceptives, Reproductive Factors and Risk of Inflammatory Bowel Disease.” Gut 62, no. 8: 1153–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd K. A., Blanchfield P. J., Mills K. H., Palace V. P., Evans R. E., Lazorchak J. M., Flick R. W.. 2007. “Collapse of a Fish Population after Exposure to a Synthetic Estrogen.” Proceedings of the National Academy of Science 104, no. 21: 8897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M. L., Morrisey M. A., Becker D. J., Gary L. C., Curtis J. R., Saag K. G., Yun H., et al. 2009. “Health Care Expenditures Associated with Skeletal Fractures among Medicare Beneficiaries, 1999-2005.” Journal of Bone and Mineral Research 24, no. 12: 2050–55. [DOI] [PubMed] [Google Scholar]
- King O. C., van de Merwe J. P., McDonald J. A., Leusch F. D.. 2016. “Concentrations of Levonorgestrel and Ethinylestradiol in Wastewater Effluents: Is the Progestin also Cause for Concern?” Environmental Toxicology and Chemistry 35, no. 6: 1378–85. [DOI] [PubMed] [Google Scholar]
- Kinsey A. C., Pomeroy W. R., Martin C. E.. 1948. Sexual Behavior in the Human Male. Philadelphia, PA: W. B. Saunders. [Google Scholar]
- Kinsey A. C., Pomeroy W.R., Martin C., Gebhard P.. 1953. Sexual Behavior in the Human Female. Philadelphia, PA: Saunders. [Google Scholar]
- Kirby M. F., Allen Y. T., Dyer R. A., Feist S. W., Katsiadaki I., Matthiessen P., Scott A. P., et al. 2004. “Surveys of Plasma Vitellogenin and Intersex in Male Flounder (Platichthys flesus) as Measures of Endocrine Disruption by Estrogenic Contamination in United Kingdom Estuaries: Temporal Trends, 1996 to 2001.” Environmental Toxicology and Chemistry 23, no. 3: 748–58. [DOI] [PubMed] [Google Scholar]
- Klaus H., Cortes M. E.. 2015. “Psychological, Social, and Spiritual Effects of Contraceptive Steroid Hormones.” The Linacre Quarterly 82, no. 3: 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostich M., Flick R., Martinson J.. 2013. “Comparing Predicted Estrogen Concentrations with Measurements in US Waters.” Environmental Pollution 178, no. July: 271–77. [DOI] [PubMed] [Google Scholar]
- Lahl J., Powell G., Farley M., Anderson R. T.. 2019. “Casualties of Surrogacy: Women for Rent, Infants for Sale, LGBT Rights for Hijacking.” https://www.heritage.org/marriage-and-family/event/casualties-surrogacy-women-rent-infants-sale-lgbt-rights-hijacking.
- Lambeth Conference Resolution Archive. 2005. “Index of Resolutions from 1930.” https://www.anglicancommunion.org/media/127734/1930.pdf.
- Lanza L. L., McQuay L. J., Rothman K. J., Bone H. G., Kaunitz A. M., Harel Z., Ataher Q., Ross D., Arena P. L., Wolter K. D.. 2013. “Use of Depot Medroxyprogesterone Acetate Contraception and Incidence of Bone Fracture.” Obstetrics and Gynecology 121, no. 3: 593–600. [DOI] [PubMed] [Google Scholar]
- Larsson D. G. J., Adolfsson-Erici M., Parkkonen J., Pettersson M., Berg A. H., Olsson P. E., Forlin L.. 1999. “Ethinyloestradiol—An Undesired Fish Contraceptive?” Aquatic Toxicology 45, no. 2–3: 91–97. [Google Scholar]
- Laumann E., Gagnon J., Michael R., Michaels S.. 1994. The Social Organization of Sexuality: Sexual Practices in the United States. Chicago: The University of Chicago Press. [Google Scholar]
- Levine H., Jørgensen N., Martino-Andrade A., Mendiola J., Weksler-Derri D., Mindlis I., Pinotti R., Swan S. H.. 2017. “Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis.” Human Reproduction Update 23, no. 6: 646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidegaard Ø., Nielsen L. H., Skovlund C. W., Løkkegaard E.2012, May10. “Venous Thrombosis in Users of Non-Oral Hormonal Contraception: Follow-Up Study, Denmark 2001–10.” British Medical Journal 344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Bakken K., Dumeaux V., Andersen V., Kumle M.. 2007. “Hormone Replacement Therapy and Breast Cancer in Former Users of Oral Contraceptives—The Norwegian Women and Cancer Study.” International Journal of Cancer 121, no. 3: 645–48. [DOI] [PubMed] [Google Scholar]
- Marks L.2012. “Same-Sex Parenting and Children’s Outcomes: A Closer Examination of the American Psychological Association’s Brief on Lesbian and Gay Parenting.” Social Science Research 41, no. 4: 735–51. [DOI] [PubMed] [Google Scholar]
- Marquardt E., Glenn N. D., Clark K.. 2010. My Daddy’s Name Is Donor. A New Study of Young Adults Conceived through Sperm Donation. New York: Institute for American Values. http://americanvalues.org/catalog/pdfs/Donor_FINAL.pdf. [Google Scholar]
- Martin J. A., Hamilton B. E., Osterman M. J. K., Driscoll A. K.. 2019. “Births: Final Data for 2018.” National Vital Statistics Reports 68, no. 13. National Center for Health Statistics. HHS, CDC. https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_13-508.pdf. [PubMed] [Google Scholar]
- Mascola L., Cates W., Jr, Reynolds G. H., Blount J. H.. 1983. “Gonorrhea and Salpingitis among American Teenagers, 1960-1981.” MMWR CDC Surveillance Summary 32, no. 3: 25–30. [PubMed] [Google Scholar]
- McLennan M. T.2014. “Interstitial Cystitis: Epidemiology, Pathophysiology, and Clinical Presentation.” Obstetrics and Gynecology Clinics of North America 41, no. 3: 385–95. [DOI] [PubMed] [Google Scholar]
- Michael R.1988. “Why Did the U.S. Divorce Rate Double Within a Decade?” Research in Population Economics 6:367–99. (Obtained from the author by request. Contact: Robert T. (Bob) Michael, rmichael@uchicago.edu) [Google Scholar]
- Michael R.2005. “Chapter 5: An Economic Perspective on Sex, Marriage, and the Family in the Contemporary United States.” In Family Transformed: Religion, Values, and Society in American Life, edited by Witte J., Jr, Tipton S., 94–119. Washington, DC: Georgetown University Press. [Google Scholar]
- Mørch L. S., Skovlund C. W., Hannaford P. C., Iversen L., Fielding S., Lidegaard Ø.2017. “Contemporary Hormonal Contraception and the Risk of Breast Cancer.” New England Journal of Medicine 377, no. 23: 2228–39. [DOI] [PubMed] [Google Scholar]
- Mosher W., Bachrach C.. 1988. “Contraceptive Use: United States, 1982.” Vital and Health Statistics, Series 23, No. 12. DHHS Pub. No. (PHS) 86. www.cdc.gov/nchs/data/series/sr_23/sr23_012.pdf. [PubMed]
- Mosher W., Pratt W.. 1990. Contraceptive Use in the United States, 1973-1988 (Advanced Data from Vital and Health Statistics, No.182). Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/data/series/sr_16/sr16_019.pdf. [Google Scholar]
- National Cancer Institute. 2020. “Financial Burden of Cancer Care” (Data up to date as of March 2020). https://www.progressreport.cancer.gov/after/economic_burden.
- National Multiple Sclerosis Society. n.d. “Who Gets MS? (Epidemiology).” https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS.
- Nichols G. A., Bell T. J., Pedula K. L., Rosetti M. O.. 2010. “Medical Care Costs among Patients with Established Cardiovascular Disease.” The American Journal of Managed Care 16, no. 3: e86–93. [PubMed] [Google Scholar]
- Nicolle L. E.2005. “Sexually Transmitted Infections.” Canadian Journal of Infectious Diseases and Medical Microbiology 16:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr S.1999. “National Data on Child Abuse and Neglect. Adoption Factbook III.” In National Council for Adoption, edited by Marshner C., Pierce W. [Google Scholar]
- Osborn C. O.2018. “10 Benefits of Birth Control beyond Preventing Pregnancy.” Healthline. https://www.healthline.com/health/birth-control-benefits.
- Park B. J., Kidd K. A.. 2009. “Effects of the Synthetic Estrogen Ethinylestradiol on Early Life Stages of Mink Frogs and Green Frogs in the Wild and in Situ.” Environmental Toxicology 24, no. 8: 2027–36. [DOI] [PubMed] [Google Scholar]
- Pendrith C., Thind A., Zaric G. S., Sarma S.. 2016. “Costs of Cervical Cancer Treatment: Population-Based Estimates from Ontario.” Current Oncology 23, no. 2: e109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington M., Gentry-Maharaj A., Karpinskyj C., Miners A., Taylor J., Manchanda R., Iyer R., et al. 2016. “Long-Term Secondary Care Costs of Endometrial Cancer: A Prospective Cohort Study Nested within the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS).” PLoS One 11, no. 11: e0165539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L.1995, February14. “Contraceptive Use in the United States: 1982-1990.” Advanced Data from Vital and Health Statistics. www.cdc.gov/nchs/data/ad/ad260.pdf.
- Popenoe D.2009. Families without Fathers: Fathers, Marriage and Children in American Society. New Brunswick, NJ: Transaction, pp. 60f. [Google Scholar]
- Provan C.1989. The Bible and Birth Control. Monongahela, PA: Zimmer Printing. [Google Scholar]
- Public Broadcasting System. n.d. “The Pill: Timeline. A Timeline of Contraception.” https://www.pbs.org/wgbh/americanexperience/features/pill-timeline/.
- Purdom C. E., Hardiman P. A., Bye V. V. J., Eno N. C., Tyler C. R., Sumpter J. P.. 1994. “Estrogenic Effects of Effluents from Sewage Treatment Works.” Chemical Ecology 8, no. 4: 275–85. [Google Scholar]
- Rao B. B., Click B. H., Koutroubakis I. E., Ramos Rivers C., Regueiro M., Swoger J., Schwartz M., et al. 2017. “The Cost of Crohn’s Disease: Varied Healthcare Expenditure Patterns across Distinct Disease Trajectories.” Inflammatory Bowel Disease 23, no. 1: 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector R., Johnson K., Fagan P.. 2002, April15. “The Effect of Marriage on Child Poverty.” http://www.heritage.org/research/reports/2002/04/the-effect-of-marriage-on-child-poverty.
- Regnerus M.2012. “How Different Are the Adult Children of Parents Who Have Same-Sex Relationships? Findings from the New Family Structures Study.” Social Science Research 41, no. 4: 252–70. [DOI] [PubMed] [Google Scholar]
- Roura E., Travier N., Waterboer T., de Sanjosé S., Bosch F. X., Pawlita M., Pala V., et al. 2016. “The Influence of Hormonal Factors on the Risk of Developing Cervical Cancer and Pre-Cancer: Results from the EPIC Cohort.” PLoS One 11, no. 1: e0147029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackman B. R., Fleishman J. A., Su A. E., Berkowitz B. K., Moore R. D., Walensky R. P., Becker J. E., et al. 2015. “The Lifetime Medical Cost Savings from Preventing HIV in the United States.” Medical Care 53, no. 4: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm W. R.2018. Same-Sex Parenting Research: A Critical Assessment. London, UK: Wilberforce. [Google Scholar]
- SEER (Surveillance, Epidemiology, and End Result Program). n.d.a. “Cancer Stat Facts: Cervical Cancer.” https://seer.cancer.gov/statfacts/html/cervix.html.
- SEER (Surveillance, Epidemiology, and End Result Program). n.d.b. “Cancer Stat Facts: Female Breast Cancer.”https://seer.cancer.gov/statfacts/html/breast.html.
- SEER (Surveillance, Epidemiology, and End Result Program). n.d.c. “Cancer Stat Facts: Ovarian Cancer.”https://seer.cancer.gov/statfacts/html/ovary.html.
- SEER (Surveillance, Epidemiology, and End Result Program). n.d.d. “Cancer Stat Facts: Uterine Cancer.”https://seer.cancer.gov/statfacts/html/corp.html.
- Sharpe R. M., Skakkebaek N. E.. 1993. “Are Oestrogens Involved in Falling Sperm Counts and Disorders of the Male Reproductive Tract?” Lancet 341, no. 8857: 1392–95. [DOI] [PubMed] [Google Scholar]
- Shivashankar R., Tremaine W. J., Harmsen W. S., Loftus E. V., Jr2017. “Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota from 1970 through 2010.” Clinical Gastroenterology Hepatology 15, no. 6: 857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek N. E., De Meyts E. R., Louis G. M., Toppari J., Andersson A. M., Eisenberg M. L., Jensen T. K., et al. 2016. “Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility.” Physiological Reviews 96, no. 1: 55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovlund C. W., Mørch L. S., Kessling L. V., Lidegaard O.. 2016. “Association of Hormonal Contraception with Depression.” JAMA Psychiatry 73, no. 11: 1154–62. [DOI] [PubMed] [Google Scholar]
- Smock P., Greenland F.. 2010. “Diversity in Pathways to Parenthood: Patterns, Implications, and Emerging Research Directions.” Journal of Marriage and Family 72, no. 3: 576–93. [Google Scholar]
- Stanczyk F. Z., Archer D. F., Bhavnani B. R.. 2013. “Ethinyl Estradiol and 17β-Estradiol in Combined Oral Contraceptives: Pharmacokinetics, Pharmacodynamics and Risk Assessment.” Contraception 87, no. 6: 706–27. [DOI] [PubMed] [Google Scholar]
- Strieder T. G., Prummel M. F., Tijssen J. G., Endert E., Wiersinga W. M.. 2003. “Risk Factors for and Prevalence of Thyroid Disorders in a Cross-Sectional Study among Healthy Female Relatives of Patients with Autoimmune Thyroid Disease.” Clinical Endocrinology (Oxford) 59:396–401. [DOI] [PubMed] [Google Scholar]
- Sumpter J. P.1995. “Feminized Responses in Fish to Environmental Estrogens.” Toxicology Letters 82–83, no. December: 737–42. [DOI] [PubMed] [Google Scholar]
- Sutton P. M., Cretella M.. 2019. “A Review and Summary of Walter Schumm’s Same-Sex Parenting Research: A Critical Assessment.” Journal of Human Sexuality 9:101–07. [Google Scholar]
- Tsilidis K. K., Allen N. E., Key T. J., Dossus L., Lukanova A., Bakken K., Lund E., et al. 2011. “Oral Contraceptive Use and Reproductive Factors and Risk of Ovarian Cancer in the European Prospective Investigation into Cancer and Nutrition.” British Journal of Cancer 105, no. 9: 1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung A., Hepp Z., Bansal A., Devine E. B.. 2017. “Characterizing Health Care Utilization, Direct Costs, and Comorbidities Associated with Interstitial Cystitis: A Retrospective Claims Analysis.” Journal of Managed Care and Specialty Pharmacy 23, no. 4: 474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.2016. “Fertility-Awareness Practice and Education in General Practice.” Australian Journal of Primary Health 22, no. 5: 375–76. [DOI] [PubMed] [Google Scholar]
- Tyler C. R., Jobling S., Sumpter J. P.. 1998. “Endocrine Disruption in Wildlife: A Critical Review of the Evidence.” Critical Reviews in Toxicology 28, no. 4: 319–61. [DOI] [PubMed] [Google Scholar]
- US Census Bureau. 2020. “Historical Living Arrangements of Children.” https://www.census.gov/data/tables/time-series/demo/families/children.html.
- US Fish and Wildlife Service. n.d. “Intersex Fish. Endocrine Disruption in Smallmouth Bass.” https://ecos.fws.gov/ServCat/DownloadFile/21216?Reference=22669.
- Ventura S. J., Abma J. C., Mosher W. D., Henshaw S.. 2003. “Revised Pregnancy Rates, 1990-1997 and New Rates for 1998-1999: United States.” National Vital Statistics Reports 52, no. 7: 1–16. [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Weeke J., Hoeck H. C., Nielsen H. K., Rungby J., Laurberg P., Mosekilde L.. 2002. “Smoking as a Risk Factor for Graves’ Disease, Toxic Nodular Goiter, and Autoimmune Hypothyroidism.” Thyroid 12, no. 1: 69–75. [DOI] [PubMed] [Google Scholar]
- Wedekind C., Penn D.. 2000. “MHC Genes, Body Odours, and Odour Preferences.” Nephrology, Dialysis, Transplantation 15, no. 9: 1269–71. [DOI] [PubMed] [Google Scholar]
- Wesley J. n.d. “Notes on the Bible, Notes on the Old Testament, Notes on the First Book Called Genesis, Commentary on Chapter 38.”http://www.ccel.org/ccel/wesley/notes.ii.ii.xxxix.ii.html.
- Williams W. V.2017. “Hormonal Contraception and the Development of Autoimmunity: A Review of the Literature.” The Linacre Quarterly 84, no. 3: 275–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., Carlson K., Mitchell L. A., Raviele K.. 2018. “Association of Combined Estrogen-Progestogen and Progestogen Only Contraceptives with the Development of Cancer.” The Linacre Quarterly 85, no. 4: 412–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodling J., Lopez E., Maldonado T., Norris D., Vajda A.. 2006. “Intersex and Other Reproductive Disruption of Fish in Wastewater Effluent Dominated Colorado Streams.” Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology 144, no. 1: 10–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-lqr-10.1177_00243639211005121 for Hormonally Active Contraceptives, Part II: Sociological, Environmental, and Economic Impact by William V. Williams, Joel Brind, Laura Haynes, Michael D. Manhart, Hanna Klaus, Angela Lanfranchi, Gerard Migeon, Michael Gaskins, Elvis I. Šeman, Lester Ruppersberger and Kathleen M. Raviele in The Linacre Quarterly