Abstract
Background:
Non-compressible hemorrhage is a leading cause of preventable death in civilian and military trauma populations. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is a promising method for controlling non-compressible hemorrhage, but safe balloon inflation parameters are not well defined. Our goal was to determine the balloon inflation parameters associated with benchtop flow occlusion and aortic/balloon rupture in ex vivo human aortas and test the hypothesis that optimal balloon inflation characteristics depend on systolic pressure and subject demographics.
Methods:
Aortic occlusion parameters in human thoracic and abdominal aortas (TAs and AAs) from 79 tissue donors (median age 52 ± 18 years, range 13-75 years, 52 M, 27 F) were recorded under 100/40, 150/40, and 200/40 mmHg flow pressures for ER-REBOA and Coda balloons. Rupture tests were done with Coda balloons only without flow.
Results:
In the TA, the average balloon inflation volumes and pressures resulting in 100/40 mmHg flow occlusion were 11.7 ± 3.8 ml and 174 ± 65 mmHg for the ER-REBOA, and 10.6 ± 4.3 ml and 94 ± 57 mmHg for the Coda balloons. In the AA, these values were 6.2 ± 2.6 ml and 110 ± 47 mmHg for the ER-REBOA, and 5.9 ± 2.2 ml and 71 ± 30 mmHg for the Coda. The average balloon inflation parameters associated with aortic/Coda balloon rupture were 39.1 ± 6.5 ml and 1284 ± 385 mmHg in the TA, and 27.7 ± 7.7 ml and 1410 ± 483 mmHg in the AA. Age, sex, and systolic pressure all had significant effects on balloon occlusion and rupture parameters.
Conclusions:
Optimal balloon inflation parameters depend on anatomical, physiological, and demographic characteristics. Pressure-guided rather than volume-guided balloon inflation may reduce the risk of aortic rupture. These results can be used to help improve the safety of REBOA procedures and devices.
Level of Evidence:
Level II
Study type:
Therapeutic/Care Management
Keywords: REBOA, balloon occlusion, aorta, aortic rupture, hemorrhage control
1. Background
Exsanguinating non-compressible hemorrhage is a major contributor to mortality from unintentional injuries.1,2 Many victims die before reaching the hospital because prehospital hemorrhage control methods are not yet fully developed.3 The emergence of endovascular techniques for aortic repair has resulted in the development of multiple, large diameter, compliant aortic balloons4-6 and the re-emergence of aortic balloon occlusion for hemorrhage control. First used in the Korean Conflict,4 aortic occlusion balloons have recently been shown to be clinically effective in managing shock due to ruptured aneurysms,6,7 post-partum hemorrhage,8 massive gastrointestinal bleeding,9 and multiorgan trauma.10-14 In many situations, the use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) may offer a more rapid non-compressible hemorrhage control compared to conventional aortic exposure and clamping.3,7,15-17
The safe and effective use of occlusion balloon technology depends on multiple factors, including the optimal balloon inflation parameters. Underinflated balloons might be ineffective in maintaining central pressure and limiting blood loss, while also potentially predisposing to downstream migration from the intended aortic occlusion zone. Conversely, overinflation can cause vessel injury or catastrophic rupture, thereby exacerbating rather than reducing hemorrhage.18 Aortic balloon occlusion and rupture characteristics have recently been reported in swine,19 but human data are lacking comparison of balloon inflation parameters between occlusion and rupture events.8,20,21 The goal of this study was to determine balloon inflation characteristics associated with benchtop flow occlusion and aortic/balloon rupture in ex vivo human aortas and test the hypothesis that optimal balloon inflation characteristics depend on systolic pressure and subject demographics. To our knowledge, this is the first study attempting to comprehensively characterize flow occlusion and rupture events in human aortas under controlled conditions.
2. Methods
2.1. Sample preparation
Human aortas from 79 tissue donors (median age 52 ± 18 years, range 13-75 years, 52 M/27 F) were procured by Live On Nebraska within 24 hours of death after obtaining consent from next of kin. Sex, age, and cause of death were recorded for each subject. Fresh aortas were freed from the surrounding tissues, and their side branches were ligated with 4-0 silk sutures or titanium clips on the benchtop. Aortic segments were then mounted on a custom-designed support structure and pre-stretched to their in-situ length measured at the time of the recovery. When the in-situ length measurement was unavailable, pre-stretch values were estimated using an aortic pre-stretch regression model22 based on the donor’s age.
2.2. Flow circuit and data acquisition
Volume-pressure-diameter relations for the unconstrained ER-REBOA and Coda balloons were characterized prior to occlusion and rupture tests (Figure S1 in the Supplemental Digital Content (SDC)). Fresh pre-stretched aortas were immersed in a heated (37 °C) PBS bath and connected to a pressurized pulsatile flow circuit (SDC Figure S2A) driven by a piston pump (Harvard Apparatus, Holliston, Massachusetts, USA). Pressures were continuously recorded with digital pressure transducers in the balloon (MCT/RAM, 0-60 psi range, < ± 0.1% accuracy/FS) and in the flow circuit (MCT/RAM, 0-15 psi range, < ± 0.1% accuracy/FS) at upstream and downstream locations from the mounted aortas. All pressure measurements were synchronized using DASYLab (Measurement Computing Corporation, Norton, MA, USA) data acquisition software. Diastolic pressure in the flow circuit was set to 40 mmHg by pressurizing a downstream PBS reservoir with air. Systolic pressures were iteratively set to 100, 150, and 200 mmHg by modulating a bypass valve or changing the stroke volume of the pump within a 30-70 cm3 range.
2.3. Occlusion and rupture tests
TA and AA occlusion tests were performed with two commonly used balloon catheters, the ER-REBOA (ER7232A, Prytime Medical, Boerne, TX, USA) and the Coda (2-10.0-35-120-40, Cook Medical, Bloomington, IN, USA) each at 100/40, 150/40, and 200/40 mmHg flow pressures. Devices were delivered to the aortic segment through a custom-made downstream delivery port (SDC Figure S2A). Coda balloons were delivered and inflated over 0.035-inch diameter guidewires, while ER-REBOA balloons were delivered and tested without guidewires. Balloons were inflated with deionized water using an insufflator, and inflation volumes were recorded. The aortic segment was considered occluded when no difference between systolic and diastolic pressures could be detected downstream from the balloon. After completing the occlusion tests, TA and AA rupture testing was performed without pulsatile flow. Because rupture tests are destructive, only one rupture test per aortic segment was performed, and it was done using only the Coda balloon. The test was stopped when either the aorta or the balloon ruptured. The aortic rupture was manifested by a sudden drop in balloon pressure and balloon protrusion through the aorta wall (SDC Figure S3B), while balloon rupture was manifested by a complete loss of balloon pressure. After rupture tests, aortic rings from the proximal and distal locations were immersed in PBS and photographed to determine the unpressurized internal diameter (ID) and wall thickness (WT).
2.4. Statistical analysis
Group comparisons were performed using two-tailed independent sample t-tests. Correlation between continuous variables was assessed by calculating Pearson’s correlation coefficient r with values closer to ± 1 indicating a stronger correlation, and values closer to zero indicating a weaker correlation. Statistical significance of the correlation was assessed by testing the null hypothesis against the alternative of having a nonzero correlation assuming statistical significance at p ≤ 0.05. The analysis was performed with MS Excel 365 (Microsoft, Redmond, WA, USA).
3. Results
3.1. Aortic occlusion and rupture as a function of age
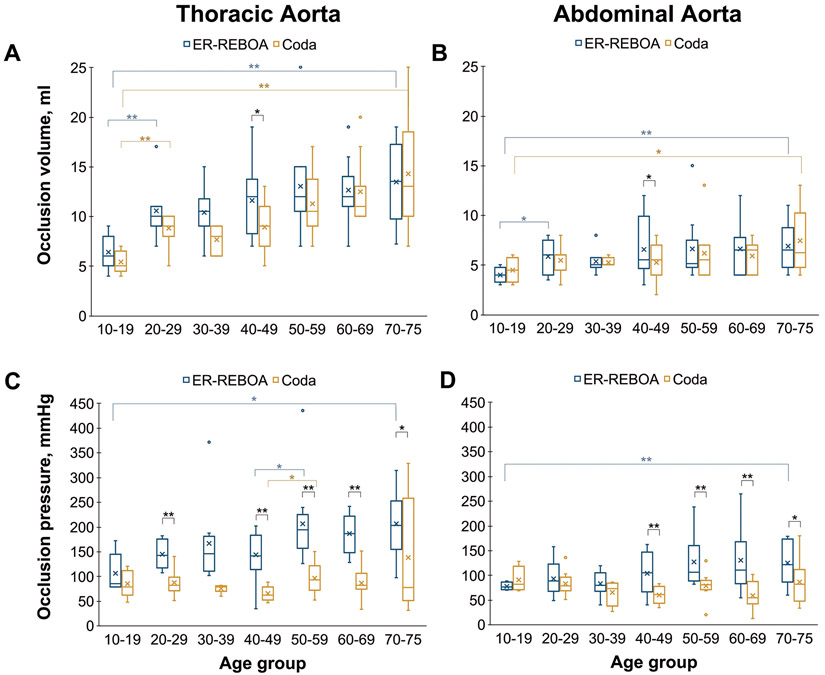
Donor information and aortic morphometry with respect to age are summarized in the SDC (Table S1, Figure S2B, Figure S4). Balloon inflation volumes required for TA occlusion (Figure 1A) increased with age for both the ER-REBOA (r = 0.44, p < 0.01) and the Coda (r = 0.61, p < 0.01) balloons. On average, it required 2.1-fold (13.4 ± 3.9 vs. 6.4 ± 1.8 ml, p < 0.01) and 2.6-fold (14.3 ± 6.0 vs. 5.4 ± 1.1 ml, p < 0.01) larger balloon volumes to occlude the TA in the oldest age group compared with the youngest when using the ER-REBOA and Coda balloons, respectively (Figure 1A). Balloon inflation pressures exhibited a stronger correlation with age for the ER-REBOA (r = 0.41, p < 0.01) than for the Coda balloon (r = 0.26, p = 0.04). Balloon inflation pressures in the oldest age group compared to the youngest were 1.9-fold (p < 0.01) higher for the ER-REBOA, and 1.6-fold (p = 0.13) higher for the Coda (Figure 1C).
Figure 1.
Balloon occlusion volumes and pressures in seven age groups for the ER-REBOA (blue) and the Coda (mustard yellow) balloon catheters at 100/40 mmHg flow pressure. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circles. Statistical significance is marked with single (*, p < 0.05) and double (**, p < 0.01) asterisks.
Across all age groups, balloon inflation volumes during AA occlusion were 55 ± 15% and 61 ± 20% of the volumes required to occlude the TA with the ER-REBOA and Coda balloons, respectively (Figure 1B). Balloon inflation volumes during AA occlusion exhibited a relatively weak correlation with age for both the ER-REBOA (r = 0.26, p = 0.04) and Coda (r = 0.32, p = 0.01) balloons. Nevertheless, balloon inflation volumes in the oldest age group were approximately 1.7-fold larger than in the youngest age group for both the ER-REBOA (6.9 ± 2.5 vs. 4.0 ± 0.8 ml, p < 0.01) and the Coda (7.5 ± 3.1 vs. 4.5 ± 1.3 ml, p = 0.03) devices, respectively. Across all age groups, balloon inflation pressures during AA occlusion (100/40 mm flow) were 70 ± 30% (ER-REBOA) and 91 ± 40% (Coda) of the inflation pressures necessary for the TA occlusion. Inflation pressures during AA occlusion exhibited a weak positive correlation with age for the ER-REBOA (r = 0.26, p = 0.04), while for the Coda balloon no significant correlation was observed (r = −0.15, p = 0.24). The mean inflation pressure during 100/40 mmHg flow occlusion in the oldest age group for the ER-REBOA was 60% higher than in the youngest age group (125 ± 42 vs. 77 ± 8 mmHg, p < 0.01), while for the Coda it was 20% lower (74 ± 45 vs. 90 ± 26 mmHg), although the latter difference was not statistically significant (p = 0.43).
Of the 132 recorded rupture events, 109 (83%) were aortic ruptures, and 23 (17%) were Coda balloon ruptures, with all aortas rupturing circumferentially. Balloon ruptures occurred at similar inflation volumes as aortic ruptures in both the TA (39.4 ± 6.7 ml vs. 37.6 ± 5.4 ml, p = 0.29) and the AA (27.7 ± 8.0 ml vs. 27.9 ± 5.8 ml, p = 0.94), but the mean inflation pressures associated with balloon rupture compared with the aortic rupture were nearly 30% higher in both the TA (1553 ± 310 mmHg vs. 1220 ± 375 mmHg, p < 0.01) and the AA (1743 ± 323 mmHg vs. 1351 ± 485 mmHg, p < 0.01). Coda balloon inflation parameters associated with balloon/aortic rupture events in all age groups are presented in Figure 2. The inflation volume associated with the balloon/aortic rupture in the TA did not correlate with age (r = 0.12, p = 0.33), while in the AA segment, the correlation was weak but statistically significant (r = −0.26, p = 0.04). Conversely, balloon inflation pressures associated with balloon/aortic rupture exhibited a negative correlation with age in both the TA (r = −0.59, p < 0.01) and the AA (r = −0.35, p < 0.01). Across all age groups, rupture events in the TA on average required larger balloon inflation volumes than in the AA (39.1 ± 6.5 ml vs. 27.7 ± 7.7 ml, p < 0.01), but the associated balloon pressures appeared to be smaller in the TA (1284 ± 385 mmHg vs. 1410 ± 483 mmHg), although this difference was not statistically significant (p = 0.07). The maximum inflation volume recommended by the manufacturer is 24 ml for the ER-REBOA balloon and 40 ml for the Coda (2-10.0-35-120-40) balloon. In this study, 8 (10%) rupture events in the TA and 35 (44%) rupture events in the AA occurred at or below 24 ml of balloon inflation volume, while 48 (61%) rupture events in the TA and 75 (95%) rupture events in the AA occurred at or below the 40 ml balloon inflation volume.
Figure 2.
Coda balloon inflation volumes (A) and pressures (B) resulting in rupture across all seven age groups. Data presented are for the TA (red) and the AA (violet) aortic segments. All rupture tests were done with a Coda balloon. Manufacturer-indicated maximum inflation volumes of the ER-REBOA and Coda balloons are shown in panel A with dashed blue and dashed mustard yellow lines. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circles. Statistical significance (p ≤ 0.01) is marked with double asterisks (**).
3.2. Aortic occlusion as a function of systolic pressure
Figure 3 summarizes the balloon inflation parameters required for aortic occlusion with the ER-REBOA and Coda balloons under various systolic flow pressures. In the TA, the mean occlusion volumes for both types of balloons increased proportionally with the increase in systolic pressure, from 11.7 ± 3.8 ml and 10.6 ± 4.3 ml at 100/40 mmHg to 14.7 ± 4.2 ml and 13.7 ± 5.2 ml at 200/40 mmHg for the ER-REBOA and Coda balloons, respectively. Despite the Coda balloon requiring a smaller inflation volume than the ER-REBOA to occlude the TA at each systolic pressure level, this difference was only statistically significant during 150/40 mmHg flow occlusion (p = 0.047). Higher systolic flow pressure also required higher balloon inflation pressures to produce stable occlusion with both devices. Specifically, to effectively occlude the 100/40 mmHg flow in the TA, the ER-REBOA balloon needed to be inflated with 174 ± 65 mmHg pressure while the Coda balloon needed only 94 ± 57 mmHg pressure, but, for 200/40 mmHg flow occlusion, these values increased to 244 ± 77 mmHg and 168 ± 72 mmHg, respectively, with ER-REBOA always requiring higher inflation pressures to produce a stable TA occlusion than the Coda (p < 0.01).
Figure 3.
Balloon inflation parameters required to occlude the TA and the AA at different systolic flow pressures using the ER-REBOA (blue) and the Coda (mustard yellow) balloons. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circles. Statistical significance is marked with single (*, p<0.05) and double (**, p<0.01) asterisks.
During AA occlusion, the inflation volumes for both balloons also increased proportionally with higher systolic pressures, from 6.2 ± 2.6 ml and 5.9 ± 2.2 ml at 100/40 mmHg to 8.2 ± 3.5 ml and 7.3 ± 2.9 ml at 200/40 mmHg for the ER-REBOA and CODA balloons, respectively. The difference between the ER-REBOA and the Coda inflation volumes in the AA was not statistically significant at all flow conditions (p = 0.12-0.33). The increase in systolic pressure also resulted in higher balloon inflation pressures required to achieve a stable occlusion in the AA, and the ER-REBOA balloon required higher inflation pressure than the Coda balloon at all flow conditions (p < 0.01), i.e., 110 ± 47 vs. 71 ± 30 mmHg at 100/40 mmHg and 179 ± 72 vs. 126 ± 39 mmHg at 200/40 mmHg.
3.3. Comparison of the Coda balloon inflation parameters between aortic occlusion and rupture events
The Coda balloon inflation parameters associated with occlusion and rupture events in the TA and AA are summarized and compared in Figure 4, demonstrating overlaps between occlusion and rupture parameters in both aortic segments. Excluding one likely outlier of AA rupture (please see details in the SDC), there were 2 (3%) cases in the TA and 0 (0%) cases in the AA when balloon inflation volume required for 100/40 mmHg flow occlusion was equal to or larger than the smallest recorded balloon volume associated with rupture in the respective segment. For the 200/40 mmHg flow occlusion, it was true in 4 (6%) cases in the TA and 2 (3%) cases in the AA. During TA occlusion experiments at 100/40, 150/40, and 200/40 mmHg flows, there were 10 (15%), 20 (31%), and 25 (37%) cases, respectively, when the required balloon inflation volumes were larger than the smallest inflation volume associated with the AA rupture (14 ml). Contrary to balloon inflation volumes, there were no overlaps in balloon inflation pressures between occlusion and rupture events in either TA or AA even at 200/40 mmHg flow occlusion, when the above-mentioned outlier was excluded, and there were no overlaps in balloon inflation pressures between any TA occlusion and AA rupture events.
Figure 4.
Balloon volumes (A) and pressures (B) during aortic occlusion and rupture events with the Coda balloon in the TA (red) and AA (violet). Data are provided for all age groups combined. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circles. Statistical significance (p ≤ 0.01) is marked with double asterisks (**).
3.4. Differences in aortic occlusion and rupture characteristics between male and female aortas
The differences in ID and WT values between male and female aortas are summarized in the SDC Figure S5. Male aortas required larger balloon inflation volumes than female aortas to produce occlusion of the 100/40 mmHg flow with the ER-REBOA device in both the TA (12.7 ± 3.7 ml vs. 9.8 ± 3.1 ml, p < 0.01) and the AA (7.0 ± 2.7 ml vs. 4.7 ± 1.3 ml, p < 0.01) segments. However, during occlusion with the Coda balloon, this was only true in the AA (6.3 ± 2.2 ml vs. 4.9 ± 1.8 ml, p = 0.014) but not in the TA (10.8 ± 3.8 ml vs. 10.1 ± 5.1 ml, p = 0.57). Male aortas also required larger balloon inflation volumes than female aortas to induce rupture in both the TA (41.1 ± 5.3 ml vs. 35.5 ± 7.0 ml, p < 0.01) and the AA (30.5 ± 6.9 ml vs. 22.2 ± 6.0 ml, p < 0.01) as illustrated in Figure 5A,B.
Figure 5.
Differences in aortic occlusion and rupture characteristics between female (F – red) and male (M – violet) aortas. A and C – balloon inflation volumes and pressures in the TA; B and D – balloon inflation volumes and pressures in the AA. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circles. Insets in panels D-F show balloon occlusion parameters at a magnified scale. Statistical significance is marked with double (**, p ≤ 0.01) asterisks.
Unlike balloon inflation volumes, balloon inflation pressures during occlusion did not show statistically significant differences between male and female aortas (Figure 5C,D). Flow occlusion with the ER-REBOA device required similar balloon inflation pressures between male and female aortas: 179 ± 72 mmHg vs. 163 ± 48 mmHg (p = 0.29) in the TA and 115 ± 48 mmHg vs. 99 ± 42 mmHg (p = 0.19) in the AA, respectively. Coda balloon inflation pressures during occlusion were also similar between male and female aortas: 89 ± 47 mmHg vs. 103 ± 73 mmHg (p = 0.40) in the TA and 73 ± 30 mmHg vs. 67 ± 28 mmHg (p = 0.41) in the AA, respectively. Balloon inflation pressures were also similar between male and female aortas during rupture events in the TA (1276 ± 352 mmHg vs. 1298 ± 445 mmHg, p = 0.84) and AA (1458 ± 431 mmHg vs. 1316 ± 572 mmHg, p = 0.31).
4. Discussion
The use of REBOA for controlling non-compressible hemorrhage both inside and outside the operating room has many challenges,17,23-28 including proper patient selection, successful navigation of the occlusion balloon, selection of the appropriate balloon inflation parameters, and mitigating issues of prolonged ischemia and reperfusion injury. As a result, the reported rates of mortality and morbidity in patients receiving REBOA vary widely between studies.29-31 Nevertheless, REBOA is gaining popularity and demonstrating utility for rapid control of catastrophic bleeding both in the hospital and in the field,32,33 and substantial efforts are focused on making it safer and more effective through enabling automated vessel access, refining inflation techniques (i.e., p-REBOA),34 or characterizing catheter advancement distances.20,28,35,36 In this study, we present a comprehensive analysis evaluating the role of age, sex, aortic morphometry, flow pressures, balloon type, and inflation volume and pressure in the safety and effectiveness of REBOA occlusion.
Our results demonstrate that the balloon inflation volumes required to achieve stable occlusion can be larger or smaller than the starting volumes recommended by Prytime Medical – 2ml for aortic Zone 3 (AA) and 8ml for aortic Zone 1 (TA). Though balloon inflation parameters are scarcely presented in the literature, a recent prospective study by Meyer et al.20 reported ER-REBOA balloon inflation volumes in trauma patients. In their clinical study, the median balloon inflation volume in the TA (aortic Zone 1) was 14 ml (range 3-24 ml) for subjects with a median age of 44 years (range 29-61 years) and a median systolic pressure of 106 (range 93-118) mmHg. Occlusion of the AA (aortic Zone 3) required 10 ml (range 4-22 ml) for subjects with a median age of 30 years (range 22-45 years) and a median systolic pressure of 118 (range 99-135) mmHg. Though these values appear somewhat larger than the median values in our study (TA: 11 ml for ER-REBOA and 10 ml for the Coda, AA: 5 ml for ER-REBOA, and 6 ml for Coda), the discrepancy might be stemming from the differences in the experimental setting (i.e. in vivo vs. in vitro), systolic pressure, and subject demographics such as age and sex.
Two parameters frequently monitored during the aortic occlusion are the patient’s blood pressure and the balloon inflation volume. An increase in systolic pressure during aortic occlusion was reported by several other studies.17,18,20,26,37,38 Our observations agree with these reports and inform the values of balloon occlusion pressure and volume for different systolic pressure conditions. We have also demonstrated that these parameters are device-dependent since the ER-REBOA balloon consistently required a larger inflation volume than the Coda in all age groups and flow conditions, although this difference was not always statistically significant. While the differences in balloon inflation characteristics (SDC Figure S1) certainly play important roles in occlusion parameters, another possible contributing factor could be the influence of the guidewire used with the Coda balloon that may have helped to secure its position and produce a stable occlusion with lower inflation parameters.17
Age and sex are known to affect the arterial diameter and mechanical properties,28,39-43 which could explain our current finding that they also affect balloon occlusion and rupture parameters. Our results demonstrate that older TAs require larger balloon inflation volumes to achieve occlusion, but this was not observed by Meyer et al.20, who reported no correlation between age and balloon inflation volume when occluding the TA. In the AA, we observed a weak correlation between donor’s age and the Coda balloon inflation volume during occlusion, but not for the ER-REBOA, which is in better agreement with the findings of Meyer et al. Importantly, the median subject age and rage were different in our study (median 52 years [range 13-75 years]) and in that of Meyer et al. (median 44 years [range 29-61 years]). Our results also demonstrate that male aortas generally have larger diameters, resulting in larger required balloon inflation volumes during occlusion. They also suggest a possible risk of aortic/balloon rupture when the balloons are inflated to their maximum capacity or misplaced in the AA and inflated to volumes intended for the TA occlusion. The risk of rupture is likely higher in older aortas because they required larger inflation volumes to achieve the occlusion and because they had a reduced aortic wall strength44 and therefore, lower rupture pressures (Figure 2B). It is also important to note that all aortas ruptured in the circumferential direction, which is in good agreement with the observations by Wasicek et al.19 done using porcine aortas.
Our experiments demonstrated that control of the balloon pressure rather than the volume during inflation could significantly reduce the risk of aortic rupture or damage since the difference between occlusion and rupture balloon pressures were over 13-fold with no overlaps. More importantly, there were no overlaps in balloon pressures between the AA rupture and the TA occlusion, suggesting that pressure-guided balloon inflation could help avoid aortic ruptures in case of balloon misplacement or migration. While we are not aware of other studies investigating balloon pressures during aortic occlusion and rupture in human aortas, McCarthy et al.45 reported balloon pressures < 200 mmHg during aortic occlusion in swine.
The presented results provide new insights into safe balloon occlusion parameters for human aortas in different age groups and both sexes, but they need to be considered in the context of study limitations. First, the lack of supporting surrounding tissues and the use of PBS instead of blood in our benchtop model make it substantially different from in vivo conditions. Second, we did not analyze aortic dissections, i.e., partial ruptures, that may have serious clinical implications. While these and other limitations are being addressed, we hope that the presented analysis will help develop safer procedures and devices for non-compressible hemorrhage control.
5. Conclusions
Balloon inflation parameters associated with human aortic flow occlusion and rupture events were characterized in a controlled benchtop setting showing dependence on donor demographic factors, anatomic location, flow pressures, and device type. Our results suggest that pressure-guided rather than volume-guided balloon inflation may reduce the risk of aortic rupture. Despite the limitations of the benchtop setting, this study provides useful information that can be used to improve the safety and efficacy of REBOA procedures and future devices.
Supplementary Material
Figure S1. Volume-pressure-diameter relations for the unconstrained ER-REBOA and Coda balloons inflated in air. Whiskers extend to one standard deviation.
Figure S2. Experimental setup (A) and distribution of aortic specimens by age groups (B). TA = Descending Thoracic Aorta (red), AA = Abdominal Aorta (violet). Please note that since some aortas only included the TA or the AA segment, the total number of donors in most age groups is higher than the number of TA and AA tests.
Figure S3. Pressure during occlusion (A) and rupture events (B) in a 64-year-old male TA using a Coda balloon. Note that the upstream systolic pressure (red) increases as the distal systolic pressure (dashed blue) decreases during the aortic occlusion (A). To compensate for this increase, pump stroke volume and balloon inflation (green) were adjusted accordingly, resulting in 100/40 mmHg systolic/diastolic flow pressure equilibrium upstream from the balloon. Red arrows in panel B indicate balloon location.
Figure S4. Unpressurized inner diameter (A) and wall thickness (B) of Thoracic (TA – red) and Abdominal (AA – violet) aortas in seven age groups. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circles. Statistical significance is marked with single (*, p ≤ 0.05) and double (**, p ≤ 0.01) asterisks.
Figure S5. Differences in unpressurized inner diameter (A) and wall thickness (B) between female (F – red) and male (M – violet) aortas. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circlesStatistical significance (p ≤ 0.01) is marked double (**) asterisks.
6. Acknowledgments
The research reported in this publication was supported in part by the U.S. Army Medical Research and Materiel Command (USAMRMC) under Award Number W81XWH-16-2-0034, Log 14361001, The National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers HL125736 and HL147128. The authors also wish to acknowledge Live On Nebraska for their help and support and express their deepest gratitude to tissue donors and their families for making this study possible. Finally, we greatly appreciate the help of Paul Deegan and Thomas Kalil in developing the aortic flow circuit and data acquisition system.
Funding disclosure
This research was partially funded by the National Heart, Lung, And Blood Institute of the National Institutes of Health.
Footnotes
Conflicts of Interest and Source of Funding
The authors declare no conflicts of interest.
List of meetings
Part of this work was previously presented (poster) in the MHSRS meeting on August 21, 2018 in Kissimmee, Florida.
References
- 1.Dorlac WC, DeBakey ME, Holcomb JB, Fagan SP, Kwong KL, Dorlac GR, Schreiber MA, Persse DE, Moore FA, Mattox KL. Mortality from isolated civilian penetrating extremity injury. J Trauma. 2005;59(1):217–222. [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma - Inj Infect Crit Care. 2006;60(6 SUPPL.):3–11. [DOI] [PubMed] [Google Scholar]
- 3.Kerby JD, Cusick MV. Prehospital emergency trauma care and management. Surg Clin North Am. 2012;92(4):823–841, vii. [DOI] [PubMed] [Google Scholar]
- 4.Hughes CW. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery. 1954;36(1):65–68. [PubMed] [Google Scholar]
- 5.Low RB, Longmore W, Rubinstein R, Flores L, Wolvek S. Preliminary report on the use of the Percluder occluding aortic balloon in human beings. Ann Emerg Med. 1986;15(12):1466–1469. [DOI] [PubMed] [Google Scholar]
- 6.Arthurs Z, Starnes B, See C, Andersen C. Clamp Before You Cut: Proximal Control of Ruptured Abdominal Aortic Aneurysms Using Endovascular Balloon Occlusion: Case Reports. Vasc Endovascular Surg. 2006;40(2):149–155. [DOI] [PubMed] [Google Scholar]
- 7.Mayer D, Pfammatter T, Rancic Z, Hechelhammer L, Wilhelm M, Veith FJ, Lachat M. 10 Years of Emergency Endovascular Aneurysm Repair for Ruptured Abdominal Aortoiliac Aneurysms: Lessons Learned. Ann Surg. 2009;249(3):510–515. [DOI] [PubMed] [Google Scholar]
- 8.Søvik E, Stokkeland P, Storm BS, Asheim P, Bolås O, Äsheim P, Bolås O. The use of aortic occlusion balloon catheter without fluoroscopy for life-threatening post-partum haemorrhage. Acta Anaesthesiol Scand. 2012;56(3):388–393. [DOI] [PubMed] [Google Scholar]
- 9.Karkos CD, Bruce I a., Lambert ME. Use of the intra-aortic balloon pump to stop gastrointestinal bleeding. Ann Emerg Med. 2001;38(3):328–331. [DOI] [PubMed] [Google Scholar]
- 10.Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78(4):721–728. [DOI] [PubMed] [Google Scholar]
- 11.Irahara T, Sato N, Moroe Y, Fukuda R, Iwai Y, Unemoto K. Retrospective study of the effectiveness of Intra- Aortic Balloon Occlusion (IABO) for traumatic haemorrhagic shock. World J Emerg Surg. 2015;10(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delamare L, Crognier L, Conil J-M, Rousseau H, Georges B, Ruiz S. Treatment of intra-abdominal haemorrhagic shock by Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Anaesth Crit Care Pain Med. 2015;34(1):53–55. [DOI] [PubMed] [Google Scholar]
- 13.Martinelli T, Thony F, Decléty P, Sengel C, Broux C, Tonetti J, Payen J-F, Ferretti G. Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma. 2010;68(4):942–948. [DOI] [PubMed] [Google Scholar]
- 14.Miura F, Takada T, Ochiai T, Asano T, Kenmochi T, Amano H, Yoshida M. Aortic Occlusion Balloon Catheter Technique Is Useful for Uncontrollable Massive Intraabdominal Bleeding After Hepato-Pancreato-Biliary Surgery. J Gastrointest Surg. 2006;10(4):519–522. [DOI] [PubMed] [Google Scholar]
- 15.Mehta M, Taggert J, Darling RC, Chang BB, Kreienberg PB, Paty PSK, Roddy SP, Sternbach Y, Ozsvath KJ, Shah DM. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg. 2006;44(1):1–8; discussion 8. [DOI] [PubMed] [Google Scholar]
- 16.Karkov CD, Harkin DW, Giannakou A, Gerassimidis TS. Mortality After Endovascular Repair of Ruptured Abdominal Aortic Aneurysms: A systematic Review and Meta Analysis. Arch Surg. 2009;144(8):770–778. [DOI] [PubMed] [Google Scholar]
- 17.Brenner M, Inaba K, Aiolfi A, DuBose J, Fabian T, Bee T, Holcomb JB, Moore L, Skarupa D, Scalea TM, et al. Resuscitative Endovascular Balloon Occlusion of the Aorta and Resuscitative Thoracotomy in Select Patients with Hemorrhagic Shock: Early Results from the American Association for the Surgery of Trauma’s Aortic Occlusion in Resuscitation for Trauma and Acu. J Am Coll Surg. 2018;226(5):730–740. [DOI] [PubMed] [Google Scholar]
- 18.Morrison JJ, Galgon RE, Jansen JO, Fficm F, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80(2):324–334. [DOI] [PubMed] [Google Scholar]
- 19.Wasicek PJ, Teeter WA, Brenner ML, Hoehn MR, Scalea TM, Morrison JJ. Resuscitative endovascular balloon occlusion of the aorta: rupture risk and implications for blind inflation. Trauma Surg Acute Care Open. 2018;3(1):e000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer DE, Mont MT, Harvin JA, Kao LS, Wade CE, Moore LJ. Catheter distances and balloon inflation volumes for the ER-REBOA™ catheter: A prospective analysis. Am J Surg. 2019;(xxxx). [DOI] [PubMed] [Google Scholar]
- 21.Gerard J, Skertich NJ, Wiegmann A, Bokhari F. Reboa catheter balloon rupture during CPR. Am Surg. 2020;86(4):E196–E197. [PubMed] [Google Scholar]
- 22.Horný L, Netušil M, Voňavková T. Axial prestretch and circumferential distensibility in biomechanics of abdominal aorta. Biomech Model Mechanobiol. 2014;13(4):783–799. [DOI] [PubMed] [Google Scholar]
- 23.Allen B, Callaway D, Gibbs M, Noste E, West K, Johnson MA, Caro D, Godwin A. Regarding the Joint Statement From the American College of Surgeons Committee on Trauma and the American College of Emergency Physicians Regarding the Clinical Use of Resuscitative Endovascular Balloon Occlusion of the Aorta. J Emerg Med. 2018;55(2):266–268. [DOI] [PubMed] [Google Scholar]
- 24.Davidson AJ, Russo RM, Reva VA, Brenner ML, Moore LJ, Ball C, Bulger E, Fox CJ, Dubose JJ, Moore EE, et al. The pitfalls of resuscitative endovascular balloon occlusion of the aorta: Risk factors and mitigation strategies. J Trauma Acute Care Surg. 2017;84(1):192–202. [DOI] [PubMed] [Google Scholar]
- 25.Brenner M, Bulger EM, Perina DG, Henry S, Kang CS, Rotondo MF, Chang MC, Weireter LJ, Coburn M, Winchell RJ, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg Acute Care Open. 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchino H, Tamura N, Echigoya R, Ikegami T, Fukuoka T. REBOA - Is it really safe? A case with massive intracranial hemorrhage possibly due to endovascular balloon occlusion of the aorta (REBOA). Am J Case Rep. 2016;17:810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doucet J, Coimbra R. REBOA: Is it ready for prime time? J vasc Bras. 2017;16(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacTaggart JN, Poulson WE, Akhter M, Seas A, Thorson K, Phillips NY, Desyatova AS, Kamenskiy AV. Morphometric roadmaps to improve accurate device delivery for fluoroscopy-free resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2016;80(6):941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison JJ, Rasmussen TE. Noncompressible Torso Hemorrhage: A Review with Contemporary Definitions and Management Strategies. Houston, TX; 2015. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro Junior MAF, Feng CYD, Nguyen ATM, Rodrigues VC, Bechara GEK, de-Moura RR, Brenner M. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J Emerg Surg. 2018;13(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howie W, Broussard M, Batoon B. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an option for uncontrolled hemorrhagic shock: Current best practices and anesthetic implications. AANA J. 2019;87(1):19–25. [PubMed] [Google Scholar]
- 32.Pasley JD, Teeter WA, Gamble WB, Wasick P, Romagnoli AN, Pasley AM, Scalea TM, Brenner ML. Bringing Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) Closer to the Point of Injury. J Spec Oper Med. 2018;18(1):33–36. [DOI] [PubMed] [Google Scholar]
- 33.Ross EM, Redman TT. Feasibility and Proposed Training Pathway for Austere Application of Resuscitative Balloon Occlusion of the Aorta. J Spec Oper Med. 2018;18(1):37–43. [DOI] [PubMed] [Google Scholar]
- 34.Russo RM, Neff LP, Johnson MA, Williams TK. Emerging Endovascular Therapies for Non-Compressible Torso Hemorrhage. Shock. 2016;46(3S):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pezy P, Flaris AN, Prat NJ, Cotton F, Lundberg PW, Caillot JL, David JS, Voiglio EJ. Fixed-distance model for balloon placement during fluoroscopy-free resuscitative endovascular balloon occlusion of the aorta in a civilian population. JAMA Surg. 2017;152(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stannard A, Morrison JJ, Sharon DJ, Eliason JL, Rasmussen TE. Morphometric analysis of torso arterial anatomy with implications for resuscitative aortic occlusion. J Trauma Acute Care Surg. 2013;75(2 SUPPL. 2):169–172. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Kim K, Jo YH, Lee JH, Kim J, Chung H, Hwang JE. Use of resuscitative endovascular balloon occlusion of the aorta in a patient with gastrointestinal bleeding. Clin Exp Emerg Med. 2016;3(1):55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner M, Teeter W, Hoehn M, Pasley J, Hu P, Yang S, Romagnoli A, Diaz J, Stein D, Scalea T. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg. 2018;153(2):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamenskiy AV, Pipinos II, Dzenis Y a, Phillips NY, Desyatova AS, Kitson J, Bowen R, MacTaggart JN. Effects of age on the physiological and mechanical characteristics of human femoropopliteal arteries. Acta Biomater. 2015;11. [DOI] [PubMed] [Google Scholar]
- 40.Anttila E, Balzani D, Desyatova A, Deegan P, MacTaggart J, Kamenskiy A. Mechanical damage characterization in human femoropopliteal arteries of different ages. Acta Biomater. 2019;90:225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamenskiy AVAV, Dzenis YYAA, Kazmi SAJSAJSAJ, Pemberton MMAAMAA, Pipinos III, Phillips NNYY, Herber K, Woodford T, Bowen RREE, Lomneth CSCSC, et al. Biaxial mechanical properties of the human thoracic and abdominal aorta, common carotid, subclavian, renal and common iliac arteries. Biomech Model Mechanobiol. 2014;13(6):1341–1359. [DOI] [PubMed] [Google Scholar]
- 42.Kamenskiy A, Miserlis D, Adamson P, Adamson M, Knowles T, Neme J, Koutakis P, Phillips N, Pipinos I, MacTaggart J. Patient demographics and cardiovascular risk factors differentially influence geometric remodeling of the aorta compared with the peripheral arteries. Surgery. 2015;158(6):1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jadidi M, Habibnezhad M, Anttila E, Maleckis K, Desyatova A, MacTaggart J, Kamenskiy A. Mechanical and structural changes in human thoracic aortas with age. Acta Biomater. 2020;103:172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Herrera CM, Atienza JM, Rojo FJ, Claes E, Guinea GV, Celentano DJ, García-Montero C, Burgos RL. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med Biol Eng Comput. 2012;50(6):559–566. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy C, Kanterman I, Trauzettel F, Jaeger HA, Goetz AA, Colvard B, Swanstrom L, Cantillon-Murphy P. Automated Balloon Control in Resuscitative Endovascular Balloon Occlusion of the Aorta. IEEE Trans Biomed Eng. 2019;66(6):1723–1729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Volume-pressure-diameter relations for the unconstrained ER-REBOA and Coda balloons inflated in air. Whiskers extend to one standard deviation.
Figure S2. Experimental setup (A) and distribution of aortic specimens by age groups (B). TA = Descending Thoracic Aorta (red), AA = Abdominal Aorta (violet). Please note that since some aortas only included the TA or the AA segment, the total number of donors in most age groups is higher than the number of TA and AA tests.
Figure S3. Pressure during occlusion (A) and rupture events (B) in a 64-year-old male TA using a Coda balloon. Note that the upstream systolic pressure (red) increases as the distal systolic pressure (dashed blue) decreases during the aortic occlusion (A). To compensate for this increase, pump stroke volume and balloon inflation (green) were adjusted accordingly, resulting in 100/40 mmHg systolic/diastolic flow pressure equilibrium upstream from the balloon. Red arrows in panel B indicate balloon location.
Figure S4. Unpressurized inner diameter (A) and wall thickness (B) of Thoracic (TA – red) and Abdominal (AA – violet) aortas in seven age groups. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circles. Statistical significance is marked with single (*, p ≤ 0.05) and double (**, p ≤ 0.01) asterisks.
Figure S5. Differences in unpressurized inner diameter (A) and wall thickness (B) between female (F – red) and male (M – violet) aortas. Whiskers extend to the minimum and maximum values, boxes represent the interquartile range (IQR), the median is shown as a horizontal band within the box, the mean value is marked with an x, and statistical outliers (values that lie 1.5 IQRs or more outside the box) are shown as hollow circlesStatistical significance (p ≤ 0.01) is marked double (**) asterisks.