Abstract
Background
The rationale for Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is to control life-threatening sub-diaphragmatic bleeding and facilitate resuscitation, however, incorporating this into the resuscitative practices of a trauma service remains challenging. The objective of this study is to describe the process of successful implementation of REBOA use in an academic urban level I trauma center. All REBOA procedures from April 2014 through December 2019 were evaluated; REBOA was implemented after surgical faculty attended a required and internally developed Advanced Endovascular Strategies for Trauma Surgeons course (AESTS). Success was defined by sustained early adoption rates.
Methods
An institutional protocol was published, and a REBOA supply cart was placed in the emergency department(ED) with posters attached to depict technical and procedural details. A focused professional practice evaluation was utilized for the first three REBOA procedures performed by each faculty member, leading to internal privileging.
Results
REBOA was performed in 97 patients by 9 trauma surgeons, which is 1% of the total trauma admissions during this time. Each surgeon performed a median of 12 REBOAs (IQR: 5,14). Blunt (77/97, 81%) or penetrating abdominopelvic injuries (15/97, 15%) comprised the main injury mechanisms; 4% were placed for other reasons (4/97) including ruptured abdominal aortic aneurysms (AAA, n=3) and pre-operatively for a surgical oncologic resection (n=1). Overall survival was 65% (63/97) with a steady early adoption trend that resulted in participation in a Department of Defense (DoD) multicenter trial.
Conclusions
Strategies for how departments adopt new procedures require clinical guidelines, a training program focused on competence, and a hospital education and privileging process for those acquiring new skills.
Level of Evidence:
Level V
Keywords: REBOA, Resuscitative Endovascular Balloon Occlusion of the Aorta, Trauma Resuscitation
Introduction
Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is a minimally invasive tool for the occlusion of the aorta for hemorrhage control and the enhancement of central perfusion to facilitate resuscitation in the setting of hemorrhage[1–10]. Traditionally, resuscitative occlusion of the aorta involved a thoracotomy, which requires operative closure. Mortality rates remain high for hypotensive patients with truncal abdominal hemorrhage who live long enough to receive a laparotomy – these rates have not changed in the last two decades[11].
Balloon occlusion techniques were initially championed by C. W. Hughes and others dating back to the Korean War[12–14]. With new devices and lower profile systems, widespread implementation of endovascular technologies for trauma have expanded. Branco et al examined endovascular procedures for trauma between 2002 and 2010 in the National Trauma Data Bank (NTDB); there was a substantial increase in endovascular procedures during the 9-year evaluation with a concomitant decrease in open operative management[15]. They also noted that the increase in endovascular procedures in trauma was associated with improved in-hospital mortality and morbidity[15]. Other studies have found similar results of an uptrend in endovascular use in trauma management and have attributed this to advancements in device developments as well as an improved understanding of vascular pathology[15, 16]. Additionally, Trauma and Surgical Critical Care fellowships have promoted focused training in vascular and endovascular skills that are beyond training from general surgery residency[17]
While the role of REBOA in resuscitative practice has been described, incorporating this tool into the workflow of a trauma service remains challenging and its use appears to remain infrequent[4, 6–8, 18–20]. There are several published articles on the strategic methods of initiating and sustaining a REBOA program in a variety of settings, but little is published on this topic for major metropolitan trauma centers[3, 9, 10]. The implementation of new technology into an established trauma program requires several key components. Essential are early adopters who garner interest and have the willingness to obtain new skill sets. Additionally, there is a requirement to establish clinical guidelines for appropriate indications and provide a consensus for a privileging process prior to the use of the new equipment. Further refinements include the dissemination of the technology to a more widespread use, including local or regional applications. The objective of this overview is to describe the process of successful implementation of REBOA use in an urban academic level I trauma center. We hypothesized that after training and safe credentialing, REBOA use steadily increases over time.
Methods:
Institutional Context
The Ernest E Moore Shock Trauma Center at Denver Health Medical Center (DH) is the urban level 1 trauma center in Denver, Colorado. DH is a core teaching facility of the University of Colorado, Denver. All surgical attendings have faculty appointments with DH and the University of Colorado Hospital. DH manages on average 3100 trauma patients annually. Of these trauma patients, 71% will require admission and over 22% will have an injury severity score above 15[21]. On average, 18% of the trauma admissions are due to penetrating wounds, 1% require a resuscitative thoracotomy in the emergency department, and 18% are taken to the operating room (OR) within 4 hours of admission. Of the aforementioned 3100 trauma patients seen annually, approximately 3% will require the OR for an emergent laparotomy.
REBOA Protocol Development
Our REBOA program was initiated in 2014 after attending trauma surgeons were trained and credentialed on the REBOA. It is crucial not only to establish guidelines and protocols prior to implementing a new technology or new program, but to have supportive multidisciplinary team members; such as surgeons, emergency department physicians, nurses, and operating room teams [3]. Our surgical team jointly developed indications for REBOA placement based on current protocols already established for emergency department thoracotomy (EDT) at DH[4].
Indications for REBOA
Prior to starting REBOA in 2014, our institution published an algorithm (Figure 2) for control of exsanguinating torso hemorrhage, which was later revised to add the management of pelvic fractures and hemorrhagic shock (Figure 3). The initial resuscitative procedure is based on a clinical evaluation of the patients’ condition and source of hemorrhage based on findings on chest radiograph (CXR), pelvic radiograph, and a focused abdominal sonography for trauma (FAST exam)[4]. The algorithm was developed through consensus of surgical attendings and literature review on the topic[22]. An institutional protocol was published and a REBOA supply cart was placed in the emergency department with posters attached to depict technical and procedural details. A focused professional practice evaluation was utilized for the first three REBOA procedures performed by each faculty member, leading to internal privileging.
Figure 2:

Denver Health Algorithm for torso hemorrhage control. EDT, emergency department resuscitative thoracotomy(4).
Figure 3:
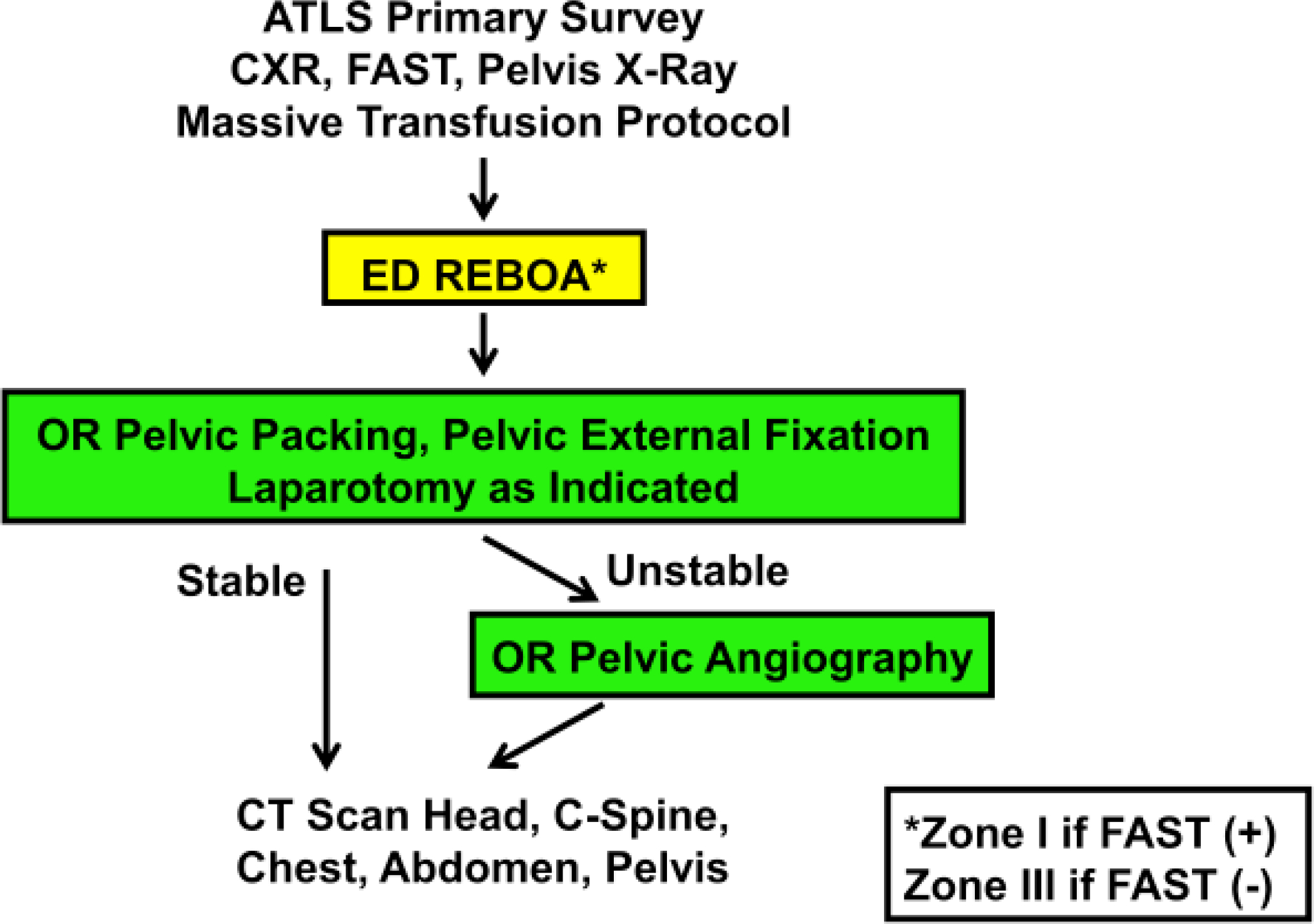
Revised Denver Health Algorithm for the management of hemodynamically unstable patients with mechanically unstable pelvic fractures. ATLS, advanced trauma life support.
REBOA Training
After protocols and indications for REBOA use were established, training and credentialing were the next key steps in establishing a successful program. Available training programs have included Basic Endovascular Skills for Trauma (BEST) and the Endovascular Skills for Trauma and Resuscitative Surgery (E-STARS) courses[5, 7]. The E-STARS course was the original REBOA training course funded by a Department of Defense (DoD) grant, developed for deploying military surgeons. A senior vascular surgeon faculty member of the E-STARS course and two trauma surgeons from DH attended this course in 2013, which they adapted to create a course specific to civilian trauma, Advanced Endovascular Skills Course for Trauma Surgeons (AESTS). The AESTS had the following learning objectives: 1) to review clinical guidelines, 2) to rehearse basic ultrasound guided vascular access techniques, and 3) to familiarize participants with the device and use of digital subtraction angiography. This course consisted of a 2-hour lecture, followed by 1-hour of simulation training, and completed with a 5-hour training with a live swine model.
The need for a one-day, on-site course was obvious as time constraints prevented providers from traveling out-of-state to attend the two-day E-STARS course, which was eventually discontinued. Additionally, our providers believed that a course with live tissue training added significant value to the understanding and use of the REBOA and this concept was adapted from the ESTARS to the AESTES course. The AESTES course remains the preferred training course at our institution.
Adapting material from the E-STARS course to develop the AESTES course required the collaboration between three surgeons over a period of approximately three months. Once the initial development phase was complete, the training aspect for staff was a single-day, on-site training. Competence in REBOA placement was determined with success at the live animal training and was further monitored with the three proctored cases. The reasoning behind three proctored cases was initially an arbitrary number following the live animal sessions, but ultimately proved to work well as the system was developed. Credentialing was completed by the medical staff office and was determined by the director of service for surgery. It was granted to providers who submitted a certificate of attendance of the AESTES course and completed a focused professional practice evaluation after these proctored REBOA cases.
REBOA Procedure Team
All REBOAs and arterial sheaths were placed by the surgical team. The decision for a surgeon-only REBOA team was multifactorial: 1) surgeons are familiar with the regional vascular anatomy and can manage complications, 2) occasionally femoral access requires a “cut-down”, which is best performed by a surgeon, 3) REBOA placement is often followed by transfer to the OR where a surgeon is required, 4) REBOAs may also be placed in the OR or in the intensive care unit(ICU) where surgeons must have the expertise for REBOA placement, 5) it was thought prudent to minimize the number of providers performing REBOA so as not to dilute the experience. This is in line with several studies that reported on their own practices or REBOA programs at high volume trauma centers, which consistently showed that the emergency department (ED) and OR were the most frequent locations of insertion, followed by radiology suite/hybrid room and field placement[8, 23]. Additionally, surgeons performing the REBOA have the required training of vascular anatomy and pathology to become board certified by the American Board of Surgery(ABS), therefore possessing the essential knowledge and skills for REBOA placement[3, 6, 9]. At our institution, ABS certification is a mandatory component to become eligible for REBOA placement either as the proceduralist or as the instructor to a trainee.
After planning, training, and credentialing but prior to the initiation of the program, it was necessary to establish a REBOA cart. This mobile cart, developed by the trauma attendings, contained all the necessary equipment and supplies to place a REBOA and was located in the ED near the trauma bays. In addition to having all the supplies in a central location, there are also step-by-step procedural depictions laminated and placed on the cart for educational reminders for any party involved in the patient resuscitation (Figure 4). This cart is built into the usual supply chain and is checked and restocked by the ED central supply team. Other studies have described the importance of developing a trauma-specific endovascular inventory supply that is organized according to equipment type[3, 24]. The cart is supplied with the REBOA kits, sterile gloves and gowns, 18-gauge needles and syringes, saline flushes, contrast, iodine, suture, a sphygmomanometer, scalpels, and gauze. With the development of the REBOA cart, in addition to educational and planning meetings, the ED staff, including the nursing teams, embraced the innovation led by our trauma team. Nursing team buy-in was of particular importance as once they embraced the concept of the REBOA, they participated in monitoring the stocking of the REBOA cart, were interested in learning about the REBOA purpose and function, and were key players in the arterial line setup and real time equipment assistance.
Figure 4:
4A: REBOA Cart located in the DH Emergency Room
4B: Top of REBOA cart with procedural pictures, algorithms, and cart contents. Attending physician personal contact number has been de-identified with white bar.
4C: Example of REBOA Cart drawer content of supplies for procedure including sterile gloves of varying sizes, suture, scalpel, manual blood pressure cuff.
4D: Example of REBOA cart drawer content of supplies containing the Prytime ER-REBOA™.
REBOA Protocol Evaluation
At the initiation of any new program, especially with new technology, there should be a physician leader in charge of a registry to document and monitor all cases in which the new technology is used. When the REBOA program was initiated at DH, a protected and encrypted database was started to track all REBOA placements within calendar year as a part of a quality improvement project. Information collected and monitored for internal evaluation include patient demographics, injury mechanism, non-traumatic indications for REBOA placement, survival, presence of pelvic fracture, ISS, hospital length of stay, REBOA location (OR or ED for placement), volume instilled in balloon (mL), catheter length, zone of inflation, occlusion time, complications related to device, and attending surgeon of record.
REBOA Protocol Implementation
Once the planning, training, and credentialing were completed, the REBOA program was initiated at DH in November of 2014. At the start of the program in 2014 and early 2015, the Cook Incorporated (Bloomington, IN USA) CODA® balloon required entry through large-bore sheaths (either 12 or 14 French) and the use of lengthy guidewires for access and balloon placement. Creation of a sterile field for use of this balloon was difficult to achieve in the busy trauma bay as a sterile field for the patient as well as for the length of the wire was required. This equipment was used until mid-2015 when the Prytime Medical ER-REBOA™ (Boerne, TX USA) became available on the market. We performed 11 placements of the CODA® balloon prior to the change in technology. The ER-REBOA™ eliminated the need for the use of wires and large-sheath access as it can be inserted through a 7 French sheath and does not require a separate operation to repair the arteriotomy. It is also thought to have fewer complications than the previously used large bore sheaths[21, 25]. Our surgeons were initially trained and credentialed on the CODA® device and participated in a separate training session when the ER-REBOA™ came into use.
Once the ER-REBOA™ became available to our institution in 2015, we quickly implemented it by 2016, similar to the national and international shifts away from the large bore sheaths in favor of the smaller arterial sheaths [8]. Additionally, we transitioned to placing 5 French arterial lines in the common femoral artery (CFA) for access for hemodynamic monitoring in the majority of all hypotensive trauma patients with sustained systolic blood pressure of <80mmHg. Established arterial access can then easily and rapidly be upsized to accommodate the 7 French sheath required for REBOA placement [1, 6].
Results
In the time since the implementation of the REBOA program, a total of nine attendings have placed 97 REBOAs for a median total of 12 REBOAs placed per attending(IQR: 5,14). Patient demographics are listed in Table 1. Overall, 81% of these were placed for blunt abdominopelvic injuries, 15% for penetrating abdominopelvic wounds, and 4% for other reasons, including ruptured AAA (n=3) and pre-operatively for a surgical oncologic tumor resection (n=1). There was some variability on the number of REBOAs placed by our surgeons; the most senior surgeons placed the most REBOAs and the surgeons recently out of fellowship placed the least amount over this time period.
Table 1:
REBOA Patient Demographics 2014–2019. (ISS = injury severity score; TBI = traumatic brain injury; ED = Emergency department; SBP = systolic blood pressure; GCS = Glasgow coma score; FAST = Focused assessment with sonography in trauma; EDT = emergency department thoracotomy; Hgb = hemoglobin; INR = international normalized ratio; Min = minutes)
| Demographic Information for REBOA | |
|---|---|
| Age (median) | 43 years (IQR28,56) |
| Sex | |
| Male | 77% |
| Female | 23% |
| ISS (median) | 34 (IQR25,43) |
| TBI total | 38 |
| Mechanism | |
| Blunt | 81.5% |
| Penetrating | 15.5% |
| Other | 3% |
| ED SBP (median) | 84mmHg (IQR70,98) |
| ED GCS (median) | 14 (IQR6,15) |
| Total FAST + | 82 |
| Total EDT | 14 |
| Hgb (median) | 12.3 g/dL (IQR10.9,14) |
| INR (median) | 1.28 (IQR1.2,1.5) |
| Lactate (median) | 5.7 mmol/L (IQR3.8,8.9) |
| Balloon Occlusion Time (median) | 33min (IQR15,52) |
As expected, there was a slow uptrend in the REBOA use followed by a substantial rise in REBOA placements. However, after a peak in 2017 of 32 total REBOA placements, DH experienced a slight downtrend in the annual number of REBOAs placed. The numbers of REBOAs placed and emergent laparotomies performed per year at our institution are depicted in Figure 1. Over this time period, the number of trauma admissions continued to increase each year. There is little published about rates of REBOA use and comparison to national or international trends are limited[18–20].
Figure 1:

Comparison of REBOA insertion, ED Thoracotomy, and Emergent Laparotomy numbers over time
We define complications associated with the REBOA as arterial or venous abnormalities occurring as a result of sheath placement or balloon inflation that were either detected clinically or through surveillance ultrasound. Complications associated with the ER-REBOA™ are listed in Table 2 (CODA® balloon n=11 were removed from complication table) and have declined over time. There was an overall complication rate of 24.4% (n=21) (37% in 2016, 26% in 2017, 23% in 2018 and 7% in 2019; p=0.33). The CODA® balloon was excluded from the complications assessment, as all placements with this device required an additional operation for arteriotomy repair (2014–2015). This complication rate of femoral artery access for sheath placement without REBOA (balloon inflation) was 6.8% (n=2), one of which was an arterial injury not requiring operative repair and the other was a hematoma associated with arterial access. Data on sheath-only femoral artery access was from 2016 forward, coinciding with the transition from the CODA® balloon to the ER-REBOA™. In total, 29 sheath-only placements have been recorded: 3 (2016), 13 (2017), 7 (2018), and 6 (2019).
Table 2:
Complications based on type of femoral arterial access. Of note, REBOA column excludes all CODA® balloon placed between 2014–2015. This data shows complications of ER-REBOA™ compared to REBOA sheath.
| ER-REBOA™ | Sheath-only | |
|---|---|---|
| Total | 86 | 29 |
| Open Arteriotomy Repair | 1 (1.2%) | 0 |
| Compartment Syndrome requiring fasciotomy | 1 (1.2%) | 0 |
| Lower Extremity Amputation | 2 (2.3%) | 0 |
| Vasospasm | 5 (5.8%) | 0 |
| Arterial Dissection | 1 (1.2%) | 0 |
| Arterial Injury not requiring repair | 1 (1.2%) | 1 (1.2%) |
| Vein injury requiring repair | 1 (1.2%) | 0 |
| Arterial Thrombus | 7 (8.1%) | 0 |
| Hematoma | 1 (1.2%) | 1 (1.2%) |
| Arterial Stenosis | 1 (1.2%) | 0 |
Barriers and Areas of Improvement
Some of the early barriers to successful implementation included the time needed to develop the AESTES course and to ensure all attending surgeon successful and safe credentialing for REBOA placement. Another early issue was the necessary use of the large bore sheath and inconvenient wires for the CODA® balloon, which was the only piece of equipment initially available. As mentioned, this equipment required a more cumbersome set-up of a sterile field in a busy trauma bay, as well as an additional arteriotomy repair upon removal of the sheath. It has also been associated with more complications than smaller sheaths[21, 25].
Over time, we became more adept at using the REBOA by mandating ultrasound for femoral arterial access as well as digital radiographs for quick assessment of balloon placement. We also learned to avoid over inflation of the balloon by instilling 8mL of contrast mixed solution for zone III and 13mL for zone I. In retrospect, we have had few barriers to the implementation of our REBOA program thanks to our surgeon champions. Additionally, we feel that the key to success is participating in a training session with live animal models as well as the development of a mobile REBOA cart in the ED to promote easy access for REBOA use.
Discussion
As we approach the seventh year since initial implementation of REBOA use in our program, interest has remained strong, but use has declined. The objective of this overview was to describe the process of successful implementation of REBOA use in an urban academic level I trauma center. We hypothesized that after training and safe credentialing, REBOA use steadily increases over time. What we found was that our REBOA rates increased over several years, reached a peak, and then experienced a decline.
With any new technology, we expected a slow rise in REBOA usage, followed by an increased adoption as the equipment and indications for use became more familiar to the attendings. The downtrend in placement observed at our institution was not surprising; as the REBOA became more ingrained in our trauma program, our staff became more comfortable with REBOA placement indications. As our program matured, the surgical team began to place pre-emptive REBOA sheaths on hypotensive patients but did not insert and/or inflate the REBOA unless clinically indicated based on the previously mentioned algorithm. At this time, placement of a pre-emptive sheath for femoral arterial access is at the discretion of the trauma attending, but access is typically established and agreed upon for the DH group for patients with a sustained systolic blood pressure of 90mmHg. If a REBOA was not ultimately placed, this sheath allowed for arterial access for hemodynamic monitoring or resuscitative measures. Another institution has described a similar “Step-up” approach where femoral access is obtained and a REBOA can later be placed if indicated by the patients’ clinical condition[6, 10]. Retrospective review of sheath-only placement is challenging, but there are at least twenty-nine sheath-only placements recorded since 2016 that were performed at the discretion of the surgical attending. These sheath-only placements are in the process of being documented in a similar database for quality improvement monitoring and evaluation. Nonetheless, information of sheath-only arterial access prior to 2016 remains challenging due to limitations with retrospective chart review and the use of the CODA® balloon until the transition to ER-REBOA™ in 2016.
As the REBOA use became more widespread, complications related to the REBOA were published and naturally our staff became more selective in their use of the technology[21, 25]. Furthermore, we now track all REBOA placements as well as all femoral artery access for potential sheath placements (even if a REBOA was not utilized). Since 2019, both retrospective and prospective information is being collected for trauma patients who undergo femoral access only versus femoral access followed by REBOA placement.
Future Direction
Formal training with residents on the REBOA use and placement, such as participation in the AESTES course offered to the DH trauma faculty. This would allow a standardized education and training for residents and the ability to participate in clinical REBOA placement.
As mentioned, while REBOA has been described in the civilian and military trauma literature, wise is still limited. Ideally, this roadmap will assist other centers in developing their own REBOA program in order to create a more widespread implementation.
Conclusion
Herein we describe a roadmap for introducing new technology into a busy urban, Level-1 trauma center. Strategies for how institutions adopt new procedures require clinical guidelines, a training program focused on competence, commitment to innovation, a hospital education and privileging process for those acquiring new skills, and a process for improvement and quality and safety assessment. Leadership and interest are key; the senior partners encouraged use of this innovative technology and garnered interest from the junior partners, the residents, and the hospital staff. Most importantly, partnership is paramount, not only between Surgery and the Emergency Department, but also between the operating room and nursing teams.
Acknowledgments
Disclosures of funding:
Prytime and Humacyte Research Support, Heamonetics Shared IP, NIH RM1, T32, UMI and DOD Grants.
Conflicts of Interest and Sources of Funding: Haemonetics, Stago, Prytime and Humacyte Research Support, Heamonetics Shared IP, NIH RM1, T32, UMI and DOD Grants. Author 3 is the editor of Journal of Trauma and Acute Care Surgery.
Conflict of interest:
Haemonetics: Authors 1, 2, 3, 11, 12, 13
Humacyte: Authors 3, 11, 12, 13, 14
Prytime Medical: Authors 3, 11, 13
Stago partnerships: Authors 2, 3, 11, 13
NIH RM1, T32 Funding: Authors 1, 2, 3, 11, 13
UMI and DoD Grants: Authors 1, 2, 3, 11, 12, 13, 14
Contributor Information
Jamie B Hadley, University of Colorado School of Medicine.
Julia R Coleman, University of Colorado School of Medicine.
Ernest E Moore, Denver Health Medical Center, University of Colorado School of Medicine.
Ryan Lawless, Denver Health Medical Center, University of Colorado School of Medicine.
Clay Cothren Burlew, Denver Health Medical Center, University of Colorado School of Medicine.
Barry Platnick, Denver Health Medical Center, University of Colorado School of Medicine.
Fredric M Pieracci, Denver Health Medical Center, University of Colorado School of Medicine.
Melanie R Hoehn, Denver Health Medical Center, University of Colorado School of Medicine.
Jamie J Coleman, Denver Health Medical Center, University of Colorado School of Medicine.
Eric Campion, Denver Health Medical Center, University of Colorado School of Medicine.
Mitchell Cohen, Denver Health Medical Center, University of Colorado School of Medicine.
Alexis Cralley, Denver Health Medical Center.
Andrew Eitel, University of Colorado School of Medicine.
Matthew Bartley, University of Colorado School of Medicine.
Navin Vigneshwar, University of Colorado School of Medicine.
Angela Sauaia, Denver Health Medical Center, University of Colorado School of Medicine.
Charles J Fox, University of Colorado School of Medicine.
References
- 1.Davidson AJ, Russo RM, Reva VA, Brenner ML, Moore LJ, Ball C, Bulgar E, Fox CJ, DuBose JJ, Moore EE, et al. , The pitfalls of resuscitative endovascular balloon occlusion of the aorta: Risk factors and mitigation strategies. J Trauma Acute Care Surg. 2018. 84(1): p. 192–202. [DOI] [PubMed] [Google Scholar]
- 2.DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, Moore L, Holcomb J, Turay D, Arbabi CN, et al. , The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016. 81(3): p. 409–19. [DOI] [PubMed] [Google Scholar]
- 3.Glaser JJ, Czerwinski A, Alley A, Keyes M, Piacentino V, and Pepe A, Implementing a REBOA program outside of the ivory tower: initial case series and lessons learned at a community trauma center. JEVTM. 2018. 2(3). [Google Scholar]
- 4.Biffl WL, Fox CJ, and Moore EE, The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg, 2015. 78(5): p. 1054–8. [DOI] [PubMed] [Google Scholar]
- 5.Villamaria CY, Eliason JL, Napolitano LM, Stansfield RB, Spencer JR, and Rasmussen TE, Endovascular Skills for Trauma and Resuscitative Surgery (ESTARS) course: curriculum development, content validation, and program assessment. J Trauma Acute Care Surg. 2014. 76(4): p. 929–35; discussion 935–6. [DOI] [PubMed] [Google Scholar]
- 6.Brenner ML, Moore L.J.m DuBose JJ, Tyson GH, McNutt MK, Albarado RP, Holcomb JB, Scalea TM, and Rasmussen TE, A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013. 75(3): p. 506–11. [DOI] [PubMed] [Google Scholar]
- 7.Brenner M, Hoehn M, Pasley J, Dubose J, Stein D, and Scalea T, Basic endovascular skills for trauma course: bridging the gap between endovascular techniques and the acute care surgeon. J Trauma Acute Care Surg. 2014. 77(2): p. 286–91. [DOI] [PubMed] [Google Scholar]
- 8.Bekdache O, Paradis T, Shen YB, Elbahrawy A, Grushka J, Deckelbaum D, Khwaja K, Fata P, Razek T, and Beckett A, Resuscitative endovascular balloon occlusion of the aorta (REBOA): indications: advantages and challenges of implementation in traumatic non-compressible torso hemorrhage. Trauma Surg Acute Care Open. 2019. 4(1): p. e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qasim Z, Bradley K, Panichelli H, Robinson J, and Zern SC, Successful Interprofessional Approach to Development of a Resuscitative Endovascular Balloon Occlusion of the Aorta Program at a Community Trauma Center. J Emerg Med. 2018. 54(4): p. 419–426. [DOI] [PubMed] [Google Scholar]
- 10.Vernamonti JP, Holcomb J, Mick NW, Falank C, Ontengco JB, Rappold J, and Sheppard FR, ‘Step Up’ approach to the application of REBOA technology in a rural trauma system. Trauma Surg Acute Care Open. 2019. 4(1): p. e000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvin JA, Maxim T, Inaba K, Martinez-Aguilar MA, King DR, Choudhry AJ, Zielinski MD, Akinyeye S, Todd SR, Griffin RL, et al. , Mortality after emergent trauma laparotomy: A multicenter, retrospective study. J. Trauma Acute Care Surg. 2017. 83(3): p. 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes CW, Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery. 1954. 36(1): p. 65–8. [PubMed] [Google Scholar]
- 13.Gupta BK, Khaneja SC, Flores L, Eastlick L, Longmore W, and Shaftan GW, The role of intra-aortic balloon occlusion in penetrating abdominal trauma. J Trauma. 1989. 29(6): p. 861–5. [DOI] [PubMed] [Google Scholar]
- 14.Wolf RK and Berry RE, Transaxillary intra-aortic balloon tamponade in trauma. J Vasc Surg. 1986. 4(1): p. 95–7. [DOI] [PubMed] [Google Scholar]
- 15.Branco BC, DuBose JJ, Zhan LX, Hughes JD, Goshima KR, Rhee P, and Mills JL Sr, Trends and outcomes of endovascular therapy in the management of civilian vascular injuries. J Vasc Surg. 2014. 60(5): p. 1297–1307.e1. [DOI] [PubMed] [Google Scholar]
- 16.Richmond BK, Judhan R, Sherrill W, Yacoub M, AbuRahma AF, Knackstedt K, Chumbe JT, Samanta D, and Thompson SN, Trends and Outcomes in the Operative Management of Traumatic Vascular Injuries: A Comparison of Open versus Endovascular Approaches. Am Surg. 2017. 83(5): p. 495–501. [PubMed] [Google Scholar]
- 17.Davis KA and Jurkovich GJ, Fellowship training in Acute Care Surgery: from inception to current state. Trauma Surg Acute Care Open. 2016. 1(1): p. e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuels JM, Sun K, Moore EE, Coleman JR, Fox CJ, Cohen MJ, Sauaia A, and MacTaggart JN, REBOA: Interest Is Wide but Use Remains Limited. J. Am. Coll. 2019. 229(4): p. e240–e241. [Google Scholar]
- 19.Matsumoto S, Hayashida K, Akashi T, Jung K, Sekine K, Funabiki T, and Moriya T, Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Severe Torso Trauma in Japan: A Descriptive Study. World J Surg. 2019. 43(7): p. 1700–1707. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto S, Hayashida K, Akashi T, Jung K, Sekine K, Funabiki T, and Moriya T, Effectiveness and Usage Trends of Hemorrhage Control Interventions in Patients with Pelvic Fracture in Shock. World J Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro Junior MAF, Feng CYD, Nguyen ATM, Rodrigues VC, Bechara GEK, Reis de-Moura R, and Brenner M, The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J. Emerg. Surg. 2018. 13: p. 20–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner M, Bulger EM, Perina DG, Henry S, Kang CS, Rotondo MF, Chang MC, Weireter LJ, Coburn M, Winchell RJ, et al. , Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). J. Trauma Surg. Acute Care Open. 2018. 3(1): p. e000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doucet J and Coimbra R, REBOA: is it ready for prime time? J Vasc Bras. 2017. 16(1): p. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen TE, Clouse WD, Peck MA, Bowser AN, Eliason JL, Cox MW, Woodward EB, Jones WT, and Jenkins DH, Development and implementation of endovascular capabilities in wartime. J Trauma. 2008. 64(5): p. 1169–76; discussion 1176. [DOI] [PubMed] [Google Scholar]
- 25.Teeter WA, Matsumoto J, Idoguchi K, Kon Y, Orita T, Funabiki T, Brenner ML, and Matsumura Y, Smaller introducer sheaths for REBOA may be associated with fewer complications. J Trauma Acute Care Surg. 2016. 81(6): p. 1039–1045. [DOI] [PubMed] [Google Scholar]



