Abstract
Context
The key gut microbial biomarkers for polycystic ovarian syndrome (PCOS) and how dysbiosis causes insulin resistance and PCOS remain unclear.
Objective
To assess the characteristics of intestinal flora in PCOS and explore whether abnormal intestinal flora can affect insulin resistance and promote PCOS and whether chenodeoxycholic acid (CDCA) can activate intestinal farnesoid X receptor (FXR), improving glucose metabolism in PCOS.
Setting and design
The intestinal flora of treatment-naïve PCOS patients and hormonally healthy controls was analyzed. Phenotype analysis, intestinal flora analysis, and global metabolomic profiling of caecal contents were performed on a letrozole-induced PCOS mouse model; similar analyses were conducted after 35 days of antibiotic treatment on the PCOS mouse model, and glucose tolerance testing was performed on the PCOS mouse model after a 35-day CDCA treatment. Mice receiving fecal microbiota transplants from PCOS patients or healthy controls were evaluated after 10 weeks.
Results
Bacteroides was significantly enriched in treatment-naïve PCOS patients. The enrichment in Bacteroides was reproduced in the PCOS mouse model. Gut microbiota removal ameliorated the PCOS phenotype and insulin resistance and increased relative FXR mRNA levels in the ileum and serum fibroblast growth factor 15 levels. PCOS stool-transplanted mice exhibited insulin resistance at 10 weeks but not PCOS. Treating the PCOS mouse model with CDCA improved glucose metabolism.
Conclusions
Bacteroides is a key microbial biomarker in PCOS and shows diagnostic value. Gut dysbiosis can cause insulin resistance. FXR activation might play a beneficial rather than detrimental role in glucose metabolism in PCOS.
Keywords: PCOS, FXR, insulin resistance, intestinal flora
Polycystic ovary syndrome (PCOS) is a common gynecological endocrine disease and a major cause of anovulatory infertility in reproductive-aged women (1). However, in addition to being a reproductive disease, PCOS is also associated with a wide range of metabolic disorders. Women with PCOS have a high risk of obesity (2), diabetes (2), metabolic syndrome (3), nonalcoholic fatty liver disease (4), cardiovascular disease (5), and endometrial cancer (6). Although increased ovarian and/or adrenal androgen secretion (7, 8), partial folliculogenesis arrest (8, 9), insulin resistance (10-12), neuroendocrine axis dysfunction (8), and genetic factors (13) have been suggested to be involved in the development of PCOS, the precise underlying triggers for these key biochemical and metabolic disturbances remain largely unclear.
In recent years, with the progress of next-generation sequencing technology and the verification of aseptic animals, intestinal flora has been shown to be directly related to many diseases and even plays a role in various diseases, such as obesity, diabetes, atherosclerosis, and inflammatory enteritis (14). Recent studies have shown that the gut microbiota of individuals with PCOS differs from those of healthy controls. However, analyses of the composition of the gut microbiota in women with PCOS have yielded inconsistent results (15-18). Beza et al. found higher relative abundances of Streptococcaceae and lower Bacteroidaceae and Porphyromonadaceae in PCOS (15). By contrast, Xinyu et al. found that Bacteroides vulgatus was markedly elevated in the gut microbiota of individuals with PCOS (17). Interestingly, other studies found that the relative abundances of the genera Catenabacterium and Kandleria (18) or Parabacteroides and Clostridium were enriched in PCOS (16). The reasons for the inconsistent results may be complex and diverse. Our group found that, among phenotypes, host location showed strong associations with microbiota variations (19). Host location may partially explain the inconsistencies in the intestinal flora in PCOS. More research is needed to determine the characteristics of the gut microbiota in PCOS.
The gut microbiota plays a key role in regulating energy balance and is involved in the development and progression of obesity, diabetes, and metabolic syndrome (20, 21). In addition, Lactobacillus has been found to be relevant to fasting blood glucose and central adiposity (22). Moreover, fecal microbiota transplantation (FMT) from obese into germ-free mice can result in an obese phenotype (23). However, the community structure and function of the intestinal flora in PCOS and the role and mechanism of the intestinal flora in the pathogenesis of PCOS remain obscure. The role of gut microbiota in PCOS needs to be further determined. Recent reports have revealed that the gut microbiota influences the farnesoid X receptor (FXR)-fibroblast growth factor 15 (FGF15) axis in mice (24). Some studies have found that intestinal FXR is involved in glucose and lipid metabolism and insulin resistance and that the activation of the FXR signalling pathway can improve glucose metabolism and lipid metabolism disorders (25, 26). After newly diagnosed type 2 diabetes was treated with metformin for 3 days, intestinal FXR was inhibited by the intestinal FXR antagonist glycoursodeoxycholic acid (GUDCA), and metabolic dysfunction, including hyperglycemia, was improved (27). The role of intestinal FXR in glucose metabolism in PCOS needs to be further determined. Bile acids are physiological ligands for FXR, and the strongest activator of FXR is the primary bile acid chenodeoxycholic acid (CDCA) (28). In the gut, primary bile acids are transformed into secondary bile acids by the metabolic activities of gut anaerobic bacteria (29). Letrozole (LET) treatment of female mice during puberty resulted in reproductive hallmarks of PCOS, including hyperandrogenemia, anovulation, and polycystic ovaries (30), and did not alter food intake (31). Therefore, the LET-induced PCOS mouse model provides an opportunity to study the relation of the gut microbiome and insulin resistance in a diet-independent setting. Our hypothesis is that gut microbiota dysbiosis can result in insulin resistance and contributes to the development of PCOS and that dysbiosis promotes glucose metabolism disorder possibly through the bile acid-intestinal FXR signalling pathway. The purpose of our study was to accumulate more data on the characteristics of the intestinal flora in PCOS, explore whether abnormal intestinal flora can affect insulin resistance and promote the development of PCOS, and determine whether activation of intestinal FXR by CDCA can improve glucose metabolism in PCOS. Our research will establish experimental evidence for the relationship among the intestinal flora, CDCA, intestinal FXR, and PCOS.
Methods
Human subjects
The study and all experimental procedures were approved by the Ethics Committee at the Zhujiang Hospital of Southern Medical University. All patients were recruited from the Gynecology and Obstetrics outpatient clinic at the Zhujiang Hospital of Southern Medical University. We recruited 56 individuals with PCOS and 31 healthy controls. Written informed consent was obtained from all participants. Women with PCOS were diagnosed according to the 2003 Rotterdam criteria, which require the presence of at least 2 of the following: (1) oligo-ovulation and/or anovulation; (2) clinical and/or biochemical signs of hyperandrogenism; and (3) polycystic ovaries. Diagnoses of PCOS were made after the exclusion of other etiologies for hyperandrogenemia or ovulatory dysfunction (Cushing syndrome, 21-hydroxylase deficiency, congenital adrenal hyperplasia, androgen-secreting tumors, thyroid disease, and hyperprolactinemia). All individuals with PCOS were first-visit patients and had not received PCOS-related treatment. The individuals had not received any antibiotic treatment for at least 1 month before the sample collection. The healthy controls were from the general community and had regular menstrual cycles, normal ovarian morphology, and normal levels of hormones. Women who were breastfeeding or pregnant within the past year or who took medication within the past 3 months were excluded from the study. Height, body weight, waist circumference, and hip circumference were measured, and the body mass index and waist-to-hip ratio were calculated. Peripheral blood samples were collected from all subjects during days 2 through 5 of menstruation after an overnight fast. Stool samples were collected using stool collection tubes and stored in a -80°C freezer.
Levels of serum FSH, LH, testosterone, and insulin were tested by an automatic chemiluminescent analyzer (Unicel DxI 800, Beckman Coulter, USA; FSH sensitivity range from 0.3 IU/L to 200 IU/L, intra-assay coefficient of variation [CV] 1.45%; LH sensitivity range from 0.3 IU/L to 200 IU/L, intra-assay CV 1.54%; testosterone sensitivity range from 0.025 μg/L to 15.0 μg/L, intra-assay CV 3.37%) at the Department of Clinical Chemistry in Zhujiang Hospital of Southern Medical University. Serum glucose was measured by an automatic biochemical analyser (BS2001, Mindray, China; sensitivity range from 0.6 mmol/L to 33.0 mmol/L, intra-assay CV 3.0%, interassay CV 5.0%). The homeostasis model assessment for insulin resistance index (HOMA-IR) and homeostasis model assessment for beta cell function (HOMA-beta) were calculated using the following formulas:
HOMA-IR = fasting plasma glucose (FPG) (mM) × fasting plasma insulin (FINS) (mIU/L)/22.5;
HOMA-beta = 20 × FINS (mIU/L)/ [FPG (mM) - 3.5] × 100%.
DNA extraction
Fecal samples were obtained for DNA extraction. A frozen aliquot (200 mg) of each fecal sample was processed using the PowerSoil DNA Extraction Kit (Shenzhen Bioeasy Biotechnologies Co., Ltd., China). The DNA concentrations were measured using a NanoDrop system (Thermo Fisher Scientific Co.).
16S sequencing and bioinformatics analysis
Real-time quantitative PCR (qPCR) analysis was performed using the SYBR Green PCR master mix (Vazyme) and the ABI ViiA 7 real-time PCR system (Applied Biosystems). We used the barcoded V4F 5′ GTGTGYCAGCMGCCGCGGTAA 3′ and V4R 5′ CCGGACTACNVGGGTWTCTAAT 3′ primers to amplify bacterial 16S rRNA V4 fragments. All qPCRs were carried out with 2 μL of template DNA in a final volume of 20 μL following the manufacturer’s instructions (Invitrogen Life Technology Co., Ltd). The amplification thermal cycling conditions were as follows: initial temperature of 94°C for 5 minutes, followed by 30 cycles of 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 45 seconds, and a final extension step of 72°C for 5 minutes.
All samples were sequenced using the paired-end strategy on the Illumina platform. Processing of the raw Illumina paired-end sequences was mainly performed on the QIIME (v1.9.1) platform (32, 33). The resulting sequences were demultiplexed, and barcodes and primers of each sequence were then removed. We performed reference-based operational taxonomic unit (OTU) clustering against the Greengenes database v13_8 with a similarity of 97% (32, 34, 35). R programming software and SPSS 24 were used for the statistical analysis, and P < 0.05 was considered statistically significant.
Animal models
Four-week-old C57BL/6 female mice were randomly divided into different groups, housed 4 to 5 per cage, and maintained under controlled temperature, lighting (12-hour light: 12-hour dark cycle), and standard laboratory conditions with free access to rodent feed and water. All animal experimental procedures were approved by the Laboratory Animal Center and Ethics Committee at the Zhujiang Hospital of Southern Medical University according to the national legislation for animal care. (1) The mice were randomly divided into 2 groups and treated for 5 weeks. The control group received vehicle only 1% aqueous solution of carboxymethylcellulose, once daily by oral gavage. The PCOS model mouse group was orally gavaged with LET (Sigma-Aldrich, catalog #PHR1540) 2.8 mg/kg-1 body weight, which was dissolved in 1% carboxymethylcellulose once daily (36, 37). (2) The mice were randomly divided into a control FMT group, a PCOS FMT group, LET+control FMT group, and LET+ PCOS FMT group. The mice were treated with an antibiotic cocktail (20 mg/mL vancomycin, 40 mg/mL neomycin sulfate, 40 mg/mL metronidazole, 40 mg/mL ampicillin) (38) for 5 days before human stool transplantation experiments. The human stool samples were mixed with saline solution (20 mg/mL), vortexed, and centrifuged to collect the supernatant. The mice received another 10 weeks of treatment by oral gavage with 100 µL fecal suspension from healthy controls for the control FMT group or gavage with 100 µL fecal suspension from PCOS patients for the PCOS FMT group. The mice received another 5 weeks of treatment by oral gavage with LET and 10 weeks of treatment by oral gavage with 100 µL fecal suspension from healthy controls for the LET+control FMT group or gavage with 100 µL fecal suspension from PCOS patients for the LET+PCOS FMT group. (3) The mice were randomly divided into a control group, a PCOS model mouse group, and a PCOS with antibiotics group and treated for 5 weeks. The PCOS with antibiotics group was administered LET plus an antibiotic cocktail (20 mg/mL vancomycin, 40 mg/mL neomycin sulfate, 40 mg/mL metronidazole, and 40 mg/mL ampicillin) once daily by oral gavage. (4) The mice were randomly divided into the PCOS model mouse group, the LET+CDCA group, and the LET+GUDCA group and treated for 5 weeks. Mice in the LET+CDCA group were administered LET and CDCA (Targetmol, catalog #T0847) at a dosage of 50 mg/kg/d (27) once daily by oral gavage. Mice in the LET+GUDCA group were administered LET and GUDCA (Sigma-Aldrich, catalog #06863) at a dosage of 50 mg/kg/d (27) once daily by oral gavage. The fecal pellets were collected from each mouse on day 35, flash frozen in liquid nitrogen, and stored at -80°C. Glucose tolerance tests (GTTs) were performed on the mice the day before the end of the experiment. At the end of the experiments, the mice were anesthetized; blood was collected via retro-orbital bleeding, centrifuged at 4000 rpm for 10 minutes at 4°C, and stored at -80°C for subsequent serum analyses; the ovaries were dissected, fixed in 4% paraformaldehyde at 4°C overnight, and processed for histology; the intestines and livers were quickly frozen and stored at -80°C.
Glucose tolerance tests
Mice were fasted for 12 hours before the GTT. Glucose levels were measured by tail vein blood sampling using an Accu-Chek Performa blood glucose analyser (Roche Diagnostics, sensitivity range from 0.3 mmol/L to 33.3 mmol/L). After measurement of fasting glucose levels, the mice were IP injected with glucose (2 g/kg-1 body weight) for the GTT, and tail blood samples were collected at 30, 60, 90 and 120 minutes after the IP injection for glucose level detection.
Serum analysis
Serum was collected to measure sex hormone and insulin levels. The levels of testosterone were tested by an automatic chemiluminescent analyser (Unicel DxI 800, Beckman Coulter; sensitivity range from 0.025 μg/L to 15.0 μg/L, intra-assay CV 3.37%). Insulin was determined by ELISA kits (MM-0579M2, Meimian, China; sensitivity range from 0.3 mIU/L to 8.0 mIU/L) for mice.
Morphology
The ovarian tissues were processed for hematoxylin and eosin staining. Ovaries were quickly collected, fixed in 4% paraformaldehyde, subjected to gradient ethanol hydration, and embedded in paraffin. The sections were prepared and stained with hematoxylin and eosin. The ovaries were longitudinally and serially sectioned into 5-μm sections (CM2016; Leica); all of the sections were mounted onto a glass slide and observed for histomorphological examination under a light microscope (NIKON DS-U3, Nikon Eclipse E100; Nikon). The numbers of cystic follicles were counted.
Real-time qPCR analysis
Phenol-chloroform extraction was performed to isolate total RNA from mouse liver and ileum tissue with TRIzol reagent (Invitrogen, catalog #15596-026). cDNA was synthesized from 2 μg of total RNA with a Reverse Transcription Kit (Takara, catalog #RR036A). Real-time qPCR analysis was performed using SYBR Green PCR master mix (Vazyme, catalog #Q111-02) with the ABI ViiA 7 real-time PCR system (Applied Biosystems). The qPCR primers used in this study were FXR-F 5′-TGGGCTCCGAATCCTCTTAGA-3′ and FXR-R 5′-TGGTCCTCAAATAAGATCCTTGG-3′. All qPCRs were carried out in a final volume of 20 μL. The amplification thermal cycling conditions were as follows: 95°C for 5 minutes; 40 cycles at 95°C for 10 seconds and 60°C for 30 seconds; and a final extension step of 95°C for 15 seconds and 60°C for 1 minute.
Metabolomics
Global metabolomics profiling was performed for cecum contents (ultra-high-performance liquid chromatograph and high-resolution mass spectrometer; Waters). Samples were separated on a chromatographic column with mobile phase A, 0.1% formic acid in water, and mobile phase B, 0.1% formic acid in acetonitrile, and analyzed in positive and negative heated electrospray ionization mode at m/z 50 to 1500 as separate injections. The injection volume was 5 μL. The flow rate was 400 μL/min with a 45°C column temperature. MassLynx identified and aligned features. Data were exported to Progenesis QI for analysis. Data analyses were performed using DataFactory. Partial least squares discriminant analysis was used to determine differential metabolites.
Statistics
GraphPad Prism version 5.0 (GraphPad Software) and SPSS version 23.0 were used for statistical analysis. The sample distribution was determined by the Kolmogorov-Smirnov normality test. One-way ANOVA was used for normally distributed data and the Kruskal-Wallis test was used for nonnormally distributed data to evaluate the statistical significance of differences among 3 groups. The data among the 3 groups were analyzed by homogeneity tests of variances. The homogeneity of variance among the 3 groups was determined with the least significant difference test, and missing variance was determined with the Dunnett test. For more than 2 groups, multiple comparisons were adjusted by the false discovery rate. A 2-tailed Student t test was used to evaluate significant differences between 2 groups. For the nonparametric tests, the 2-tailed Mann-Whitney U test was used to evaluate significant differences between 2 groups. Data are shown as the means ± SDs or as medians with interquartile ranges. P < 0.05 was considered statistically significant.
Results
Bacteroides is increased significantly in PCOS patients, and the microbial characteristics have potential diagnostic value
Laboratory, anthropometric, and patient history data are summarized in Table 1. Treatment-naïve PCOS patients had significantly higher waist circumference, hip circumference, and waist-to-hip ratio (P = 0.001, 0.024, and 0.015, respectively) levels than controls, whereas no difference was found for age, weight, height, body mass index, or HOMA-beta (P = 0.069, 0.090, 0.660, 0.072, and 0.749, respectively). HOMA-IR, FPG, and FINS were higher in the treatment-naïve PCOS group (P = 0.001, 0.029, and 0.0001, respectively). Treatment-naïve PCOS patients showed a characteristic dysregulation of LH and testosterone secretion, with higher LH and testosterone levels than controls (P = 0.000 and 0.033). FSH was not significantly different between the 2 groups (P = 0.580), whereas LH/FSH was higher in treatment-naïve PCOS patients than in controls (P = 0.000).
Table 1.
Characteristics of the study participants and hormone levels
| Parameters | Control (n = 31) | PCOS (n = 56) | P values |
|---|---|---|---|
| Age, y | 26.00 (24.00, 27.00) | 24.00 (22.00, 27.00) | 0.069 |
| Height, cm | 160.23 ± 3.40 | 160.69 ± 5.36 | 0.660 |
| Weight, kg | 52.00 (48.50, 54.00) | 55.60 (47.63, 64.88) | 0.090 |
| Waist circumference, cm | 69.06 ± 5.23 | 74.72 ± 10.23 | 0.001 |
| Hip circumference, cm | 89.24 ± 3.62 | 92.62 ± 9.84 | 0.024 |
| BMI, kg/m2 | 19.81 (18.76, 21.16) | 21.07 (18.75, 24.41) | 0.072 |
| WHR | 0.77 ± 0.06 | 0.81 ± 0.06 | 0.015 |
| HOMA-IR | 1.03 (0.80, 1.38) | 1.61 (0.94, 2.33) | 0.001 |
| HOMA-β | 90.92 ± 122.88 | 96.88 ± 49.14 | 0.749 |
| FPG, mmol/L | 4.95 ± 0.46 | 5.18 ± 0.46 | 0.029 |
| FINS, mIU/L | 4.96 (3.53, 5.99) | 7.01 (4.27, 10.21) | 0.001 |
| FSH, IU/L | 6.82 ± 2.86 | 7.07 ± 1.45 | 0.580 |
| LH, IU/L | 4.50 (3.03, 5.96) | 8.67 (4.77, 12.97) | <0.001 |
| Testosterone, μg /L | 0.53 ± 0.15 | 0.64 ± 0.27 | 0.033 |
| LH/FSH | 0.69 (0.49, 1.03) | 1.36 (0.78, 1.85) | <0.001 |
Data are shown as the means ± SDs or as medians with interquartile ranges. Multiple comparisons were significantly different (P < 0.05). P values obtained from ANOVA for normally distributed variables or from the nonparametric Wilcoxon test for nonnormally distributed variables. The parameters of height, waist circumference, hip circumference, WHR, FPG, FSH and testosterone were normally distributed. BMI, body mass index; WHR, waist-hip ratio; FPG, fasting plasma glucose; FINS, fasting plasma insulin; HOMA-IR, homeostasis model assessment for insulin resistance index; HOMA-beta, homeostasis model assessment for beta cell function.
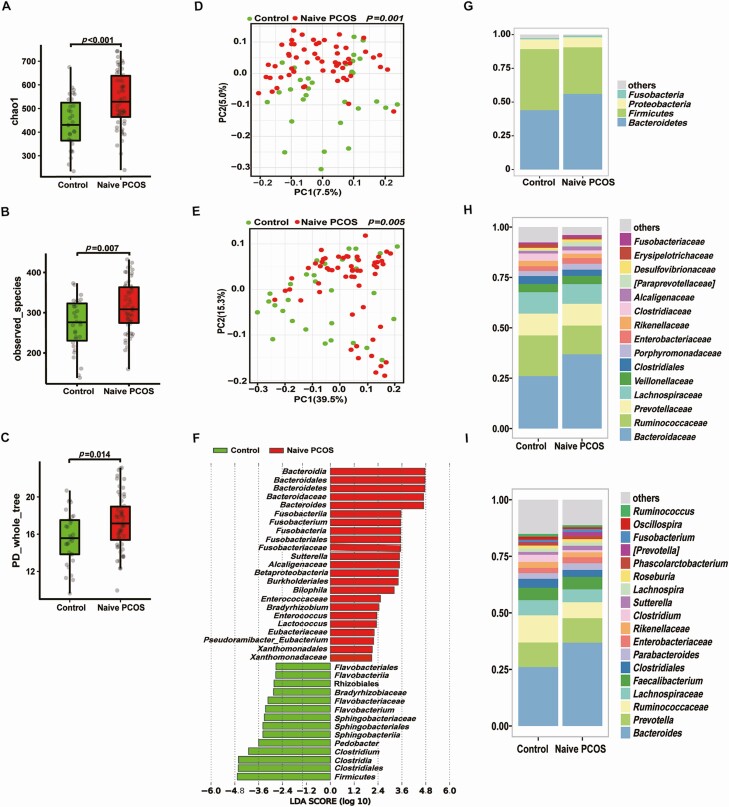
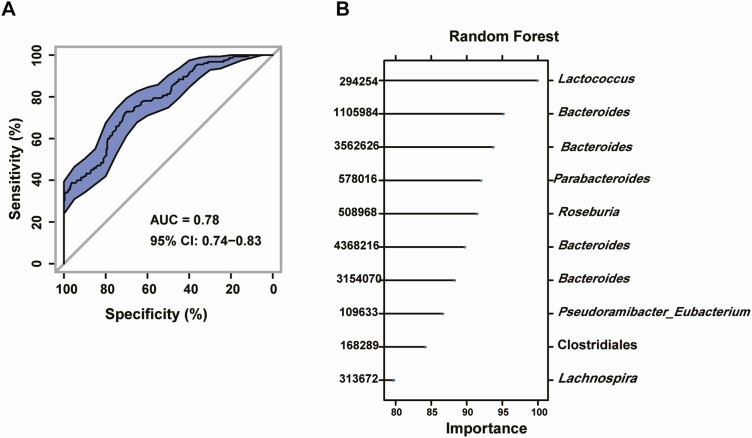
There was a significant difference in bacterial alpha diversity between treatment-naïve PCOS patients and healthy controls (Fig. 1A-C). Beta (β) diversity of the microbial communities based on principal coordinate analysis (PCoA, unweighted UniFrac) of the UniFrac metric (Fig. 1D, E, P = 0.001 and 0.005) revealed a separation between treatment-naïve PCOS patients and healthy controls. Furthermore, the abundance of Bacteroides was markedly higher in treatment-naïve PCOS patients than in healthy controls (Fig. 1F-I). To explore the potential ability of the intestinal flora to identify treatment-naïve PCOS, we constructed a random forest model based on the intestinal flora. The performance of the model was assessed using receiver operating characteristic analysis, achieving an area under the curve (AUC) value of 0.78 (95% CI, 0.74-0.83). Subsequently, we further analyzed which bacteria have the ability to identify PCOS. According to the ranking of importance scores in the random forest models, the results showed that the top 10 OTUs with the potential to identify PCOS had 4 OTUs belonging to Bacteroides (Fig. 2A, B).
Figure 1.
Characteristics of the gut microbiota in individuals with PCOS. Comparison of the stool microbiome of women with treatment-naïve PCOS with that of healthy controls (treatment-naïve PCOS patients: 56; controls: 31). (A-C) α-diversity of the microbiota in the feces. Data are presented as the mean ± SEM, P < 0.05, Wilcoxon rank-sum test. (D, E) Two-dimensional plot of PCoA for the microbiota. (F) Differentially abundant taxa identified using LEfSe analysis, P < 0.05, Kruskal-Wallis rank sum test. (G-I) The gut microbiota average relative abundance of predominant bacterial taxa at the phylum, family, and genus levels. PCoA, principal coordinate analysis; PCOS, polycystic ovarian syndrome.
Figure 2.
The gut microbiota signature can be used to discriminate between treatment-naïve PCOS patients and healthy controls. A random forest model based on the intestinal flora of treatment-naïve PCOS patients and healthy controls was used to explore the potential ability of the intestinal flora to identify treatment-naïve PCOS patients (treatment-naïve PCOS patients: 56; controls: 31). (A) A random forest model assessed using receiver operating characteristic analysis (area under the curve [AUC] = 0.78). (B) The top 10 OTUs with the potential to identify treatment-naïve PCOS patients. OTU, operational taxonomic unit; PCOS, polycystic ovarian syndrome.
High abundance of Bacteroides, insulin resistance, and cecum farnesol increase in PCOS model mice
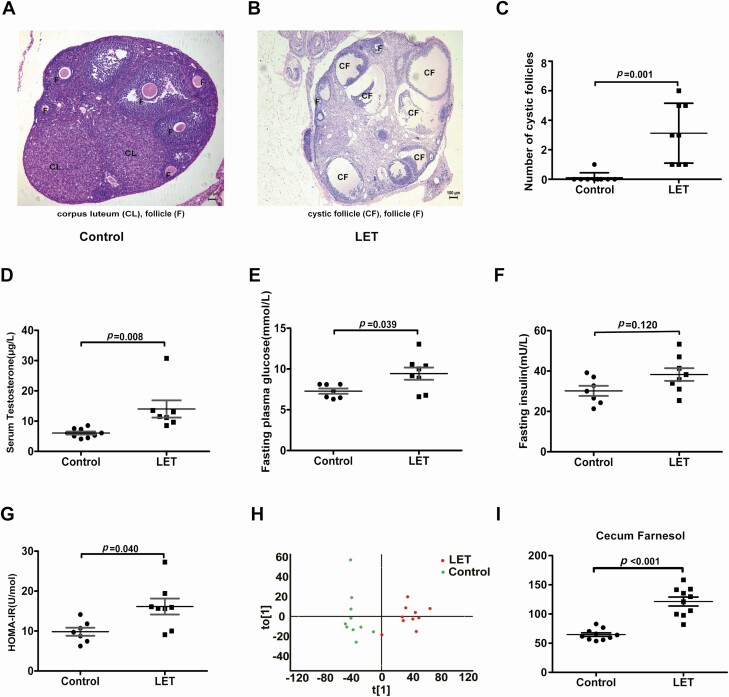
There was a significant difference in bacterial alpha diversity between the PCOS model mice and controls (Fig. 3A-C). The β diversity of the microbial communities (based on PCoA, unweighted UniFrac) of the UniFrac metric (Fig. 3D, E) revealed a separation between PCOS model mice and controls. Furthermore, the abundance of Bacteroides was markedly higher in PCOS model mice than in controls, and this finding was similar to the intestinal flora of PCOS patients (Fig. 3F-G). Under light microscopy, the control mouse ovaries exhibited follicles in various stages and fresh corpora lutea. The granulosa within the follicles showed multiple layers (Fig. 4A). The PCOS mouse ovaries follicles showed a disorganized granulosa cell compartment with irregular granulosa cell layer thickness, characteristic of atretic antral follicles. The PCOS mouse ovaries appeared to lack corpora lutea and have more antral follicles. Many cystic follicles showed no granulosa layer or scant granulosa (Fig. 4B). The numbers of cystic follicles, testosterone levels, fasting blood glucose, and HOMA-IR in PCOS model mice were significantly higher than those in controls (Fig. 4C-G). To investigate the possible causes of insulin resistance resulting from intestinal flora disorder in PCOS model mice, we collected cecum contents from PCOS model mice and controls for the metabolomics test. Metabolomics analysis further revealed that cecum farnesol increased significantly in PCOS model mice (Fig. 4H-I).
Figure 3.
Characteristics of the gut microbiota in PCOS model mice. Comparison of the stool microbiome between letrozole (LET)-induced PCOS model mice and controls (PCOS model mice: 8; controls: 9). (A-C) α-diversity of the microbiota in the feces. Data are presented as the mean ± SEM, P < 0.05, Wilcoxon rank-sum test. (D, E) Two-dimensional plot of PCoA for the microbiota. (F) The gut microbiota average relative abundance of the predominant bacterial taxa at the genus level. (G) Differentially abundant taxa identified using LEfSe analysis, P < 0.05, Kruskal-Wallis rank sum test. PCoA, principal coordinate analysis; PCOS, polycystic ovarian syndrome; SEM, standard error of the mean.
Figure 4.
Insulin resistance and cecum farnesol are increased in PCOS model mice. Comparing the phenotype and cecal contents by global metabolomics between letrozole (LET)-induced PCOS model mice and controls (PCOS model mice: 8; controls: 9). (A, B) Hematoxylin and eosin staining of ovaries, corpus luteum (CL), cystic follicle (CF), and follicle (F). (C) Quantitative analysis of cystic follicles. (D) Serum testosterone levels. (E) Fasting blood glucose. (F) Fasting insulin. (G) HOMA-IR. (H) Orthogonal partial least squares-discriminate analysis (OPLS-DA) of cecum content global metabolomics. (I) Cecum farnesol. (C-I) P values were determined by 2-tailed Student t test, and P < 0.05 was considered statistically significant. HOMA-IR, homeostasis model assessment for insulin resistance index; PCOS, polycystic ovarian syndrome.
Intestinal flora is a key factor in insulin resistance and contributes to the development of PCOS
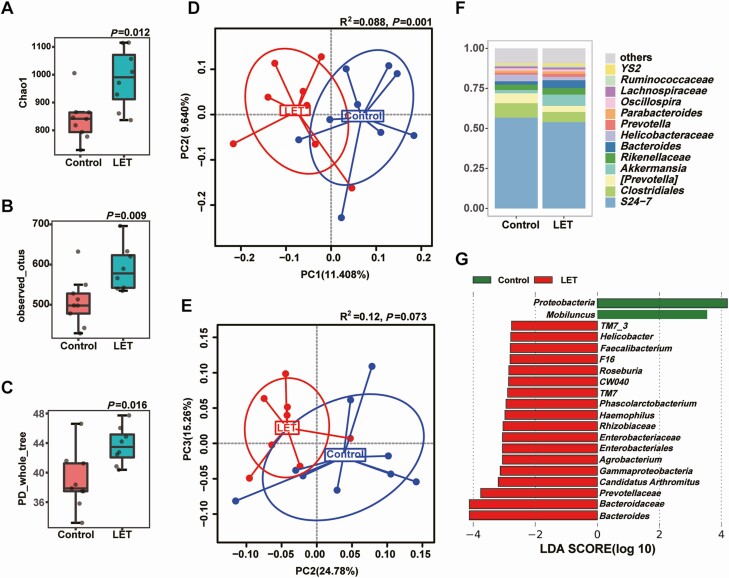
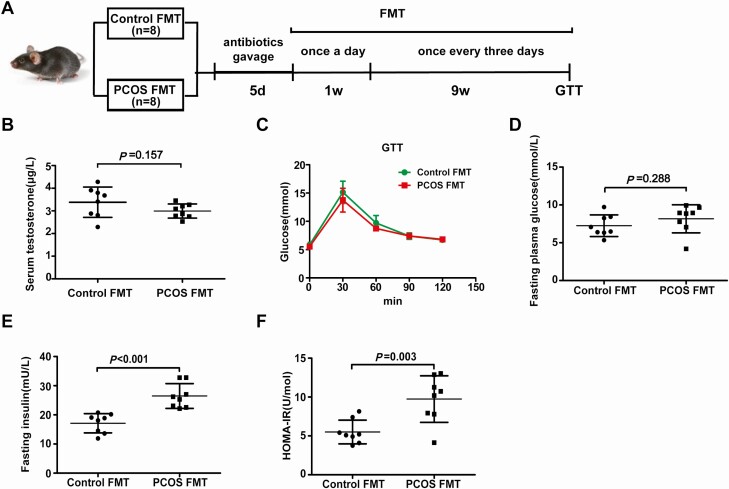
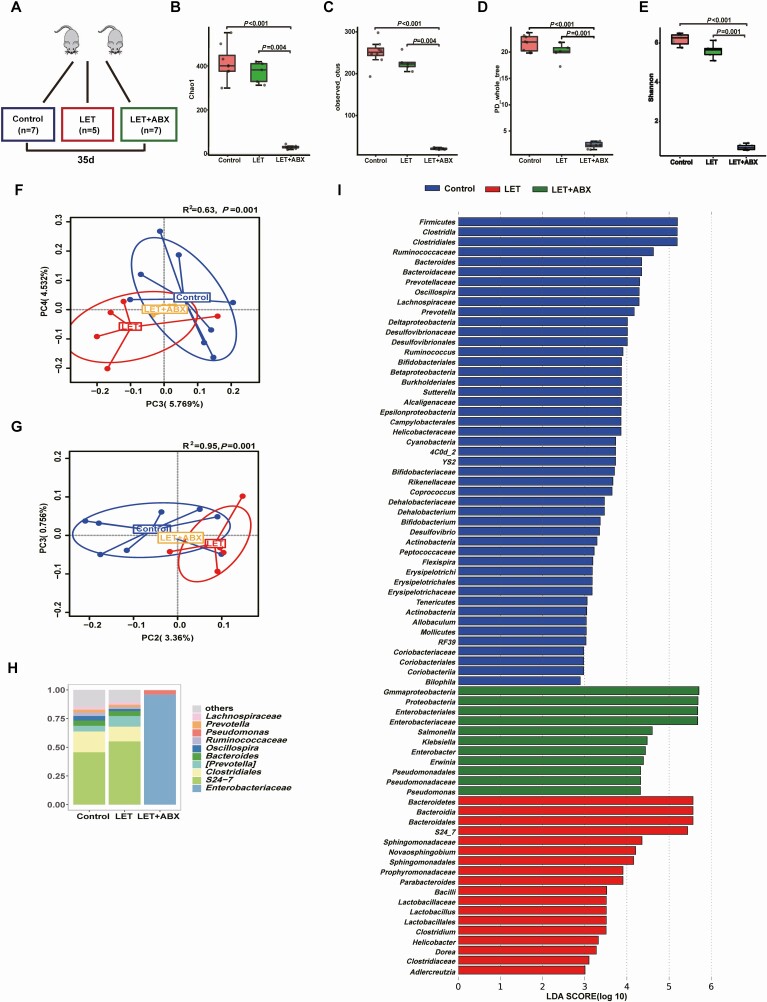
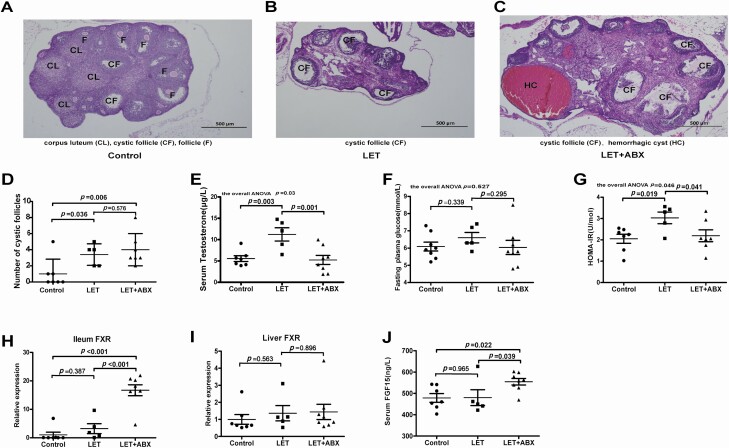
To investigate the effect of the gut microbiota on insulin sensitivity in PCOS, stools from healthy controls or individuals with PCOS were transplanted into mice by oral gavage for 10 weeks (Fig. 5A). The testosterone levels were not different between the mice transplanted with stool from healthy controls and the mice transplanted with stool from individuals with PCOS (Fig. 5B). Compared with mice transplanted with stool from healthy controls, mice transplanted with stool from individuals with PCOS displayed insulin resistance but not disordered glucose metabolism (Fig. 5C-F). However, there was no difference between PCOS model mice transplanted with stool from healthy controls and PCOS model mice transplanted with stool from individuals with PCOS (39). To further determine the role of the intestinal flora in the PCOS phenotype in the host, a continuous antibiotic cocktail was administered by gavage for 35 days (Fig. 6E). Microbial diversity and composition were also apparently altered by antibiotic cocktail treatment from the results of the chao1, observed_otus, PD_whole_tree, and Shannon indexes; the PCoA of the Bray-Curtis distances; and species comparison (Fig. 6A-D, F-G). Bacteroidetes and Firmicutes in PCOS mice were removed completely after antibiotic cocktail treatment (Fig. 6H-I). Under light microscopy, the antibiotic-treated PCOS model mouse ovaries still showed more antral follicles. Large cysts showed no granulosa layer or scant granulosa (Fig. 7A-D). The testosterone levels in the antibiotic-treated PCOS model mice were significantly lower than those in the PCOS model mice (Fig. 7E). Administration of antibiotic cocktail to the mice improved insulin resistance (Fig. 7F-G). FXR mRNA levels in the ileum were significantly increased in the presence of the antibiotic cocktail, but no differences were observed in the liver. Moreover, we found that administration of antibiotic cocktails increased serum FGF15 levels (Fig. 7H-J). This finding indicated that the removal of Bacteroidetes or Firmicutes affected intestinal FXR expression in PCOS.
Figure 5.
Effects of PCOS fecal microbiota transplantation on the disruption of insulin sensitivity. Mice transplanted with stool suspensions from healthy controls and women with PCOS were defined as the control-FMT and PCOS-FMT groups, respectively. After treatment with an antibiotic cocktail (20 mg/mL vancomycin, 40 mg/mL neomycin sulfate, 40 mg/mL metronidazole, 40 mg/mL ampicillin intragastrically once daily) for 5 days, the mice were orally gavaged with stool suspensions once a day for 1 week and once every 3 days for 9 weeks (control-FMT: 8; PCOS-FMT: 8). (A) Schematic representation of the experimental design. (B) Serum testosterone levels. (C) GTT. (D) Fasting blood glucose. (E) Fasting insulin. (F) HOMA-IR. (B-E) P values were determined by 2-tailed Student t test, and P < 0.05 was considered statistically significant. FMT, fecal microbiota transplantation; GTT, glucose tolerance test; HOMA-IR, homeostasis model assessment for insulin resistance index; PCOS, polycystic ovarian syndrome.
Figure 6.
Characteristics of the gut microbiota in PCOS mice after treatment with antibiotic cocktails. Intestinal flora analysis of PCOS model mice after antibiotic treatment (20 mg/mL vancomycin, 40 mg/mL neomycin sulfate, 40 mg/mL metronidazole, 40 mg/mL ampicillin intragastrically once daily) removed the intestinal flora for 35 days (control: 7; LET: 5; LET+ABX: 7). (A) Schematic representation of the above experimental design. (B-E) α-diversity of the microbiota in the feces. Data are presented as the mean ± SEM, P < 0.05, Wilcoxon rank-sum test. (F, G) Two-dimensional plot of PCoA for the microbiota. (H) The gut microbiota average relative abundance of the predominant bacterial taxa at the genus level. (I) Differentially abundant taxa identified using LEfSe analysis, P < 0.05, Kruskal-Wallis rank-sum test. ABX, antibiotic treatment; LET, letrozole; PCoA, principal coordinate analysis; PCOS, polycystic ovarian syndrome; SEM, standard error of the mean.
Figure 7.
Removal of the intestinal flora can improve insulin resistance and suppress the development of PCOS. Phenotype analysis of PCOS model mice after antibiotic treatment (20 mg/mL vancomycin, 40 mg/mL neomycin sulfate, 40 mg/mL metronidazole, 40 mg/mL ampicillin intragastrically once daily) removed the intestinal flora for 35 days (control: 7; LET: 5; LET+ABX: 7). (A-C) Hematoxylin and eosin staining of the ovary, corpus luteum (CL), cystic follicle (CF), and hemorrhagic cyst (HC). (D) Quantitative analysis of cystic follicles. (E) Serum testosterone levels. (F) Fasting blood glucose. (G) HOMA-IR. (H) mRNA levels of ileum FXR. (I) mRNA levels of liver FXR. (J) Serum FGF15 levels. (D-J) P values were determined by 1-way ANOVA for normally distributed data and the Kruskal-Wallis test for nonnormally distributed data. P < 0.05 was considered statistically significant. ABX, antibiotic treatment; FGF15, fibroblast growth factor 15; FXR, farnesoid X receptor; HOMA-IR, homeostasis model assessment for insulin resistance index; LET, letrozole; PCOS, polycystic ovarian syndrome.
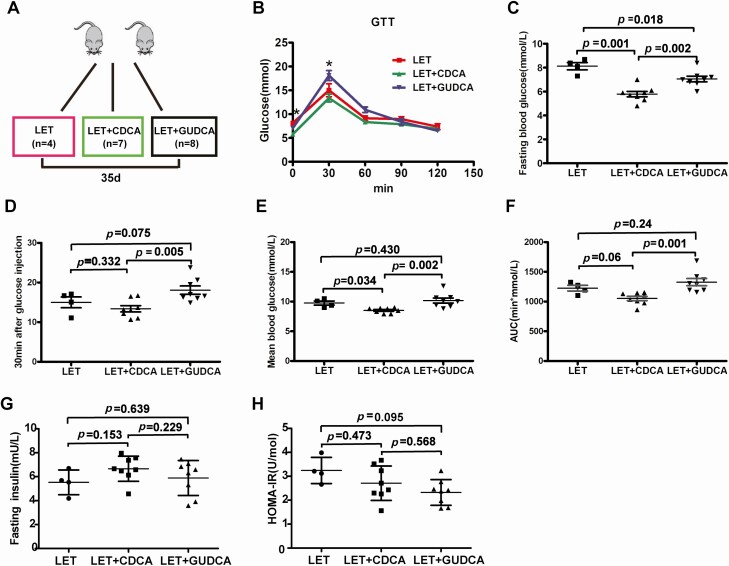
Activation of FXR improves glucose metabolism in PCOS model mice
Next, we measured the effects of intestinal FXR on glucose metabolism in the PCOS model mice (Fig. 8A). We found that administration of the FXR agonist CDCA to LET-treated mice affected glucose metabolism, as revealed by the GTT (Fig. 8B). The fasting blood glucose and mean blood glucose from the GTT in the CDCA-treated PCOS model mice were significantly lower than those in the PCOS model mice (Fig. 8C-F). There were no differences in fasting insulin levels and HOMA-IR among the PCOS model group, LET+CDCA group, and LET+GUDCA group (Fig. 8G-H). Compared with PCOS model mice treated with CDCA, mice treated with the FXR antagonist GUDCA displayed significantly higher fasting blood glucose, glucose 30 minutes after glucose injection, mean blood glucose, and AUC of the GTT. These results confirmed that CDCA therapy can improve glucose metabolism in PCOS model mice.
Figure 8.
Intestinal FXR plays an important role in glucose metabolism in PCOS model mice. Mice were divided into 3 groups (LET, LET+CDCA, and LET+GUDCA) and were treated for 35 days to explore the effects of intestinal FXR (LET: 4, LET+CDCA: 7, and LET+GUDCA: 8). (A) Schematic representation of the experimental design. (B) GTT. (C) Fasting blood glucose. (D) Thirty minutes after glucose injection. (E) Mean blood glucose. (F) Area under the curve (AUC) of the GTT. (G) Fasting insulin. (H) HOMA-IR. (B-H) P values were determined by 1-way ANOVA with the least significant difference (LSD) multiple comparison post hoc test. P < 0.05 was considered statistically significant. CDCA, chenodeoxycholic acid; FXR, farnesoid X receptor; GTT, glucose tolerance test; GUDCA, glycoursodeoxycholic acid; HOMA-IR, homeostasis model assessment for insulin resistance index; LET, letrozole; PCOS, polycystic ovarian syndrome.
Discussion
In this study, we identified dysbiosis of the intestinal flora in treatment-naïve PCOS patients. First, we observed an altered gut microbial pattern in treatment-naïve PCOS patients when compared with the gut microbial patterns of controls. Second, the LEfSe tool revealed that the treatment-naïve PCOS patients had a high relative abundance of Bacteroidetes; this finding was in accordance with a recent study investigating the intestinal flora in individuals with PCOS (40, 41). Third, 4 of the top 10 OTUs with the highest importance in random forest model belonged to Bacteroides. The combination of these 10 OTUs discriminated PCOS patients from healthy controls with high accuracy. We noted that the gut microbiome-based analysis could be used to predict the disease as a classifier with an AUC of 0.78, implying that the microbial signature identified may be a potentially powerful tool for the diagnosis of PCOS and that integrating clinical markers and microbial profiles may further improve the discriminative ability.
PCOS model mice had dysbiosis of the intestinal flora compared to the intestinal flora of the control mice. We used LEfSe to determine differentially abundant bacterial taxa in PCOS model mice and controls and then found that the PCOS model mice had a high relative abundance of Bacteroidetes, which is in accordance with the findings among PCOS patients. A high relative abundance of Bacteroidetes may be characteristic of PCOS intestinal flora. This result may provide more data on the intestinal flora characteristics of PCOS.
In our work, compared with controls, women with PCOS and PCOS model mice had insulin resistance, in accord with previous studies that considered that insulin resistance plays a significant etiological role in PCOS (42, 43). Moreover, the intestinal flora disorder of women with PCOS and that of PCOS model mice are similar. Evidence is accumulating that the gut microbiota is involved in the etiology of insulin resistance (44, 45). Mice transplanted with stool from individuals with PCOS displayed insulin resistance only and did not show abnormal glucose metabolism, contradicting a recent study that showed abnormal glucose metabolism in mice transplanted with stool from individuals with PCOS for 3 weeks and suggesting the need for further experimental verification (17). However, no difference was found between the PCOS mice transplanted with fecal microbiota from control women and the PCOS mice transplanted with fecal microbiota from women with PCOS. This result may be because the drugs used for PCOS modelling were too strong or the time for FMT was insufficient. When we removed Bacteroidetes and Firmicutes from the intestinal flora via a continuous 35-day antibiotic cocktail treatment, the PCOS model mice exhibited improved phenotypes and insulin resistance, which was similar to the finding that a prominent reduction in the abundance of Firmicutes and Bacteroidetes with vancomycin and bacitracin would ameliorate insulin resistance in diet-induced obesity (46). The gut microbiota appeared to be an interrelated key factor in insulin resistance and may promote the pathogenesis of PCOS.
Furthermore, we identified and analyzed cecum metabolites and found that cecum farnesol was significantly increased in PCOS model mice. Farnesol is a natural ligand of FXR; however, the activity of FXR activated by farnesol is very weak. FXR can be activated only by a superphysiological dose of farnesol (47), which means that farnesol is an antagonist of FXR. Intestinal L-cell secretion of GLP-1 decreased, and blood glucose and HOMA-IR increased after FXR was suppressed (48, 49). Cecum farnesol-FXR may be related to insulin resistance in PCOS model mice.
FXR is widely distributed in organs such as the liver, kidney, and intestines (28). FXR mRNA levels in the ileum, but not in the liver, were significantly increased in antibiotic-treated PCOS mice compared with those in the PCOS model mice. The serum FGF15 level was significantly increased synchronously. Intestinal FXR activation induces the expression of FGF15/19, and it has been demonstrated that FGF15/19 affects glucose and energy homeostasis (48, 49). FGF19 transgenic mice showed increased hepatic β oxidation, reduced adipose tissue weight, and improved glucose tolerance and insulin sensitivity (50). Our results suggest that intestinal flora may play an important role in the pathogenesis and insulin resistance of PCOS via the FXR signalling pathway.
Bile acids are physiological ligands for FXR and can regulate multiple metabolic diseases by binding to FXR (28, 29). The primary bile acid CDCA is a strong agonist of FXR (51), and the secondary bile acid GUDCA, transformed from primary bile acids by the metabolic activities of gut anaerobic bacteria, is an antagonist of FXR (27). In our study, we observed that oral administration of CDCA improved glucose intolerance. Oral GUDCA supplementation did not affect glucose metabolism in PCOS mice. In the gut, primary bile acid CDCA is successively converted by microbial bile salt hydrolase, an enzyme expressed predominantly by Bacteroides, Lactobacillus, Bifidobacteria, and Clostridium, and by bacterial 7α-dehydroxylase, an enzyme mainly expressed by Eubacterium and Clostridium, into the secondary bile acid lithocholic acid (52). The intestinal flora affects the properties and functions of bile acids. The abundance of Bacteroidetes, which can hydrolyze CDCA by microbial bile salt hydrolases, is high in PCOS patients and PCOS model mice. We considered that the metabolism of CDCA mediated by Bacteroides was enhanced in PCOS patient and PCOS model mice so that activated intestinal FXR was downregulated. Thus, the metabolic disorders of PCOS may in part be due to a lack of intestinal FXR activation. The observations of similar metabolic effects of antibiotics and CDCA in PCOS model mice suggest that the intestinal flora–bile acid–intestinal FXR signalling pathway might be an important mechanism of glucose metabolic disorder in PCOS. However, no differences in fasting insulin levels and HOMA-IR were found among the PCOS model group (LET), LET+CDCA group, and LET+GUDCA group. Thus, the mechanisms by which the gut microbiota affect insulin resistance are indeed complex and might involve individual susceptibility, which should be investigated further.
The present study explored the characteristics of the intestinal flora in individuals with PCOS and the effect of the intestinal flora on PCOS. We concluded that a high relative abundance of Bacteroidetes may be characteristic of the PCOS intestinal flora. In addition, we found that removing the gut microbiota decreased serum testosterone levels, ameliorated insulin resistance, and increased relative FXR mRNA levels in the ileum. PCOS stool-transplanted mice exhibited insulin resistance at 10 weeks. Treating the PCOS model mice with CDCA improved fasting blood glucose and mean blood glucose levels. The intestinal flora is a key factor in the development of insulin resistance in PCOS, and it promotes the glucose metabolism disorder of PCOS possibly through the Bacteroidetes–bile acid–intestinal FXR signalling pathway; moreover, FXR activation may have a beneficial, rather than detrimental, effect on PCOS glucose metabolism. The intestinal flora shows promise as a potential target for PCOS treatment.
Limitations
Women with PCOS were diagnosed according to the 2003 Rotterdam criteria; PCOS subtypes were not identified. Subtype analyses can determine if the microbiome is different between different PCOS subtypes. In this study, we recruited 56 individuals with PCOS and 31 healthy controls. We did not perform a subtype analysis because the sample size for each subtype was too small to produce reliable results; future studies with larger sample sizes should be conducted to explore the influence of PCOS subtypes. When studying insulin resistance induced by intestinal flora, the addition of bile acid supplementation and antibiotic treatments with the PCOS fecal transplantation experiment would have further strengthened our findings. Antibiotic treatment of PCOS model mice can decrease serum testosterone levels, ameliorate insulin resistance, and increase relative FXR mRNA levels in the ileum; treating the PCOS model mice with CDCA improved fasting blood glucose and mean blood glucose levels, but the addition of bile acid supplementation and antibiotic treatments to the control mice would have better demonstrated our experimental results.
Our study would have been improved by strictly excluding vegetarians, and those with probiotic, prebiotic, and antibiotic use before specimen collection. However, it is difficult to require participants to standardize their diet before collecting the stool samples. We reduced the impact of diet on the fecal samples by strictly following the standard that the individuals could not have received treatment with any antibiotic for at least 1 month before collecting the fecal samples. Although none of the fecal samples were verified by collecting 2 samples from the same woman, all fecal samples were collected in strict accordance with the same fecal collection standards.
Acknowledgments
Financial Support: This study was supported by the National Key R&D Program of China (2019YFA0802300 and 2017YFC1310600), the National Natural Science Foundation of China (NSFC31570497, NSFC 31322003 and NSFC 81800746), the National Projects of Major Infectious Disease Control and Prevention (2017ZX10103011), the Science and Technology Program of Guangzhou, China (201904010091), and the Natural Science Foundation of Hunan Province (2018JJ3467).
Glossary
Abbreviations
- AUC
area under the curve
- CDCA
chenodeoxycholic acid
- CV
coefficient of variation
- FGF15
fibroblast growth factor 15
- FINS
fasting plasma insulin
- FMT
fecal microbiota transplantation
- FPG
fasting plasma glucose
- FXR
farnesoid X receptor
- GTT
glucose tolerance test
- GUDCA
glycoursodeoxycholic acid
- HOMA-beta
homeostasis model assessment for beta cell function
- HOMA-IR
homeostasis model assessment for insulin resistance index
- LET
letrozole
- OTU
operational taxonomic unit
- PCoA
principal coordinate analysis
- PCOS
polycystic ovary syndrome
- qPCR
quantitative PCR
Accession numbers: Gut microbiome 16S rRNA gene sequences have been deposited in the European Nucleotide Archive under the accession numbers PRJEB38647 and PRJEB38648.
Additional Information
Disclosures: The authors declare that there are no conflicts of interest.
Data Availability
The data used to support the findings of this study are available from the corresponding author on reasonable request.
References
- 1. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270-284. [DOI] [PubMed] [Google Scholar]
- 2. Jacewicz-Święcka M, Kowalska I. Polycystic ovary syndrome and the risk of cardiometabolic complications in longitudinal studies. Diabetes Metab Res Rev. 2018;34(8):e3054. [DOI] [PubMed] [Google Scholar]
- 3. Spinedi E, Cardinali DP. The polycystic ovary syndrome and the metabolic syndrome: a possible chronobiotic-cytoprotective adjuvant therapy. Int J Endocrinol. 2018;2018:1349868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumarendran B, O’Reilly MW, Manolopoulos KN, et al. . Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. Plos Med. 2018;15(3):e1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajuk Studen K, Jensterle Sever M, Pfeifer M. Cardiovascular risk and subclinical cardiovascular disease in polycystic ovary syndrome. Front Horm Res. 2013;40:64-82. [DOI] [PubMed] [Google Scholar]
- 6. Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78(8):782-785. [DOI] [PubMed] [Google Scholar]
- 7. Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol. 2015;145:213-225. [DOI] [PubMed] [Google Scholar]
- 8. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism. Endocr. Rev. 2016;37(5):467-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garg D, Tal R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod Biomed Online. 2016;33(1):15-28. [DOI] [PubMed] [Google Scholar]
- 10. Miller WL, Tee MK. The post-translational regulation of 17,20 lyase activity. Mol Cell Endocrinol. 2015;408:99-106. [DOI] [PubMed] [Google Scholar]
- 11. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu S, Divall S, Nwaopara A, et al. . Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63(4):1270-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crespo RP, Bachega TASS, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018;62(3):352-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagpal R, Yadav H, Marotta F. Gut microbiota: the next-gen frontier in preventive and therapeutic medicine? Front Med (Lausanne). 2014;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jobira B, Frank DN, Pyle L, et al. . Obese adolescents with PCOS have altered biodiversity and relative abundance in gastrointestinal microbiota. J. Clin. Endocrinol. Metab. 2020;105(6):dgz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Sun Z, Jiang S, et al. . Probiotic bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut-brain axis. mSystems. 2019;4(2): e00017-e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qi X, Yun C, Sun L, et al. . Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019;25(9):1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552-2562. [DOI] [PubMed] [Google Scholar]
- 19. He Y, Wu W, Zheng HM, et al. . Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24(10):1532-1535. [DOI] [PubMed] [Google Scholar]
- 20. Parekh PJ, Arusi E, Vinik AI, Johnson DA. The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. Front Endocrinol (Lausanne). 2014;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim MY, You HJ, Yoon HS, et al. . The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut. 2017;66(6):1031-1038. [DOI] [PubMed] [Google Scholar]
- 23. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027-1031. [DOI] [PubMed] [Google Scholar]
- 24. Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7(1):12-18. [DOI] [PubMed] [Google Scholar]
- 25. Gadaleta RM, van Erpecum KJ, Oldenburg B, et al. . Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60(4):463-472. [DOI] [PubMed] [Google Scholar]
- 26. Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51(4):771-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun L, Xie C, Wang G, et al. . Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24(12):1919-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gege C, Makishima M, Okamoto AY, et al. . Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362-1365. [DOI] [PubMed] [Google Scholar]
- 29. Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368(1-2):17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torres PJ, Skarra DV, Ho BS, et al. . Letrozole treatment of adult female mice results in a similar reproductive phenotype but distinct changes in metabolism and the gut microbiome compared to pubertal mice. BMC Microbiol. 2019;19(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skarra DV, Hernández-Carretero A, Rivera AJ, Anvar AR, Thackray VG. Hyperandrogenemia induced by letrozole treatment of pubertal female mice results in hyperinsulinemia prior to weight gain and insulin resistance. Endocrinology. 2017;158(9):2988-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McDonald D, Price MN, Goodrich J, et al. . An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. Isme J. 2012;6(3):610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caporaso JG, Kuczynski J, Stombaugh J, et al. . QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460-2461. [DOI] [PubMed] [Google Scholar]
- 35. He Y, Caporaso JG, Jiang XT, et al. . Stability of operational taxonomic units: an important but neglected property for analyzing microbial diversity. Microbiome. 2015;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kauffman AS, Thackray VG, Ryan GE, et al. . A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103-108. [DOI] [PubMed] [Google Scholar]
- 38. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229-241. [DOI] [PubMed] [Google Scholar]
- 39. Yuelian Y. Data from: Effects of PCOS faecal microbiota transplantation on the disruption of insulin sensitivity in PCOS model mice. Harvard Dataverse. 2020. Deposited October 7, 2020. doi: 10.7910/DVN/CAILIS. [DOI] [Google Scholar]
- 40. Qi X, Yun C, Sun L, et al. . Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019;25(8):1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu R, Zhang C, Shi Y, et al. . Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324-332. [DOI] [PubMed] [Google Scholar]
- 43. Hutchison SK, Harrison C, Stepto N, Meyer C, Teede HJ. Retinol-binding protein 4 and insulin resistance in polycystic ovary syndrome. Diabetes Care. 2008;31(7):1427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. ; MetaHIT Consortium . Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376-381. [DOI] [PubMed] [Google Scholar]
- 45. Khan MT, Nieuwdorp M, Bäckhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20(5):753-760. [DOI] [PubMed] [Google Scholar]
- 46. Hwang I, Park YJ, Kim YR, et al. . Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. Faseb J. 2015;29(6):2397-2411. [DOI] [PubMed] [Google Scholar]
- 47. Forman BM, Goode E, Chen J, et al. . Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687-693. [DOI] [PubMed] [Google Scholar]
- 48. Kir S, Beddow SA, Samuel VT, et al. . FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331(6024):1621-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taoka H, Yokoyama Y, Morimoto K, et al. . Role of bile acids in the regulation of the metabolic pathways. World J Diabetes. 2016;7(13):260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tomlinson E, Fu L, John L, et al. . Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143(5):1741-1747. [DOI] [PubMed] [Google Scholar]
- 51. Jung D, Podvinec M, Meyer UA, et al. . Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology. 2002;122(7):1954-1966. [DOI] [PubMed] [Google Scholar]
- 52. Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol. 2018;9:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author on reasonable request.