Summary:
Some patients with bland smooth muscle tumors in the uterus have synchronous or asynchronous smooth muscle tumors in the peritoneum and/or the retroperitoneum. It is usually assumed that the uterine tumor is the primary lesion, and the extrauterine neoplasm represents its metastasis. Thus, they are designated as low-grade leiomyosarcomas because they lack the diagnostic features of a conventional spindle cell leiomyosarcoma. Nineteen such cases were retrieved from the files of the Department of Pathology at The University of Texas M.D. Anderson Cancer Center, covering a period of 18 yr. Institutional Review Board approval was obtained before the initiation of this study. In addition, 31 cases of conventional uterine leiomyosarcomas of a high grade were reviewed for comparison. Clinicopathologic features such as patients’ age, tumor location, histologic features, stage, treatment, and follow-up were recorded. Immunohistochemical stains for estrogen receptor (ER), progesterone receptor (PR), p53, Ki-67, and WT-1 were performed in the initially detected tumor and the subsequent neoplasm of all cases with available material in the low-grade group and selected cases in the high-grade group. Compared with high-grade leiomyosarcomas, the low-grade group cases were found at an early age (45 vs. 52.8 yr), had a longer median time of "recurrence" (42mo for the low-grade group vs. 12mo for high-grade leiomyosarcomas), longer median survival (165mo for the low-grade group vs. 41mo for the high-grade group), and a much better overall survival (84% vs. 13%). Three (16%) patients died of disease in the low-grade group versus 27 (87%) patients in the high-grade leiomyosarcoma group. We also found a difference in the location of the extrauterine tumors. Most cases of low-grade tumors were found in the pelvis, abdomen, or retroperitoneum, whereas most high-grade leiomyosarcomas involved the lung. In the low-grade tumors, there were some differences in the immunophenotype between the uterine and the extrauterine neoplasms, but in the high-grade tumors, there were no differences in the immunohistochemistry between the primary tumor and the metastasis. In addition to these differences between the 2 groups in the age of the patients, sites of recurrences, and the immunophenotype of the uterine and extrauterine tumor, neither the uterine nor the extrauterine low-grade lesions had histologic features of malignancy. On the basis of these differences, the possibility that the extrauterine lesions in the low-grade group represent independent primaries involving the secondary mullerian system is proposed.
Keywords: Uterus, Ovary, Peritoneum, Retroperitoneum, Female genital tract, Leiomyosarcoma, Low-grade leiomyosarcoma, Smooth muscle tumor of uncertain malignant potential, Smooth muscle tumor
Most leiomyosarcomas of the uterus and ovary are a homogeneous group of high-grade tumors characterized by at least 2 of the following features: coagulative tumor cell necrosis, significant nuclear atypia, and numerous mitotic figures. In addition, they commonly metastasize to the lungs. In contrast, patients with low-grade smooth muscle tumors of the gynecologic tract who develop smooth muscle tumors outside the gynecologic tract fall into 2 different groups. On one side of the spectrum, we have disseminated peritoneal leiomyomatosis, which is characterized by the presence of small (< 1 cm) smooth muscle tumors in the omentum and peritoneum, probably derived from multipotential submesothelial mesenchymal cells under the influence of a hormonal stimulus (1–5). On the other side, we have patients with a previous hysterectomy for leiomyomas who subsequently develop large smooth muscle tumors in the peritoneum or the retroperitoneum. These cases usually raise a nomenclature dilemma and are commonly designated as metastatic, low-grade leiomyosarcomas, despite the absence of the aforementioned typical features of malignancy in the "primary tumor" or in its alleged "metastasis." Alternatively, some authors have referred to these lesions as benign metastasizing leiomyomas (6–8).
In this study, we present our experience with a group of patients with low-grade smooth muscle tumors of the uterus and ovary with synchronous or asynchronous tumors involving the peritoneum and retroperitoneum, and propose multicentricity as their pathogenetic mechanism.
MATERIAL AND METHODS
Fifty smooth muscle tumors of the gynecologic tract were reviewed. These tumors included 19 cases designated as low-grade leiomyosarcomas and 31 high-grade leiomyosarcomas. The cases were retrieved from the files of the Department of Pathology at The University of Texas M.D. Anderson Cancer Center and the files of one of the authors (E.G.S.). These cases had been originally diagnosed between May 1989 and January 2007. Slides from the original tumor and also from the subsequent tumor were reviewed. The number of slides reviewed ranged from 1 to 28 (median 6 and mean 8.9) for the original tumor, and from 1 to 19 (median 3, mean 4.9) for the subsequent tumor.
The tumors designated as low-grade leiomyosarcomas included 18 from the uterus and 1 from the ovary. These cases had been initially diagnosed as smooth muscle tumors of uncertain malignant potential (12 cases), low-grade leiomyosarcomas (3 cases), and leiomyomas (4 cases). The 3 cases initially diagnosed as low-grade leiomyosarcomas were the only cases that had a tumor outside the gynecologic tract at presentation. The other 16 cases developed a tumor outside the uterus after the hysterectomy. Thirty-one cases of conventional uterine leiomyosarcoma diagnosed by the World Health Organization criteria (9) were also retrieved for comparison.
Clinical information, including the age of the patients, treatment, International Federation of Gynecology and Obstetrics stage, and follow-up, was obtained from the patients’ charts. The following gross and histologic features were recorded: tumor size, the presence or absence of significant atypia, the presence or absence of coagulative tumor cell necrosis, and mitotic index (MI) per 10 high-power fields (HPF) (determined in the most mitotically active area). Immunohistochemical studies were performed in the initial and subsequent tumors in 10 low-grade leiomyosarcomas and 5 high-grade leiomyosarcomas, using the avidin biotin peroxidase method, with antisera to WT-1 (1:40 dilution, Dako Carpinteria, CA), Ki-67 (1:100 dilution, Dako), ER (1:35 dilution, Leica, Bannockburn, IL), PR (1:200 dilution, Neomarkers, Freemont, CA), and p53 (1:100 dilution, Dako). The degree of staining was evaluated semiquantitatively as follows: 1 + (1%–25%), 2 + + (26%–50%), 3 + + + (51%–75%), and 4 + + + + (>75%) (Table 2). WT-1 was considered positive in cases of nuclear and cytoplasmic staining.
TABLE 2.
Discordant immunohistochemistry results between the uterine and the extrauterine cases in 7 low-grade lesions
| Estrogen receptors |
Progesterone receptors |
WT-1 |
||||
|---|---|---|---|---|---|---|
| Case # | Uterine | Extrauterine | Uterine | Extrauterine | Uterine | Extrauterine |
|
| ||||||
| 1 | + + | − | ||||
| 2 | + | − | + + | − | ||
| 3 | + | − | ||||
| 5 | − | + | ||||
| 6 | + + | − | − | + | ||
| 7 | − | + | ||||
| 9 | + | − | ||||
Clonality studies were attempted on 6 cases; however, they were unsuccessful due to problems in DNA extraction.
RESULTS
Pathology Findings
Gross Findings
The uterine "low-grade leiomyosarcoma" ranged in tumor size from 4.0 to 19.0 cm (mean 12.9 cm), and the ovarian tumor size was 9 cm. Recurrent tumors ranged in size from 2.0 to 30.0 cm (mean 11.5 cm).
Histologic Findings
Low-grade group:
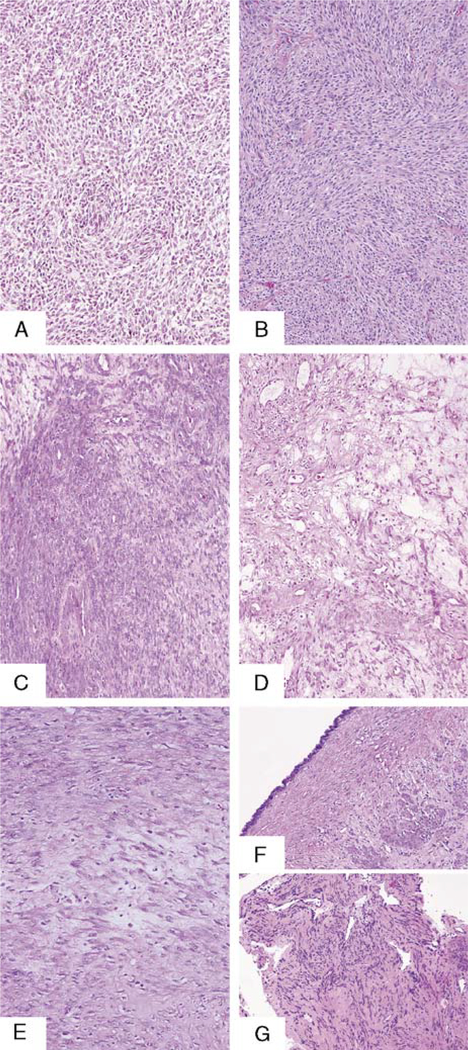
All tumors showed absent or mild cytologic atypia (Figs. 1A–G). Six of the uterine cases had areas of coagulative tumor cell necrosis mixed with hyaline foci, mainly at the periphery of the necrosis. Only 1 of these 6 cases had coagulative tumor cell necrosis mixed with hyaline foci, in the extragenital tumor. The "primary" tumor had a mean MI of 5 mitoses per 10 HPF (range, 1–12 mitoses per 10 HPF). In the extragenital tumors, the mean MI was 6 mitoses per 10 HPF (range, 1–14 mitoses per 10 HPF). The diagnostic features required to make the diagnosis of conventional leiomyosarcoma were not seen in the uterine or ovarian main tumors in any of the cases. Of the 19 cases of low-grade leiomyosarcoma, 12 were diagnosed as smooth muscle tumors of uncertain malignant potential on the basis of the presence of mild atypia (10 cases) or necrosis (mixed coagulative and hyaline, 2 cases) and 5 mitoses or more. Currently, some of these tumors would be diagnosed as mitotically active leiomyomas! Three tumors were diagnosed as "low-grade leiomyosarcomas" despite their bland appearance, because they were detected with synchronous bland smooth muscle tumors in the pelvis. The other 4 cases were originally diagnosed as leiomyomas, but because there was a "recurrence" in the peritoneum, they were designated as low-grade leiomyosarcomas. All the cases in this group developed a smooth muscle tumor in the peritoneum or the retroperitoneum, synchronous (3 cases), or after the resection of the uterine/ovarian smooth muscle tumor (16 cases). In all except 1 case, the extragenital tumor had the bland histologic appearance seen in the main tumors and rare mitotic figures. In 1 case, the extragenital smooth muscle tumor had moderate cytologic atypia, 3 mitotic figures per 10 HPF, and absence of coagulative necrosis.
FIG. 1.
(A and B) Uterine low-grade leiomyosarcoma in 2 patients with a remarkable bland histology (100 ×) and their corresponding pelvic recurrences (C and D) (100 ×). (E) Uterine low-grade leiomyosarcoma in 1 patient (100 ×) and her pelvic recurrence (F); note benign mullerian-type epithelium lining the pelvic tumor (100 ×), lung recurrence (G) in the same patient (100 ×).
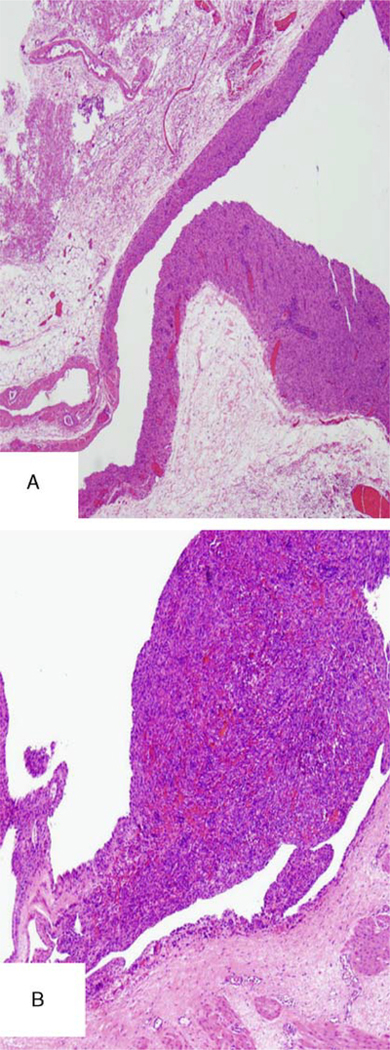
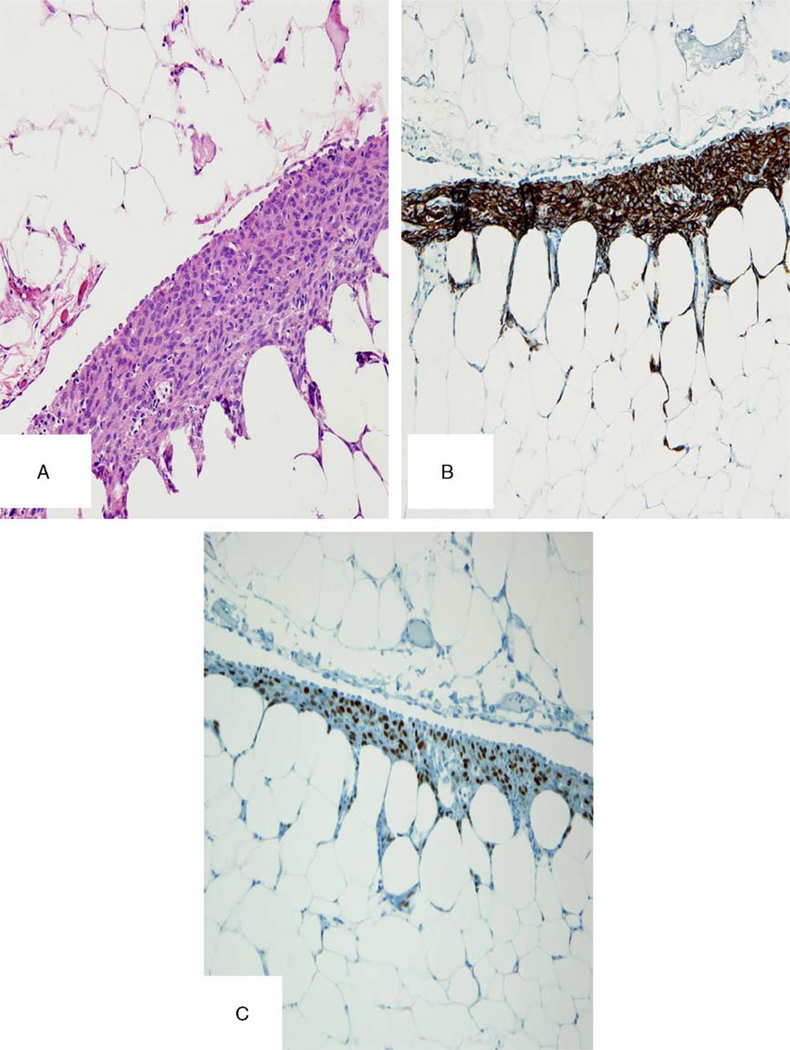
In this group of peritoneal and retroperitoneal possible recurrences, there were 3 cases with unusual features including: (1) 1 case with a benign mullerian type of serous epithelium focally lining the pelvic tumor (Fig. 1F), diagnostic of endosalpingiosis; (2) 1 case with a proliferation of smooth muscle cells in a vessel wall of a recurrent smooth muscle tumor in the pelvis (Fig. 2); and (3) 1 case with foci of proliferating submesothelial cells in the pelvic peritoneum with a smooth muscle immunophenotype (Fig. 3) near the areas of recurrent tumor.
FIG. 2.
(A and B) A smooth muscle proliferation involving the wall of a vessel in a smooth muscle tumor found in the pelvis.
FIG. 3.
(A) A proliferation of smooth muscle cells in the omentum, under the mesothelial surface. (B) Desmin-positive stain in these cells. (C) Estrogen receptor positive.
All the cases of high-grade leiomyosarcoma fulfilled at least 2 of the diagnostic criteria required for leiomyosarcoma (i.e. diffuse significant cytologic atypia, coagulative tumor cell necrosis, and an MI of at least 10 mitoses per 10 HPF). One patient in this group also had peritoneal leiomyomatosis disseminata.
Immunohistochemical Findings
Immunohistochemical studies performed in 10 tumors of the "low-grade group," in both the initial and the recurrent tumors, showed that both had a low proliferating index, < 500 positive cells per 10HPF, as demonstrated by the Ki-67 immunostaining. p53 stain, a marker usually positive in malignant smooth muscle tumors, was positive in only 2 cases and negative in 8 cases. PR, a marker frequently negative in malignant cases, was positive in all cases. ER and/or WT-1, markers that indicate an origin in the mullerian system, were positive in 8 of the 10 "recurrences" (10,11). In 7 of 10 cases, there were some differences in the immunoprofile between the uterine tumor and the extrauterine neoplasm.
In the 5 high-grade leiomyosarcomas, 2 cases were positive for WT-1 and PR, and 3 cases were negative for these markers. Four cases were positive for p53, and all cases had a high Ki-67 (over 2000 positive cells per 10HPF). There were no immunohistochemical differences between the primary tumor and their metastases in the 3 cases where primary tumor and metastases were examined.
Clinical Findings
The mean age of initial presentation was 45 yr (range, 37–58 yr) for patients with low-grade neoplasms versus 52.8 yr (range, 35–86 yr) for patients with high-grade leiomyosarcomas.
All of the patients in the low-grade group had undergone total abdominal hysterectomies and resections of the recurrent tumors. In addition, 7 patients had received chemotherapy; 5 patients had received a combination of chemotherapy and radiation. One patient had received only radiation therapy.
Thirty of the high-grade leiomyosarcoma patients had undergone total abdominal hysterectomies and 1 had undergone a vaginal hysterectomy. All these patients, except 1 with liver metastasis, had undergone resections of the recurrent tumors. Fifteen patients had received chemotherapy and 10 patients had received a combination of chemotherapy and radiation.
Two of the 19 patients in the low-grade group also had a second malignancy; 1 had stage IV colonic carcinoma and the other had acute myelogenous leukemia. One patient with high-grade leiomyosarcoma had a renal cell carcinoma, Fuhrman nuclear Grade 2, limited to the kidney.
Follow-up was available in 14 low-grade cases and in all 31 high-grade leiomyosarcoma cases.
The follow-up ranged from 1 to 285mo (mean 129.46 mo). Significant clinical differences were found between the 2 groups, such as the location of the first metastatic lesion. Metastases were first found in the pelvis in 79% of the low-grade group versus 19% of the high-grade leiomyosarcoma group and in the lung in 16% of the low-grade group versus 70% of the high-grade leiomyosarcoma group. In 1 case of low-grade neoplasm, the first recurrent tumor was an axillary mass, discovered before the pelvic recurrence. Six low-grade cases that had pelvic involvement later developed distally located recurrent tumors (lung, axilla, and vertebra). In the low-grade group, there were more soft tissue metastases than organ metastases (79% vs. 21%); in high-grade leiomyosarcoma, there were 19% soft tissue metastases and 81% organ metastases. Nine (64%) of 14 patients with low-grade neoplasm and follow-up available are still alive (6 alive with no evidence of disease, 3 alive with disease). Deaths due to low-grade neoplasms occurred in 3 (16%) patients: 1 patient with progressive pelvic disease and 2 patients with combined progressive pelvic disease and distant disease including liver, lung, and vertebral recurrent tumors, respectively. Of interest, one of the patients in the low-grade group had several pelvic recurrences in a period of 86 mo. The patient died 3mo after developing a vertebral recurrence that showed high-grade histology. Only 1 of the 3 patients had lung metastases. Two patients died of a second malignancy and 5 patients were lost to follow-up. In the high-grade leiomyosarcomas, 27 patients died of disease, 17 patients died of a combination of distant pelvic and recurrences, 4 patients died of progressive abdominal-pelvic disease, and 6 patients died of distant metastases. Twenty-two of the 27 patients had lung metastases. Four patients with high-grade leiomyosarcoma are alive with disease.
Recurrence and Survival
Significant differences were found between the groups. The median time of recurrence was 42mo (low-grade neoplasm) versus 12mo (high-grade leiomyosarcoma). The mean time of recurrence was 52mo (low-grade neoplasm) versus 19.3mo (high-grade leiomyosarcoma). The median time of survival was 165mo (low-grade neoplasm) versus 41mo (high-grade leiomyosarcoma). In the low-grade group, only 3 patients (16%) died of disease. In the high-grade group, 27 patients (87%) died of disease.
DISCUSSION
A summary of the main clinicopathologic findings is presented in Table 1 and a summary of the immunohistochemical differences is presented in Table 2. Smooth muscle tumors from the soft tissue or from different organs have similar features. However, uterine smooth muscle tumors are easier to classify and, except for the unusual group of uncertain malignant lesions, more predictable. Soft tissue smooth muscle tumors are less predictable and the criteria for malignancy are different in both groups (12–14). A soft tissue smooth muscle tumor might be considered malignant because of focal atypia, but in the uterus it would be an atypical leiomyoma. These differences have made it difficult to evaluate an extrauterine smooth muscle tumor in a patient with a uterine smooth muscle tumor. When both lesions are high-grade malignancies, the uterine tumor is a leiomyosarcoma and the extrauterine tumor could represent a metastasis, through lymphatic vessels or transtubal, or could represent a recurrent tumor as explained below in point 8. However, if both tumors are low-grade lesions and the extrauterine tumor involves the pelvis, abdomen, or retroperitoneum, they have been evaluated as soft tissue smooth muscle tumors. Recent studies have shown that tumors arising in the secondary mullerian system, including the peritoneum and the retroperitoneum, might be evaluated as uterine tumors and that their classification as mullerian can be supported by positive WT-1 and ER stains (10,11,15).
TABLE 1.
Differences between low-grade tumors and high-grade leiomyosarcomas
| Low-grade tumors (19) | High-grade leiomyosarcomas (31) | |
|---|---|---|
|
| ||
| Mean age (y) | 45 | 52 |
| Nuclear atypia | Mild | Marked |
| Coagulative necrosis | Mixed with hyaline areas | Present, without hyaline areas |
| Mitoses (per 10HPF) | 5 | 13 |
| First site of metastases | ||
| Pelvis | 79% | 19% |
| Lung | 16% | 70% |
| Metastases in organs | 21% | 81% |
| Mean time to recurrence (mo) | 52 | 19 |
| Patients who died of disease | 3 (16%) | 27 (87%)* |
P value <0.0001 (Fisher exact test).
If the smooth muscle tumors are multiple, small, and involve the peritoneum, they are diagnosed as leiomyomatosis peritonealis disseminate (1–5). In most reports, leiomyomatosis peritonealis disseminata is an entity in which each one of the lesions is smaller than 1 cm in the largest dimension. However, some reports have also reported lesions measuring 3, 7, and 12 cm (2,3). When the lesions have a benign appearance, are single or multiple but involve the lungs, they are designated as benign metastasizing leiomyomas; however, some lesions involving the pelvis or skeletal muscle have also been designated as benign metastasizing leiomyomas (7,16). We have also observed the development of smooth muscle tumors in the peritoneum or the abdominal wall near the wound of a previously resected smooth muscle tumor, which, because of the large size, was resected in small parts. Most probably, during the surgical resection, there was contamination of the wound. Despite all these possibilities, frequently, benign-appearing extrauterine smooth muscle tumors that could be related to uterine smooth muscle tumors found in a previous myomectomy or hysterectomy are designated smooth muscle tumors of uncertain malignant potential or low-grade leiomyosarcoma. The size of the recurrences in the 19 cases in the low-grade group ranged from 2.0 to 30.0 cm, with a mean of 11.5 cm, and these were diagnosed as metastatic low-grade leiomyosarcomas even when neither the uterine nor the extrauterine tumors had features of malignancy. Applying the current diagnostic criteria for smooth muscle tumors of mullerian origin, these uterine and extrauterine tumors would be designated as leiomyomas. We believe that on the basis of the cases included in this study and the review of the literature, there is evidence supporting a multicentric origin for these tumors.
Evidence from our cases:
"Recurrences" in 15 of the 19 cases developed first in the peritoneum and not in the lung, like most high-grade leiomyosarcomas. The peritoneum is considered the secondary mullerian system and capable of developing epithelial (serous) and stromal (endometrial stroma, smooth muscle) mullerian proliferations. In addition, in 1 case, the tumor also had areas of endosalpingiosis overlying the smooth muscle proliferation (Fig. 1F).
All tumors were histologically benign (Fig. 1). Most of them had been diagnosed as smooth muscle tumors of uncertain malignant potential or leiomyosarcoma because the tumor in the peritoneum was considered to be a recurrence and because, before the recent immunohistochemistry studies, most pathologists evaluated them as soft tissue tumors. The latter requires less atypia or a small number of mitoses to be considered smooth muscle tumors of uncertain malignant potential or leiomyosarcoma. In these cases, WT-1 and ER, markers of mullerian tumors, were positive in 8 of 10 cases.
In the low-grade group of tumors involving the uterus, 6 of 9 tumors were positive for WT-1 and 9 of 10 were positive for ER. In the same group of low-grade neoplasms but in extrauterine locations, 5 of 9 tumors were positive for WT-1 and 8 of 10 were positive for ER. WT-1 and ER are well-known markers of mullerian/smooth muscle neoplasms (10,11). This is very important because if the extrauterine tumors are from the mullerian system, they are probably induced by the same factors as the uterine tumors and they should be evaluated using the same criteria as those used for uterine tumors. Criteria for malignancies in smooth muscle tumors of the soft tissue, including the retroperitoneum, are different (11,12,14,17).
Immunostains showed some differences in 7 of 10 cases between the uterine and the extrauterine tumors, supporting the possibilities of independent lesions.
"Metastasis" in soft tissues was seen in 79% of the cases and in organs such as the lungs in 21% of the cases. Because smooth muscle tumors metastasize through blood vessels, lung metastasis should be more common. Soft tissue "metastases" could represent independent lesions.
In 1 case, we found a proliferation of submesothelial spindle cells that had a smooth muscle immunophenotype. This has been advocated as a possible origin for the proliferation of cells that are responsible for the development of leiomyomatosis peritonealis disseminate (1–3,5,18,19).
In 1 case, we have observed in the area of the smooth muscle tumor, in the pelvis, a proliferation of the smooth muscle cells in the wall of the vessel, similar to the proliferation sometimes seen in some smooth muscle tumors, indicating a possible origin from the vessel wall (20). This feature would support an origin at the extrauterine site.
Of 12 patients with high-grade leiomyosarcoma who developed tumors in the pelvis, peritoneum, or retroperitoneum and had relevant information on the chart, the peritoneal lesions in 8 patients could be explained by different possibilities other than metastasis. These include positive margins of resection, tumor only within vessels, tumor that penetrated the uterine serosa and involved the peritoneum, contamination through the fallopian tube lumen due to preoperative procedures, and vaginal recurrence that extended to the peritoneum. None of these features were found in the low-grade leiomyosarcomas.
One high-grade leiomyosarcoma had metastasis in the lung, but also peritoneal leiomyomatosis disseminata, indicating that even if one of the smooth muscle tumors is malignant, a patient might develop multiple benign smooth muscle tumors in the peritoneum.
Evidence of multicentricity from the literature:
Most disseminated peritoneal leiomyomatosis are characterized by small lesions; however, there are reports of cases with lesions measuring up to 7 or 12 cm (2,3). In addition, in some cases of disseminated peritoneal leiomyomatosis, foci of leiomyosarcomas have been reported (3,19). Until recently, it seemed that the study of the clonality of different sites would be the final answer to determine whether different lesions are metastatic or independent primaries. However, recent studies looking for additional explanations for the monoclonality found in tumors in which it was extremely difficult to accept them as metastases, such as multiple papillary tumors of the bladder, or from the head and neck area, or from the thyroid, have proposed that these are multicentric neoplasms arising from a patch of cells with the same clones (21–24). Recently, a similar concern was raised regarding a case of uterine and ovarian endometrioid carcinomas that showed the same clonality by a common method (25). Applying similar concepts to gynecologic tumors derived from the mullerian duct, we could explain unusual findings like monoclonality in leiomyomatosis peritonealis disseminata, a lesion that is difficult to view as metastatic (18). We believe that until we have a complete understanding of the meaning of clonality, we should use common sense when reviewing lesions that could be multicentric.
There are examples of patients with smooth muscle tumors at different sites. Multiple smooth muscle tumors from the soft tissue have been reported in immunocompromised patients and in patients who have an autosomal dominant syndrome that results from heterozygous germline mutations in the fumarate hydratase gene that may function as a tumor suppressor. These patients may also have renal cell carcinoma and uterine smooth muscle tumors (8,26,27).
Multicentricity in peritoneal smooth muscle tumors in patients with uterine smooth muscle tumors has been previously proposed in 1 case that would be difficult to explain otherwise (28).
SUMMARY
On the basis of these facts, we believe that some patients have the propensity to develop multiple smooth muscle tumors in the mullerian tract and eventually some of these could become malignant.
These multicentric lesions might develop due to an abnormal hormonal background, genetic defect, or most probably both. Chemotherapy treatment should be reserved only for lesions where malignant features are clearly present. Alternatively, hormonal treatment may be offered to some patients. In addition, if our hypothesis of multicentricity is correct, the concept of smooth muscle tumor of uncertain malignant potential needs to be reevaluated because most of the rare recurrences of cases diagnosed as smooth muscle tumors of uncertain malignant potential are in the pelvis, peritoneum, and retroperitoneum (29–31), and therefore they might represent multicentric tumors.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Lorena Posligua, Department of Pathology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas
Elvio G. Silva, Department of Pathology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas
Michael T. Deavers, Department of Pathology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas
Maria J. Merino, National Institute of Health, Bethesda, Maryland
Anais Malpica, Department of Pathology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas
REFERENCES
- 1.Fujii S, Okamura H, Nakashima N, et al. Leiomyomatosis peritonealis disseminata. Obstet Gynecol 1980;55:(3 suppl): 79S–83S. [DOI] [PubMed] [Google Scholar]
- 2.Hardman WJ III, Majmudar B. Leiomyomatosis peritonealis disseminata: clinicopathologic analysis of five cases. South Med J 1996;89:291–4. [PubMed] [Google Scholar]
- 3.Raspagliesi F, Quattrone P, Grosso G, et al. Malignant degeneration in leiomyomatosis peritonealis disseminata. Gynecol Oncol 1996;61:272–4. [DOI] [PubMed] [Google Scholar]
- 4.Robles-Frias A, Severin CE, Robles-Frias MJ, et al. Diffuse uterine leiomyomatosis with ovarian and parametrial involvement. Obstet Gynecol 2001;97:(5 part 2): 834–5. [PubMed] [Google Scholar]
- 5.Tavassoli FA, Norris HJ. Peritoneal leiomyomatosis (leiomyomatosis peritonealis disseminata): a clinicopathologic study of 20 cases with ultrastructural observations. Int J Gynecol Pathol 1982;1:59–74. [PubMed] [Google Scholar]
- 6.Egberts JH, Schafmayer C, Bauerschlag DO, et al. Benign abdominal and pulmonary metastasizing leiomyoma of the uterus. Arch Gynecol Obstet 2006;274:319–22. [DOI] [PubMed] [Google Scholar]
- 7.Jo JH, Lee JH, Kim DC, et al. A case of benign metastasizing leiomyoma with multiple metastasis to the soft tissue, skeletal muscle, lung and breast. Korean J Intern Med 2006;21:199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet 2003;73:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavassoli FA, Devilee P, eds. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon, IARC Press; 2003. [Google Scholar]
- 10.Deavers MT, Silva EG, Euscher ED, et al. WT-1 expression may differentiate mullerian from non-mullerian smooth muscle tumors. Mod Pathol 2006;19:176A. [Google Scholar]
- 11.Paal E, Miettinen M. Retroperitoneal leiomyomas: a clinicopathologic and immunohistochemical study of 56 cases with a comparison to retroperitoneal leiomyosarcomas. Am J Surg Pathol 2001;25:1355–63. [DOI] [PubMed] [Google Scholar]
- 12.Billings SD, Folpe AL, Weiss SW. Do leiomyomas of deep soft tissue exist? An analysis of highly differentiated smooth muscle tumors of deep soft tissue supporting two distinct subtypes. Am J Surg Pathol 2011;25:1134–42. [DOI] [PubMed] [Google Scholar]
- 13.Hornick JL, Fletcher CD. Criteria for malignancy in non-visceral smooth muscle tumors. Ann Diagn Pathol 2003;7:60–6. [DOI] [PubMed] [Google Scholar]
- 14.Weiss SW. Smooth muscle tumors of soft tissue. Adv Anat Pathol 2002;9:351–9. [DOI] [PubMed] [Google Scholar]
- 15.He H, Luthringer DJ, Hui P, et al. Expression of CD56 and WT1 in ovarian stroma and ovarian stromal tumors. Am J Surg Pathol 2008;32:884–90. [DOI] [PubMed] [Google Scholar]
- 16.Tori M, Akamatsu H, Mizutani S, et al. Multiple benign metastasizing leiomyomas in the pelvic lymph nodes and biceps muscle: report of a case. Surg Today 2008;38:432–5. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi K, Yabe H, Mukai M, et al. Multiple smooth muscle tumors arising in deep soft tissue of lower limbs with uterine leiomyomas. Am J Surg Pathol 1998;22:897–901. [DOI] [PubMed] [Google Scholar]
- 18.Quade BJ, McLachlin CM, Soto-Wright V, et al. Disseminated peritoneal leiomyomatosis. Clonality analysis by X chromosome inactivation and cytogenetics of a clinically benign smooth muscle proliferation. Am J Pathol 1997;150:2153–66. [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Chaturvedi KU, Gupta R, et al. Leiomyomatosis peritonealis disseminata with malignant change in a postmenopausal woman. Gynecol Oncol 2004;95:742–5. [DOI] [PubMed] [Google Scholar]
- 20.Farshid G, Pradhan M, Goldblum J, et al. Leiomyosarcoma of somatic soft tissues: a tumor of vascular origin with multivariate analysis of outcome in 42 cases. Am J Surg Pathol 2002;26:14–24. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic L, Delahunt B, Mclver B. Thyroid gland clonality revisited: the embryonal patch size of the normal human thyroid gland is very large, suggesting X-chromosome inactivation tumor clonality studies of thyroid tumors have to be interpreted with caution. J Clin Endocrinol Metab 2003;88:3284–91. [DOI] [PubMed] [Google Scholar]
- 22.Lindgren D, Gudjonsson S, Jee KJ, et al. Recurrent and multiple bladder tumors show conserved expression profiles. BMC Cancer 2008;30:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louhelainen J, Wijkström H, Hemminki K. Allelic losses demonstrate monoclonality of multifocal bladder tumors. Int J Cancer 2000;87:522–7. [PubMed] [Google Scholar]
- 24.Worsham MJ, Wolman SR, Carey TE, et al. Common clonal origin of synchronous primary head and neck squamous cell carcinomas: analysis by tumor karyotypes and fluorescence in situ hybridization. Hum Pathol 1995;26:251–261. [DOI] [PubMed] [Google Scholar]
- 25.Matias-Guiu X, Bussaglia E, Catasus L, et al. Correspondence re: W. M. Lin et al., loss of heterozygosity and mutational análisis of the PTEN/MMAC1 gene in synchronous endometrial and ovarian carcinomas. Clin Cancer Res 2000;6:1598–600. [PubMed] [Google Scholar]
- 26.Garg K, Tickoo SK, Soslow RA, et al. Morphologic features of uterine leiomyomas associated with heredity leiomyomatosis and renal cell carcinoma syndrome: a case report. Am J Surg Path 2011;35:1235–37. [DOI] [PubMed] [Google Scholar]
- 27.Sudarshan S, Linehan WM, Neckers L. HIF and fumarate hydratase in renal cancer. Br J Cancer 2007;96:403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho KR, Woodruff JD, Epstein JI. Leiomyoma of the uterus with multiple extrauterine smooth muscle tumors: a case report suggesting multifocal origin. Hum Pathol 1989;20:80–3. [DOI] [PubMed] [Google Scholar]
- 29.Atkins KA, Arronte N, Darus CJ, et al. The use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol 2008;32:98–102. [DOI] [PubMed] [Google Scholar]
- 30.Guntupalli SR, Ramirez PT, Anderson ML, et al. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol 2009;113:324–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol 2009;33: 992–1005. [DOI] [PubMed] [Google Scholar]