Abstract
Introduction:
Cognitive deficits occur in Parkinson’s disease (PD). Cardiorespiratory fitness (CRF) is associated with better cognitive performance in aging especially in executive function (EF) and memory. The association between CRF and cognitive performance is understudied in people with PD. Brain structures underlying associations also remains unknown. This cross-sectional study examined the associations between CRF and cognitive performance in PD. We also examined associations between CRF and brain structures impacted in PD. Mediation analysis were conducted to examine whether brain structures impacted in PD mediate putative associations between CRF and cognitive performance.
Methods:
Individuals with PD (N=33) underwent magnetic resonance imaging (MRI), CRF evaluation (estimated VO2max), and neuropsychological assessment. Composite cognitive scores of episodic memory, EF, attention, language, and visuospatial functioning were generated. Structural equation models were constructed to examine whether MRI volume estimates (thalamus and pallidum) mediated associations between CRF and cognitive performance (adjusting for age, education, PD disease duration, sex, MDS-UPDRS motor score, and total intracranial volume).
Results:
Higher CRF was associated with better episodic memory (Standardized β=0.391; p=0.008), EF (Standardized β=0.324; p=0.025), and visuospatial performance (Standardized β=0.570; p=0.005). Higher CRF was associated with larger thalamic (Standardized β=0.722; p=0.004) and pallidum (Standardized β=0.635; p=0.004) volumes. Thalamic volume mediated the association between higher CRF and better EF (Indirect effect=0.309) and episodic memory (Indirect effect=0.209) performance (p<0.05). The pallidum did not significantly mediate associations between CRF and cognitive outcomes.
Conclusion:
The thalamus plays an important role in the association between CRF and both EF and episodic memory in PD.
Keywords: thalamus, cardiorespiratory fitness, cognition, structural magnetic resonance imaging, MRI, imaging, exercise
1. Introduction
Motor deficits, such as gait and balance dysfunction, are the most frequently recognized characteristics of Parkinson’s disease (PD); however, non-motor features such as cognitive impairment are prominent and associated with poor quality of life. Approximately 20 to 40% of patients in early PD meet criteria for Mild Cognitive Impairment (MCI) while almost 80% of individuals with PD will develop dementia eight years after PD diagnosis [1, 2]. Although Executive function (EF) is the most common cognitive domain affected by PD; other cognitive domains such as visuospatial function, episodic memory, attention and language are also impacted. The pathophysiological basis for cognitive decline in PD includes alterations in dopamine, serotonin, acetylcholine, and glutamate neurotransmission, as well as the neurodegeneration of a number of important cortical structures such as the prefrontal cortex (PFC) and cerebellum. Hallmark neuropathology in PD is evidenced by changes in deep grey matter structures, in the basal ganglia (caudate, putamen, and pallidum), hippocampus, anterior cingulate, and thalamus [3, 4]. Furthermore, in persons with PD, impairments in EF are associated with decreased volumetric size in the PFC, as well as the basal ganglia. Increasing evidence supports the important role of the thalamus and its connections with the PFC and associated cognition, since thalamic lesions are concomitant with EF impairments observed in PFC neurodegeneration. Functional and structural pathophysiological changes in the thalamus have also been associated with impaired EF, attention, memory, and visual spatial processing and overall cognitive functioning in PD. Currently there are no effective treatments for cognitive deficits in PD. Thus, identifying therapeutic interventions that may attenuate cognitive decline and the onset of dementia and slow pathophysiological changes in affected brain regions, such as deep grey matter structures, including the thalamus, may improve quality of life [5].
In PD, different forms of physical exercise, including aerobic exercise (e.g. treadmill training) and motor skill training (e.g. yoga), improve motor performance particularly gait and balance. However, the impact of exercise on cognitive function in PD remains relatively understudied. A small number of aerobic exercise intervention studies using brisk walking, treadmill training, or recumbent bike-training have reported improvements in CRF along with moderate improvement in EF [6–10]. In healthy older adults, both aerobic exercise and CRF have been demonstrated to benefit EF and memory along with improvements in attention and visuospatial function [11–15]. In addition, investigations in cognitive aging have also revealed that cognitive benefits may be due in part to the association of CRF (defined as V02max) on grey matter volumetric (GMV) changes in the basal ganglia, PFC, hippocampus, and thalamus [16]. However, the role of aerobic exercise, and in particular the association of CRF and domain-specific cognitive performance such as attention, memory, visuospatial function, and language remain relatively unexplored in PD [6]. Furthermore, the underlying exercise- and fitness-related structural brain changes that may account for these cognitive benefits have yet to be elucidated in PD.
The aim of this cross-sectional study was to investigate the association of CRF (defined as estimated VO2max) and performance in the cognitive domains of attention, language, visuospatial function, EF, and episodic memory in individuals with PD. We hypothesized a significant association would be seen between CRF and EF, as well as CRF and episodic memory, given reports of significant associations in cognitive aging. Next, we examined the association of CRF and GMV in brain structures associated with cognitive deficits in PD. Regarding brain structures of interest, we focused on deep grey matter structures (caudate nucleus and putamen, nucleus accumbens, globus pallidus, thalamus, anterior cingulate, and hippocampus), as well as the PFC, and cerebellum. These brain regions were selected based on at least one of the following: (i) associated neuropathological changes observed in PD; (ii) regions of exercise-induced neuroplasticity as reported in animal models; and (iii) association with increased CRF and GMV reported in cognitive aging populations. Mediation analyses were conducted to investigate whether brain regions of interested implicated in PD mediated putative associations between CRF and cognitive performance.
2. Methods
2.1. Participants
The study sample consisted of 33 individuals with idiopathic PD who were enrolled in a cross-sectional study investigating associations between CRF, cognitive performance, and neuroanatomical volumetrics using magnetic resonance imaging (MRI). All participants had a diagnosis of PD using criteria used by the UK Brain Bank criterium [17]. Participants completed a structured interview and the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [18] with a movement disorder specialist (GP). Exclusion criteria included deep brain stimulation surgery, or a diagnosis of dementia based on MDS-PD dementia criteria [1]. Participants were recruited from the University of Southern California (USC) Movement Disorder Clinic, the Michael J. Fox trial finder, radio advertisements, and local PD patient support groups. All participants were optimally medicated during the completion of the study assessments. The USC Institutional Review Boards (IRB) approved study procedures and participants provided written informed consent.
2.2. Fitness assessment
CRF was quantified by estimating the maximum oxygen uptake volume (estimated VO2max) as measured by the Ebbeling Single Stage Submaximal Treadmill Walking Fitness Test [19], a reliable and valid way to estimate VO2max [20]. Briefly, participants first underwent a 4-minute warm-up, walking at a brisk, but comfortable pace ranging from 2.0 to 4.5 mph, and 0% grade, eliciting a heart rate within 50 to 70% of age-predicted maximum, followed by a single testing stage of treadmill walking for 4 minutes at 5% grade. The steady state heart rate was determined from the average of the final 30 seconds at 5% grade. The equation to estimate VO2max (ml kg-1 min-1) from a single stage 4-minute, 5% grade submaximal treadmill test is as follows: VO2max = 15.1 + 21.8*SPEED (mph) - 0.327*HEART RATE (bpm) - 0.263*SPEED*AGE (yr) + 0.00504*HEART RATE*AGE + 5.98*GENDER (0 = female; 1 = male).
2.3. Neuropsychological assessment
The neuropsychological battery administered followed the Level-II guidelines from the PD-MCI MDS taskforce for assessing cognition [21]. The neuropsychological battery consisted of tests that measured cognitive performance across multiple domains including attention, language, visuospatial function, EF, and episodic memory. To reduce the number of comparisons a composite score was created capturing performance within each domain. Scores were transformed as needed so that for all tests higher scores represented better performance. Composite and raw scores on each test were z-score standardized based on the sample mean and standard deviation. Attention was measured by (i) the Delis Kaplan Executive Function System (D-KEFS), Color Word Interference Test (CWIT), with the number of seconds to complete the color naming and word reading subtests, (ii) the total score for the forward digit span condition of the Adaptive Digit Ordering Test (DOT-A), and (iii) the total raw score from the digit sequencing condition of the DOT-A. Language ability was measured by (i) the total raw score from the 4th edition of the Wechsler Adult Intelligence Scale (WAIS-IV) Similarities subtest, (ii) the total correct number of words produced on the D-KEFS Letter Fluency test, and (iii) the total correct number of words on the D-KEFS Category Fluency test. Episodic memory was measured by (i) the total number of correct words recalled across the five immediate recall trials of the 2nd Edition of the California Verbal Learning Test (CVLT-II), (ii) the total number of words recalled on the long-delayed free recall condition of the CVLT-II, (iii) the total points on the immediate recall subtest of the Wechsler Memory Scale-Third Edition Visual Reproduction test (WMS-III), and (iv) the total points on the delayed recall condition of the WMS-III Visual Reproduction test. Visuospatial ability was measured by (i) total raw score on the Judgment of Line Orientation Test (JLO) and (ii) the total raw score on the Hooper Visual Organization Test (HVOT). EF was measured using the number of errors on the Wisconsin Card Sorting Test (WCST), (ii) the number of seconds to complete the color-word inhibition subtest of the D-KEFS CWIT, (iii) the number of seconds to complete the inhibition/switching subtest from the D-KEFS CWIT, (iv) the number of correct words produced on the verbal fluency switching subtest from the D-KEFS verbal fluency test, and (v) the total move score from the Tower of London-Drexel University test. For descriptive purposes participants were classified as meeting criteria for mild cognitive impairment (PD-MCI) by consensus (VF, AP, MG) based on Level II MDS Task Force Criteria.
2.4. Structural Magnetic Resonance Imaging (MRI) Acquisition
The structural MRI was completed on a 3.0 Tesla MRI system (Siemens, Erlangen, Germany) fitted with a 12-channel receive-only phased array head coil at the UCLA Staglin IMHRO Center for Cognitive Neuroscience. A high-resolution whole-brain structural MRI scan was acquired using a T1-weighted 3-dimensional (3D) magnetization prepared rapid gradient echo (MPRAGE) protocol with the following parameters: TR = 2300 ms, TE = 2.98 ms, flip angle = 15°, 176 axial slices; 1 mm slice thickness, 256 × 256 matrix; 256 mm field of view. Participants were instructed to remain still as possible during the MRI acquisition to obtain good image quality. FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/; version v 5.1.0) was used for volumetric measurements. Briefly, preprocessing steps included conversion of MPRAGE images to FreeSurfer format, motion, non-uniformity and intensity correction, skull stripping using a hybrid watershed/surface deformation procedure, automated registration to a standard Talairach atlas space, and tissue segmentation (white matter, grey matter and cerebrospinal fluid). The white/gray matter boundary (i.e., white surface) and gray matter/cerebrospinal fluid boundary (i.e., pial surface) were identified and transformed into surfaces and the distance between the white and pial surfaces calculated at each point across the curtail mantle. Each participant’s cortex was anatomically parcellated, labeled, and aligned to the FreeSurfer’s standard averaged cortical surface template (FSaverage template) and smoothed on the level of the sphere using a 5-mm full-width half-maximum Gaussian spatial smoothing kernel. No obvious errors in parcellation and automatic labelling were observed for any participant upon inspection, and so no manual intervention was performed. Following automatic cortical and subcortical segmentation (i.e., recon-all function in FreeSurfer), volumetric measurements were collected for dorsolateral PFC, anterior cingulate, caudate nucleus, putamen, nucleus accumbens, pallidum, thalamus, cerebellum, and hippocampus based on the Desikan-Killiany atlas and were extracted from FreeSurfer for further statistical analysis. All brain volumes of interest were bilateral and scaled with respect to total intracranial volume (TICV) using the ICV-ANCOVA approach [22].
2.5. Statistical analysis
A multivariable linear regression analyses was used to first estimate associations between CRF and performance within each cognitive domain. The CRF was z-score standardized (mean=0; standard deviation=1) based on the sample mean and standard deviation and used for all statistical analysis. Cognitive domain estimates were adjusted for age, sex, years since PD diagnosis, UPDRS Motor Score, and years of education. Multivariable linear regression analyses were conducted to examine the association between CRF and brain volumes of interest (including PFC, anterior cingulate, caudate nucleus, putamen, nucleus accumbens, pallidum, thalamus, cerebellum, and hippocampus). Extracted brain volumes of interest were corrected for intracranial volume and z-score standardized using the sample mean and standard deviation. The effect of CRF on brain volumes of interest were adjusted for age, sex, years of education, UPDRS motor score, and years since PD diagnosis. To decrease the false-discovery rate a Benamini-Hochberg (B-H) p-value adjustment was applied.
Structural equation models (SEMs) were constructed to estimate whether structural MRI brain volumes of interest mediated any putative associations between CRF and cognitive performance. The SEM tested mediation using standard approaches by estimating three parameter effects of CRF on cognitive performance termed the Direct, Indirect, and total effects. The Direct effect (denoted by the pathway “c’” in Figure 1 and 2) is defined as the effect of CRF on cognitive performance that is independent of brain volume of interest and covariates. The Indirect effect of CRF on cognitive performance is defined as effect of CRF on cognitive performance mediated by the structural MRI brain volumes of interest. The Indirect effect is a product of the effect of CRF on structural MRI brain volumes of interest (represented by Figure 1 and 2 path “a”) and MRI brain volumes of interest on cognitive performance (Figure 1 and 2 path “b”). The total effect of CRF on individual cognitive performance is calculated by adding the Direct and Indirect effects. All parameter effect estimates were adjusted for covariates, including age, sex, years since PD diagnosis, UPDRS motor score, and years of education. The 95% confidence interval of the Indirect effect was estimated using bootstrapping sampling (with 5,000 samples). Analyses were run with the SEM modeling software MPLUS in the R environment using the MPLUS Automation package.
Figure 1. Thalamic Volume Mediates the Association Between VO2max and Episodic Memory and Executive Function.
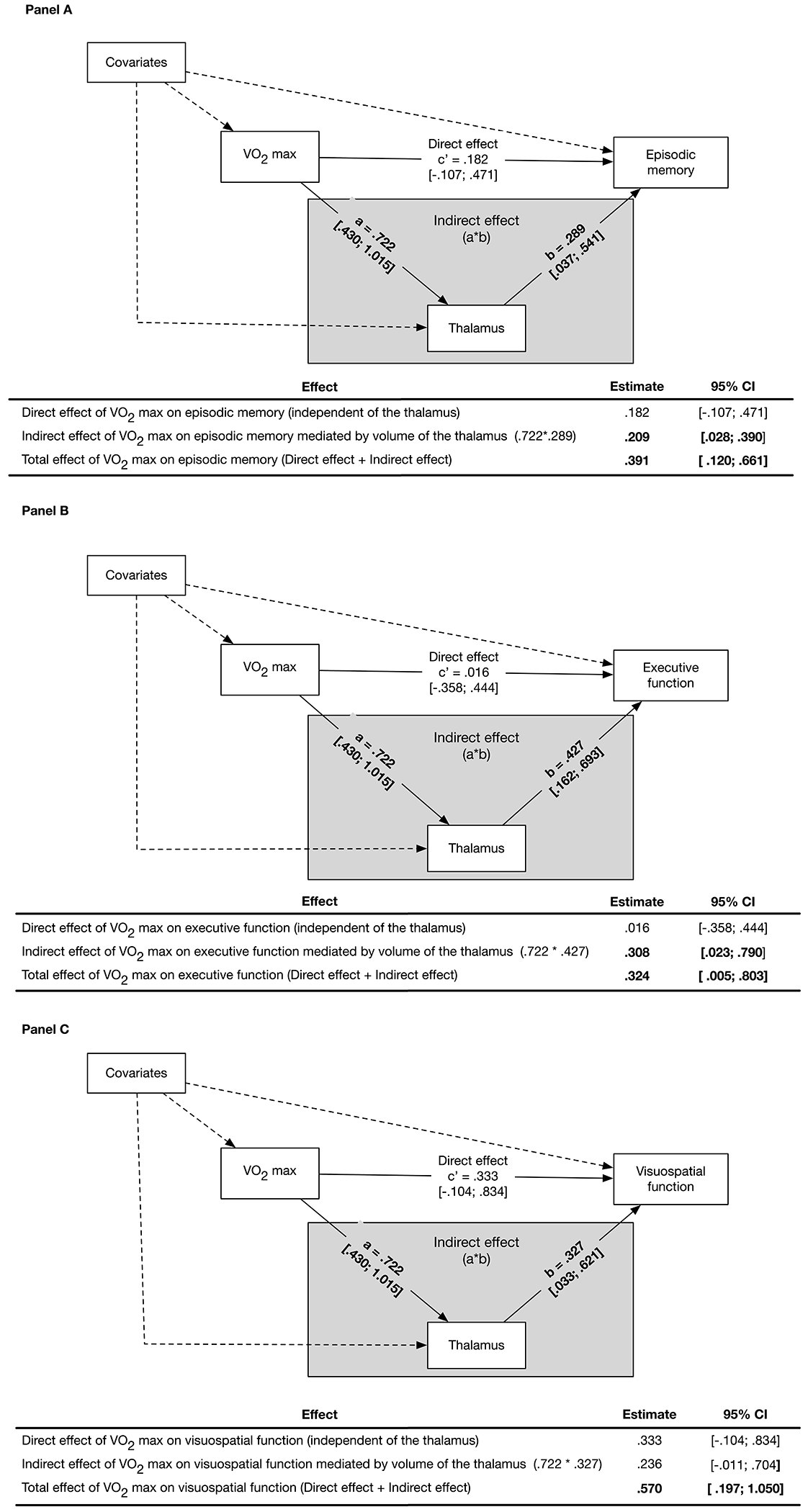
Panel A illustrates the structural equation mediation model with parameter effects, estimates, and 95% confidence intervals (CI). Thalamic volume is a significant mediator of the association between VO2max and episodic memory, as demonstrated by the significant Indirect effect (grey box) of the thalamus on this association. The Indirect effect is the product of the effect of VO2max on the thalamic volume (Path a) and the effect of thalamic volume on episodic memory (Path b). The Direct effect (Path c’) is not significant and is defined as the effect of VO2max on episodic memory while adjusting for the effect of thalamic volume and covariates. The total effect of VO2max on episodic memory is significant and is calculated by adding the Direct and Indirect effects. Episodic memory is a composite of the total number of correct words recalled on the immediate and long-delay free recall trials from the California Verbal Learning Test-II, and visual reproduction one and two scores from the Wechsler Memory Scales. Panel B illustrates a similar structural equation mediation model as in Panel A and demonstrates that thalamic volume is a significant mediator (Indirect effect, grey box) of VO2max on executive function. The total effect of VO2max on executive function is also significant. Executive function is a composite of the number of seconds to complete the inhibition and inhibition-switching subtests of the D-KEFS Color-Word Interference test, the number of errors on the Wisconsin Card Sorting Test, total number of correct words on the switching fluency subtest from the D-KEFS Verbal Fluency test, and the total move score on the Tower of London-Drexel University test. Panel C illustrates a similar structural equation mediation model as in Panel A and demonstrates that thalamic volume is not a significant mediator (Indirect effect, grey box) of the association between VO2max on visuospatial function. The total effect of VO2max on visuospatial function is significant. Visuospatial function is a composite score of visuospatial ability consisting of the Judgement of Line Orientation and Hooper Visual Organization Test. All parameter effect estimates were adjusted for covariates. Dashed pathways represent effects of covariates Bolded estimates are significant at p < .05. The 95% confidence intervals for parameter estimates are presented in brackets.
Figure 2. Pallidum Volume Does Not Mediate the Association Between VO2max and Cognitive Performance.
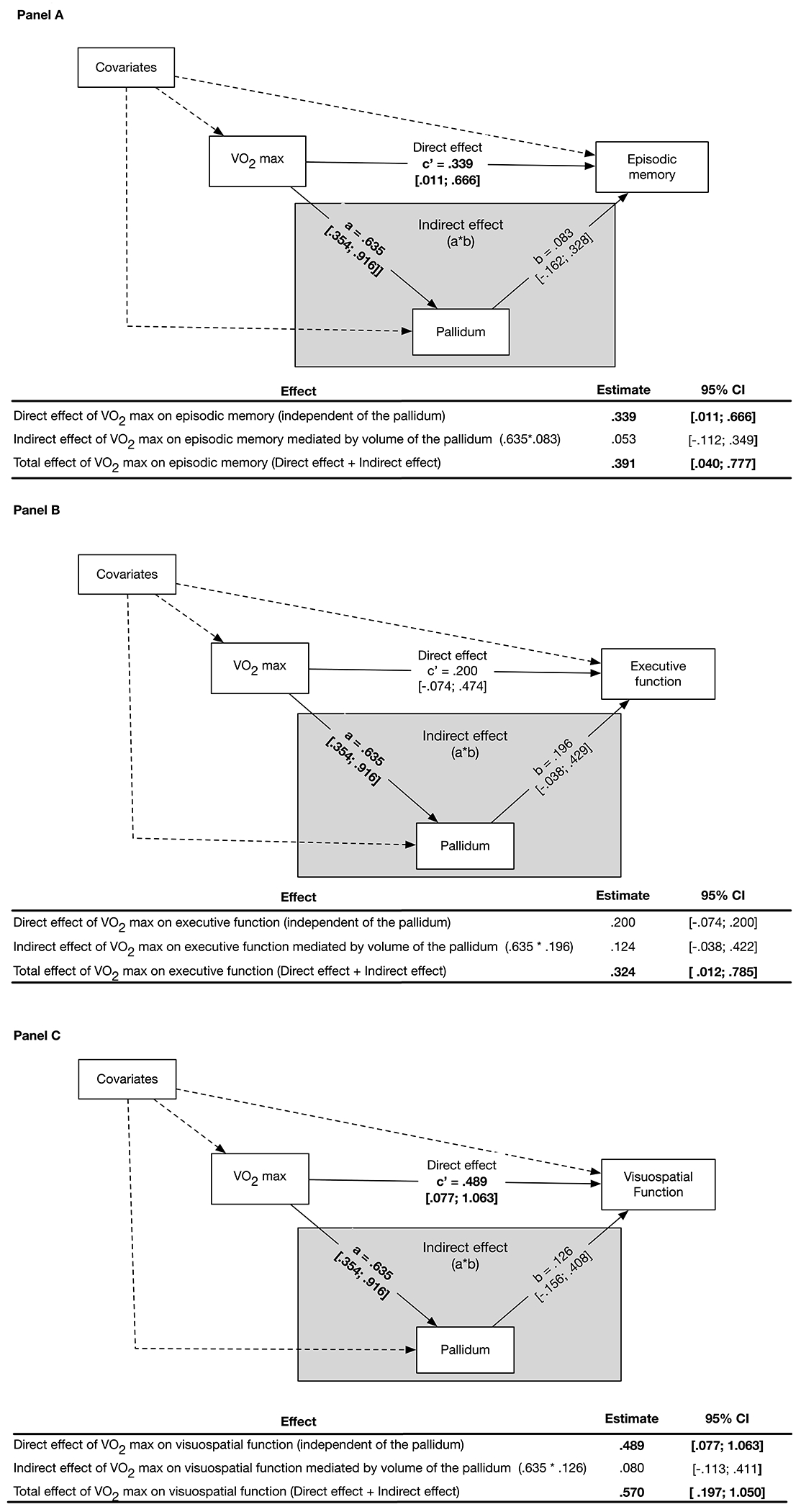
Panel A illustrates the structural equation mediation model with parameter Effects, Estimates, and 95% Confidence Intervals (CI). Pallidum volume does not significantly mediate the association between VO2max and episodic memory, as demonstrated by the non-significant Indirect effect (grey box) of the pallidum on this association. The Indirect effect is the product of the effect of VO2max on the pallidum volume (Path a) and the effect of pallidum volume on episodic memory (Path b). The Direct effect (Path c) is significant and is defined as the effect of VO2max on episodic memory while adjusting for the effect of pallidum volume and covariates. The total effect of VO2max on episodic memory is significant and is calculated by adding the Direct and Indirect effects. Episodic memory is a composite of the total number of correct words recalled on the immediate and long-delay free recall trials from the California Verbal Learning Test-II, and visual reproduction one and two scores from the Wechsler Memory Scales. Panel B illustrates a similar structural equation mediation model as in Panel A and demonstrates that pallidum volume is not a significant mediator (Indirect effect, grey box) of VO2max on executive function. The total effect of VO2max on executive function is significant. Executive function is a composite of the number of seconds to complete the inhibition and inhibition-switching subtests of the D-KEFS Color-Word Interference test, the number of errors on the Wisconsin Card Sorting Test, total number of correct words on the switching fluency subtest from the D-KEFS Verbal Fluency test, and the total move score on the Tower of London-Drexel University test. Panel C illustrates a similar structural equation mediation model as in Panel A and demonstrates that pallidum volume is not a significant mediator (Indirect effect, grey box) of the association between VO2max on visuospatial function. The Direct effect of VO2max on visuospatial function is significant. The total effect of VO2max on visuospatial function is significant. Visuospatial function is a composite score of visuospatial ability consisting of the Judgement of Line Orientation and Hooper Visual Organization Test. All parameter effect estimates were adjusted for covariates. Dashed pathways represent effects of covariates bolded estimates are significant at p < .05. The 95% confidence intervals for parameter estimates are presented in brackets.
3. Results
Participants were on average 64.6 (SD=9.6) years old, 70% male, diagnosed with PD approximately five years (SD=3.7) prior to the study and had an MDS-UPDRS motor score of 24.15 (SD=9.8) (Table 1). Fifty two percent met criteria for PD-MCI. The age-based normative performance on individual cognitive tests within each cognitive domain are shown on Supplement Table S1. Multivariable linear regression analyses revealed that higher CRF was associated with better performance on tests of visuospatial function (standardized β=0.570; 95% confidence interval (CI) = .238, .901; Benjamini-Hochberg adjusted [B-H] p=0.005), episodic memory (standardized β=0.391; 95% CI = .129, .653; B-H p=0.008) and EF (standardized β=0.324; 95% CI = .064, .585; B-H p=0.025), (Table 2A). Associations were all adjusted for covariates. CRF was not significantly associated with performance on tests of attention or language ability. Regarding brain structures of interest, CRF was positively associated with larger volumes of the thalamus (standardized β=0.722; 95% CI = .430, 1.015; B-H p=0.004) and pallidum (standardized β=0.635; 95% CI = .354, .916; B-H p=0.004) (Table 2B). CRF was not significantly associated with volumes of the caudate nucleus, putamen, PFC, nucleus accumbens, anterior cingulate, hippocampus, or cerebellum.
Table 1.
Sample characteristics (N = 33)
| Mean or N | SD or % | Min | Max | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 64.61 | 9.55 | 49 | 88 |
| Sex N (%) | ||||
| Male | 23 | 70% | ||
| Female | 10 | 30% | ||
| Years of education | 16.88 | 1.95 | 13 | 20 |
| Clinical characteristics | ||||
| Years since PD diagnosis | 4.98 | 3.69 | 1 | 15 |
| Hoehn and Yahr stage N (%) | ||||
| Two | 29 | 88% | ||
| Three | 4 | 12% | ||
| Part 3 of MDS-UPDRS | 24.15 | 9.88 | 4 | 45 |
| Dopamine equivalence | 10.12 | 7.73 | 1 | 23 |
| Meets MDS criteria for PD-MCI N (%) | 17 | 52% | ||
| Cardiorespiratory fitness | ||||
| Maximum VO2 (ml/min/kg) | 30.35 | 4.51 | 22.90 | 40.83 |
Bolded estimates denote p < .05
UPDRS=Unified Parkinson’s Disease Rating Scale
Table 2.
Associations between maximum VO2a and domain-specific cognitive performanceb are presented in panel A while association between maximum VO2 and structural magnetic resonance imaging derived brain volumes are presented in panel B (N = 33).
| Panel A: Associations between max VO2 and domain-specific cognitive performance | 95% Confidence Interval | |||
|---|---|---|---|---|
| Estfc | Lower | Upper | B-H p d | |
| Executive functiond | .324 | .064 | .585 | .025 |
| Episodic memorye | .391 | .129 | .653 | .008 |
| Visuospatialf | .570 | .238 | .901 | .005 |
| Attentiong | .081 | −.241 | .403 | .623 |
| Languageh | .243 | −.164 | .651 | .302 |
| Panel B: Associations between max VO2 and structural magnetic resonance imaging derived brain volumese | 95% Confidence Interval | |||
| Estc | lower | upper | B-H p d | |
| Thalamus | .722 | .430 | 1.015 | .004 |
| Pallidum | .635 | .354 | .916 | .004 |
| Caudate nucleus | .155 | −.260 | .571 | .464 |
| Putamen | −.248 | −.585 | .088 | .395 |
| Dorsolateral prefrontal cortex | .095 | −.326 | .516 | .813 |
| Nucleus accumbens | .119 | −.242 | .479 | .813 |
| Anterior cingulate | −.113 | −.409 | .183 | .813 |
| Hippocampus | −.081 | −.511 | .348 | .813 |
| Cerebellum | .024 | −.245 | .294 | .859 |
Bolded estimates denote p < .05
maximum VO2 was estimated by the Ebbling Single State Submaximal Treadmill Walking Test and z-score standardized (mean = 0; standard deviation=1) based on the sample mean and standard deviation.
all cognitive outcomes were z-score standardized (mean = 0; standard deviation =1).
parameter estimates were adjusted for covariates.
p value represents the Benjamini-Hochberg adjusted p value to correct for multiple comparisons.
brain volumes were derived from structural magnetic resonance imaging, were corrected for intracranial volume and were z-score standardized using the sample mean and standard deviation.
Mediation analyses were conducted to examine whether thalamic volume mediated putative associations between CRF and performance on tests of episodic memory (Figure 1, Panel A), EF (Figure 1, Panel B), and visuospatial function (Figure 1, Panel C). The Indirect effect of CRF on episodic memory was statistically significant demonstrating that the thalamus partially mediated observed associations between higher CRF and better episodic memory. In addition, the Indirect effect of CRF on EF was statistically significant, demonstrating that the thalamus partially mediated observed associations between higher CRF and better EF. The Indirect effect of CRF on visuospatial function mediated by the thalamus was a trend to significance as indicated by the 95% confidence interval.
In contrast to the thalamus, the pallidum was not significantly associated with performance on either EF, episodic memory, or visuospatial function (Figure 2, Panels ABC). In addition, the resulting Indirect effect of CRF on performance across the three domains mediated by the pallidum was not statistically significant.
4. Discussion
The relationship between CRF and brain structure has been extensively studied in the context of cognitive aging, through its contribution in neurodegenerative disorders such as PD has been relatively under-investigated. Findings from our study showed that greater CRF is associated with better performance on tests of EF, episodic memory, and visuospatial function in individuals with PD. Although the associations between CRF with EF and episodic memory are well established in older adults without PD [15], our findings are the first to link CRF with specific cognitive domains impacted in PD, including EF, memory, and visuospatial function. Higher CRF was also associated with greater GMV of the thalamus, consistent with studies in cognitive aging [23], and in the pallidum as reported in children [24]. Unlike findings in older adults without PD, we did not observe an association between CRF and hippocampal or PFC volumes, which could be an artefact of the relative sparing of the hippocampus in the pathogenesis of PD, compared to subcortical regions.
We also found that thalamic volume mediated the association of higher CRF and EF and episodic memory, but not visuospatial function in PD. The thalamus and its connections to the PFC and the hippocampus are well known to play an important role in cognition [25]. In PD and its animal models, thalamic volume loss has been associated with behavioral deficits, including cognitive impairment [4, 26]. Published studies in animal models demonstrating that aerobic exercise can elicit functional reorganization of several brain regions including the thalamus, may provide insight to the mechanisms by which CRF may influence thalamic volume [27, 28]. Studies by Holschneider and colleagues [29] have demonstrated that exercised rats compared to their sedentary counterparts show increased functional activation during motor challenge in the mediodorsal thalamus (MD). The MD demonstrates dense connections to frontal cortical regions, including prefrontal, anterior cingulate and premotor cortex, and plays a central role in behavioral flexibility, memory, and working memory [25]. In addition, exercise increases functional activation in the midline thalamus (inter-mediodorsal, retrouniens, rhomboid nuclei) and intralaminar thalamus (central medial, central lateral, paracentral, parafascicular), whose nuclei have strong connections to the medial PFC, striatum, and hippocampus, and play a role in spatial working memory, the temporal organization of memory, and executive functions [30–32]. Exercise also elicits functional adaptations in motor thalamic nuclei (ventrolateral, ventromedial, central medial) [29] which may contribute to the association of CRF with thalamic volume. Adaptations of the motor thalamus have been proposed to reflect neuroplasticity in the cerebellar-thalamocortical circuit, with changes noted especially at the level of the ventrolateral nucleus (VL), which constitutes a target for efferent fibers from the cerebellum [33, 34]. Similar functional adaptations to exercise in these motor nuclei of the thalamus are also observed in the 6-OHDA rat model of Parkinsonism [35], with evidence for exercise-related increased functional connectivity between the motor thalamic nuclei and their connections to primary motor cortex, basal ganglia and cerebellum [28]. Prior research from Greenough and colleagues has demonstrated increased cerebral angiogenesis in healthy rats undergoing aerobic exercise [36]. Although the precise role of angiogenesis in neuroplasticity and structural change remains to be fully elucidated, exercise-induced changes in regional blood flow may reflect localized molecular changes in enhanced neuronal synaptic connectivity including dendritic spine morphology, metabolism, as well as non-neuronal alterations in astrocyte structure and function.
While there was an association between CRF and the pallidum, there was no significant association between the pallidum with EF, memory, or visuospatial function. Thus, there was no mediating effect of the pallidum on CRF and its association with cognitive performance. Differences between the mediating effects observed with thalamus but not pallidum volume may indicate that pathophysiological changes of the pallidum contribute more to PD motor deficits than to cognitive deficits [37]. There was also no association between CRF and volumes of the hippocampus and PFC. This is in contrast to findings in healthy aging studies involving principally aerobic exercise interventions where increases in CRF and both hippocampal volume and PFC were reported [38]. In this case, the cross-sectional nature of our study design may be one limitation to our findings, and support the need for larger, long-term aerobic exercise studies. Alternatively, differences between studies may be related to the level of cognitive function as over half of our participants were experiencing some decline in cognitive function and met diagnostic criteria for PD-MCI. However, our novel findings could potentially highlight focal specificity of CRF effects in PD patients but warrants replication in a larger PD sample.
The present study suffers from certain limitations which include: (i) cross-sectional aspect of the study which limits the ability to make any inferences of causality, (ii) we used an automated ROI approach to analyzing sMRI data which may not be sensitive to examining subtle effects compared to high-dimensional neuroimaging approaches, and (iii) participants were mostly Caucasian and well-educated potentially limiting the generalizability of our results, thereby limited the generalizability of our findings to other cultures, ethnicities, and socioeconomic groups.
In summary, we demonstrate that higher CRF is associated with better cognitive performance in multiple domains, including memory, visuospatial function, and EF in individuals with PD. While the underlying mechanisms by which CRF impacts the thalamus are unknown, individuals with PD who had higher CRF demonstrated better cognitive performance and larger GMVs of the thalamus and pallidum. The thalamus partially mediated the observed associations between CRF and both episodic memory and EF. These findings support the role of CRF and the critical importance of increasing physical aerobic exercise in promoting neurocognitive health in PD to maintain quality of life and daily functioning.
Supplementary Material
Acknowledgments:
The authors would like to thank all the study participants for their contributions, as well as the contributions of friends of the PD Research Program at USC. Special thanks to lab members for their helpful discussions.
Funding sources:
Don Roberto Gonzalez Family Foundation (GP); Parkinson’s Foundation (GP); US Army Medical Research and Material Command Parkinson Research Program, Grant/Award Numbers: W81XWH18-0665 (GP), W81XWH18-00443 (MJ), W81XWH18-1-0666 (DPH), NIMH Mentored Research Scientist Career Development Award K01 MH099431 (SM); VA RR&D MERIT Award 1I01RX001691-01A1 (DMS).
Footnotes
Declarations of interest: The authors have no financial disclosures and no conflicts of interest concerning the research related to this manuscript.
Data availability statement:
All data has been de-identified and stored as a RedCap data base at the USC-CTSI. Any requests for data should be directed to the principal investigator (Giselle M. Petzinger petzinge@med.usc.edu)
References
- [1].Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Troster AI, Weintraub D, MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI, Mov Disord 26(10) (2011) 1814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P, Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study, Arch Neurol 60(3) (2003) 387–92. [DOI] [PubMed] [Google Scholar]
- [3].Gao Y, Nie K, Huang B, Mei M, Guo M, Xie S, Huang Z, Wang L, Zhao J, Zhang Y, Wang L, Changes of brain structure in Parkinson’s disease patients with mild cognitive impairment analyzed via VBM technology, Neurosci Lett 658 (2017) 121–132. [DOI] [PubMed] [Google Scholar]
- [4].Li MG, He JF, Liu XY, Wang ZF, Lou X, Ma L, Structural and Functional Thalamic Changes in Parkinson’s Disease With Mild Cognitive Impairment, J Magn Reson Imaging 52(4) (2020) 1207–1215. [DOI] [PubMed] [Google Scholar]
- [5].Lang AE, Espay AJ, Disease Modification in Parkinson’s Disease: Current Approaches, Challenges, and Future Considerations, Mov Disord 33(5) (2018) 660–677. [DOI] [PubMed] [Google Scholar]
- [6].Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, Rizzo M, Newman SR, Mehta S, Grabowski TJ, Bruss J, Blanchette DR, Anderson SW, Voss MW, Kramer AF, Darling WG, Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting, Neurology 83(5) (2014) 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Duchesne C, Gheysen F, Bore A, Albouy G, Nadeau A, Robillard ME, Bobeuf F, Lafontaine AL, Lungu O, Bherer L, Doyon J, Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson’s disease individuals, NeuroImage. Clinical 12 (2016) 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tanaka K, Quadros AC Jr., Santos RF, Stella F, Gobbi LT, Gobbi S, Benefits of physical exercise on executive functions in older people with Parkinson’s disease, Brain Cogn 69(2) (2009) 435–41. [DOI] [PubMed] [Google Scholar]
- [9].Picelli A, Varalta V, Melotti C, Zatezalo V, Fonte C, Amato S, Saltuari L, Santamato A, Fiore P, Smania N, Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson’s disease: a pilot, single-blind, randomized controlled trial, Funct Neurol 31(1) (2016) 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM, Goldman JG, Comella CL, Vaillancourt DE, Corcos DM, Exercise Improves Cognition in Parkinson’s Disease: The PRET-PD Randomized, Clinical Trial, Mov Disord 30(12) (2015) 1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Colcombe S, Kramer AF, Fitness effects on the cognitive function of older adults: a meta-analytic study, Psychol Sci 14(2) (2003) 125–30. [DOI] [PubMed] [Google Scholar]
- [12].Wittfeld K, Jochem C, Dörr M, Schminke U, Gläser S, Bahls M, Markus MRP, Felix SB, Leitzmann MF, Ewert R, Bülow R, Völzke H, Janowitz D, Baumeister SE, Grabe HJ, Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population, Mayo Clin Proc 95(1) (2020) 44–56. [DOI] [PubMed] [Google Scholar]
- [13].Erickson KI, Leckie RL, Weinstein AM, Physical activity, fitness, and gray matter volume, Neurobiol Aging 35Suppl 2 (2014) S20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B, Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis, Br J Sports Med 52(3) (2018) 154–160. [DOI] [PubMed] [Google Scholar]
- [15].Kramer AF, Colcombe S, Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study-Revisited, Perspectives on psychological science : a journal of the Association for Psychological Science 13(2) (2018) 213–217. [DOI] [PubMed] [Google Scholar]
- [16].Verstynen TD, Lynch B, Miller DL, Voss MW, Prakash RS, Chaddock L, Basak C, Szabo A, Olson EA, Wojcicki TR, Fanning J, Gothe NP, McAuley E, Kramer AF, Erickson KI, Caudate Nucleus Volume Mediates the Link between Cardiorespiratory Fitness and Cognitive Flexibility in Older Adults, J Aging Res 2012 (2012) 939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases, J Neurol Neurosurg Psychiatry 55(3) (1992) 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results, Mov Disord 23(15) (2008) 2129–70. [DOI] [PubMed] [Google Scholar]
- [19].Ebbeling CB, Ward A, Puleo EM, Widrick J, Rippe JM, Development of a single-stage submaximal treadmill walking test, Med Sci Sports Exerc 23(8) (1991) 966–73. [PubMed] [Google Scholar]
- [20].Nakagaichi M, Tanaka K, Development of a 12-min treadmill walk test at a self-selected pace for the evaluation of cardiorespiratory fitness in adult men, Appl Human Sci 17(6) (1998) 281–8. [DOI] [PubMed] [Google Scholar]
- [21].Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P, Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease, Brain 134(Pt 2) (2011) 359–74. [DOI] [PubMed] [Google Scholar]
- [22].O’Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ, Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods, Psychiatry Res 193(2) (2011) 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fletcher MA, Low KA, Boyd R, Zimmerman B, Gordon BA, Tan CH, Schneider-Garces N, Sutton BP, Gratton G, Fabiani M, Comparing Aging and Fitness Effects on Brain Anatomy, Front Hum Neurosci 10 (2016) 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chaddock L, Erickson KI, Prakash RS, Vanpatter M, Voss MW, Pontifex MB, Raine LB, Hillman CH, Kramer AF, Basal Ganglia Volume Is Associated with Aerobic Fitness in Preadolescent Children, Dev Neurosci 32(3) (2010) 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Halassa MM, Kastner S, Thalamic functions in distributed cognitive control, Nat Neurosci 20(12) (2017) 1669–1679. [DOI] [PubMed] [Google Scholar]
- [26].Halliday GM, Thalamic changes in Parkinson’s disease, Parkinsonism Relat Disord 15Suppl 3 (2009) S152–5. [DOI] [PubMed] [Google Scholar]
- [27].Ding Y, Li J, Lai Q, Azam S, Rafols JA, Diaz FG, Functional improvement after motor training is correlated with synaptic plasticity in rat thalamus, Neurol Res 24(8) (2002) 829–36. [DOI] [PubMed] [Google Scholar]
- [28].Wang Z, Guo Y, Myers KG, Heintz R, Peng YH, Maarek JM, Holschneider DP, Exercise alters resting-state functional connectivity of motor circuits in parkinsonian rats, Neurobiol Aging 36(1) (2015) 536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holschneider DP, Yang J, Guo Y, Maarek JM, Reorganization of functional brain maps after exercise training: Importance of cerebellar-thalamic-cortical pathway, Brain Res 1184 (2007) 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dolleman-van der Weel MJ, Griffin AL, Ito HT, Shapiro ML, Witter MP, Vertes RP, Allen TA, The nucleus reuniens of the thalamus sits at the nexus of a hippocampus and medial prefrontal cortex circuit enabling memory and behavior, Learn Mem 26(7) (2019) 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cholvin T, Hok V, Giorgi L, Chaillan FA, Poucet B, Ventral Midline Thalamus Is Necessary for Hippocampal Place Field Stability and Cell Firing Modulation, J Neurosci 38(1) (2018) 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Varela C, Thalamic neuromodulation and its implications for executive networks, Front Neural Circuits 8 (2014) 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aumann TD, Rawson JA, Finkelstein DI, Horne MK, Projections from the lateral and interposed cerebellar nuclei to the thalamus of the rat: a light and electron microscopic study using single and double anterograde labelling, J Comp Neurol 349(2) (1994) 165–81. [DOI] [PubMed] [Google Scholar]
- [34].Haroian AJ, Massopust LC, Young PA, Cerebellothalamic projections in the rat: an autoradiographic and degeneration study, J Comp Neurol 197(2) (1981) 217–36. [DOI] [PubMed] [Google Scholar]
- [35].Wang Z, Myers KG, Guo Y, Ocampo MA, Pang RD, Jakowec MW, Holschneider DP, Functional reorganization of motor and limbic circuits after exercise training in a rat model of bilateral parkinsonism, PLoS One 8(11) (2013) e80058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT, Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats., Proc Natl Acad Sci U S A. 87(14) (1990) 5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Magrinelli F, Picelli A, Tocco P, Federico A, Roncari L, Smania N, Zanette G, Tamburin S, Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation, Parkinsons Dis 2016 (2016) 9832839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF, Exercise training increases size of hippocampus and improves memory, Proc Natl Acad Sci U S A 108(7) (2011) 3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data has been de-identified and stored as a RedCap data base at the USC-CTSI. Any requests for data should be directed to the principal investigator (Giselle M. Petzinger petzinge@med.usc.edu)


