Abstract
Pulmonary hypertension (PH) is often diagnosed late in its course when it purports a particularly poor prognosis. Exercise effectively unmasks early forms of several cardiopulmonary diseases but the role of performing pulmonary arterial pressure measurements during exercise in the evaluation of PH remains unclear. Whether pulmonary arterial pressure-flow relationships during exercise may provide a window into earlier diagnosis of functionally significant pulmonary arterial hypertension and left ventricular dysfunction,1 or add incrementally to our armentarium of diagnostic tests and prognostic indicators in PH, is the topic of active ongoing investigation. Evidence is emerging that abnormal pulmonary arterial pressure response patterns to exercise, when properly indexed to increased blood flow, may help to identify early forms of heart failure and pulmonary arterial hypertension. This article will discuss approaches to performing hemodynamic measurements during exercise as well as the potential clinical utility of identifying normal and abnormal pulmonary vascular response patterns to exercise.
The earliest manifestations of cardiovascular diseases often are evident during exercise. For example, exercise-induced systemic hypertension and myocardial ischemia predict future onset of resting hypertension and myocardial infarction, respectively.2, 3 Pulmonary arterial pressure (PAP) responses to exercise were first characterized over 60 years ago,4 yet uncertainties remain about the role of performing exercise PAP measurements in the evaluation and treatment of pulmonary hypertension (PH).
PH was previously defined in United States and European guidelines as a mean PAP (PAPm) >25 mmHg at rest or >30 mmHg during exercise.5, 6 In 2008 the Working Group on Diagnosis and Assessment of Pulmonary Arterial Hypertension from the 4th World Symposium on PH (Dana Point, CA) appropriately concluded that PAPm >30 mmHg should be abandoned as a diagnostic criteria for PH. This decision was based on the age-dependent nature of exercise PAPm7 and limited data on normal subjects derived from heterogeneous levels, types, and postures during exercise testing.8
However, in patients with PH exercise remains a highly relevant physiologic stressor in that exertional dyspnea is the most common symptom that leads to the diagnosis of PH,9 exercise capacity predicts prognosis and characterizes responses to therapies,10, 11 and exercise probes the reserve capacity of the right ventricular-pulmonary vascular unit to accommodate increased blood flow. Moreover, resting PAPm does not closely correlate with symptoms or exercise capacity. Whether exercise hemodynamic measurements may provide a window into earlier diagnosis of functionally significant PH and left ventricular dysfunction,1 or add incrementally to our armentarium of diagnostic tests and prognostic indicators in PH, is the topic of active ongoing investigation. This article will examine the current state of knowledge of pulmonary vascular response patterns to exercise in normals and in individuals with cardiopulmonary diseases to begin to frame the role of measuring pulmonary hemodynamics in the evaluation of patients with PH.
Methods for Measurement of Pulmonary Hemodynamics During Exercise
Invasive hemodynamic measurements during exercise:
Right heart catheterization (RHC) with invasive hemodynamic monitoring is the gold standard for evaluating the pulmonary vasculature during exercise, as it is at rest. Exercise testing with invasive hemodynamic monitoring has been successfully performed using upright and supine cycle ergometry,7 leg-presses,4, 12 and treadmill protocols.13, 14 A detailed description of methods utilized in our laboratory at Massachusetts General Hospital,15, 16 where ~150 cardiopulmonary exercise studies with invasive hemodynamic measurements are performed annually, is described by Drs. Odem and Systrom in this issue of Advances in Pulmonary Hypertension (Insert Ref, pg. X-Y).
Upright cycle ergometry offers the advantage of simulating upright posture employed in activities of daily living, while allowing precise continuous ramp incremental work and eliciting near maximum exercise capacity without excessive upper body movement during hemodynamic measurements. A multi-site oxygen saturation run should be performed upon insertion of the right heart catheter if left-to-right shunting is suspected, particularly when measuring Fick cardiac outputs (CO) during exercise. High fidelity micromanometer-tipped catheters, which can be advanced via fluid filled catheters inserted for routine RHC, are preferred when available to maximize accuracy of pressure measurements. Pulmonary capillary wedge pressure (PCWP) should be verified based on characteristic waveforms, systemic oxygen saturation and/or appearance on fluoroscopy. The critical extravascular closure pressure imposed by the lung parenchyma that contains the pulmonary vasculature is typically below that of the PCWP during exercise,13, 17 and therefore PCWP can be used as the downstream pressure in order to determine transpulmonary pressure gradients (TPG=PAPm-PCWP) and pulmonary vascular resistance (PVR=TPG/CO). Care should be taken to maintain consistent upright posture relative to the leveled transducers throughout exercise. Serial measurements of PAPm, pulmonary capillary wedge pressure (PCWP), and CO should be performed at regular intervals (i.e. every minute) during incremental exercise to characterize pressure-flow relationships. In light of increased thoracic pressure changes with exercise, particularly in overweight or deconditioned patients, it is important to uniformly measure pressures at end-expiration, when intrathoracic pressure most closely approximates atmospheric pressure, in order to ensure consistency in data interpretation.
Assessment of exercise-induced increases in PAPm should be interpreted relative to increases in blood flow (i.e. ΔPAP/ΔCO) and specific work rates rather than relying on a single absolute PAP threshold (i.e. 30 mmHg) or a peak exercise PAPm, in order to account for inter-individual variability in peak exercise intensity levels achieved. Determination of relative contributions to exercise PAPm from increases in left sided hydrostatic pressure (i.e. PCWP or LVEDP) and pre-capillary pulmonary arterial pressure (i.e. the transpulmonary gradient, [TPG]) is also critical in order to discern the etiology of exercise limitation.
Non-invasive measurements during exercise:
Doppler echocardiography is increasingly utilized as a screening procedure for PAH18, 19 and may be performed during exercise to estimate right ventricular systolic pressure, which approximates systolic PAP (PASP) in the absence of pulmonary valve stenosis or RV outflow tract stenosis.20 One report found an excellent correlation with catheter measurements obtained simultaneously (R=0.98)21 and recently echocardiographic estimates of cardiac output and PAPm have been combined with reasonable approximation of normal PAP-flow relationships in healthy individuals (Table 1).22
Table 1.
Pulmonary Arterial Pressure Responses to Exercise
| Condition | Author | N | Age | Exercise Protocol | Work rate (Watts) |
CO at Rest (L/min) |
CO with Ex (L/min) |
PAPm at Rest (mmHg) |
PAPm with Ex (mmHg) |
ΔPAP ΔCO |
|---|---|---|---|---|---|---|---|---|---|---|
| NORMAL | ||||||||||
| Hickam4 | 8 | 23±7 | Supine leg press | 20±7 | 7.1±1.8 | 10.4±3.5 | 11±3 | 13±1 | 0.9 | |
| Lonsdorfer36 | 7 | 31±8 | Upright cycle | 276±50 | 5.4±1.5 | 20.0±3.3 | 14±2 | 27±6 | 0.9 | |
| Degre32 | 11 | 41±5 | Upright cycle | NA | 5.0±0.9 | 13.3±1.7 | 11±4 | 23±5 | 1.4 | |
| Slonim12 | 5 | 22±2 | Supine leg press | 20 ± 2 | 4.9±0.6 | 7.2±0.8 | 15±2 | 19±2 | 1.6 | |
| Wagner33 | 8 | 30 ±6 | Upright cycle | 180 | 6.9±2.0 | 20.5±2.5 | 13 ±3 | 30±6 | 1.2 | |
| Damato14 | 24 | 31±6 | Upright treadmill | 160±23 | 5.1±0.8 | 17.5±2.0 | 15±5 | 29±8 | 1.1 | |
| Reeves35 | 9 | Upright cycle | 240 | 6.7±1.2 | 24.7±3 | 15±2 | 29±8 | 0.8 | ||
| Tolle1 | 16 | 46±15 | Upright cycle | 156±43 | 5.8±1.0 | 15.5±3.2 | 14±3 | 27±4 | 1.4 | |
| Argiento22 | 25 | 36±14 | 45° cycle | 170±51 | 4.7±1.0 | 18.0±4.2 | 14±3 | 30±7 | 1.4 | |
| Kovacs7 | 116 | >50 | Supine | NA | 6.2±1.5 | 11.2±2.0 | 15±4 | 29±8 | 2.8 | |
| COPD | ||||||||||
| Hickam4 | 5 | 51±10 | Supine leg press | 10 | 6.2±1.0 | 7.8±2.3 | 18±5 | 25±2.5 | 4.0 | |
| Saito91 | 14 | 67±7 | Supine cycle | 27 | 4.3±0.7 | 8.8±1.1 | 19±4 | 41±10 | 4.6 | |
| COPD+PH | Blanco74 | 20 | 64±7 | Upright cycle | NA | 4.9±1.0 | 9.0±2.2 | 27±10 | 56±14 | 7.0* |
| HEART FAILURE | ||||||||||
| HFrEF | Lewis15 | 13 | 47±9 | Upright cycle | 83±10 | 3.2±0.2 | 4.8±0.3 | 28±4 | 36±4 | 5.0 |
| HFrEF | Lewis65 | 30 | 56±4 | Upright cycle | 75±11 | 3.5±0.3 | 6.8±0.4 | 30±3 | 49±3 | 4.9 |
| HFrEF Survivors Non-surviv |
Mancini69 |
49 16 |
53±10 52±8 |
Upright cycle |
NA |
4.0±1.1 4.1±0.8 |
7.7±2.1 7.1±2.3 |
28±11 31±10 |
46±12 55±8 |
4.9 8.0 |
| HFrEF | Janicki13 | 42 | Upright treadmill | NA | 3.7±1.3 | 5.9 | ||||
| HFpEF | Borloug26 | 32 | 65±13 | Supine cycle/arm | 47±19 | 5.2±1.1 | 9.1±1.9 | 19±4 | 43±7 | 6.1 |
| PAH | ||||||||||
| Janicki13 | 9 | Upright treadmill | NA | 3.4±0.7 | 8.9±2.7 | 43±16 | 81±16 | 6.1 | ||
| Blumberg60 | 16 | 55±8 | 45° cycle | 25–50 | 3.7±1 | 5.8±2.4 | 45±8 | 70±13 | 11.9 |
CO indicates cardiac output; PAPm indicates mean pulmonary arterial pressure; ΔPAP/ΔCO indicates the slope of the pulmonary arterial pressure-flow relationship during exercise.
However, tricuspid regurgitant velocity to estimate PASP is more challenging to ascertain during exercise21 and both overestimation and underestimation of pulmonary artery systolic pressure have been reported to occur with echocardiography,23, 24 particularly with exercise.20 In addition, exercise echocardiography has limited capacity to measure left ventricular filling pressures during exercise in order to exclude the common condition of impaired LV filling mediating pulmonary venous hypertension during exercise.25, 26 Finally, although resting echocardiographic indices of RV structure and function play a very important role in characterizing PH, and emerging echo-based measurements such as strain-rate imaging, eccentricity and Tei indices and TAPSE offer valuable information,27 echocardiographic indices of RV function have not been widely validated during exercise. Therefore, further investigation is needed to bring exercise echocardiography to the forefront of our evaluation of PH.
Pulmonary vascular responses to exercise in healthy subjects:
The relationship between PAP and blood flow during exercise in normal individuals and in patients with cardiopulmonary diseases was first reported by Drs. Hickam and Cargill in 1948 and was among the first observations in human cardiac catheterization.4 In 8 healthy subjects (age 23±7 years) undergoing low-level supine leg press-exercise (~20 watts) they observed a modest increase in PAPm (2±2mmHg) in the context of a 3.5±2.8L increase in CO. Subsequent studies in healthy individuals have employed various exercise modalities, postures, and protocols resulting in widely variable absolute PAPm values achieved during exercise.
A recent meta-analysis was conducted that consisted of 47 studies describing 72 populations of healthy volunteers who underwent right heart catheterization with invasive measurement of PAP at rest and during exercise.7 Importantly, data were stratified by sex, age, type of exercise (i.e. cycle ergometry, treadmill exercise), body position (upright vs. supine), and exercise levels (slight, submaximal, and maximal) as defined by categories of heart rate, work rate, and oxygen uptake. Normal resting PAPm was 14±3 mm Hg. The upper limit of normal (ULN) was 21 mmHg. During slight exercise, the mean and ULN for PAPm was 21±7 and 33 (supine) and 20±4 and 29 mmHg (upright). During maximal exercise, the ULN was 37 (supine) and 35 mm Hg (upright).
The size of this study permitted several important observations regarding relationships between exercise PAP and potential covariates. Age ≥50 years was associated with slightly higher PAPm at rest (15±4 mmHg vs. 13±3 mmHg in subjects <50 years old), and markedly higher PAPm during slight exercise (29 ±8 vs. 20±5 mmHg, P<0.0001). Gender did not significantly influence resting or exercise PAPm and upright position was associated with a modest reduction in exercise PAPm and cardiac output along with increased heart rate. One limitation of this study was the lack of information about the influence of body mass index on exercise PAP, due to the paucity of overweight and obese subjects studied. Population based studies have indicated higher PASP in individuals with higher BMI,28 suggesting that exercise PAP relative to CO is likely to be greater in obese individuals.
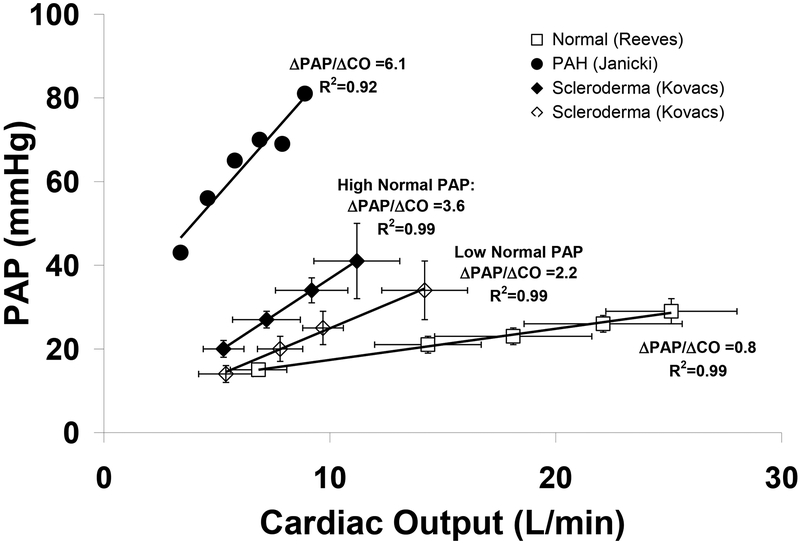
In comparing studies of exercise pulmonary hemodynamics derived from heterogeneous exercise protocols and intensity levels, it is helpful to index exercise-induced increases in PAP to increases in blood flow (i.e. ΔPAP/ΔCO, Table 1). Linear pressure-flow relationships have been observed across a wide range of flows in isolated lung preparations,29, 30 in tact animal models,31 and in the majority of humans who underwent serial PAP and CO measurements during exercise (Fig 1).7, 13, 32–35 Accordingly, an average PAP increment of ~1 mmHg/Liter of cardiac output in young, healthy controls was evident in 3 high intensity exercise protocols (240–276W),33, 35, 36 and in exercise protocols with lower intensity exercise (~20W).4, 12, 37
Figure 1.
Pulmonary arterial pressure (PAP)-flow relationships based on serial measurements of mean PAP and cardiac output during incremental exercise. Normal subjects (□), patients with scleroderma with PAP in the lower normal range (◊) and upper normal range (♦), and patients with resting PAH (•) demonstrate approximately linear pressure-flow responses during exercise with progressively higher ΔPAP/ΔCO that may be indicative of early pulmonary vasculopathy in the scleroderma groups. Adapted with permission from (□) Reeves,35 Kovacs (◊,♦),54 and (•)Janicki.13
Early studies of pulmonary hemodynamics during exercise often did not measure exercise PCWP to permit assessement of post-capillary, left ventricular hydrostatic pressure contributions to increases in exercise PAP.4, 12, 14, 32 Studies in which serial measurements of PAPm and PCWP were performed during upright exercise in normal young individuals indicate a disproportionate contribution of ΔPCWP compared to ΔTPG to ΔPAPm with a ratio of approximately 2:1 (i.e. ΔPCWP=0.67 mmHg/L CO and ΔTPG=0.33 mmHg/L CO).33–35 Older individuals have greater augmentation in PCWP relative to blood flow during exercise but similar relative contributions of changes in TPG and PCWP to changes in PAPm during exercise.7 The modest increment in TPG relative to CO during exercise results in a decrement in PVR, which is attributable to passive recruitment and distention of a compliant pulmonary circulationand active flow-mediated vasodilation.38, 39 The absolute value for exercise PVR should be below 1 Wood unit.1
From investigations to date in normal individuals, it can be concluded that the compliant pulmonary vasculature can accommodate large increases in blood flow during exercise with a proportionate modest increment in PAPm and a fall in PVR. When assessing PAPm, it is critical to account for CO augmentation, and therefore determination of ΔPAPm/ΔCO or PVR is preferable to PAPm alone.
Exercise Hemodynamic Measurements to Unmask Early Forms of PAH and Left Ventricular Dysfunction
When advanced, heart failure with preserved ejection fraction (HFpEF) and PAH are readily apparent through routine clinical evaluation and diagnostic testing performed at rest. However, the diagnosis of earlier stages of HFpEF and PAH may be challenging, as exertional dyspnea is not specific for either condition, and biomarkers and hemodynamic indicators in “early stages” of these conditions may be unremarkable at rest.26 Therefore, individuals who experience dyspnea on exertion despite normal resting hemodynamics (i.e. PCWP or LVEDP<15mmHg40 and PAPm<25mmHg) may benefit from confrontational testing with exercise with particular attention to relative increases in PCWP and TPG components of PAP.
Exercise-induced Heart Failure with Preserved Left Ventricular Ejection Fraction
HFpEF constitutes approximately 40% of the HF population and is associated with a similar prognosis to HF with reduced LVEF.41 Current guideline-based diagnostic criteria for HFpEF include objective evidence of elevated cardiac filling pressures based upon cardiac catheterization, echocardiography, or naturetic-peptide assays.42 The potential for over-diagnosis of HFpEF based on these criteria has garnered significant attention43–45 but less is known about the potential under-diagnosis of HFpEF in symptomatic patients without overt hypervolemia.
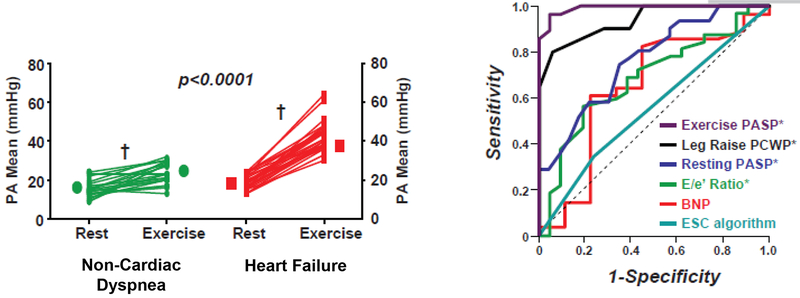
Borloug et al. reported hemodynamic responses to exercise in 55 euvolemic subjects with exertional dyspnea, normal naturetic peptide levels, and normal resting hemodynamic measurements.26 Supine exercise with cycle ergometry (N=42) or arm weights (N=13) was performed, with an exercise PCWP threshold of ≥25 mmHg for the diagnosis of HFpEF. This PCWP value was chosen based on previous studies in normal controls have shown that peak PCWP and LVEDP during supine exercise are <20–23 mmHg46, 47 and <25 mmHg48, respectively. HFpEF subjects experienced significantly greater exercise-induced increases in PAPm (i.e. from 19±4 to 43±7 mmHg) than subjects with non-cardiac dyspnea (i.e. from 15±4 to 23±5 mmHg, Figure 2A) despite achieving lower peak COs. Exaggerated increases in PAP in HFpEF were related exclusively to elevations in left ventricular hydrostatic pressure as evidenced by similar increments in TPG and reductions in PVR from rest to exercise in HFpEF and non-cardiac dyspnea patients. Exercise-induced changes in PCWP and PAP in patients with HFpEF remained significantly higher than those in patients with non-cardiac dyspnea following adjustment for age, BMI, cardiac output, and work rate.
Figure 3.
(Panel A) Mean pulmonary arterial pressures (PAP) increased to a greater extent during exercise in patients with heart failure and preserved ejection fraction (HFpEF) compared to patients with non-cardiac dyspnea.
(Panel B) Clinical measures (B-type naturetic peptide levels and echocardiographic E/e’) and the European Society of Cardiology (ESC) diagnostic algorithm42 did not robustly distinguish HFpEF from non-cardiac dyspnea. In contrast, PCWP with leg raise and exercise PASP showed excellent discrimination between HFpEF and NCD.
Borloug and colleagues further examined the discriminatory capacity of exercise PAP for the diagnosis of HFpEF. They focused on exercise PASP because it was closely related to exercise PCWP (R=0.76, P<0.0001) and is amenable to non-invasive estimation by echo Doppler. PASP >45 mmHg identified HFpEF with 96% sensitivity and 95% specificity (Figure 2B). Strict exclusion of patients with pulmonary vascular disease (i.e. PAPm>25 mmHg at rest or >30 mmHg during exercise with a PCWP<15 mmHg) likely inflated the specificity of exercise PASP for the diagnosis of HFpEF, compared to an unselected population of patients with exertional dyspnea, but exercise PASP outperformed resting PASP, naturetic peptide levels, and echocardiographic indicators for diagnosing HFpEF.
Kitzman et al. similarly found that compensated outpatients with HFpEF had normal resting PCWP but marked increases in exercise PCWP, suggesting that HFpEF may initially manifest with only intermittent elevations in cardiac filling pressures.49 Both studies indicate the incremental value of exercise hemodynamics in the diagnosis of HFpEF and may provide a window into earlier diagnosis of the condition. However, prior to labeling elevations in exercise hemodynamics as an “early forms” of HFpEF, more information is needed on the natural history of patients with exercise elevations in PAP and PCWP to determine the rate at which they go on to develop overt HF.
Exercise-induced Pulmonary Arterial Hypertension
Analogous to HFpEF, PAH is often diagnosed late in its course when in purports a particularly poor prognosis. Whether exercise-induced elevations in PAPm provide a window into the diagnosis of early, potentially more treatable form of PAH remains controversial.6, 50, 51 Definitions of exercise-induced PAH (EIPAH) and the rigor with which pulmonary venous hypertension has been excluded in studies performed to date varies,1, 52, 53 and follow up to determine natural history of EIPAH is lacking. However, there is emerging evidence that EIPAH, if properly defined may represent an important intermediate PAH phenotype.1, 54
Tolle and colleagues conducted a comprehensive study of 406 sequential patients undergoing incremental cardiopulmonary exercise testing with invasive hemodynamic monitoring to evaluate dyspnea on exertion and thereby compiled the largest EIPAH experience reported to date. “Exercise-induced PAH” was defined as resting PAPm <25 mmHg coupled with exercise PCWP<20 mmHg, PAPm>30 mmHg and PVR>80 dyne▪s▪cm−5. Patients with EIPAH (N=78) were compared to patients with normal exercise capacity and hemodynamics (N=16) and patients with resting PAH (PAPm>25 mmHg, PCWP<15 mmHg, N=15). The inclusion of an exercise PVR minimum in the definition of EIPAH was particularly important in light of the subsequently published meta-analysis by Kovacs indicating that PAPm=30 mmHg is often surpassed during exercise in normals performing maximum exercise,7 particularly when high workloads are achieved.33, 35 In addition, a PCWP<20 mmHg during maximum exercise34 represents a relatively strict threshold to exclude a primary contribution of elevated left sided hydrostatic pressures to PH during exercise and further helps to validate the findings from this study.
Tolle and colleagues found that the percent predicted peak VO2 (on the basis of age, sex, height, and weight)55 was lowest in resting PAH (55.8±20.3%), intermediate in exercise-induced PAH (66.5±16.3%), and highest in normals (91.7±13.7%), whereas mean pulmonary artery pressure (48±11 versus 37±6 versus 27+4 mm Hg) and pulmonary vascular resistance (294±158 versus 161±60 versus 62±20 dyne s cm–5, respectively; all P<0.05) followed an opposite pattern. This data suggests that EIPAH is an intermediate phenotype between normal subjects and those with resting PAH. Of note, the upper limit of normal PAPm in the upright position is likely closer to 20 mmHg instead of 25 mmHg, but Tolle et al. noted that resting PAPm 21–25 mmHg in this study did not predict EIPAH. The important findings of this study in characterizing EIPAH merit further exploration of the natural history of EIPAH to determine its prognostic implications and responsiveness to therapy.
Subsequent studies have provided further evidence of the functional significance of exercise-induced elevations in PAP. Kovacs and colleagues studied 29 patients with systemic sclerosis, an “at risk population” for PAH, in whom resting PAPm was less than 25 mmHg. Stratification by median PAPm at rest (PAPm=17mmHg) and during exercise at 25 and 50 watts (median PAP=23 mmHg and 28 mmHg, respectively), indicated that higher rest PAP within the normal range, and particularly higher exercise PAP was associated with reduced six minute walk distance and reduced peak workload.54 Notably, the PVR failed to fall in the above-median PAP, EIPAH group (rest: 168±47 dyne▪s▪cm−5, 25W: 161±36 dyne▪s▪cm−5, 50W: 166±41 dyne▪s▪cm−5). These values are strikingly similar to the PVR values reported by Tolle et al. in EIPAH (161±60 dyne▪s▪cm−5). A second recent publication in patients with systemic sclerosis demonstrated the utility of exercise hemodynamics in identifying the primary mediator of potentially multi-factorial exercise limitation.56
Exercise induced PH and left ventricular dysfunction:
The studies described above compartmentalize patients as having either HFpEF or PAH unmasked by exercise. However, in some patients exercise will elicit exaggerated increases in both TPG and PCWP. The entity of “PH out of proportion to LV dysfunction”, alternatively named “PH-LVD” or “mixed PH”, as identified by abnormal resting hemodynamics, is being increasingly recognized in patients with both HFpEF57 and HF with reduced LVEF.58 For example, Lam et al recently described a wide spectrum of PASPs in patients with HFpEF, with higher PASP potently predicting worse outcomes.57, 59 The identification of exercise-induced mixed PH, with partitioning of relative contributions of PCWP and TPG, may help to further phenotype patients with dyspnea and eventually inform targeted interventions directed at either the left ventricle or the pulmonary vasculature.
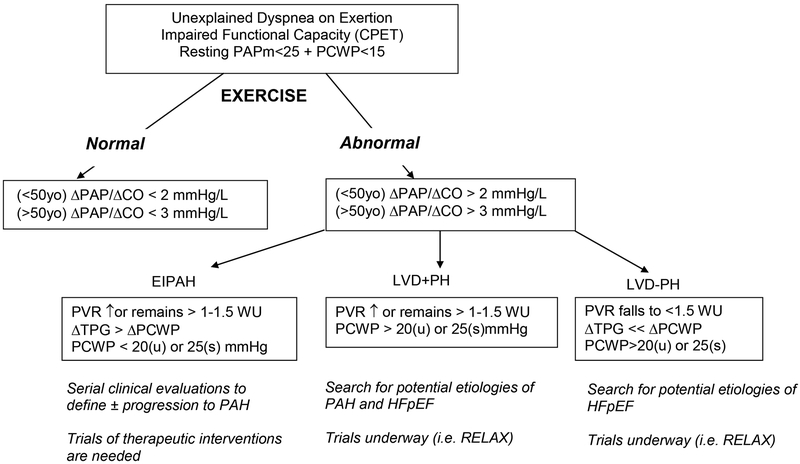
Based on studies to date in normal individuals and in dyspneic patients in the studies described above, a suggested algorithm is proposed below (Figure 2) whereby patients can be further classified on the basis of exercise hemodynamic values. The initial branch point is based on marked differences in pressure-flow relationships in the pulmonary vasculature in normals relative to individuals with HF or PAH (Table 1).
Figure 2.
Diagnostic algorithm for evaluation of pulmonary vascular response patterns to exercise in the setting of normal resting filling pressures. CPET indicates cardiopulmonary exercise test. ΔPAP/ΔCO indicates relative changes in pulmonary arterial pressure and cardiac output during exercise. WU indicates Wood units; TPG indicates transpulmonary gradient; PCWP indicates pulmonary capillary wedge pressure; (u) and (s) indicate upright and supine, respectively.
Pulmonary Vascular Responses to Exercise in Established Cardiopulmonary Diseases
Pulmonary Arterial Hypertension
Patients with confirmed PAH demonstrate steep pressure-flow relationships during exercise (Table 1).60 When exercise-induced increases in PAP are analyzed relative to work rate or VO2 during maximum incremental exercise, a plateau pattern in PAP has been observed.1, 61 This pattern of a steep initial increment in PAP coupled with inability to augment PAP throughout exercise may be indicative of dynamic right ventricular dysfunction. Hence, while exercise hemodynamic measurements are not necessary to confirm the diagnosis of PAH, they may aid in probing the compensatory capacity of the right ventricular-pulmonary vascular unit.
Heart Failure with Reduced LV Ejection Fraction
Resting pulmonary hypertension (PH) is present in the majority of patients with LVSD and is associated with right ventricular dysfunction, reduced exercise tolerance, and poor prognosis.58, 62, 63 There is a strong inverse relationship between resting PVR and exercise capacity as measured by peak VO2.58, 64 Despite the recognized importance of resting PH and right ventricular dysfunction in LVSD, less is known about the PAP response patterns during exercise in subjects with LVSD, and their relationship to exercise capacity and outcomes.
In our laboratory we studied two cohorts of 15 and 30 subjects with LVSD who underwent CPET with measurements of PAP, PCWP, Fick CO, invasive systemic blood pressure are recorded each minute during incremental exercise.15, 65 We observed an exaggerated increase in PAP relative to CO during exercise of 4.9mmHg/L and 5.0mmHg/L in two separate studies. Despite uniform increases in PCWP, PVR failed to fall normally with exercise, remaining in excess of 3 Wood units on average during exercise in both studies. Exercise PVR was strongly associated with 3 established prognostic indicators in HF: peak VO2, RVEF, and VE/VCO2 slope.66, 67 Janicki et al. similarly observed an increment in PAP relative to CO of 5.9mmHg/L13 in 42 subjects with HF. Notably, patients with resting PAPm<19 mmHg at rest, indicating well compensated HF, had an average increment in PAP of 4.1 mmHg/L whereas those with resting PAPm>19 mmHg had an increment in PAP of 6.8 mmHg/L. Unlike our group, Janicki observed a modest decrement in PVR in both subgroups during exercise, but the average PVR remained above 2 Wood units during exercise.
Two studies have examined whether invasive hemodynamic measurements during exercise,68, 69 including PAPm, provide incremental prognostic information in HFrEF. Mancini et al. examined rest and exercise hemodynamics and gas exchange variables in 65 patients who underwent cardiac transplantation evaluation and stratified them into two groups: those who died or required urgent transplant (N=16), and those who survived (N=49) over an average follow-up of 8±4 months. Nonsurvivors did not differ from survivors in resting hemodynamics, including PAPm and CO. However, during exercise nonsurvivors had higher PAPm (55±8mmHg) than survivors (46±12mmHg, P=0.01, Table 1) and higher increment in PAPm per liter of CO (8.0 vs. 4.9mmHg/L). In a similar study, Metra et al. evaluated 219 patients (181 survivors and 38 non-survivors), and found that both rest and exercise PAP were higher in non-survivors than in survivors, despite lower workloads achieved in nonsurvivors.68 Furthermore, exercise PVR, but not rest PVR, distinguished survivors from non-survivors. Both studies indicate superior prognostic value of measuring exercise pulmonary hemodynamics compared to resting measurements alone. Peak exercise PAPm, however, was not retained in multivariate analyses of predictors of survival in either study. Whether this finding was attributable to limited incremental prognostic value of exercise PAPm, failure to account for lower workloads in non-survivors, or to inclusion of interrelated exercise hemodynamic variables into the multivariate models (i.e. stroke work index) requires further investigation.
Several mechanisms may account for the heightened PAPm/CO relationship observed in HF. Increased hydrostatic pressures during exercise due to LV dysfunction are to be expected, but persistent elevations in PVR reflect exaggerated increases in TPG during exercise as well. This may be due to maximally recruited pulmonary vasculature at rest on account of elevated resting hydrostatic pressures, thus limiting passive recruitment and distension during exercise observed in normals. Alternatively, subnormal mixed venous oxygen saturation in HF during exercise may contribute modestly to hypoxic-vasoconstriction.70 Finally, in one echocardiographic study, PH in HFrEF was closely related to the severity of functional mitral regurgitation and diastolic dysfunction, both at rest and during exercise.71, 72 Trials are currently underway to evaluate the role of pulmonary vasodilator therapy in LV dysfunction with disproportionate PH (i.e. LEPHT–LEft ventricular systolic dysfunction associated with Pulmonary Hypertension Riociguat Trial).73 Further defining the extent of disproportionate PH (i.e. ΔPVR) versus LV dysfunction (i.e. ΔPCWP) during exercise may offer improved patient phenotyping to inform targeted interventions.
Chronic Obstructive Pulmonary Disease
Pulmonary hypertension is a well known complication of COPD. Descriptions of PAPm response patterns to exercise in chronic obstructive pulmonary disease are limited by the heterogeneity in the small cohorts studied. In Hickam’s original series, despite normal average resting PAPm (18±4mmHg) the average increment in PAPm relative to CO was steeper in patients with emphysema (4.0±1.7) relative to normals (0.9±1.4). Blanco et al. found a steeper increment in PAPm relative to CO of 7.0 mmHg/L in patients with COPD selected on the basis of resting PAPm >20 mmHg.74
In a third study of 10 subjects undergoing lung volume reduction surgery with mild PH (resting PAPm=26±6 mmHg), PAP at rest and during exercise were examined relative to the degree of thickening of excised small (100–200 μm diameter) muscular pulmonary arteries (i.e. itima + media as a percentage of vessel diameter) as an indicator of pulmonary arterial remodeling. Interestingly, there was no relationship between resting PAPm and arterial wall thickness, but exercise PAP and change in PAP with standardized exercise (25W) were strongly related to wall thickness (R=0.72 and R=0.90, respectively, both P<0.05). The authors acknowledge the multiple potential mechanisms mediating PH in emphysema beyond vascular remodeling. However, based on these findings exercise PH may be indicative of reduced recruitability and distensibility of pulmonary vessels and the degree of the remodeling can not be estimated by resting PAPm.
Mitral Valve Disease
The development of PH in the presence of mitral stenosis (MS) and mitral regurgitation (MR) has been well described for over 40 years,75, 76 yet mechanisms mediating heterogeneity in pulmonary vascular responses to left atrial hypertension among patients with mitral valve disease at rest and during exercise remain incompletely understood.77, 78 Schwammenthal et al. showed that elevated PAP at rest and during exercise in MS is closely related to net atrioventricular compliance, to a greater extent than mitral valve area.79 Reduced compliance (<4 ml/mmHg) predicted need for mitral valve replacement (MVR) or commissurotomy and defines an MS cohort in which PH is closely linked to functional capacity.80
Pharmacotherapies for Treatment of Pulmonary Arterial Hypertension Alter Exercise Pulmonary Arterial Pressure
The observed improvements in exercise capacity with short-term aerosolized iloprost exposure, despite minimal changes in resting hemodynamics,81 led Blumberg and colleagues to hypothesize that iloprost would elicit more favorable effects on pulmonary hemodynamics during exercise than at rest. Indeed, one-time dose of iloprost was associated with a 6±8% reduction in resting PAP and a 18±11% fall in exercise PAP at a standardized submaximum workload (25 or 50W, P<0.05) in a cohort consisting of primarily Group 1 PH.60 One limitation of this study was the short duration between exercise studies that raised the possibility of reduced PAP responses on the post-intervention study on account of repeated testing. A second small study (n=5) of PAH patients with implantable haemodynamic monitors (ChronicleR IHM, Model 9520, Medtronic) also showed reduction in PAP with iloprost during submaximal exercise.61
Calcium channel antagonists have been shown to reduce absolute values of exercise PAPm and PVR and to blunt the increment in PAPm during exercise in PAH82 and PH due to COPD83, 84 despite patients achieving a greater workload. In contrast, hydralazine did not reduce exercise PAP in a similar, small study, but did reduce PVR because CO increased.85 This study is an example of why PAPm alone is insufficient, as greater CO with hydralazine resulted in equal PAPm at peak exercise but lower PVR. Further work is needed to ascertain whether there are treatment-specific approaches that differentially modulate pulmonary hemodynamics during exercise.
The influence of PDE5 inhibitors on exercise pulmonary hemodynamics has been studied in secondary forms of PH. Our group demonstrated that sildenafil, administered over 12 weeks in a randomized, double-blind placebo controlled trial (average dose of 50mg every 8 hours), led to improved peak VO2 and a reduction in exercise PVR. The magnitude of reduction in exercise PVR in this study was strongly related to the degree of improvement in peak VO2. Subsequent studies have found similar improvements in exercise capacity in HFrEF.
However, large scale outcomes trials of other pulmonary vasodilators for the treatment of HF have been disappointing to date.86, 87 These studies did not stratify HF subjects by rest and/or exercise pulmonary hemodynamics to focus on subjects with disproportionate elevations in PVR relative to PCWP.
Future Directions
Right ventricular-pulmonary vascular interactions:
Characterization of pulmonary vascular response patterns to exercise to date have largely focused on changes in PAP and PVR, which is simply a measure of opposition to the mean component of flow. However, the pulmonary vasculature is a low resistance, high compliance system, making the pulsatile component of hydraulic load and wave reflection important to consider as well.88 Impedence is the measure of opposition to pulsatile components of flow and provides a more complete indicator of right ventricular afterload.89 Several studies have described changes in resting impedence arising from large-artery stiffening and remodeling in PH.90 This abnormal pulsatile load may have detrimental effects on ventricular–vascular coupling, unfavorably loading the still-ejecting RV. Measurements of impedence in the frequency domain, and pressure-wave forms in the time-domain during exercise are needed to more completely characterize RV-PA interactions during exercise. Whether right ventricle responses to increased pulsatile flow during exercise predict future development of RV dysfunction or response to specific PH therapies merits further investigation as well.
Further development and validation of multi-modality non-invasive modalities to characterize RV-PA structure and function during exercise hold promise in furthering our understanding of pulmonary vascular responses to exercise. Finally, future studies are needed to define the natural history of “early” forms of PH unmasked by exercise (i.e. exercise induced PAH and HFpEF) and the extent to which these conditions may be amenable to treatment.
REFERENCES
- 1.Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118(21):2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh JP, Larson MG, Manolio TA, et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99(14):1831–1836. [DOI] [PubMed] [Google Scholar]
- 3.Benbassat J, Froom P. Blood pressure response to exercise as a predictor of hypertension. Arch Intern Med. 1986;146(10):2053–2055. [PubMed] [Google Scholar]
- 4.Hickam JB, Cargill WH. Effect of Exercise on Cardiac Output and Pulmonary Arterial Pressure in Normal Persons and in Patients with Cardiovascular Disease and Pulmonary Emphysema. J Clin Invest. 1948;27(1):10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galie N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25(24):2243–2278. [DOI] [PubMed] [Google Scholar]
- 6.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S–34S. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894. [DOI] [PubMed] [Google Scholar]
- 8.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S55–66. [DOI] [PubMed] [Google Scholar]
- 9.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–1665. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161(2 Pt 1):487–492. [DOI] [PubMed] [Google Scholar]
- 11.Wensel R, Opitz CF, Anker SD, et al. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106(3):319–324. [DOI] [PubMed] [Google Scholar]
- 12.Slonim NB, Ravin A, Balchum OJ, Dressler SH. The effect of mild exercise in the supine position on the pulmonary arterial pressure of five normal human subjects. J Clin Invest. 1954;33(7):1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janicki JS, Weber KT, Likoff MJ, Fishman AP. The pressure-flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation. 1985;72(6):1270–1278. [DOI] [PubMed] [Google Scholar]
- 14.Damato AN, Galante JG, Smith WM. Hemodynamic response to treadmill exercise in normal subjects. J Appl Physiol. 1966;21(3):959–966. [DOI] [PubMed] [Google Scholar]
- 15.Lewis GD, Lachmann J, Camuso J, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115(1):59–66. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz DH, Systrom DM. Diagnosis of pulmonary vascular limit to exercise by cardiopulmonary exercise testing. J Heart Lung Transplant. 2004;23(1):88–95. [DOI] [PubMed] [Google Scholar]
- 17.Fowler NO. The normal pulmonary arterial pressure-flow relationships during exercise. Am J Med. 1969;47(1):1–6. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. [DOI] [PubMed] [Google Scholar]
- 19.Grunig E, Janssen B, Mereles D, et al. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation. 2000;102(10):1145–1150. [DOI] [PubMed] [Google Scholar]
- 20.Sadaniantz A, Katz A, Wu WC. Miscellaneous use of exercise echocardiography in patients with chronic pulmonary disease or congenital heart defect. Echocardiography. 2004;21(5):477–484. [DOI] [PubMed] [Google Scholar]
- 21.Himelman RB, Stulbarg M, Kircher B, et al. Noninvasive evaluation of pulmonary artery pressure during exercise by saline-enhanced Doppler echocardiography in chronic pulmonary disease. Circulation. 1989;79(4):863–871. [DOI] [PubMed] [Google Scholar]
- 22.Argiento P, Chesler N, Mule M, et al. Exercise stress echocardiography for the study of the pulmonary circulation. Eur Respir J.35(6):1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vachiery JL, Brimioulle S, Crasset V, Naeije R. False-positive diagnosis of pulmonary hypertension by Doppler echocardiography. Eur Respir J. 1998;12(6):1476–1478. [DOI] [PubMed] [Google Scholar]
- 24.Oudiz RJ, Ginzton L. Pulmonary artery systolic pressure estimated by echocardiogram vs catheterization in patients awaiting lung transplantation. J Heart Lung Transplant. 2003;22(7):832–833; author reply 833. [DOI] [PubMed] [Google Scholar]
- 25.Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351(11):1097–1105. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120(11):992–1007. [DOI] [PubMed] [Google Scholar]
- 28.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104(23):2797–2802. [DOI] [PubMed] [Google Scholar]
- 29.Mitzner W, Sylvester JT. Hypoxic vasoconstriction and fluid filtration in pig lungs. J Appl Physiol. 1981;51(5):1065–1071. [DOI] [PubMed] [Google Scholar]
- 30.Graham R, Skoog C, Oppenheimer L, Rabson J, Goldberg HS. Critical closure in the canine pulmonary vasculature. Circ Res. 1982;50(4):566–572. [DOI] [PubMed] [Google Scholar]
- 31.Hyman AL. Effects of large increases in pulmonary blood flow on pulmonary venous pressure. J Appl Physiol. 1969;27(2):179–185. [DOI] [PubMed] [Google Scholar]
- 32.Degre S, de Coster A, Messin R, Denolin H. Normal pulmonary pressure-flow relationship during exercise in the sitting position. Int Z Angew Physiol. 1972;31(1):53–59. [DOI] [PubMed] [Google Scholar]
- 33.Wagner PD, Gale GE, Moon RE, et al. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol. 1986;61(1):260–270. [DOI] [PubMed] [Google Scholar]
- 34.Reeves JT, Moon RE, Grover RF, Groves BM. Increased wedge pressure facilitates decreased lung vascular resistance during upright exercise. Chest. 1988;93(3 Suppl):97S–99S. [DOI] [PubMed] [Google Scholar]
- 35.Reeves JT, Groves BM, Sutton JR, et al. Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol. 1987;63(2):531–539. [DOI] [PubMed] [Google Scholar]
- 36.Lonsdorfer-Wolf E, Richard R, Doutreleau S, et al. Pulmonary hemodynamics during a strenuous intermittent exercise in healthy subjects. Med Sci Sports Exerc. 2003;35(11):1866–1874. [DOI] [PubMed] [Google Scholar]
- 37.Laskey WK, Ferrari VA, Palevsky HI, Kussmaul WG. Pulmonary artery hemodynamics in primary pulmonary hypertension. J Am Coll Cardiol. 1993;21(2):406–412. [DOI] [PubMed] [Google Scholar]
- 38.Borst HG, McGregor M, Whittenberger JL, Berglund E. Influence of pulmonary arterial and left atrial pressures on pulmonary vascular resistance. Circ Res. 1956;4(4):393–399. [DOI] [PubMed] [Google Scholar]
- 39.Hopkins RA, Hammon JW Jr., McHale PA, Smith PK, Anderson RW. An analysis of the pulsatile hemodynamic responses of the pulmonary circulation to acute and chronic pulmonary venous hypertension in the awake dog. Circ Res. 1980;47(6):902–910. [DOI] [PubMed] [Google Scholar]
- 40.Libby P, Bonow RO, Mann DL, Zipes DP. Cardiac Catheterization. In: Braunwald E, ed. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 8th Edition ed.Philadelphia: Saunders Elsevier; 2007:449. [Google Scholar]
- 41.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. [DOI] [PubMed] [Google Scholar]
- 42.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–2550. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee P, Banerjee T, Khand A, Clark AL, Cleland JG. Diastolic heart failure: neglected or misdiagnosed? J Am Coll Cardiol. 2002;39(1):138–141. [DOI] [PubMed] [Google Scholar]
- 44.Azevedo A, Bettencourt P, Pimenta J, et al. Clinical syndrome suggestive of heart failure is frequently attributable to non-cardiac disorders--population-based study. Eur J Heart Fail. 2007;9(4):391–396. [DOI] [PubMed] [Google Scholar]
- 45.Ingle L, Cleland JG, Clark AL. Perception of symptoms is out of proportion to cardiac pathology in patients with “diastolic heart failure”. Heart. 2008;94(6):748–753. [DOI] [PubMed] [Google Scholar]
- 46.Thadani U, Parker JO. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Cardiol. 1978;41(1):52–59. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida A, Kadota K, Kambara H, et al. Left ventricular responses to supine bicycle exercise assessed by radionuclide angiocardiography and a Swan-Ganz catheter. Jpn Circ J. 1985;49(7):661–671. [DOI] [PubMed] [Google Scholar]
- 48.McCallister BD, Yipintsoi T, Hallermann FJ, Wallace RB, Frye RL. Left ventricular performance during mild supine leg exercise in coronary artery disease. Circulation. 1968;37(6):922–931. [DOI] [PubMed] [Google Scholar]
- 49.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17(5):1065–1072. [DOI] [PubMed] [Google Scholar]
- 50.Proudman SM, Stevens WM, Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: the need for early detection and treatment. Intern Med J. 2007;37(7):485–494. [DOI] [PubMed] [Google Scholar]
- 51.Raeside DA, Chalmers G, Clelland J, Madhok R, Peacock AJ. Pulmonary artery pressure variation in patients with connective tissue disease: 24 hour ambulatory pulmonary artery pressure monitoring. Thorax. 1998;53(10):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James KB, Maurer J, Wolski K, et al. Exercise hemodynamic findings in patients with exertional dyspnea. Tex Heart Inst J. 2000;27(2):100–105. [PMC free article] [PubMed] [Google Scholar]
- 53.Raeside DA, Smith A, Brown A, et al. Pulmonary artery pressure measurement during exercise testing in patients with suspected pulmonary hypertension. Eur Respir J. 2000;16(2):282–287. [DOI] [PubMed] [Google Scholar]
- 54.Kovacs G, Maier R, Aberer E, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180(9):881–886. [DOI] [PubMed] [Google Scholar]
- 55.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 Pt 2):S49–55. [DOI] [PubMed] [Google Scholar]
- 56.Walkey AJ, Ieong M, Alikhan M, Farber HW. Cardiopulmonary Exercise Testing with Right-heart Catheterization in Patients with Systemic Sclerosis. J Rheumatol. [DOI] [PubMed] [Google Scholar]
- 57.Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34(6):1802–1806. [DOI] [PubMed] [Google Scholar]
- 59.Lewis GD. The role of the pulmonary vasculature in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;53(13):1127–1129. [DOI] [PubMed] [Google Scholar]
- 60.Blumberg FC, Riegger GA, Pfeifer M. Hemodynamic effects of aerosolized iloprost in pulmonary hypertension at rest and during exercise. Chest. 2002;121(5):1566–1571. [DOI] [PubMed] [Google Scholar]
- 61.Wonisch M, Fruhwald FM, Maier R, et al. Continuous haemodynamic monitoring during exercise in patients with pulmonary hypertension. Int J Cardiol. 2005;101(3):415–420. [DOI] [PubMed] [Google Scholar]
- 62.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37(1):183–188. [DOI] [PubMed] [Google Scholar]
- 63.Costard-Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19(1):48–54. [DOI] [PubMed] [Google Scholar]
- 64.Franciosa JA, Baker BJ, Seth L. Pulmonary versus systemic hemodynamics in determining exercise capacity of patients with chronic left ventricular failure. Am Heart J. 1985;110(4):807–813. [DOI] [PubMed] [Google Scholar]
- 65.Lewis GD, Shah R, Shahzad K, et al. Sildenafil Improves Exercise Capacity and Quality of Life in Patients With Systolic Heart Failure and Secondary Pulmonary Hypertension. Circulation. 2007:CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 66.Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1(4):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555–1562. [DOI] [PubMed] [Google Scholar]
- 68.Metra M, Faggiano P, D’Aloia A, et al. Use of cardiopulmonary exercise testing with hemodynamic monitoring in the prognostic assessment of ambulatory patients with chronic heart failure. J Am Coll Cardiol. 1999;33(4):943–950. [DOI] [PubMed] [Google Scholar]
- 69.Mancini D, Katz S, Donchez L, Aaronson K. Coupling of hemodynamic measurements with oxygen consumption during exercise does not improve risk stratification in patients with heart failure. Circulation. 1996;94(10):2492–2496. [DOI] [PubMed] [Google Scholar]
- 70.Marshall C, Marshall B. Site and sensitivity for stimulation of hypoxic pulmonary vasoconstriction. J Appl Physiol. 1983;55(3):711–716. [DOI] [PubMed] [Google Scholar]
- 71.Enriquez-Sarano M, Rossi A, Seward JB, Bailey KR, Tajik AJ. Determinants of pulmonary hypertension in left ventricular dysfunction. J Am Coll Cardiol. 1997;29(1):153–159. [DOI] [PubMed] [Google Scholar]
- 72.Tumminello G, Lancellotti P, Lempereur M, D’Orio V, Pierard LA. Determinants of pulmonary artery hypertension at rest and during exercise in patients with heart failure. Eur Heart J. 2007;28(5):569–574. [DOI] [PubMed] [Google Scholar]
- 73. http://clinicaltrials.gov/ct2/show/NCT01065454?term=LEPHT&rank=1.
- 74.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med.181(3):270–278. [DOI] [PubMed] [Google Scholar]
- 75.Braunwald E, Braunwald NS, Ross Jm Jr., Morrow AG. Effects of Mitral-Valve Replacement on the Pulmonary Vascular Dynamics of Patients with Pulmonary Hypertension. N Engl J Med. 1965;273:509–514. [DOI] [PubMed] [Google Scholar]
- 76.Reeve R, Selzer A, Popper RW, Leeds RF, Gerbode F. Reversibility of pulmonary hypertension following cardiac surgery. Circulation. 1966;33(4 Suppl):I107–114. [DOI] [PubMed] [Google Scholar]
- 77.Adams W, ed. Pulmonary circulation; an international symposium.. New York: Grune; 1959. The vasoconstrictive factor in pulmonary hypertension; No. 1. [Google Scholar]
- 78.Alexopoulos D, Lazzam C, Borrico S, Fiedler L, Ambrose JA. Isolated chronic mitral regurgitation with preserved systolic left ventricular function and severe pulmonary hypertension. J Am Coll Cardiol. 1989;14(2):319–322. [DOI] [PubMed] [Google Scholar]
- 79.Schwammenthal E, Vered Z, Agranat O, et al. Impact of atrioventricular compliance on pulmonary artery pressure in mitral stenosis: an exercise echocardiographic study. Circulation. 2000;102(19):2378–2384. [DOI] [PubMed] [Google Scholar]
- 80.Kim HK, Kim YJ, Hwang SJ, et al. Hemodynamic and prognostic implications of net atrioventricular compliance in patients with mitral stenosis. J Am Soc Echocardiogr. 2008;21(5):482–486. [DOI] [PubMed] [Google Scholar]
- 81.Wensel R, Opitz CF, Ewert R, Bruch L, Kleber FX. Effects of iloprost inhalation on exercise capacity and ventilatory efficiency in patients with primary pulmonary hypertension. Circulation. 2000;101(20):2388–2392. [DOI] [PubMed] [Google Scholar]
- 82.Olivari MT, Levine TB, Weir EK, Cohn JN. Hemodynamic effects of nifedipine at rest and during exercise in primary pulmonary hypertension. Chest. 1984;86(1):14–19. [DOI] [PubMed] [Google Scholar]
- 83.Muramoto A, Caldwell J, Albert RK, Lakshminarayan S, Butler J. Nifedipine dilates the pulmonary vasculature without producing symptomatic systemic hypotension in upright resting and exercising patients with pulmonary hypertension secondary to chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;132(5):963–966. [DOI] [PubMed] [Google Scholar]
- 84.Franz IW, Van Der Meyden J, Schaupp S, Tonnesmann U. The effect of amlodipine on exercise-induced pulmonary hypertension and right heart function in patients with chronic obstructive pulmonary disease. Z Kardiol. 2002;91(10):833–839. [DOI] [PubMed] [Google Scholar]
- 85.Rubin LJ, Peter RH. Oral hydralazine therapy for primary pulmonary hypertension. N Engl J Med. 1980;302(2):69–73. [DOI] [PubMed] [Google Scholar]
- 86.Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134(1):44–54. [DOI] [PubMed] [Google Scholar]
- 87.Kalra PR, Moon JC, Coats AJ. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol. 2002;85(2–3):195–197. [DOI] [PubMed] [Google Scholar]
- 88.Kussmaul WG, Noordergraaf A, Laskey WK. Right ventricular-pulmonary arterial interactions. Ann Biomed Eng. 1992;20(1):63–80. [DOI] [PubMed] [Google Scholar]
- 89.O’Rourke MF. Vascular impedance in studies of arterial and cardiac function. Physiol Rev. 1982;62(2):570–623. [DOI] [PubMed] [Google Scholar]
- 90.Huez S, Brimioulle S, Naeije R, Vachiery JL. Feasibility of routine pulmonary arterial impedance measurements in pulmonary hypertension. Chest. 2004;125(6):2121–2128. [DOI] [PubMed] [Google Scholar]
- 91.Saito S, Miyamoto K, Nishimura M, et al. Effects of inhaled bronchodilators on pulmonary hemodynamics at rest and during exercise in patients with COPD. Chest. 1999;115(2):376–382. [DOI] [PubMed] [Google Scholar]