Abstract
The clinical management of melanoma patients has been rapidly evolving with the introduction of new targeted immuno-oncology (IO) therapeutics. The current diagnostic paradigms for melanoma patients begins with the histopathologic confirmation of melanoma, initial staging of disease burden with imaging and surgical approaches, treatment monitoring during systemic cytotoxic chemotherapy or IO therapeutics, restaging after completion of adjuvant systemic, surgical and/or external radiation therapy, and the detection of recurrent malignancy/metastatic disease following therapy. New and evolving imaging approaches with positron-emission tomography (PET) imaging technologies, imaging methodologies, image reconstruction and image analytics will likely continue to improve tumor detection, tumor characterization and diagnostic confidence enabling novel precision nuclear medicine practices for managing melanoma patients. This review will examine current concepts and challenges with existing PET imaging diagnostics for melanoma patients and introduce exciting new opportunities for PET in the current era of IO therapeutics.
Keywords: Positron emission tomography, Computed tomography, Immuno-oncology, Immunotherapy, Melanoma, Key Points
1. Introduction:
Melanoma remains the most deadly form of skin cancer with an incidence that has risen faster than nearly any other cancer in the last 50 years [1–3]. For stage I cutaneous malignant melanoma, the 5-year survival rate is 90% whereas it is 15–20% for Stage IV melanoma with distant metastatic disease [4]. In the metastatic setting, it can spread to distant organs of the body through vascular and/or lymphatic spread. In addition, disease recurrence occurs in 50–80% of melanoma patients with locoregional metastatic involvement and almost all patients with distant metastases [5]. An estimated 100,350 new cases of cutaneous melanoma were projected in 2020 with 6,850 deaths. While the number of cases has been increasing, mortality rates are declining most likely due to promising new systemic therapies for the treatment of locally advanced and metastatic disease. In recent years, the development and clinical use of new IO therapeutics (i.e., targeted small molecule inhibitors and immunotherapy) have improved survival in melanoma patients. From 2013 until 2017, in men and women (ages 20 to 64 years), the overall mortality from melanoma dropped by 7% annually. During that same time for patients 65 years of age and older, mortality rates were declining by 5–6% per year, while prior to 2013, mortality rates were increasing [6]. Imaging to assess for malignant/metastatic disease is a vital component of the work-up of patients with newly diagnosed lymph node-positive or recurrent melanoma so that the most appropriate therapy can be selected and delivered. Similarly, imaging plays a critical role in subsequently assessing treatment response in patients with recurrent malignancy and/or metastatic disease.
Positron emission tomography with computed tomography (PET/CT) is clinically used for the detection and assessment of malignant/metastatic lesions in patients with melanoma as well as many other cancers [7]. In the case of melanoma, 18F-Fluorodeoxyglucose (FDG) PET/CT imaging enables a whole-body assessment of physiologic and pathophysiologic glucose metabolism in order to identify metabolically reprogramed cancer lesions, which demonstrate increased FDG uptake relative to the normal tissues nearby. FDG PET/CT can also provide insight into therapeutic responses of tumors to cytotoxic chemotherapy. Whereas conventional diagnostic imaging approaches with CT and magnetic resonance imaging (MRI) use anatomic changes in tumor size as the measure of treatment response, FDG PET/CT can provide the additional functional insight by evaluating the metabolic activity of the tumor as another measure of treatment response. In particular, FDG PET/CT enables visual/qualitative assessment of glucose utilization throughout the body as well as semi-quantitative measurement for the evaluation of metabolic response to therapy [8]. The most widely used PET method for quantifying FDG activity is the standardized uptake value (SUV) [8]. In general, FDG PET/CT demonstrates high sensitivity and specificity for detecting and staging melanoma lesions when compared with CT and its improved accuracy can impact clinical and therapeutic management of melanoma patients [9].
The purpose of this review is to provide an overview of the current perspectives and future opportunities for PET imaging in the management of melanoma. Herein, we describe the current role of FDG PET/CT in the clinical management of melanoma including initial staging, treatment monitoring during therapy, restaging following therapy, and the subsequent detection of recurrent malignancy/metastatic disease. We also provide an overview of new developments in PET imaging technology including new and emerging imaging technologies, novel imaging approaches, and emerging concepts. Finally, we provide brief perspective on clinical trials investigating the use of PET in melanoma patients to assess response to therapies focusing primarily on immunotherapy.
2. Role of FDG PET in Management of Melanoma:
A. Initial staging
In order to determine the most optimal therapeutic plan for a newly diagnosed melanoma patient, the detection and localization of all sites of malignant/metastatic disease is essential [10]. While the primary melanoma site and locoregional metastases may be detected on clinical examination, the detection of distant disease (including visceral metastases) is often more challenging and may not present until the disease is quite advanced and causing clinical symptoms. Furthermore, melanoma spreads distantly in an often atypical pattern with a high frequency of metastatic spread to the spleen, adrenal glands, and small bowel when compared to other malignancies [11]. FDG PET/CT adds value as part of the comprehensive diagnostic evaluation for patients with advanced stage or high-risk melanoma. Early studies evaluating the ability of FDG PET to detect distant metastatic disease are limited by the inclusion of patients with all stages of melanoma (i.e., Stages I-IV). In general, these studies showed FDG PET sensitivity ranging from 84–94%, and specificity ranging from 83–97%, compared to CT sensitivity of 55–58% and specificity of 70–84% [12, 13]. Rodriguez Rivera et al. performed a meta-analysis on the use of FDG PET in patients with only stage III cutaneous melanoma [14]. In this meta-analysis, the overall sensitivity and specificity of FDG PET in detecting metastatic disease was 89% and 89%, respectively, with a change in stage and/or management noted in 22% of patients. A systematic review from Krug et al. evaluating the utility of FDG PET for initial staging of cutaneous malignant melanoma included 2,905 patients and patients with both early stage and advanced disease [15]. The pooled sensitivity and specificity for detection of metastasis by FDG PET was 83% and 85%, respectively, with disease management changes in 33% of patients. FDG PET was determined to be most helpful in patients with stages III and IV disease and especially in the detection of deep soft tissue, lymph node, and visceral metastases. Similarly, the diagnostic accuracy of FDG PET/CT was even higher for stage III and IV malignant melanoma when compared to stages I and II [16].
While the majority of evidence is retrospective with multiple systematic reviews and meta-analyses, there is limited prospective evidence highlighting the utility of FDG PET in initial staging of patients with cutaneous melanoma. In fact, a prospective non-randomized clinical trial of 144 patients with early stage cutaneous melanoma showed no benefit with the addition of FDG PET to standard clinical workup [17]. Bastiaannet et al. performed a prospective comparison of FDG PET alone to CT in melanoma patients with palpable lymph node metastases [18]. While FDG PET and CT tended to upstage patients by identifying the presence of distant metastases at similar rates in the study, FDG PET was able to identify more metastatic sites including the presence of bone and subcutaneous metastases when compared to CT alone. Hybrid FDG PET/CT imaging was then demonstrated to have a higher sensitivity than either FDG PET alone or CT in a separate meta-analysis evaluating multiple tumor histologies including melanoma [19].
Another prospective multicenter registry study was performed in Ontario evaluating the clinical utility of FDG PET/CT in patients with potentially resectable localized high-risk melanoma or recurrent disease being considered for metastasectomy [20]. Of 319 patients included in this study, 18% were upstaged to M1 status following FDG PET/CT, which had a subsequent impact on surgical management of these patients. Another study demonstrates clinical impact on patient management when using FDG PET/CT for melanoma patients being evaluated for metastectomy. In this study, half of the patients were subsequently spared surgery due to the detection of additional unresectable metastases and about 25% of patients had no change in the intended management plan [10]. Therefore, FDG PET/CT can play an important role in initial surgical staging for those melanoma patients evaluated for potential metastectomy. For the detection of intracranial metastatic melanoma lesions, FDG PET performs poorly when compared with contrast-enhanced MRI and CT [21] although larger metastatic lesions may be detectable on FDG PET.
Sentinel lymph node biopsy is standard of care for initial staging of melanoma patients. It has been reported that the false negative rate of sentinel lymph node biopsy is 6–29% and therefore new imaging approaches may be helpful in further detecting and quantifying metastatic nodal involvement [22]. The status of the sentinel lymph node is the single most important predictor of survival in node-negative melanoma [23]. To this end, FDG PET has a sensitivity of 17%, a positive predictive value of 50%, and a negative predictive value of 82% [24]. A systematic review of pooled data from eight studies showed that FDG PET, in comparison to sentinel lymph node biopsy SLNB, has a positive likelihood ratio (LR) of 1.33, a negative LR of 1.00, and a diagnostic odds ratio of 1.2 [25]. The Cochrane Collaboration has analyzed 4 studies evaluating PET/CT prior to SLNB, and found that PET/CT has a sensitivity of 10% and a specificity of 97%, which is inferior to a combination of ultrasound with fine needle aspirate of concerning lymph nodes prior to SLNB [26]. It should be noted that these earlier studies likely utilized analog photomultiplier tube-based PET detector imaging systems and these reported poor performance characteristics for PET detection of metastatic lymph nodes should not be surprising. Immunohistochemical evaluation of the excised sentinel lymph node(s) is capable of detecting isolated metastatic tumor cells which would be below the detection limit of conventional analog PET (cPET) and cPET/CT systems. As such, FDG cPET and FDG cPET/CT systems did not improve the detection of metastatic lymph nodes when compared with sentinel lymph node biopsy. More recently, the use of combined modality imaging with integrated FDG PET/MRI with diffusion weighted imaging also did not reliably differentiate metastatic lymph nodes from benign lymph nodes when correlated with sentinel lymph node biopsy. It is proposed that very small metabolic tumor volumes within metastatic lymph nodes may account for this historical poor sensitivity of FDG PET and therefore higher definition PET imaging approaches with smaller voxel volumes may improve detection of subcentimeter metastatic deposits [22].
Given the limited prospective clinical evidence, imaging guidelines from the National Comprehensive Cancer Network (NCCN) suggest that cross-sectional imaging, including FDG PET/CT, should be considered in those melanoma patients with stage III disease for baseline staging and in patients with stage IV or recurrent disease [27].
B. Treatment monitoring during therapy & restaging following completion of therapy
As metastatic melanoma patients have poor prognosis [28], diagnostic imaging again serves as the non-invasive approach for evaluating treatment response to various oncologic therapies. In particular, FDG PET/CT readily assists in routine detection and response assessment of distant extracranial melanoma metastasis [29, 30]. In the current era of immunotherapy/immune checkpoint inhibitor-based immuno-oncology (IO) treatments for melanoma patients, imaging assessment of response to IO treatment has become more complex [31]. These IO therapeutics have introduced new challenges for the interpretation of therapeutic response when compared with historical conventional cytotoxic chemotherapeutics. For example, small molecular IO inhibitors may improve patient survival while demonstrating minimal anatomic tumor size changes on follow-up diagnostic imaging. Due to the unconventional or delayed anatomic tumor responses of IO therapies on CT and/or MRI imaging, this challenge highlights the importance of adapting or developing new immune-related imaging response criteria strategies in patients treated with IO as opposed to cytotoxic chemotherapy. In the IO treatment setting, pseudo-progression is a phenomenon that presents as an initial enlargement of tumor size followed by a subsequent reduction in size. These initially enlarging tumors may result from immune-mediated tumor infiltrates and consequently these tumor infiltrates can increase FDG uptake on early PET imaging. It is important to recognize these early potential tumor pseudo-progression events in melanoma patients on IO therapies and help to distinguish it from actual tumor disease progression on follow-up imaging. Some other imaging findings that can suggest therapy-related inflammatory response are reactive uptake in the lymph node drainage basins for malignant/metastatic lesions as well as diffusely increased FDG uptake in the spleen. These observations has resulted in the development of the immune-related Response Evaluation Criteria in Solid Tumor (irRECIST) [9].
It is important to note that traditional methods to evaluate treatment response have focused on WHO, RECIST and EORTC criteria, which were developed for cytotoxic therapy regimens as opposed to IO regimens [32]. Per RECIST 1.1 criteria, PET/CT studies cannot independently be used for treatment response assessment because the attenuation-correction CT component of the PET/CT image acquisition are often deemed of inferior diagnostic quality when compared to dedicated diagnostic CT imaging owing to the lower radiation dose and lack of intravenous contrast administration on the attenuation-correction CT imaging for PET [33]. Consequently, the majority of the seminal, prospective, therapeutic trials evaluating systemic therapies for stage III and stage IV melanoma (such as the Checkmate and KEYNOTE series) did not evaluate the role of PET in assessing clinical outcomes. Multiple clinical trials are now underway to address the role of PET in melanoma detection and response assessment. Table 1 highlights the current clinical trials that are assessing PET imaging at various time points and with various PET radiopharmaceuticals in melanoma patients treated with different IO therapeutics. Table 2 highlights the current international clinical trials incorporating PET imaging into the response assessment during IO therapy or the surveillance period following IO therapy for melanoma.
Table 1:
Clinical trials investigating PET at various time points and with various PET radiopharmaceuticals in melanoma patients treated with different IO therapeutics as registered at www.clinicaltrials.gov at the time of submission.
| Study ID | Radiopharmaceutical(s) | IO Therapy | Primary endpoint | Imaging Time points |
|---|---|---|---|---|
| NCT03356470 | FDG and FLT | Nivolumab or Pembrolizumab |
Correlate baseline and posttreatment molecular imaging biomarkers of response to immunotherapy | Baseline and 1012 weeks posttherapy |
| NCT03089606 | FDG and [11C]AMT | Pembrolizumab | Association of SUVmax with objective response rate |
Baseline and 12 weeks posttherapy |
| NCT03888950 | FDG | Nivolumab or Pembrolizumab |
Quantify changes in FDG uptake by PERCIST criteria | Baseline , day 21- 31, and 3 months post-therapy |
| NCT04272658 | FDG | Nivolumab or Pembrolizumab or combo Ipilimumab/Nivoluma b |
Differentiate progression vs pseudoprogressio n using 4D bodyto-whole dynamic acquisition | Not specifie d |
| NCT03584334 | FDG | Nivolumab or Pembrolizumab |
Threshold of FDG retention index to distinguish progression versus pseudoprogression | Baseline , 7 weeks, and 3 months posttherapy |
| NCT02716077 | FDG | Pembrolizumab | Disease-free survival | Not specifie d |
| NCT04221438 | FLT | Encorafenib and Binimetinib |
Change in SUVmax |
Baseline and 8–9 weeks posttherapy |
| NCT04462406 | FDG | Nivolumab or Pembrolizumab or combo with | Event-free survival – active surveillance | Baseline and 12 months |
| Ipilimumab | following negative PET or positive PET but negative biopsy | posttherapy | ||
| NCT03520634 | [18F]PD-L1 | Nivolumab | Determine optimal dose of tracer and timing of imaging | Baseline and 6 weeks posttherapy |
| NCT02591654 | FLT | Pembrolizumab | Prevalence of lesion detection | Baseline and 6 weeks posttherapy |
Table 2:
Clinical trials integrating and investigating incorporating PET imaging into the response assessment during IO therapy or the surveillance period following IO therapy for melanoma patients as registered at www.clinicaltrials.gov at the time of submission.
| Study ID | Brief description | Country enrolling |
|---|---|---|
| patients | ||
| NCT03356470 | Comparing FDG PET and FLT PET, along with blood and tissue biomarkers | USA |
| NCT03888950 | Evaluate whether FDG PET predicts therapeutic response after 2 cycles of PD-1 directed therapy | France |
| NCT04272658 | Determine the value of 4D body-to-whole dynamic acquisition in FDG PET for immunotherapy monitoring | France |
| NCT03584334 | Using FDG PET to distinguish tumor progression versus pseudo-progression in patients with melanoma or non-small cell lung cancer | France |
| NCT02716077 | Early evaluation of response to pembrolizumab in patients with melanoma | USA |
| NCT04478318 | Determine the minimum FDG PET scan duration on a total-body versus conventional scanner | USA |
| NCT04462406 | Determine how FDG PET may allow early discontinuation of PD-1 directed therapy in unresectable stage IIIB-IV melanoma | USA |
| NCT03116412 | Prospective randomized multicenter trial to assess the role of imaging during follow up after resection of Stage IIb-c and III melanoma | Sweden |
| NCT03554083 | Determine the role of FDG PET in assessing response to neoadjuvant combination targeted and immunotherapy for patients with high-risk stage III melanoma | USA |
| NCT02621021 | Determine the role of FDG PET in assessing response to talimogene laherparepvec with or without radiation for patients with advanced melanoma, Merkel cell carcinoma or other solid tumors with skin metastasis | USA |
| NCT02575404 | Determine the role of FDG PET in assessing response to GR-MD-02 plus pembrolizumab for patients with advanced melanoma, nonsmall cell lung cancer or head and neck squamous cell cancer | USA |
| NCT04165967 | Determine the role of FDG PET in assessing response to adoptive tumor infiltrating lymphocyte transfer plus nivolumab for patients with metastatic melanoma that failed immunotherapy | Switzerland |
| NCT03311308 | Correlate hypoxia measurements in tumor via FDG PET in advanced melanoma patients treated with pembrolizumab with or without metformin | USA |
| NCT03161756 | Determine the role of FDG PET in assessing response to denosumab plus nivolumab with or without ipilimumab for patients with | Australia |
| metastatic melanoma | ||
| NCT04207086 | Determine the role of FDG PET in assessing response to neoadjuvant pembrolizumab plus levatinib for patients with resectable stage III/IV melanoma |
Australia |
| NCT03475134 | Determine the role of FDG PET and 68GaNODAGA-RGD PET in assessing response to tumor infiltrating lymphocyte therapy plus nivolumab rescue for patients with unresectable locally advanced or metastatic melanoma | Switzerland |
| NCT02858921 | Determine the role of FDG PET in assessing response to neoadjuvant dabrafenib, trametinib, and/or pembrolizumab for patients with BRAF mutant resectable stage III melanoma | Australia |
It has been proposed that FDG PET may be able to detect and assess early metabolic responses in melanoma lesions to IO therapies as well as quantify changes in the whole-body metabolic tumor burden. On the other hand, persistently stable or increasing FDG avidity in tumor lesions treated with IO therapeutics may be an indicator of tumor resistance. It is likely that current and future clinical trials will also need to identify, characterize and distinguish response patterns on FDG PET/CT for tumor response as well as non-target tissue/organ toxicities that may develop during IO therapy. Such toxicities include dermatitis, inflammatory endocrinopathies, inflammatory esophageal/gastrointestinal manifestations, pneumonitis, hepatitis, etc. Many of these potential toxicities may be easily detectable on FDG PET/CT imaging in clinically asymptomatic patients undergoing routine imaging assessment [9].
More specifically, PET/CT may play a more important role in determining the functional/metabolic impacts of these IO therapies especially when correlated with conventional anatomic imaging findings with CT and MRI. A systematic review and meta-analysis by Ayati et al. highlights the utility of various baseline PET parameters (i.e., SUVpeak, metabolic tumor volume, and total lesion glycolysis) as predictors of the final response to IO in patients with metastatic melanoma. Furthermore, PET-based response assessments using these various PET parameters improved sensitivity and specificity when compared to conventional imaging based response criteria [34]. PERCIST, PERCIMT, PECRIT, and EORTC 1999 criteria are other imaging assessment tools that have emerged which integrate PET/CT but are less frequently incorporated into clinical trials than RECIST [35]. These PET criteria integrate various PET-specific metrics to determine treatment response, including features of target versus non-target lesions, and the maximum voxel value of standardized uptake value (SUVmax) [30, 36].
New PET response assessment concepts and strategies for patients treated with IO therapeutics have led to a renewed interest in early interval PET imaging to better predict clinical response. On the interim FDG PET/CT imaging during IO, the presence of stable appearing anatomic disease and relatively increased FDG avidity (i.e., pseudo-progression) can represent early tumor inflammatory response (i.e., favorable outcome), which will eventually demonstrate imaging findings on subsequent scans that are more consistent with tumor treatment/regression. In fact, evolving and new response assessment strategies, which integrate both anatomic and functional/metabolic metrics, may be more predictive of early and/or eventual response to IO [37].
Cho et al. performed a prospective study in patients with advanced melanoma treated with IO by serially monitoring treatment response via FDG PET/CT at days 21–28 and again at 4 months after the initiation of IO therapy [37]. These authors used a combination of anatomic and functional imaging data collected at the early time points to develop criteria predictive of response to IO with 100% sensitivity, 93% specificity, and 95% accuracy. A similar study was performed by Sachpekidis et al. who evaluated the utility of interim FDG PET/CT performed following 2 cycles (6 weeks) of IO with response assessment based on tumor metabolic activity rather than anatomical tumor dimensions [38]. A subsequent study from the same group demonstrated a threshold of 4 new FDG-avid tumor lesions on subsequent post-treatment FDG PET/CT imaging was a reliable indicator of IO treatment failure. Furthermore, as these new FDG-avid tumor lesions demonstrated diameters exceeding 1 cm in size, the sensitivity and specificity for treatment failure approached 90% [32]. Similarly, modifications to the traditional PERCIST response assessment for patients treated with IO (i.e., immunotherapy-modified PERCIST or imPERCIST) have demonstrated that new lesions, even in the setting of partial metabolic response or even stable metabolic response, are metastatic in 55% of cases. Therefore, the detection of any new lesion should be considered indeterminate as opposed to immediately progressive disease and closely monitored on follow-up imaging or biopsied as clinically indicated [39].
In 2017, the PET/CT Criteria for early prediction of Response to Immune checkpoint inhibitor Therapy (PECRIT) and PET Response Evaluation Criteria for Immunotherapy (PERCIMT) were developed and proposed [8]. In 2017, the revised Response Evaluation Criteria in Solid Tumor (RECIST) criteria for evaluation of immunotherapy response (iRECIST) was also proposed [8]. Annovazzi et al. assessed the predictive value of FDG PET/CT performed 3–4 months after initiation of IO and compared various PET metrics and response criteria [40]. This retrospective study cohort consisted of patients treated with IO using either ipilimumab or with PD-1 inhibitors. Interestingly, for patients treated with ipilimumab, the metabolic tumor volume combined with PERCIMT criteria was the most reliable predictor for best overall response at 6 months, while for patients treated with PD-1 inhibitors, multiple PET metrics were found to be reliable predictors of response. Other authors have suggested that even earlier imaging time points following initiation of IO may be potentially predictive of IO treatment response (e.g., 2 weeks following initiation of anti-PD1 therapy for advanced melanoma). The ability to quickly and reliably assess IO treatment efficacy or treatment resistance would enable the clinical determination of when stop an ineffective IO therapy and switch to another therapy, thus reducing financial burden and risk of future immune-related adverse events (irAEs) in those patients with treatment-resistant disease [9, 41, 42]. FDG PET/CT is again useful in detecting the presence and resolution of irAEs. A retrospective review of 147 patients treated with IO for advanced melanoma who underwent either contrast-enhanced CT scan or FDG PET/CT, irAEs were detected with imaging in 31% of patients. Follow-up imaging was also helpful in monitoring for the resolution of irAEs [43].
Within the NeoCombi trial, which evaluated the role of perioperative dabrafenib and trametinib therapy in resectable stage IIIB-C melanoma patients, 18 patients had evidence of a metabolic complete response on preoperative PET/CT [44]. In addition, 11 of these 18 had both a complete response by RECIST criteria and a pathologic complete response in the resection specimen whereas 6 patients with a metabolic complete response on PET/CT did not have a pathologic complete response in the resected specimen. In a different single institution Phase Ib trial, a subset of 6 patients with resectable stage III/IV melanoma underwent pre-treatment FDG PET/CT followed by just 1 dose of neoadjuvant pembrolizumab, follow-up FDG PET/CT 3 weeks later and then surgical resection. In this subset of patients, a 20% decrease in tumor diameter using RECIST at 3 weeks following single dose pembrolizumab was associated with treatment response in the surgical specimen but the change in FDG-avidity at 3 weeks following the single dose IO administration was not yet predictive. [45]. While complete resolution of metastatic melanoma lesions on post-treatment imaging is the most comforting in terms of patient prognosis, data from a retrospective analysis of 104 patients treated with PD-1 directed IO for metastatic melanoma showed that CT imaging alone might be too conservative to predict treatment success. In this study, patients with a complete metabolic response on FDG PET/CT and a partial response on CT had comparable progression-free survival to patients with a complete response on CT [46]. An example of using FDG PET/CT to monitor response to IO in metastatic melanoma is shown in Figure 1.
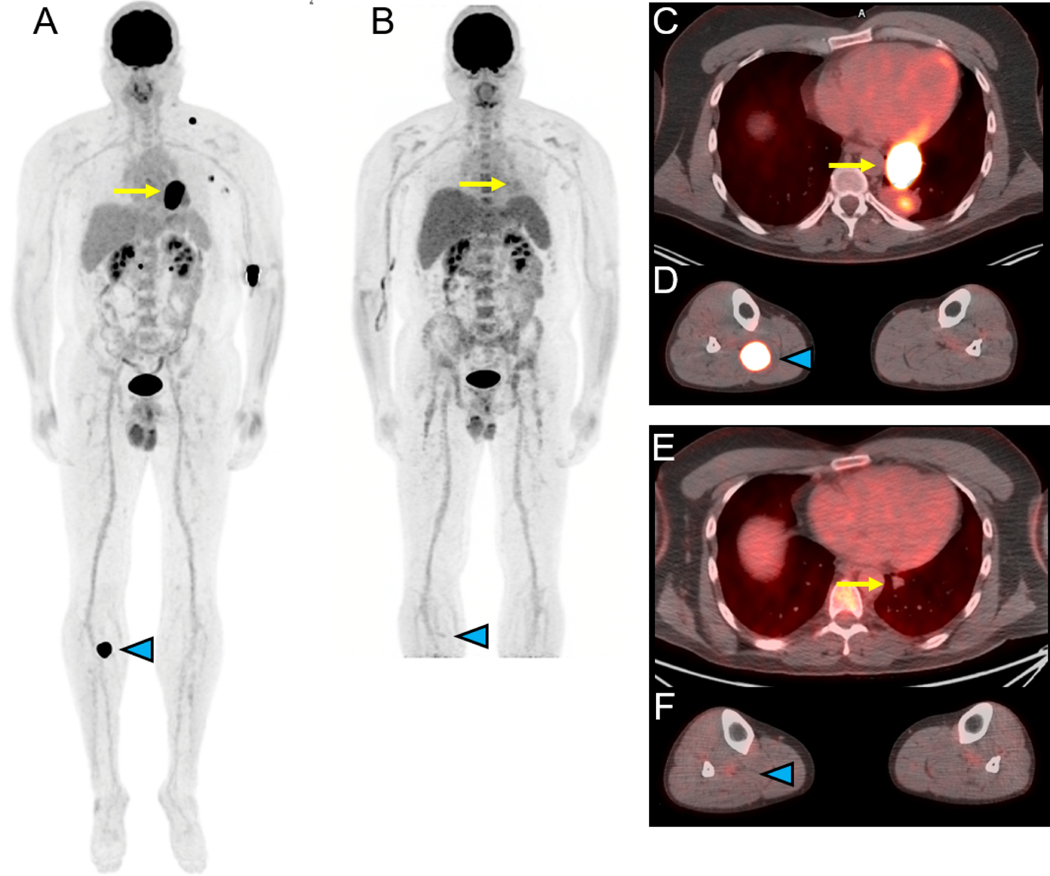
Figure 1.
18F-FDG-PET/CT for assessing response to immune checkpoint therapy (ipilimumab/nivolumab) in a 44 year-old-man with metastatic melanoma of unknown primary. Selected images prior to therapy are shown in panels A (maximal intensity projection (MIP) image), C and D (fusion images of the chest and lower extremities) while selected images obtained approximately 4 months later after starting therapy are shown in panels B (MIP), E and F (fusion images of the chest and lower extremities). The MIP images (A, B) demonstrate resolution of increased FDG uptake associated with multiple metastases with the fusion images demonstrating response in a left lower lobe mass (yellow arrow, C and E) and in a right proximal calf lesion (blue arrowhead, D and F). Courtesy of Jonathan McConathy, MD, PhD, at the University of Alabama at Birmingham (UAB).
In addition to assessing tumor response to systemic IO therapies, FDG PET/CT has been shown to be a useful imaging modality for assessing extracranial metastasis following stereotactic body radiation therapy (SBRT) [28]. Youland et al. reported on 80 extracranial metastases in 48 patients treated with SBRT who completed pre-treatment and post-treatment FDG PET/CT, which were evaluated using PERCIST (version 1.0). This study also suggested that the optimal interval between completion of SBRT and obtaining the first post-treatment FDG PET/CT scan should be more than 2 months in order to minimize radiation therapy-related inflammation observed at earlier time points. In this SBRT treatment study, an initial increase in tumor SUV corrected for lean body mass was observed in 14 metastatic lesions resulting in a classification of progressive disease. However, in this study, this increase in SUV was not associated with risk of metastasis control failure, progression-free survival, or overall survival. Response assessment following SBRT is sometimes challenging due to resultant treatment-related fibrosis and scarring in the target lesion(s) as well as the surrounding tissues. Post-radiation inflammatory changes in adequately treated tumor lesions and surrounding tissues can contribute to increased FDG activity in these regions on PET/CT imaging immediately following therapy. As noted, such transient increases in FDG avidity in post-treatment lesions/tissues can be minimized by performing restaging FDG PET/CT imaging 2–3 months after completion of SBRT [28].
C. Detection of recurrent malignancy and metastatic disease
Approximately 50% of patients treated for melanoma will relapse and these relapse events present as local recurrence (20%), locoregional nodal metastases (50%), and distant metastases (30%) [7]. Surveillance imaging recommendations are also confounded by the fact that while most melanoma recurs within 2 years of initial treatment, a significant proportion of patients may remain disease-free for decades. According to the NCCN guidelines, periodic assessment in the post-treatment setting for Stage IIb-IV melanoma patients should be considered for 5 years using appropriate radiographic, CT, MRI and FDG PET/CT approaches. FDG PET can detect malignant/metastatic lesions in post-treatment and clinically asymptomatic melanoma patients. It should be noted that FDG PET/CT is generally not considered in the surveillance imaging recommendations for most cancers despite its capability to detect recurrent malignancy/metastases [47].
Consensus for surveillance imaging following completion of definitive treatment for melanoma is lacking with limited evidence highlighting the utility of FDG-PET/CT as a routine cancer surveillance methodology. Prior to the development of IO-based therapies for melanoma, few effective systemic therapies for melanoma were available and this greatly reduced the clinical opportunities for investigating imaging surveillance approaches in these patients. However, in the current era of IO-based therapies for melanoma, there is now renewed interest in the prompt detection of recurrent/relapsed melanoma as it may result in earlier initiation of new IO treatments. This new clinical paradigm justifies a reassessment of the utility and timing of surveillance imaging for the detection of asymptomatic recurrent malignancy and metastatic disease. Bleicher et al. performed a retrospective study of 580 patients with stage II melanoma treated definitively [48]. In this retrospective analysis, over 25% of recurrences were found on follow-up surveillance imaging with over 40% of the recurrences in stage IIC disease detected on surveillance imaging. While bone and brain metastases were typically discovered following the onset of clinical symptoms, follow-up surveillance imaging was also helpful in detecting extracranial metastases.
Several studies have reported on the use of follow-up surveillance FDG PET/CT in melanoma patients after curative resection. Following the resection of Stage IIIB/C melanoma, one retrospective study examined surveillance FDG PET/CT imaging at 6-month intervals and described that PET/CT was an effective approach to detecting recurrent disease in asymptomatic melanoma patients during the first year following surgical resection [49]. Lewin et al. reported on 170 patients with stage III melanoma who completed follow-up surveillance FDG PET/CT with stage IIIA patients completing scans at 6 and 18 months and stage IIIB/C patients completing scans at 6 month intervals for the first 2 years and an additional scan at 36 months following completion of therapy [50]. Recurrent disease was detected in 38% of patients with 69% of relapses being asymptomatic. False positive FDG PET/CT findings also occurred in 7% of patients. Positive predictive values (PPV) of individual scans were 56–83% while negative scans had predictive values (NPV) of 89–96% for true non-recurrence. A negative FDG PET/CT at 18 months had negative predictive values of 80–84% for true non-recurrence at any time over the 47-month follow-up period of this study. Overall, 52% of patients with recurrence underwent curative-intent resection. In this setting for Stage III melanoma patients, a negative FDG PET/CT study is the most predictive finding. At present, conventional imaging surveillance approaches for recurrent melanoma has not yet demonstrated improved outcomes or suggested healthcare cost-saving/economic benefit.
In a separate study from Denmark, where FDG PET/CT was included as standard-of-care follow-up at 6, 12, and 24 months following treatment for stage IIB (and greater) melanoma patients, Vensby et al. reported on the value of FDG PET/CT following surgical resection in 238 patients [7]. In 526 FDG PET/CT studies, 25% were positive for recurrent disease, 69% were negative, and 5% had equivocal findings. Sensitivity was 89%, specificity was 92%, and PPV and NPV were 78% and 97%, respectively. The authors found no statistically significant difference in diagnostic accuracy in patients completing scans with or without clinical concern for recurrent disease. This study highlighted the high NPV of FDG PET/CT as part of follow-up surveillance despite the limitation of a false positivity rate of 9%.
A retrospective study from Mayo Clinic reported on 299 patients with stage III-IV melanoma followed with surveillance FDG PET/CT imaging following resection [51]. Overall, 52% of patients developed recurrent disease with the first recurrence presenting as clinically occult in 60% of patients. Both patients with clinically occult as well as those with clinically evident recurrent malignant/metastatic disease underwent curative-intent salvage therapy at similar rates (66% vs. 75%, P = 0.240). FDG PET/CT again had high sensitivity (88%), specificity (90%), and NPV (99%), but this study’s PPV of 37% emphasized the need for histologic confirmation of suspected recurrent malignancy/metastasis based on abnormal PET findings. An example of restaging melanoma with FDG PET/CT is shown in Figure 2.
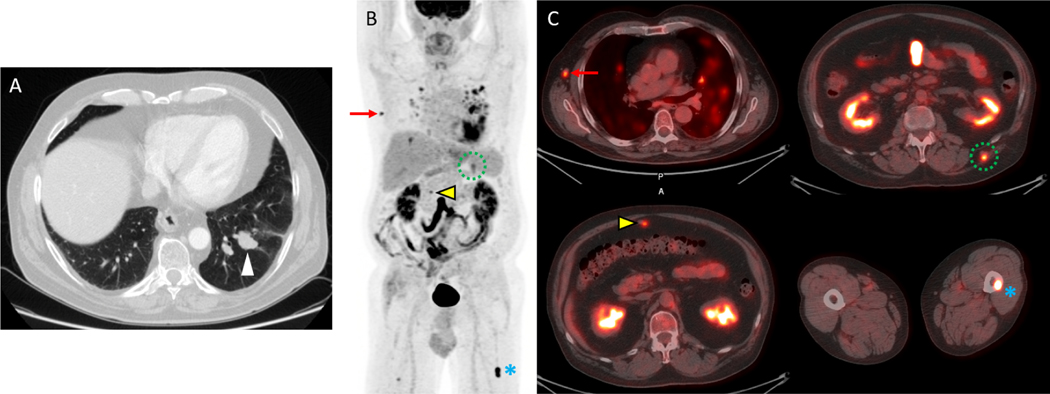
Figure 2.
18F-FDG-PET/CT detects multiple metastases in a 71 year-old-man with recurrent melanoma. A) An axial imaging from a diagnostic chest CT demonstrates a suspicious left lower lobe nodule which was subsequently biopsied bronchoscopically and shown to be a melanoma metastasis. The remainder of the diagnostic CT examination of the chest, abdomen and pelvis demonstrated an equivocal omental nodule but no definite metastatic disease. Based on these results, the patient began evaluation for a wedge resection of this metastasis which included restaging with FDG PET/CT. B) The maximal intensity projection (MIP) imaging from an FDG-PET study demonstrates multiple metastases as well as inflammation in the lungs. C) Fused PET/CT images demonstrate metastases with increased FDG uptake in the right axilla (red arrow), omentum (yellow arrow), left back musculature (dotted green circle) and left femur (blue asterisk). Surgical biopsy confirmed the right axillary lymph node metastasis, and the patient was treated with systemic therapy rather than metastectomy. Courtesy of Jonathan McConathy, MD, PhD, at the University of Alabama at Birmingham (UAB).
Stahlie et al. reported on an expanded cohort of a pilot study evaluating the utility of follow-up FDG PET/CT surveillance in asymptomatic high-risk stage III melanoma patients following surgical resection [52]. FDG PET/CT was completed every 6 months for 2 years following resection with the final scan completed at 3 years. Overall, 34% of patients developed a recurrence with 20% detected on the first follow-up FDG PET/CT study and no false positives were reported. Sensitivity and specificity of detecting recurrence in asymptomatic patients was 92% and 100%, respectively, with PPV of 100% and NPV of 99%. It should be noted that multiple studies have reported a wide range of metrics for sensitivity, specificity, NPV, and PPV for follow-up surveillance FDG PET/CT in melanoma and this is likely due to a variety of different inclusion criteria as well as variable follow-up imaging intervals.
Although the early detection of recurrent/metastatic melanoma may enable earlier therapeutic interventions, prospective randomized clinical trials are still needed to demonstrate that early detection and intervention prolongs survival [7]. In high-risk patients, the NPV of FDG PET/CT for detecting melanoma disease relapse is high (97%) but not perfect and FDG PET/CT was more likely to miss cutaneous disease recurrences which reiterates the importance of routine visual clinical skin inspection [7, 47]. A meta-analysis performed by Xing et al. investigated the utility of various imaging methods for the staging and surveillance of melanoma patients [53]. FDG PET/CT had the highest sensitivity (86%), specificity (91%), and diagnostic odds ratio (67) for detecting distant metastatic disease with a PPV of 80% for patients considered at high risk of distant spread. The practical concerns for FDG PET/CT surveillance in melanoma patients include false positive PET findings, which can lead to patient anxiety, additional follow-up imaging and even direct tissue sampling for histopathologic assessment [50, 54]. It has been recommended that surveillance by FDG PET/CT should be prospectively compared with other imaging/clinical approaches as well as with no surveillance at all [50].
3. Recent advances in PET: New technologies, approaches and analytics
Although FDG PET/CT in oncology patients is typically interpreted qualitatively, it also has the ability to accurately quantify physiologic and pathophysiologic processes. This is an opportunity for radiologists and nuclear medicine physicians to develop, refine and validate new PET approaches and analytical strategies for more personalized and precise nuclear medicine practices. In particular, current nuclear medicine and PET practices must advance to further improve (1) lesion detectability and disease burden quantification, (2) lesion characterization to distinguish between benign versus malignant processes, and (3) diagnostic confidence with existing or new imaging biomarkers in order to best align multi-disciplinary therapeutic management and minimize treatment-related toxicity.
New PET/CT imaging and analytic approaches for improving the accurate detection and characterization of small cutaneous and subcutaneous melanoma recurrences, metastatic nodal involvement (especially in nodes < 15 mm) and subcentimeter distant metastatic lesions is still an unmet clinical need. Addressing this need for melanoma is essential because improved lesion detectability allows for identifying melanoma lesions at the smallest and often earliest stage. Likewise, improved characterization of otherwise indeterminate lesions into either benign or malignant will likely contribute to reduced patient anxiety, fewer diagnostic imaging studies or biopsies, and shorter time from scan to treatment. Similarly, consistent and accurate quantification of whole-body disease burden will allow oncologists to more effectively personalize therapies [55].
A recent technical innovation introduced solid-state digital photon counting PET detectors into the latest generation of digital PET/CT (dPET/CT) systems and have replaced the conventional analog photomultiplier tube-based PET detectors (cPET) [56, 57]. Intra-individual comparison observations with dPET/CT systems have highlighted improved performance with the improved visualization of subcentimeter radiotracer-avid lesions on the dPET, which were not as visually conspicuous on cPET. There was also improved delineation of normal physiologic radiotracer activity within normal small tissues and organs (e.g., orbits, pituitary gland and adrenal glands) on dPET when compared with cPET. These new dPET detectors also allowed for radically new PET imaging systems (e.g., total body PET), image acquisition approaches and image reconstruction methods to be developed. The authors have performed > 200 intra-individual comparison studies between dPET/CT and cPET/CT systems in oncology patients (NCT02283125) and we will highlight some initial experiences with dPET/CT as well as new opportunities enabled by dPET detector technology that will help to address the unmet clinical needs for melanoma patients [58]. Specifically, dPET technologies enable improved PET image quality with higher definition PET reconstructions, lower radiotracer doses for diagnostic PET imaging (in accordance with ALARA), and shorter PET image acquisitions times for patients with symptomatic disease burden [57].
A. Higher Definition PET and Lesion Detectability
A current challenge for nuclear medicine physicians and radiologists who interpret PET/CT studies is the detection and visualization of small metastatic lesions (< 15 mm). It is important to understand that the size of the lesion, its FDG-avidity and the patient’s body-mass index are notable biological factors that significantly contribute to a lesion’s detectability on PET. In terms of technical factors that influence lesion detectability on PET, partial volume effects are particularly important and sometimes make it very challenging to distinguish tumor-specific radiotracer uptake from normal background activity. Other technical factors that influence lesion detectability include the radiotracer dose, radiotracer injection-to-PET scan time, PET image acquisition time, and PET image reconstruction. In general, cPET systems employ standard definition PET image reconstructions with voxel lengths of 3–4 mm (matrix sizes between 144–200) for standard-of-care diagnostic imaging protocols. On the other hand, dPET systems enable higher definition PET reconstructions with smaller voxel lengths of 1–2 mm (matrix sizes exceeding 200–400) and possibly even smaller. Higher definition dPET reconstructions lead to decreased PET voxel volumes, reduced partial volume effects, increased visual conspicuity of radiotracer-avid lesions for the interpreting physician, and more precise quantification of the lesion’s radiotracer avidity. Higher definition dPET reconstructions are again especially helpful for visualizing and assessing small lesions. In current clinical dPET/CT system implementations, higher definition PET imaging is feasible without prolonged PET image acquisition times but do require optimized PET reconstruction approaches when compared with standard definition cPET approaches [55, 58]. It has become evident that cPET and dPET image reconstruction optimization can greatly enhance the nuclear medicine physician’s ability to visualize and quantify small FDG avid lesions [55, 57]. Such reconstruction optimization approaches for improved detection and visualization of melanoma lesions on dPET have been described [59].
B. Improved PET Characterization of Indeterminate Lesions
Indeterminate lesions detected on anatomic diagnostic CT or MRI or even low-dose attenuation-correction CT imaging represent another unmet clinical need that dPET technologies may help to address. In general, FDG cPET imaging has been used to further characterize indeterminate lesions detected on anatomic imaging as benign or malignant but its diagnostic performance often relies on lesion size. Indeterminate lesions are also detected on FDG PET/CT given that FDG uptake is not cancer-specific and may relate to the patient’s underlying tumor biology, acute or chronic inflammation (e.g., post-vaccination, postoperative changes, and during/ after radiation or IO therapy), acute or chronic infectious processes, and altered radiotracer biodistribution. In such cases of indeterminate PET findings, correlation with the patient’s history and symptoms, physical examination, additional imaging and direct tissue sampling may be needed to determine if a finding is benign or malignant. The development of new dPET imaging approaches with FDG or other disease-specific PET radiopharmaceuticals may help address this clinical dilemma of indeterminate PET findings and especially when these findings are associated with smaller lesions on anatomic imaging [55].
The utilization of higher definition dPET image reconstruction is one approach to improve lesion characterization. Again, higher definition reconstruction allows for more precise localization of radiotracer activity within the smaller voxel volumes, reduces partial volume effects and more accurately quantifies the radiopharmaceutical activity within small lesions (Figures 3 & 4). This reduction of partial volume effects with higher definition reconstructions may also make small lesions more visually conspicuous on PET and demonstrate higher quantitative PET metrics (i.e., higher SUVmax values). Similarly, the use of higher definition reconstructions for large heterogeneous lesions may allow for the improved visualization and identification of regions of high-grade tumor versus low-grade tumor within a partially necrotic mass.
Figure 3:
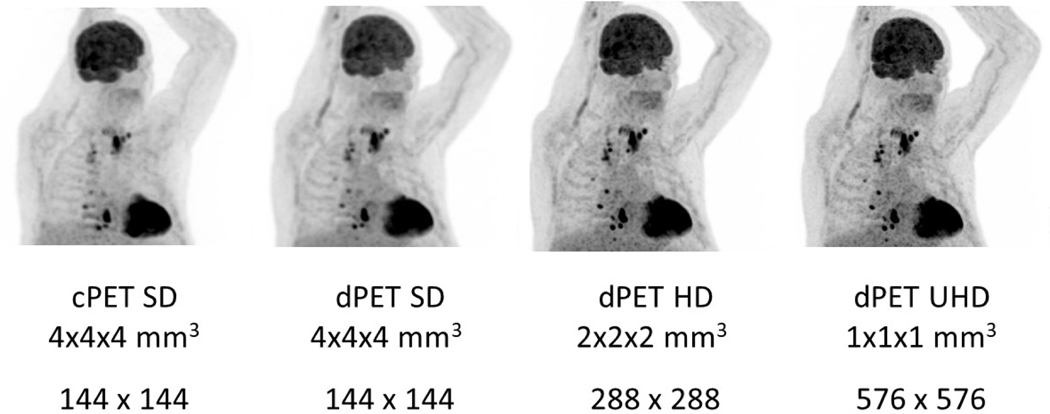
Intra-individual comparison in a patient imaged using a conventional photomultiplier-tube based PET/CT (cPET) (Gemini 64 ToF, Philips) system and a digital photon counting PET/CT (dPET) (Vereos, Philips) system with different reconstruction matrix/voxel volume sizes. This case demonstrates the capabilities of higher definition dPET to improve lesion detectability in subcentimeter metastatic nodal lesions without significant impact on background image quality. The patient was intravenously administered a standard dose of 478 MBq of FDG and then underwent imaging on the dPET/CT system at 53 minutes and the cPET/CT system at 81 minutes post injection. Both cPET and dPET emission scans were acquired with 90 seconds per bed position. There are multiple FDG-avid lesions in the base of neck and thoracic regions which are visually more conspicuous on dPET imaging and become even more suspicious with higher definition dPET reconstructions. Left to right: Maximum intensity projection images from standard definition cPET (SD, matrix size = 144 × 144, voxel length = 4 mm, voxel volume = 4 × 4 × 4 mm3), standard definition dPET (SD, matrix size = 144 × 144, voxel length = 4 mm, voxel volume = 4 × 4 × 4 mm3), high definition dPET (HD, 288 × 288, 2 mm, 2 × 2 × 2 mm3), and ultrahigh definition dPET (UHD, 576 × 576, 1 mm, 1 × 1 × 1 mm3).
Figure 4:
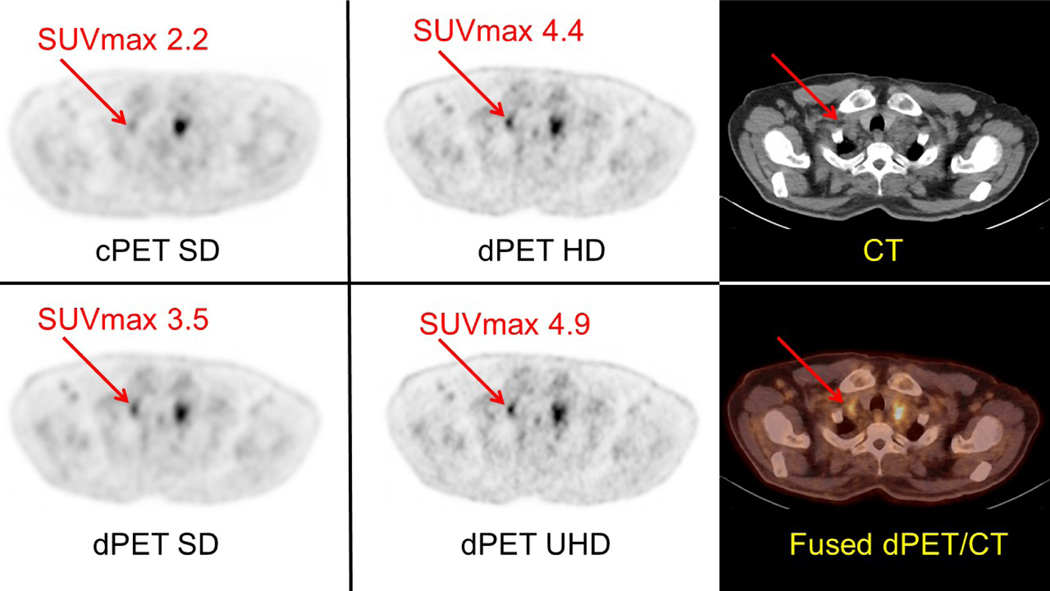
Intra-individual comparison in a patient imaged using a cPET/CT (Gemini 64 ToF, Philips) system and a dPET/CT (Vereos, Philips) system with different reconstruction matrix/voxel volume sizes. This case further demonstrates the capabilities of higher definition dPET reconstructions to reduce partial volume effects, more precisely localize FDG activity especially within small lesions, and increase the visual conspicuity of FDG-avid lesions. In addition, higher definition dPET reconstructions enable more precise measurement of SUVmax in small lesions (i.e., <15 mm in short axis). The patient was intravenously administered a standard dose of 478 MBq of FDG and then underwent imaging on the dPET/CT system at 53 minutes and the cPET/CT system at 81 minutes post injection. Both cPET and dPET emission scans were acquired with 90 seconds per bed position. Left and Middle: Axial images taken at the level of a right supraclavicular lymph node (red arrows) are shown with associated SUVmax value. Although there is a FDG-avid soft tissue lesion mass noted in the left supraclavicular region on both cPET and dPET, there is a small lymph node in the right supraclavicular regions (red arrow) which is visually more conspicuous on dPET images when compared with cPET and becomes more suspicious with higher definition dPET reconstructions. Right: Corresponding attenuation correction CT image and fused dPET/CT image at the level of the right supraclavicular lymph node.
Likewise, quantitative PET assessment of FDG uptake within a lesion has also been used to better characterize the visually detected FDG-avid lesion. Multiple quantitative PET assessment approaches have been developed and utilized in order to provide objective metrics for comparison of lesions within and between patients. The most clinically utilized quantitative FDG PET parameters is the maximum SUV (SUVmax). Advanced and evolving PET image feature analytics and approaches are also promising for melanoma patients. Beyond SUV metrics like SUVmax, SUVmean, and SUVpeak, there are FDG PET metrics like the MTV (metabolic tumor volume) and TLG (total lesion glycolysis) which provide a comprehensive whole-body assessment of tumor metabolic disease burden [60]. In addition, an assessment of tumor lesion heterogeneity in melanoma patients using FDG PET imaging has been described and FDG-PET tumor heterogeneity is associated with overall survival in those patients treated with immunotherapy [60, 61]. Given that melanoma lesions can be highly heterogeneous in terms of the tumor cells and the tumor microenvironment, more precise PET imaging assessment of tumor heterogeneity may provide insights into treatment resistance, disease progression or recurrence [61].
In addition, bone marrow-to-liver SUVmax ratio (BLR) and the spleen-to-liver ratio (SLR) have been described as potential FDG-PET imaging biomarkers in melanoma patients being treated with IO. With continued advances in imaging analytics and quantitative imaging biomarker approaches, multiple pre-treatment tumor imaging biomarkers may help to guide therapy selection as well as response assessment to therapies. These approaches apply to individual tumor lesions, whole-body tumor burden, and even normal hematopoietic tissues/organs (e.g., bone marrow and spleen). In terms of normal hematopoietic tissue/organ assessments in melanoma patients treated with immunotherapy, it has been shown that those patients with high pre-treatment BLR and SLR were associated with poorer outcomes [60].
Another new dPET-enabled approach for improved lesion characterization is Dynamic PET Perfusion Imaging (DPPI) at the time of radiopharmaceutical injection and throughout the early uptake period (e.g., 0–20 min post-injection). Early dynamic PET imaging of target lesions allows for further qualitative and quantitative assessment of the lesion’s immediate perfusion (hypoperfused versus isoperfused versus hyperperfused) and early radiotracer uptake kinetics which may help to distinguish viable tumor from inflammatory change when correlated with the later whole-body PET imaging (e.g., 60–70 min post-injection of FDG). In the future, these dPET-enabled approaches using higher definition reconstructions and/or DPPI to better characterize both indeterminate and malignant lesions before and after therapy may further improve diagnostic confidence, treatment stratification, and imaging response assessment [58]. Early dynamic FDG cPET/CT of the chest/abdomen only in melanoma patients undergoing immunotherapy failed to find any single FDG PET parameter that was predictive of which patients that would derive clinical benefit from IO and which patients would not. [8]. To date, no study has described dynamic FDG dPET assessment in melanoma patients treated with conventional cytotoxic therapy or immunotherapy.
Another approach to improve lesion characterization is the use of respiratory-gated PET imaging to reduce respiratory motion artifact (i.e., blurring of discrete FDG-avid lesions) as well as better visualize and quantify small tumor lesions in the thorax and upper abdomen. In fact, tumor SUVmax values in the chest and abdomen using breath holding FDG PET imaging approaches can be 30–40% higher than with free breathing PET [62]. When the burden of melanoma metastatic disease is limited (i.e., oligometastatic), accurate detection and characterization of malignant/metastatic lesion allows for potential surgical resection and/or targeted external radiation therapy which confers survival benefits to melanoma patients at the cost of procedure-related morbidity [49].
C. Reducing PET Radiotracer Dose and Imaging Faster with dPET
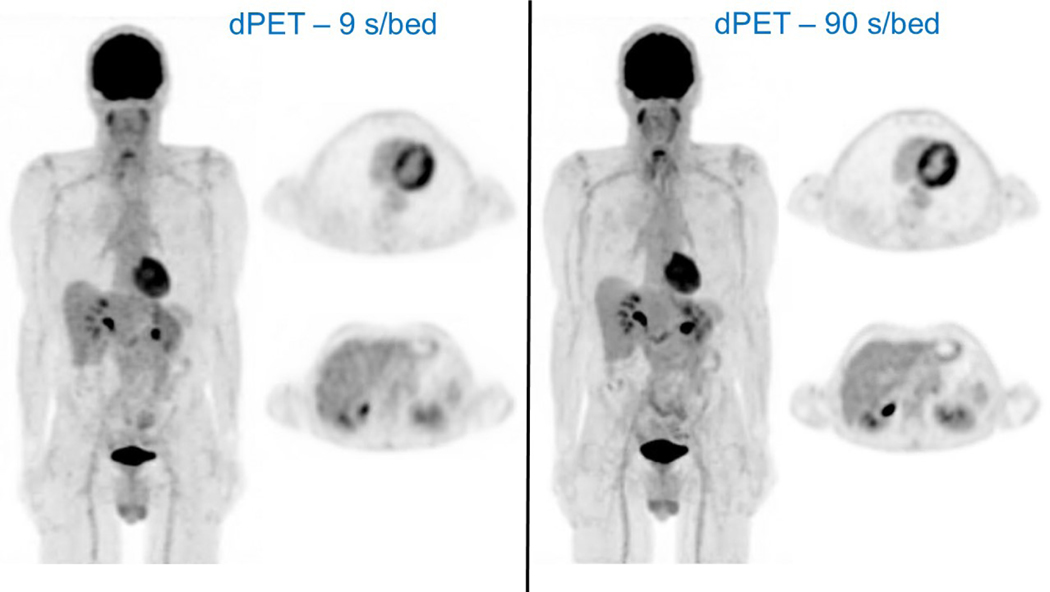
Digital PET systems also facilitate significant radiopharmaceutical dose reductions for whole-body PET imaging with no significant impact on overall image quality, background quality, lesion conspicuity and quantification when compared with cPET systems. In keeping with ALARA, this new capability for whole-body dPET imaging at significantly reduced radiopharmaceutical doses translates into new clinical and research paradigms for patients to undergo multiple serial dPET/CT studies during a treatment or follow-up surveillance period with only a fraction of the total radiation dose needed for traditional cPET/CT imaging. Likewise, dPET/CT systems allow for even faster whole-body PET imaging at standard radiopharmaceutical doses without affecting image quality, lesion detectabilty and quantification. This new capability of dPET/CT systems to facilitate faster whole-body PET imaging will also further minimize patient motion/misregistration artifacts as well as reduce the table time needed for PET imaging in patients with symptomatic lesions. Our team has again demonstrated the feasibility of >50% radiopharmaceutical dose reductions or faster whole-body dPET acquisition times by >50% without affecting PET image quality and quantification (Figure 5) [58].
Figure 5:
Intra-individual comparison in a patient imaged using a dPET/CT (Vereos, Philips) system and acquired with different dPET image acquisition times (ie, standard = 90 seconds per bed position, and ultra-fast = 9 seconds per bed position). This case demonstrates the capabilities of dPET technology to facilitate ultra-fast PET imaging with markedly reduce PET image acquisition times (1/10th of the standard acquisition time) while generating visually comparable image quality. The patient was intravenously administered a standard dose of 484 MBq of FDG and then underwent ultra-fast imaging (LEFT - 9 seconds per bed with a total PET acquisition <2 minutes) on the dPET/CT system at 53 minutes post injection followed by standard imaging (RIGHT - 90 seconds per bed with a total PET acquisition ~16 minutes) at 57 minutes post injection. For each acquisition, a maximum intensity projection image from SD dPET using optimized reconstruction methodologies are shown along with representative axial dPET SD images from taken at the levels of the heart and liver. The ultra-fast whole body dPET image acquisition produced visually comparable image quality when compared with the standard whole-body dPET image acquisition. In addition, the physiologic FDG activity is qualitatively and quantitatively similar on the ultra-fast and standard dPET acquisitions at the levels of the heart and liver. FDG uptake in the normal liver has a SUV mean = 1.9 for both dPET acquisitions.
D. Need for increased integration of advanced PET imaging and PET image analytics within clinical trials
For large prospective multi-center clinical trials, PET image acquisition and image reconstruction approaches can vary between and even within institutions. At present, there remains a need to continue to standardize PET image acquisition, image reconstruction and image analytics within multi-center therapeutic clinical trials to minimize variability in PET image quality, quantitative PET metrics and PET image features for the purpose of response assessment. To this end, harmonization efforts for multi-center PET imaging trials routinely use standardized PET phantoms to establish site-specific SUV quantification correction factors for participating institutions and can readily be implemented into future therapeutic clinical trials for melanoma. This is especially true as institutions begin to replace cPET/CT systems with newer dPET/CT systems with improved performance characteristics. As new and emerging PET radiopharmaceuticals are also integrated into clinical trials, consideration needs to be made to develop, validate and establish standardization approaches for each radiopharmaceutical in the multi-center clinical trial setting [55].
Radiomics is the process of identifying, extracting and quantifying image features from diagnostic images that can provide new insights into disease processes. As such, routine diagnostic imaging (i.e., CT, MRI and PET) may contain additional information with disease and therapeutic relevance that may not be currently appreciated in clinical practice. The development and validation of new imaging analytics and software tools are needed for PET/CT in order to begin extracting, quantifying and correlating simultaneous PET and CT imaging features with patient-specific tumor characteristics and disease-specific treatment outcomes. Image feature tools will likely facilitate new precision nuclear medicine practices for PET in terms of lesion segmentation, lesion characterization, and quantification of whole-body disease burden. Radiomics and image feature analysis for PET/CT will also play major role in developing future imaging response assessments to cancer-specific therapies (e.g., IO). A current challenge for PET/CT radiomics is the extent to which PET/CT imaging features are influenced by the various PET radioisotopes, PET radiopharmaceuticals and subsequent biodistribution, range of radiopharmaceutical doses administered, PET image acquisition, attenuation correction CT image acquisition, and PET and CT image reconstruction techniques. As already mentioned, PET/CT standardization and harmonization within multi-center clinical trials will be required to minimize image feature variability between patients and institutions in order to facilitate robust radiomic analyses and discover new imaging biomarkers with insights into disease characterization and management [55].
The current growth and development of advanced PET imaging technologies, PET radiopharmaceuticals, and PET imaging analytics is taking place simultaneously with growth and development in translational medicine technologies for the exquisitely sensitive detection of cancer specific markers from biological samples in cancer patients. Liquid biopsy-driven PET/CT imaging approaches for the detection, localization and treatment of recurrent malignant/metastatic disease in asymptomatic patients are already being developed. For example, the use of S-100B as a blood-based liquid biomarker for melanoma recurrence in asymptomatic patients. Out of all of the S-100B biomarker tests performed in one study, ~3% triggered a FDG PET/CT examination. In those patients for which FDG PET detected recurrent disease, 23% were otherwise asymptomatic and only had abnormal S-100B. Although not yet perfect, this liquid biopsy biomarker screening strategy contributed to the subsequent detection of recurrent melanoma in otherwise asymptomatic patients [63]. Future prospective clinical trials that correlate and compare serial liquid biopsy biomarkers of disease recurrence with concurrent imaging studies are needed and especially in the surveillance setting (i.e., liquid biopsy-driven imaging versus standardized scheduled surveillance imaging).
4. Conclusion
Throughout the body with the exception of the brain, FDG PET plays an important clinical role in the staging of melanoma, therapeutic response assessment, detection of treatment resistance/failure, detection of treatment-related toxicities, and detection of disease progression. With increasing use of IO therapeutics in melanoma as well as other cancer patients, it remains a clinical challenge to assess and quantify treatment response by conventional anatomic imaging alone. Melanoma lesions tends to demonstrate high glucose utilization and therefore FDG PET/CT is highly sensitive and ideally suited for detecting, monitoring and quantifying these lesions [4].
New PET imaging technologies and approaches, new PET image reconstruction techniques and image analytics, and possibly new PET radiopharmaceuticals will likely provide additional insights into the underlying tumor biology before, during and after therapy regardless of anatomic changes in tumor size, extent and burden. Although treatment-related pseudo-progression can cause some confusion on early treatment imaging, more research and clinical trial validation is needed to establish optimal time points for FDG PET/CT imaging in the setting of IO therapy for melanoma [9]. Newer immune-related response criteria have been developed to begin addressing these new treatment-related effects. In addition, future studies will likely need to incorporate both early-treatment as well as long-term FDG PET/CT imaging (e.g., 1 year follow-up after completion of IO) when therapy has been stopped in patients who have radiographically stable residual lesions in order to determine if it can provide useful prognostic information. At present, FDG PET findings of a complete metabolic response to immunotherapy is the most predictive biomarker for predicting long-term patient benefit and may allow for consideration of maintenance IO therapy discontinuation [21].
PET/CT image features may serve an early imaging biomarker of tumor response to therapy or even tumor resistance in order to help guide treating physicians. PET may also provide treating physicians with high yield tissue targets when pursuing residual tumor biopsies. Given that the optimal duration for IO therapy for various cancers remains unknown, new imaging strategies need to be developed and clinically validated to determine when complete pathologic response has been achieved versus persistent residual viable tumor. In the presence of stable but residual soft tissue lesions after long-term maintenance IO therapy, PET may allow for therapy to be stopped (and therefore minimize any immune-related toxicities or adverse events) and the patients to be monitored in a surveillance setting. It has been argued that surveillance imaging is only effective if it leads to effective therapeutic strategies and survival benefits as opposed to lead-time bias. Further prospective clinical trials in surveillance FDG PET/CT imaging are therefore needed to support its role in follow-up surveillance for asymptomatic melanoma patients.
In the future, radiologists and nuclear medicine physicians will need to better understand the impact of IO therapeutics on their patients, develop strategies for assessing treatment response during IO, determine if early predictive biomarkers of treatment response or even treatment resistance exist, and recognizing early imaging features suggestive treatment-related toxicities. It should be noted that different IO therapeutics may even have different capabilities for early PET prediction of response to therapy [64]. As our knowledge and understanding of melanoma and IO therapeutics grows, we will need to develop new tools and reporting structures to better guide medical decision-making. FDG PET has an important clinical role in the staging, therapeutic response assessment and clinical management of melanoma patients. More recently, PET technology, imaging approaches, image reconstruction and image analytics has advanced whereas the role of FDG as the primary PET radiotracer in melanoma patients has not changed. As highlighted in this review, there are some challenges but many exciting opportunities to advance FDG PET into new precision nuclear medicine strategies for patients with melanoma.
Acknowledgments:
CLW and MVK acknowledge research support: Wright Center of Innovation in Biomedical Imaging and ODSA TECH 09–028, 10–012, and 13–060 and the National Institutes of Health R01CA195513 and 5U24CA180803. CLW also acknowledges research time support: NCI UG1CA233331
Abbreviations:
- 11C-AMT
11Carbon-methyl-L-tryptophan
- BLR
bone marrow-to-liver SUVmax ratio
- cPET
Conventional analog photomultiplier-tube based positron-emission tomography
- CT
Computed tomography
- dPET
Digital position-emission tomography
- DPPI
Dynamic PET perfusion imaging
- EORTC
European Organisation for Research and Treatment of Cancer
- FDG
18F-Fluorodeoxyglucose
- FET
18F- fluoroethyltyrosine
- FLT
18F- fluorothymidine
- IO
Immuno-oncology
- imPERCIST
Immunotherapy-modified PERCIST
- irAE
Immune-related adverse events
- iRECIST
Response Evaluation Criteria in Solid Tumor criteria for evaluation of immunotherapy response
- irRECIST
Immune-related Response Evaluation Criteria in Solid Tumor
- MRI
Magnetic resonance imaging
- MTV
Metabolic tumor volume
- NCCN
National Comprehensive Cancer Network
- NPV
Negative predictive value
- PECRIT
PET/CT Criteria For Early Prediction Of Response To Immune Checkpoint Inhibitor Therapy
- PERCIMT
PET Response Evaluation Criteria for Immunotherapy
- PERCIST
Positron Emission Tomography Response Criteria in Solid Tumors
- PET
Positron-emission tomography
- PPV
Positive predictive value
- RECIST
Response Evaluation Criteria in Solid Tumor
- SBRT
Stereotactic body radiation therapy
- SLR
spleen-to-liver SUVmax ratio
- SUV
Standardized uptake value
- TLG
Total lesion glycolysis
Footnotes
Clinics Care Points
Conflicts of Interest:
CLW and MVK – none
EM – none
CC - none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erdmann F, et al. , International trends in the incidence of malignant melanoma 1953–2008--are recent generations at higher or lower risk? Int J Cancer, 2013. 132(2): p. 385–400. [DOI] [PubMed] [Google Scholar]
- 2.Linos E, et al. , Increasing burden of melanoma in the United States. J Invest Dermatol, 2009.129(7): p. 1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen CM, et al. , Trends in Melanoma Incidence Rates in Eight Susceptible Populations through 2015. J Invest Dermatol, 2019. 139(6): p. 1392–1395. [DOI] [PubMed] [Google Scholar]
- 4.Plouznikoff N and Arsenault F, Clinical relevance of 18F-FDG PET/CT lower-limb imaging in patients with malignant cutaneous melanoma. Nucl Med Commun, 2017. 38(12): p. 1103–1108. [DOI] [PubMed] [Google Scholar]
- 5.Mena E, et al. , Precision Medicine and PET/Computed Tomography in Melanoma. PET Clin, 12(4): p. 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2020. CA Cancer J Clin, 2020. 70(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 7.Vensby PH, et al. , The value of FDG PET/CT for follow-up of patients with melanoma: a retrospective analysis. Am J Nucl Med Mol Imaging, 2017. 7(6): p. 255–262. [PMC free article] [PubMed] [Google Scholar]
- 8.Sachpekidis C, et al. , Longitudinal studies of the (18)F-FDG kinetics after ipilimumab treatment in metastatic melanoma patients based on dynamic FDG PET/CT. Cancer Immunol Immunother, 67(8): p. 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong ANM, et al. , The Advantages and Challenges of Using FDG PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. Eur J Nucl Med Mol Imaging, 2017. 44(Suppl 1): p. 67–77. [DOI] [PubMed] [Google Scholar]
- 10.Forschner A, et al. , Impact of (18)F-FDG-PET/CT on surgical management in patients with advanced melanoma: an outcome based analysis. Eur J Nucl Med Mol Imaging, 2017. 44(8): p. 1312–1318. [DOI] [PubMed] [Google Scholar]
- 11.Trout AT, et al. , Melanoma metastases in the abdomen and pelvis: Frequency and patterns of spread. World J Radiol, 2013. 5(2): p. 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holder WD Jr, et al. , Effectiveness of positron emission tomography for the detection of melanoma metastases. Annals of surgery, 1998. 227(5): p. 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swetter SM, et al. , Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Annals of Surgical Oncology, 2002. 9(7): p. 646–653. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez Rivera AM, et al. , Value of positron emission tomography scan in stage III cutaneous melanoma: a systematic review and meta-analysis. Surg Oncol, 2014. 23(1): p. 11–6. [DOI] [PubMed] [Google Scholar]
- 15.Krug B, et al. , Role of PET in the initial staging of cutaneous malignant melanoma: systematic review. Radiology, 2008. 249(3): p. 836–44. [DOI] [PubMed] [Google Scholar]
- 16.Schroer-Gunther MA, et al. , F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: a systematic review. Syst Rev, 2012. 1: p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner JD, et al. , Inefficacy of F-18 fluorodeoxy-D-glucose-positron emission tomography scans for initial evaluation in early-stage cutaneous melanoma. Cancer, 2005. 104(3): p. 570–9. [DOI] [PubMed] [Google Scholar]
- 18.Bastiaannet E, et al. , Prospective comparison of [18F]fluorodeoxyglucose positron emission tomography and computed tomography in patients with melanoma with palpable lymph node metastases: diagnostic accuracy and impact on treatment. J Clin Oncol, 2009. 27(28): p. 4774–80. [DOI] [PubMed] [Google Scholar]
- 19.Gao G, Gong B, and Shen W, Meta-analysis of the additional value of integrated 18FDG PET-CT for tumor distant metastasis staging: comparison with 18FDG PET alone and CT alone. Surg Oncol, 2013. 22(3): p. 195–200. [DOI] [PubMed] [Google Scholar]
- 20.Singnurkar A, et al. , 18F-FDG-PET/CT in the Staging and Management of Melanoma: A Prospective Multicenter Ontario PET Registry Study. Clin Nucl Med, 2016. 41(3): p. 189–93. [DOI] [PubMed] [Google Scholar]
- 21.Tan AC, et al. , FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol, 2018. 29(10): p. 2115–2120. [DOI] [PubMed] [Google Scholar]
- 22.Schaarschmidt BM, et al. , Can integrated 18F-FDG PET/MR replace sentinel lymph node resection in malignant melanoma? Eur J Nucl Med Mol Imaging, 2018. 45(12): p. 2093–2102. [DOI] [PubMed] [Google Scholar]
- 23.Morton DL, et al. , Final trial report of sentinel-node biopsy versus nodal observation in melanoma. New England Journal of Medicine, 2014. 370(7): p. 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner JD, et al. , Prospective Study of Fluorodeoxyglucose–Positron Emission Tomography Imaging of Lymph Node Basins in Melanoma Patients Undergoing Sentinel Node Biopsy. Journal of Clinical Oncology, 1999. 17(5): p. 1508–1508. [DOI] [PubMed] [Google Scholar]
- 25.Krug B, et al. , Role of PET in the initial staging of cutaneous malignant melanoma: systematic review. Radiology, 2008. 249(3): p. 836–844. [DOI] [PubMed] [Google Scholar]
- 26.Dinnes J, et al. , Ultrasound, CT, MRI, or PET‐CT for staging and re‐staging of adults with cutaneous melanoma. Cochrane Database of Systematic Reviews, 2019(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coit DG, et al. , Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 2019. 17(4): p. 367–402. [DOI] [PubMed] [Google Scholar]
- 28.Youland RS, et al. , 18F-FDG PET response and clinical outcomes after stereotactic body radiation therapy for metastatic melanoma. Adv Radiat Oncol, 2017. 2(2): p. 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swetter SM, et al. , Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol, 2002. 9(7): p. 646–53. [DOI] [PubMed] [Google Scholar]
- 30.Wahl RL, et al. , From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med, 2009. 50 Suppl 1: p. 122S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seymour L, et al. , iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol, 2017. 18(3): p. e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anwar H, et al. , Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging, 2018. 45(3): p. 376–383. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer, 2009. 45(2): p. 228–247. [DOI] [PubMed] [Google Scholar]
- 34.Ayati N, et al. , The value of (18)F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging, 2020. [DOI] [PubMed] [Google Scholar]
- 35.Amrane K, et al. , Prediction of response to immune checkpoint inhibitor therapy using 18F-FDG PET/CT in patients with melanoma. Medicine, 2019. 98(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho SY, et al. , Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18 F-FDG PET/CT Imaging in Patients with Advanced Melanoma. Journal of Nuclear Medicine, 2017. 58(9): p. 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SY, et al. , Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point (18)F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J Nucl Med, 2017. 58(9): p. 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachpekidis C, et al. , The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging, 2018. 45(8): p. 1289–1296. [DOI] [PubMed] [Google Scholar]
- 39.Ito K, et al. , (18)F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J Nucl Med, 2019. 60(3): p. 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annovazzi A, et al. , Comparison of 18F-FDG PET/CT Criteria for the Prediction of Therapy Response and Clinical Outcome in Patients With Metastatic Melanoma Treated With Ipilimumab and PD-1 Inhibitors. Clin Nucl Med, 2020. 45(3): p. 187–194. [DOI] [PubMed] [Google Scholar]
- 41.Seith F, et al. , Is there a link between very early changes of primary and secondary lymphoid organs in 18F-FDG-PET/MRI and treatment response to checkpoint inhibitor therapy? Journal for immunotherapy of cancer, 2020. 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seith F, et al. , 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. European Journal of Nuclear Medicine and Molecular Imaging, 2018. 45(1): p. 95–101. [DOI] [PubMed] [Google Scholar]
- 43.Tirumani SH, et al. , Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer immunology research, 2015. 3(10): p. 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long GV, et al. , Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB–C, BRAFV600 mutation-positive melanoma (NeoCombi): a single-arm, open-label, single-centre, phase 2 trial. The Lancet Oncology, 2019. 20(7): p. 961–971. [DOI] [PubMed] [Google Scholar]
- 45.Huang AC, et al. , A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nature medicine, 2019. 25(3): p. 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan AC, et al. , FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Annals of Oncology, 2018. 29(10): p. 2115–2120. [DOI] [PubMed] [Google Scholar]
- 47.Lee HH, et al. , Recurrence of Melanoma After Initial Treatment: Diagnostic Performance of FDG PET in Posttreatment Surveillance. Nucl Med Mol Imaging, 2018. 52(5): p. 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleicher J, et al. , Recurrence patterns in patients with Stage II melanoma: The evolving role of routine imaging for surveillance. J Surg Oncol, 2020. [DOI] [PubMed] [Google Scholar]
- 49.Madu MF, et al. , PET/CT surveillance detects asymptomatic recurrences in stage IIIB and IIIC melanoma patients: a prospective cohort study. Melanoma Res, 2017. 27(3): p. 251–257. [DOI] [PubMed] [Google Scholar]
- 50.Lewin J, et al. , Surveillance imaging with FDG-PET/CT in the post-operative follow-up of stage 3 melanoma. Ann Oncol, 2018. 29(7): p. 1569–1574. [DOI] [PubMed] [Google Scholar]
- 51.Leon-Ferre RA, et al. , Association between the use of surveillance PET/CT and the detection of potentially salvageable occult recurrences among patients with resected high-risk melanoma. Melanoma Res, 2017. 27(4): p. 335–341. [DOI] [PubMed] [Google Scholar]
- 52.Stahlie EHA, et al. , The use of FDG-PET/CT to detect early recurrence after resection of high-risk stage III melanoma. J Surg Oncol, 2020. [DOI] [PubMed] [Google Scholar]
- 53.Xing Y, et al. , Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst, 2011. 103(2): p. 129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nijhuis AAG, et al. , False-Positive Results and Incidental Findings with Annual CT or PET/CT Surveillance in Asymptomatic Patients with Resected Stage III Melanoma. Ann Surg Oncol, 2019. 26(6): p. 1860–1868. [DOI] [PubMed] [Google Scholar]
- 55.Wright CL, et al. , Advancing Precision Nuclear Medicine and Molecular Imaging for Lymphoma. PET Clinics, 2017. 12(1): p. 63–82. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Maniawski P, and Knopp MV, Performance evaluation of the next generation solid-state digital photon counting PET/CT system. EJNMMI Res, 2018. 8(1): p. 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright CL, et al. , Advanced Functional Tumor Imaging and Precision Nuclear Medicine Enabled by Digital PET Technologies. Contrast Media & Molecular Imaging, 2017: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright CL, et al. , Emerging Opportunities for Digital PET/CT to Advance Locoregional Therapy in Head and Neck Cancer. Semin Radiat Oncol, 2019. 29(2): p. 93–101. [DOI] [PubMed] [Google Scholar]
- 59.Aljared A, Alharbi AA, and Huellner MW, BSREM Reconstruction for Improved Detection of In-Transit Metastases With Digital FDG-PET/CT in Patients With Malignant Melanoma. Clin Nucl Med, 2018. 43(5): p. 370–371. [DOI] [PubMed] [Google Scholar]
- 60.Seban RD, et al. , Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging, 2019. 46(11): p. 2298–2310. [DOI] [PubMed] [Google Scholar]
- 61.Sanli Y, et al. , Tumor Heterogeneity on FDG PET/CT and Immunotherapy: An Imaging Biomarker for Predicting Treatment Response in Patients With Metastatic Melanoma. AJR Am J Roentgenol, 2019: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 62.Barwolf R, Zirnsak M, and Freesmeyer M, Breath-hold and free-breathing F-18-FDG-PET/CT in malignant melanoma-detection of additional tumoral foci and effects on quantitative parameters. Medicine (Baltimore), 2017. 96(2): p. e5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deckers EA, et al. , S-100B as an extra selection tool for FDG PET/CT scanning in follow-up of AJCC stage III melanoma patients. J Surg Oncol, 2019. 120(6): p. 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seith F, et al. , Is there a link between very early changes of primary and secondary lymphoid organs in (18)F-FDG-PET/MRI and treatment response to checkpoint inhibitor therapy? J Immunother Cancer, 2020. 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]