Abstract
The activation of T helper 17 signaling plays a critical role in psoriasis pathogenesis, and systemically-administered IL-17 inhibitors are highly effective therapy for moderate-to-severe disease. We generated topically-delivered gene-regulating nanoconstructs, comprised of spherically-arrayed antisense DNA (liposomal spherical nucleic acids [L-SNAs]), which are able to penetrate human skin to knock down cutaneous gene targets. Topically-applied L-SNAs targeting the gene encoding the mouse IL-17A receptor (Il17ra) reversed the development of psoriasis clinically, histologically, and transcriptionally in imiquimod-treated psoriasis-like mouse skin. Il17ra L-SNAs reduced the modified PASI by 74% versus controls and decreased epidermal thickness by 56%. Il17ra L-SNA reduced Il17ra protein expression by 75% and significantly decreased the mRNA expression of psoriasis markers, including Defb4, Il17c, S100a7, Pi3, Krt16, and Tnfa versus scrambled spherical nucleic acid (Scr SNA) controls. A human IL17RA L-SNA penetrates 3-dimensional cultures and normal human explants to knock down IL17RA mRNA by 63% and 66%, respectively. After topical application to psoriatic 3-dimensional rafts, anti-human IL17RA L-SNAs reduced the expression of IL17RA (by 72%) and the IL-17-induced genes IL17C (by 85%), DEFB4 (by 83%), TNFA (by 77%), and PI3 (by 65%) versus scrambled L-SNA and vehicle controls (all P < 0.001). Taken together, these data suggest that targeted suppression of IL17RA is a promising new topical treatment strategy for psoriasis.
Introduction
Psoriasis pathogenesis depends on activation of the T helper 17 signaling axis, which is initiated by IL-17A binding to its receptor, IL17RA (Chiricozzi et al., 2011, Di Cesare et al., 2009, Girolomoni et al., 2012). Although IL17RA is expressed by both keratinocytes and immune cells in psoriatic infiltrates, studies of the cell-specific knockout of IL17RA have confirmed that keratinocytes are the critical target for psoriasis development in the imiquimod (IMQ)-induced mouse model of psoriasis (Moos et al., 2019).
The use of monoclonal antibodies targeting IL-17 (e.g., secukinumab and ixekizumab) and IL17RA (e.g., brodalumab) is among the most effective treatments for psoriasis (Hueber et al., 2010, Leonardi et al., 2012, Papp et al., 2012a, Papp et al., 2012b). However, the high cost and increased risk of infection associated with the systemic delivery of these drugs restricts their use to moderate-to-severe cases of psoriasis. For milder forms of psoriasis (more than 80% of patients), topical corticosteroids remain the predominant option (Greb et al., 2016, Kravvas and Gholam, 2018). Monoclonal antibodies are unable to penetrate the psoriatic epidermis for topical use, and topical approaches using small molecule inhibitors have not advanced for psoriasis. There is an unmet need to develop more targeted topical therapeutics for psoriasis.
Gene silencing to suppress protein synthesis is more specific and potent than small molecule protein inhibition. However, the development of nucleic-acid based therapeutics has faced challenges with respect to cellular entry, delivery of intact oligonucleotides, and efficacy. In addition, un-complexed oligonucleotides are unable to cross the epidermal barrier, limiting their potential topical use. Spherical nucleic acids (SNAs), nanoparticle constructs with oligonucleotides projected in a dense spherical array, penetrate the epidermal barrier and suppress target gene expression after topical application (Zheng et al., 2012). SNAs enter keratinocytes and immune cells, resist nuclease degradation, exhibit high specificity and low toxicity, and require no additional agents for skin penetration (Massich et al., 2009, Seferos et al., 2009, Wang et al., 2014). We have engineered gapmer antisense oligonucleotide liposomal IL17RA-targeting SNAs (IL17RA L-SNAs) for topical delivery and show their ability to reverse the clinical, histologic, and transcriptional psoriasis-like changes in the IMQ-induced mouse model, as well as IL-17-induced transcriptional changes in a human 3-dimensional cytokine-induced psoriasis skin model.
Results
Il17ra L-SNA inhibits Il17ra expression and improves the clinical and histological psoriasis-like phenotype in the imiquimod-induced mouse model
Three liposomal spherical nucleic acids (L-SNAs) targeting mouse Il17ra were tested for their capacity to knock down Il17ra mRNA expression in mouse fibroblasts at a concentration of 100 nM (all references to concentration reflect oligonucleotide concentration). The best-performing L-SNA showed dose-dependent knockdown of Il17ra, with a knockdown of 77.7% achieved with 1 μM L-SNA versus the scrambled (Scr) SNA control, which does not recognize any human or mouse gene (both P < 0.001) (Supplementary Figure S1a and b). This Il17ra L-SNA was advanced to comparative studies in the IMQ-induced psoriasis-like mouse model.
In previous studies, the application of Scr SNAs (vs. the vehicle) did not alter the severity of psoriasis-like skin lesions when applied 10 minutes before IMQ (Lewandowski et al., 2017), suggesting the compatibility of the SNAs and IMQ and leading to the adoption of this regimen for concurrent application. To test the ability of the Il17ra L-SNAs to inhibit the development of the psoriasis-like phenotype, 50 μM Il17ra L-SNA in a proprietary hydrogel base was applied concurrently with IMQ daily to the back of a mouse for six days (Supplementary Figure S2a). The control groups were mice treated with: i) IMQ and Scr SNAs, ii) IMQ and SNA vehicle, and iii) SNA vehicle only as a normal skin comparator (“No IMQ”).
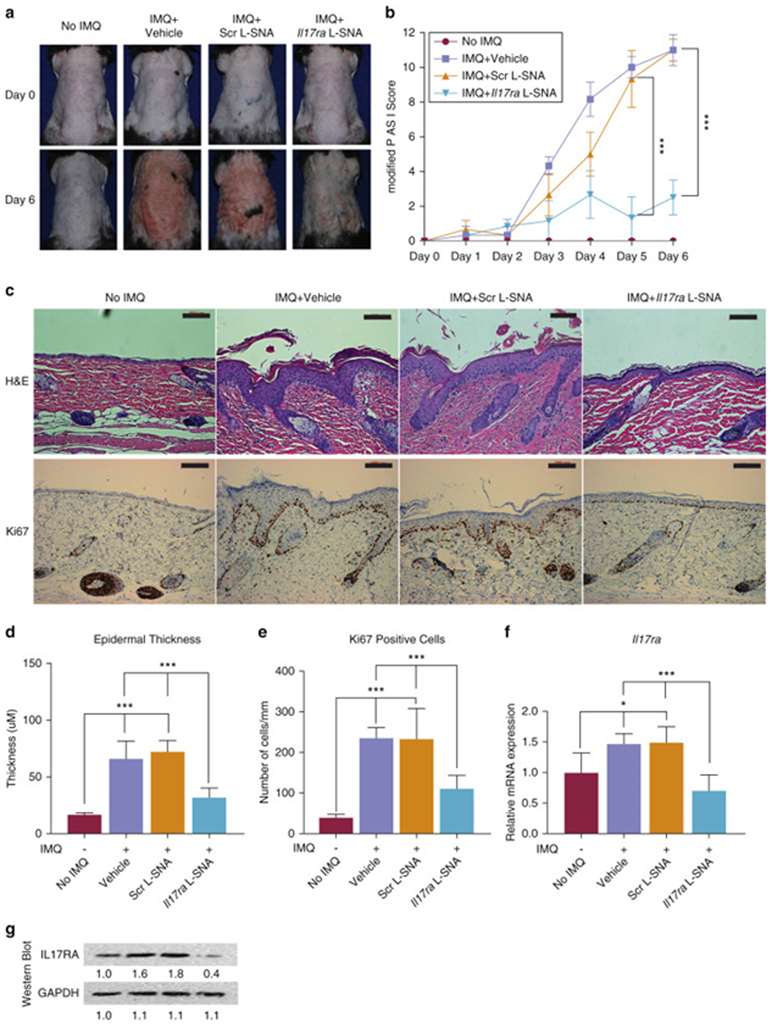
At day 6 (one day after the final treatment), the modified PASI score was significantly reduced by the Il17ra-targeting L-SNA (mean reduction, 74.2%) when compared to scrambled liposomal spherical nucleic acid (Scr L-SNA) and vehicle (both P < 0.001) (Figure 1a and b). All the subscores of the modified PASI (erythema, scaling, and thickness) were significantly reduced as well (82.6%, 69.6%, and 70.0%, respectively; all comparisons vs. Scr L-SNA, P < 0.001). The treated skin was harvested at day 6 for histologic and transcriptional evaluation. By day 6, Il17ra L-SNA treatment largely prevented the development of psoriatic histologic characteristics, including parakeratosis, acanthosis, immune cell infiltration (Figure 1c, top row), and keratinocyte hyperproliferation (Figure 1c, bottom row). Histologically-measured epidermal thickness was reduced by 56.3% (Figure 1c and d) and Ki67 staining by 52.7% (Figure 1c and e), compared to the vehicle and Scr SNA treatments, respectively. No increase in apoptotic cells was detected using TUNEL staining after 6 days of treatment with Scr or Il17ra L-SNA (Supplementary Figure S2b). The clinical and histological improvement in the psoriatic phenotype was associated with a reduction in the harvested tissue of Il17ra mRNA (by 57.1%), as measured by quantitative real-time reverse transcriptase–PCR(qRT-PCR) (Figure 1f), and Il17ra protein expression (by 75.4%), as detected by western blotting (Figure 1g).
Figure 1. Il17ra L-SNAs improve the clinical and histological psoriatic phenotype induced by imiquimod inC57BL/6 mice.
(a) Representative clinical pictures on day 6 before harvesting of back skin. (b) Mean reduction in daily modified Psoriasis Area and Severity Index , as assessed by three blinded reviewers. (c) Routine H&E (above) and Ki67 (below) staining of day 6-harvest mouse skin. Bars = 100 μm. (d) Epidermal thickness from the stratum granulosum to the epidermal-dermal junction. (e) Number of Ki67 + cells per linear length of basal epidermis. (f) Ratio of qRT-PCR levels compared to mouse skin with only vehicle and no IMQ. (g) Representative western blot from harvested skin. Values reported are the mean ± standard error of the mean from three separate experiments. *P < 0.05, ***P < 0.001. H&E, hematoxylin and eosin; IL-17RA, IL-17A receptor; IMQ, imiquimod; qRT-PCR, quantitative real-time reverse transcriptase–PCR; Scr L-SNA, scrambled liposomal spherical nucleic acid.
Il17ra L-SNA improves the transcriptional phenotype in the imiquimod-induced psoriasis-like mouse model
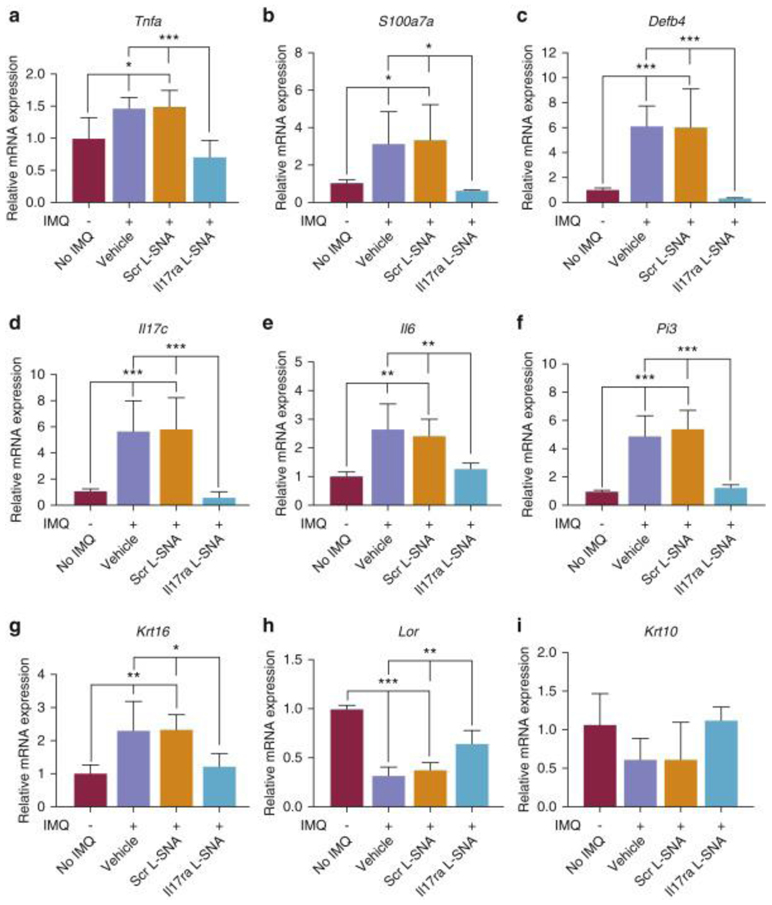
The treatment of mouse skin with IMQ significantly increased the expression of several psoriasis-related immune and proliferation markers (e.g., Tnfa [encoding the T helper 17-synergistic pathway cytokine Tumor necrosis factor-α], S100a7a [encoding psoriasin], Defb4 [encoding β-defensin 2], Il17c, Il6, Pi3 [encoding elafin], and Krt16), while decreasing the markers of keratinocyte differentiation (e.g., Lor, Krt10), as measured by qRT-PCR (Figure 2a-i, column 1 vs. column 2). Il17ra L-SNAs normalized these markers to the level of skin that was not treated with IMQ (Figure 2a-i). Significant reductions of Defb4 (mean, 94.8%), Il17c (mean,89.0%), S100A7 (mean,82.9%), Pi3 (mean,75.9%), Krt16 (mean,52.6%), Tnfa (mean,47%) and Il6 (mean,47.2%), as well as increases in Lor (mean,48.6%) and Krt10 (mean,82.8%) versus Scr L-SNA were observed. There was no significant difference between the markers in the skin treated with IMQ and Il17ra L-SNAs versus no treatment with IMQ (Figure 2a-i).
Figure 2. Il17ra L-SNA suppresses mRNA expression of inflammatory and proliferation markers and increases mRNA expression of genes promoting cell differentiation.
qRT-PCR of immune (a-f) proliferation (g) and differentiation (h, i) biomarkers associated with psoriasis. Expression of mouse 60S acidic ribosomal protein P0 and glyceraldehyde-3-phosphate dehydrogenase were averaged as a normalization control. Skin without imiquimod (IMQ) treatment was set as the comparator (no IMQ =1.0). Values are expressed as mean + standard error of the mean. *P < 0.05, **P < 0.01, ***P < 0.001. IL-17RA, IL-17A receptor; IMQ, imiquimod; qRT-PCR, quantitative real-time reverse transcriptase–PCR; Scr L-SNA, scrambled liposomal spherical nucleic acid.
IL17RA L-SNAs knock down IL-17RA expression in human keratinocytes and 3-dimensional rafts
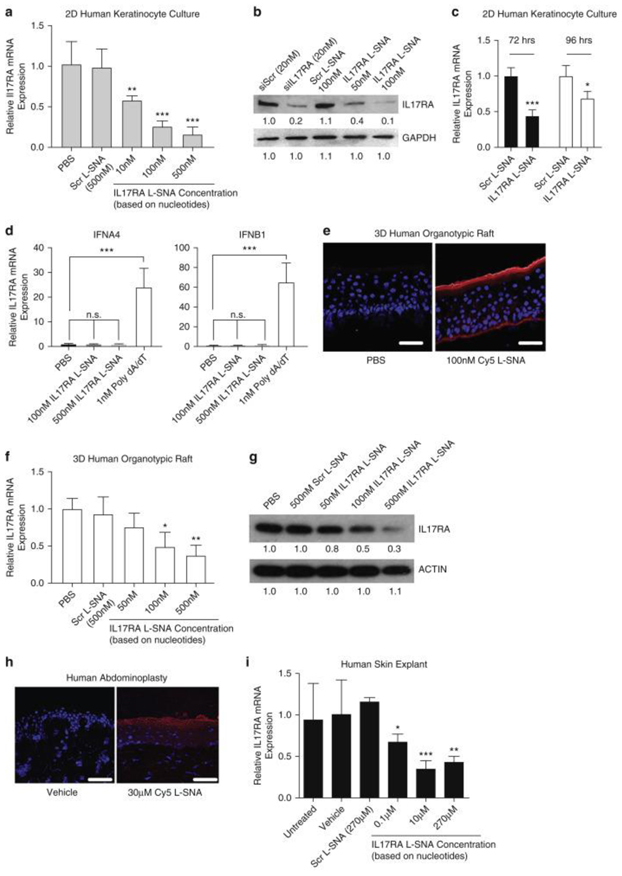
To better assess the translatability of the results from our psoriasis-like mouse model to human psoriasis prior to the clinical trials, L-SNAs were generated to knock down human IL17RA. The best performing compound was selected based on the extent and dose-dependency of the gene knockdown based on RT-PCR in foreskin-derived cultured normal human epidermal keratinocytes (NHEKs). The selected IL17RA L-SNA showed RNA knockdown at 48 hours after addition to NHEK cultures treated with as little as 10 nM IL17RA L-SNA (P < 0.01), with progressively greater IL17RA knockdown to 77.7% with 500 nM (P < 0.001) (Figure 3a). Consistently, western blots showed a 90.9% reduction in the target protein with 100 nM IL17RA L-SNA versus Scr L-SNA at 48 hours (Figure 3b). IL17RA mRNA levels remained significantly reduced at 72 (56%, P < 0.001) and 96 (32%, P < 0.05) hours after the initial treatment with 500 nM IL17RA L-SNA versus the scrambled controls (Figure 3c). To test if IL17RA L-SNA induced anti-viral responses in an IRF-3 dependent pathway, we evaluated mRNA expression of the downstream gene targets of IRF-3 activation (IFNA4 and IFNB1) using 1 nM poly dA/dT as a positive control. A dose of 100 nM and 500 nM of IL17RA L-SNA did not promote the gene expression of IFNA4 or IFNB1 in 2-dimensional keratinocytes (Figure 3c).
Figure 3. IL17RA L-SNA knocks down IL17RA expression in human cultures, rafts, and explants.
(a, b) NHEKs were treated and harvested after 48 hrs for qRT-PCR (a) and western blots (b; siIL17RA is free small interfering RNA). (c) IL17RA knockdown in proliferating NHEKs through 96 hrs. (d) qRT-PCR of NHEK IFNA4 and IFNB1 after 24 hrs treatment with 100 nM or 500 nM IL17RA L-SNA or 1 nM poly dA/dT. (e) Immunofluorescence microscopy of 3D rafts 48 hrs after 100 nM Cy5-L-SNA application. Bars = 50μm. (f, g) IL17RA knockdown 48 hrs after 3D raft treatment. (h) Immunofluorescence microscopy 24 hrs after 30μM Cy5 L-SNA application to human explants. Bars = 50μm. (i) Dose-dependent IL17RA knockdown 24 hrs after human explant treatment. Values mean ± standard error of the mean. *P < 0.05, **P < 0.01, ***P < 0.001. Red, Cy5 L-SNA; Blue, DAPI. 2D, 2-dimensional; 3D, 3-dimensional; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hrs, hours; IL17RA, IL-17A receptor; IL17RA L-SNA, IL-17A receptor liposomal spherical nucleic acid; L-SNA, liposomal spherical nucleic acid; NHEK, normal human epidermal keratinocytes; PBS, phosphate buffered saline; qRT-PCR, quantitative real-time reverse transcriptase–PCR; Scr L-SNA, scrambled liposomal spherical nucleic acid; siIL17RA, small interfering IL-17A receptor; siScr, small interfering scrambled.
To evaluate the effectiveness of the IL17RA SNA in a 3-dimensional humanized skin model, we first tested the penetration of Cy5-labeled SNA and found that 100 nM Cy5-L-SNA, applied to the surface in phosphate buffered saline (PBS), reached the basal cell layer within 24 hours of application (Figure 3e). Knockdown was evaluated by qRT-PCR 48 hours after application at the raft surface for 10-500 nM IL17RA L-SNA. IL17RA L-SNAs reduced IL17RA mRNA expression by 50.0% (P < 0.05) and 62.7% (P < 0.01) at 100 nM and 500 nM concentrations, respectively, versus Scr L-SNA (Figure 3f). Western blotting showed a reduction in target protein level by 51% and 74% at 100 nM and 500 nM concentrations, respectively, versus Scr L-SNA treatment (Figure 3g). Penetration and knockdown in human abdominoplasty explants was also tested. A total of 30 μM Cy5 L-SNA in a hydrogel vehicle penetrated abdominoplasty skin within 24 hours of application to the surface (Figure 3h). Significant dose-dependent knockdown of IL17RA by IL17RA L-SNA was observed 24 hours after the application of as little as 0.1 μM IL17RA L-SNA, peaking at 10 μM in explant studies (P < 0.001) (Figure 3i).
IL17RA L-SNA reverses psoriasis-related cytokine expression in human psoriatic 3-dimensional rafts
Psoriasis-like human skin models were generated by treating 3-dimensional organotypic rafts with a cytokine mix consisting of TNF-α, IL-17A, and IL-22 every other day, each at a concentration of 1 ng/ml, beginning at 9 days after the lifting of the cultured cells. Harvesting occurred 13 days after lifting (Supplementary Figure S3a and b). Within 4 days of the initiation of the cytokine mix treatment, the 3-dimensional raft exhibited transcriptional signs of psoriasis when compared to the non-treated rafts, as measured by qRT-PCR, with increased expression of TNFA, DEFB4, and PI3, and reduced expression of LOR (Supplementary Figure S3c). Using this model, we tested the ability of human IL17RA L-SNAs to improve the psoriatic phenotype. A total of 50-250 nM IL17RA L-SNA, as well as 250 nM Scr L-SNA or PBS, was applied to the surface of 3-dimensional rafts on days 7, 9, and 11 after lifting the raft to the liquid/air interface. The cytokine mix was added to the culture medium on days 9 and 11 (Supplementary Figure S3d for experimental design). Upon harvesting on day 13, IL17RA L-SNAs reduced IL-17RA mRNA expression in a dose-dependent manner (Figure 4a and b) compared with Scr L-SNA, reaching 72.0% (mRNA) and 82% (protein) knockdown with 250 nM. The application of 250 nM IL17RA L-SNAs also significantly reduced the expression of the genes encoding antimicrobial peptides and cytokines known to be directly activated by the IL-17 pathway, such as PI3 (by 65.1%) and DEFB4 (by 82.6%) (Figure 4c and d), and IL17C (by 84.6%) (Figure 4e), as well as the T helper 17-synergistic pathway cytokine TNFA (by 76.7%) (Figure 4f) (all P < 0.001). LOR was also significantly increased (by 6.49-fold) (Figure 4g), but abnormalities in the expression of KRT10 and S100A7 were not corrected by the treatment (Supplementary Figure S4a and b), and histologic sections from rafts treated with IL17RA L-SNA were not visibly different from those treated with vehicle or Scr L-SNA (Supplementary Figure S4c), likely reflecting the direct effect of IL-22 on mRNA expression and histology. No activation of the IRF-3 dependent immune response was induced by the application of IL17RA L-SNAs (Supplementary Figures S4d and e).
Figure 4. Human IL17RA L-SNA reduces psoriatic cytokine production by keratinocytes in 3D organotypic rafts.
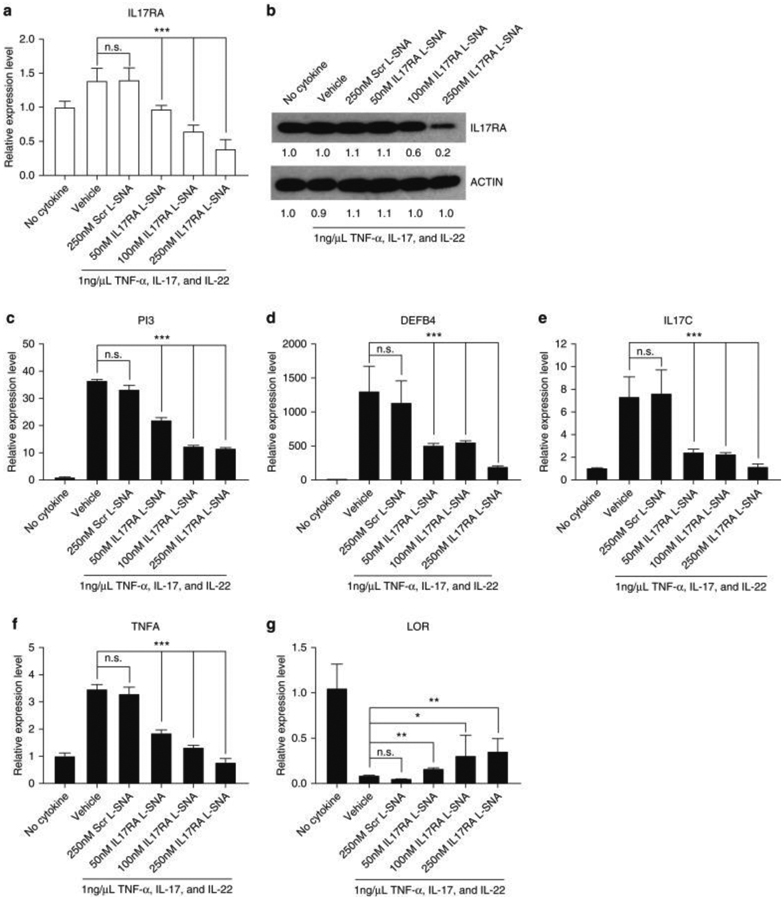
3D models were treated with L-SNAs or PBS every other day starting 7 days after lifting. The cytokine mix was added 9 days after lifting, concurrent with L-SNAs (Suppl. Fig. 3d) and harvested on day 13. (a, b) Knockdown of IL17RA was determined by qRT-PCR and western blotting. Dose-dependent knockdown of psoriasis-associated genes by IL17RA L-SNAs (vs. controls) in 3D rafts: (c) PI3, (d) DEFB4, (e) IL17C, and (f) TNFA. (g) LOR, which is reduced in psoriasis, is partially rescued by IL17RA SNA treatment. Values reported as mean ± standard error of the mean. * P < 0.05, ** P < 0.01, *** P < 0.001. 3D, 3-dimensional; IL17RA L-SNA, IL-17A receptor liposomal spherical nucleic acid; L-SNA, liposomal spherical nucleic acid; n.s, not significant; PBS, phosphate buffered saline; qRT-PCR, quantitative real-time reverse transcriptase–PCR; Scr L-SNA, scrambled liposomal spherical nucleic acid; TNF-α, tumor necrosis factor α.
Discussion
In this study, we validated the strategy of knocking down the expression of the IL-17A receptor using the topically-delivered liposome-based DNA SNA (L-SNA) nanoparticle platform, which we have shown is able to penetrate intact mouse skin, as well as normal and psoriatic human skin (Lewandowski et al., 2017, Randeria et al., 2015). The L-SNA targeting the IL-17A receptor knocked down IL17RA and reduced the clinical, histological, and transcriptional signs of IMQ-induced psoriasis-like disease in mice.
Our result mirrors our demonstration that tumor necrosis factor-α L-SNA improves these signs in the IMQ-induced model. We initially predicted that IL17RA targeting L-SNA would outperform tumor necrosis factor (TNF) L-SNA, given the superior results of IL-17RA targeting biologics. However, in our topical delivery platform, TNF L-SNA performed similarly to our IL17RA L-SNA, despite application at effectively half-dose (50 nM oligonucleotide concentration, but subsequently diluted 1:1 in the vehicle) and being applied every other day (vs. the daily dosing with the IL17RA L-SNA). While the difference may reflect the superior knockdown efficiency in cultured keratinocytes of our TNF L-SNA (69.8% reduction of TNFA) versus IL17RA L-SNA (57.1% reduction of IL17RA), the greater potency of TNF inhibition with topical delivery of TNF L-SNA (vs. the systemic delivery of biologics) may implicate the important role of the epidermis-derived TNF in amplifying the psoriatic response. In addition, the turnover rate of the interleukin receptor protein (receptor t1/2 traditionally 2-3 hours) (Dokter et al., 1992, Gerhartz et al., 1994) is slower than that of TNF (20-30 minutes) (Oliver et al., 1993, Zahn and Greischel, 1989), so that the anticipated need for a longer period before full efficacy may have played a role in this short-term study. We are currently developing and testing a combinational, bi-specific L-SNA that simultaneously targets tumor necrosis factor-α and IL17RA. We expect the synergistic effects of bi-specific SNA will allow a quicker response to treatment and increased the potency for treating more severe cases of psoriasis.
The extrapolation of preclinical results to human disease is necessarily limited by the validity of the models used. The IMQ-induced psoriasis-like mouse model was chosen because of its ease of use and the key role of T helper 17 signaling in the generation of its psoriasis-like phenotype. The standard model of IMQ-induced changes is limited to 7 days in duration owing to systemic inflammation, dehydration, and weight loss observed in mice treated for longer periods (Hawkes et al., 2017, Flutter and Nestle, 2013, Rabeony et al., 2015). As a result, our study was limited to preventing of the development of psoriasis-like disease. We are currently testing alterations in the IMQ-induced psoriasis-like inflammation model (e.g., intraperitoneal hydration, every other day application of IMQ) (Hawkes et al., 2017) to extend the duration of psoriasis-like changes to 2-3 weeks and reduce potential toxicity, allowing reversal experiments.
However, testing in a human model was more challenging. The cytokine-induced psoriasis-like human raft model requires the addition of TNF, IL-17, and IL-22. In our experience, the addition of IL-22 is vital for the generation of the psoriasis phenotype in this model. In contrast to IL-22, which is produced by immune cells in vivo (absent in the model) in response to IL-17A, TNF is upregulated in keratinocytes by IL-17A through the IL-17A receptor. As such, we expected that the knockdown of the IL-17A receptor in keratinocytes would be unable to reverse the abnormalities in the epidermal expression of the genes induced by IL-22 (i.e., increased S100A7; decreased KRT10, LOR) (Boniface et al., 2005, Wolk et al., 2006), as we observed. However, targeting IL17RA in the human keratinocyte 3-dimensional raft model of psoriasis reduced PI3 and DEFβ4 mRNA expression, as well as the mRNA expression of psoriasis-associated, keratinocyte-produced cytokines IL17C and TNFα, all of which are increased in expression by IL-17. These data suggest that expression of these cytokines is not significantly influenced by IL-22. Although the use of psoriasis explant models was considered as an alternative to the 3-dimensional organotypic model, the limited availability of explants from patients and their short viability in culture precluded having sufficient time to observe the efficacy of topically applied IL17RA L-SNA.
To date, no toxicity has been demonstrated in preclinical or early phase clinical trials. In a 28-day dermal toxicity study, human IL17RA L-SNA (XCUR17) gel was applied twice daily to the 10% body surface area of minipigs. Liver and kidney samples were collected at terminal necropsy (one day after the last dose or day 29) for an analysis of the concentration of the oligonucleotide portion of the SNA (XCUR17 DS). Based on prior work with structurally related oligonucleotides, the oligonucleotide portion of the SNA was predicted to distribute largely to the liver and kidneys if it penetrated to reach the bloodstream. Only 0.025% and 0.01% of the topically applied dose of the oligonucleotide portion of the SNA was detected in the kidneys and liver, respectively.
The daily administration of XCUR17 has been evaluated in a 26-day Phase 1 randomized, double-blinded, placebo-controlled trial in 21 patients with mild-to-moderate chronic plaque psoriasis. The trial was designed to assess the safety of XCUR17 formulated as a topical gel and to evaluate early signs of efficacy. All patients received three strengths of XCUR17 gel, a vehicle gel, and a positive comparator (Daivonex cream), which were all applied daily to the same subject with psoriasis to templated areas of similar severity. No adverse safety events related to treatment with XCUR17 were observed.
In conclusion, these studies provide additional support for the potential value of topically-applied SNAs in targeting the activation of specific pathways as a strategy for treating skin diseases. The expression of genes implicated in the pathogenesis of psoriasis in the human model was suppressed and significant improvements clinically, histologically, and transcriptionally in the IMQ-induced mouse model were observed, providing support for advancing topically-applied SNAs to the clinical setting. To this end, human IL17RA L-SNA (XCUR17, Exicure,Skokie, IL) has moved forward to Phase I human trials for mild-to-moderate psoriasis in Europe. As additional pathogenesis-based targets for other skin disorders are discovered, SNAs should be considered to be a promising topically-administered technology.
Materials and Methods
Generation of SNAs
A total of 20 nm 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) liposomes were synthesized using a high-pressure homogenizer (Avestin, Ottawa, OH). Liposome size and concentration were calculated as previously described (Lewandowski et al., 2017). Mouse IL17RA, human IL17RA, and Cy5 L-SNAs were synthesized by adding a 30-fold molar excess of cholesterol-conjugated oligonucleotides to liposomes in 1 × PBS and incubated overnight at 4°C to obtain approximately 30 oligonucleotides per liposome. The SNAs were mixed 1:1 (v/v %) with 2 × concentrated proprietary hydrogel vehicle.
Two-dimensional human epidermal keratinocytes culture and SNA-mediated gene knockdown
Human epidermal keratinocytes were isolated from neonatal foreskin as previously described (Arnette et al., 2016). Patient consent was not required for neonatal foreskin collection because US laws consider human tissue left over from surgery as discarded material. NHEKs were grown and maintained in 154CF media from Cascade Biologics (Cascade Biologics, Portland, OR), supplemented with 1% Human Keratinocyte Growth Supplements (Thermofisher Scientific, Waltham, MA), and 0.07 mM CaCl2. At 60% confluency, the desired concentration of IL17RA L-SNA nanoparticle and Scr SNA control was added to the cell culture media, and the cells were harvested for qRT-PCR and western blot analysis after 48 hours. As a positive control for gene knockdown using an established technique for in vitro knockdown, the NHEKs were transfected with 20 nM free IL17RA small interfering RNA using Lipofectamine RNAiMax (Thermofisher Scientic) for 48 hours according to the manufacturer’s instructions. Scrambled small interfering RNA (siScr, SIC001-10NMOL) and IL17RA small interfering RNA (siIL17RA, SASI_Hs01_00021030 and SASI_Hs01_00021031 in a 50%:50% ratio) were purchased from Sigma-Aldrich (St. Louis, MO). To measure the persistence of IL17RA mRNA knockdown by IL17RA L-SNA in proliferating keratinocytes, 500 nM of Scr L-SNA or IL17RA L-SNA was added to the cell culture media at 30% confluency. The cell culture media was replaced after 24 hours. The NHEKs were harvested at 48, 72, and 96 hours after the addition of the L-SNAs for qRT-PCR analysis.
Measuring IRF3 dependent immune activation in NHEKs
IL17RA L-SNA (or PBS) was added to the NHEK medium, with 1 nM poly(deoxyadenylic-deoxythymidylic) acid sodium salt (poly dA/dT) (Invitrogen, Carlsbad, CA) as a positive control, and the cells were harvested 24 hours later for qRT-PCR analysis. The primers for IFNA4 (Hs01681284_sH) and IFNb1 (Hs01077958_s1) were purchased from ThermoFisher (Thermofisher Scientific).
Induction of psoriasis-like inflammation in mice by imiquimod and SNA treatment
The mouse studies were approved by Northwestern’s Animal Care and Use Committee (Chicago, IL). C57BL/6 mice were shaved and 24 hours later, 62.5 mg/cm2 imiquimod (IMQ) cream (5%) (Taro, Hawthorne, NY) was applied to the back of 6-week-old C57BL/6 mice for six consecutive days (day 0 – day 5) (van der Fits et al., 2009, Lewandowski et al., 2017). At least six mice were included for each condition. IL17RA or Scr L-SNAs were dissolved in hydrogel (Exicure) and applied (40 μL of 50 μM L-SNA) 10 minutes before the IMQ application (Lewandowski et al., 2017). The disease severity was assessed by three blinded reviewers using a scoring system modified from the PASI, a composite score of erythema, scaling, and skin thickening, each scored independently as no visible phenotype (0) to severe (4) for a total of 12 possible points (modified PASI). The back skin of the mouse was harvested after 6 days of IMQ/SNA treatment. The samples were fixed in 10% formalin and paraffin-embedded for hematoxylin and eosin staining or immunohistochemistry using Ki67 antibody (cell proliferation) or TUNEL (apoptosis detection). Histologic assessments were performed at Northwestern’s Skin Disease Research Center and Mouse Histology and Phenotyping Core. Sections of mouse skin tissue were snap-frozen and stored at −80 °C for subsequent qRT-PCR and western blot analysis.
Topical treatment of IL17RA L-SNA on 3-dimensional psoriatic organotypic rafts
Three-dimensional organotypic rafts were generated as previously described (Arnette et al., 2016; Gordon et al., 2013; Lewandowski et al., 2017). Nine days after lifting to the air-liquid interface, 1 ng/ml each of tumor necrosis factor-α, IL-22, and IL-17A (Thermofisher Scientific) was added to the culture media. The PBS vehicle, IL17RA L-SNA (50 nM and 100 nM) or Scr SNA (100 nM) was applied to the raft surface. The SNA or PBS application and the cytokine mix were repeated every other day (Supplementary Figure 3d). All the studies were performed 3-4 times in triplicate. The raft was split into three sections upon harvesting (day 13 after lifting) for qRT-PCR, western blot, and histologic analyses.
Knockdown of IL17RA in human explants
Full-thickness skin human skin from fresh abdominoplasty tissue was obtained using a dermatome (Zimmer 666-01, Warsaw, IN) and mounted in jacketed Franz diffusion chambers (Laboratory Glass Apparatus, CA). Receptor chambers were filled with culture medium at 32 °C as previously described (Smith et al., 2016). Forty microliters of compound (0.1 μM/10 μM and 270 μM SNA concentrations were tested separately, each with untreated and hydrogel vehicle controls, N = 3) was topically applied to skin in donor chambers for 24 hours.
Statistical Significance
Significance was calculated using a one-way analysis of variance (when comparing multiple variables), and Student’s t-test (when comparing two variables) using Graphpad Prism 7 (Graphpad Software, San Diego, CA). P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This research is funded by the National Psoriasis Foundation Discovery Grant. We thank the Northwestern Skin Diseases and Research Center (NIAMS P30AR057216) for generating 3-dimensional raft models for in vitro testing and processing histologic samples.
Histology services were also provided by the Northwestern University Mouse Histology and Phenotyping Laboratory (NCI P30-CA060553). The authors appreciate Dr. Wilson Liu and the Center for Advanced Microscopy (NCI CCSG P30CA060553) for technical assistance in image analysis.
Abbreviations:
- IL17RA
IL-17A receptor
- IMQ
imiquimod
- L-SNA
liposome- based DNA spherical nucleic acid
- NHEK
normal human epidermal kerati- nocyte
- PASI
Psoriasis Area and Severity Index
- PBS
phosphate buffered saline
- qRT-PCR
quantitative real-time reverse transcriptaseePCR
- Scr
scrambled
- Scr L-SNA
scrambled liposomal spherical nucleic acid
- SNA
spherical nucleic acid
- TNF
tumor necrosis factor
- XCUR17
human Il17RA L-SNA
Footnotes
Conflict of Interest
BA, WD, DG, RK, SN, AS and RA are employees of Exicure, Inc. AP is a member of the Exicure Scientific Advisory Board and has received Exicure Inc stock options. The other authors state no conflict of interest. This research was supported by a Discovery Grant from National Psoriasis Foundation.
Data availability statement
No datasets were generated or analyzed during the current study.
References
- Arnette C, Koetsier JL, Hoover P, Getsios S, Green KJ. In vitro model of the epidermis: connecting protein function to 3d structure. Methods Enzymol 2016;569:287e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005;174: 3695e702. [DOI] [PubMed] [Google Scholar]
- Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 2011;131:677e87. [DOI] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immuno- pathogenesis of psoriasis. J Invest Dermatol 2009;129:1339e50. [DOI] [PubMed] [Google Scholar]
- Dokter WH, Borger P, Hendriks D, van der Horst I, Halie MR, Vellenga E. Interleukin-4 (IL-4) receptor expression on human T cells is affected by different intracellular signaling pathways and by IL-4 at transcriptional and posttranscriptional level. Blood 1992;80:2721e8. [PubMed] [Google Scholar]
- Flutter B, Nestle FO. TLRs to cytokines: mechanistic insights from the imi- quimod mouse model of psoriasis. Eur J Immunol 2013;43:3138e46. [DOI] [PubMed] [Google Scholar]
- Gerhartz C, Dittrich E, Stoyan T, Rose-John S, Yasukawa K, Heinrich PC, et al. Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur J Biochem 1994;223:265e74. [DOI] [PubMed] [Google Scholar]
- Girolomoni G, Mrowietz U, Paul C. Psoriasis: rationale for targeting inter- leukin-17. Br J Dermatol 2012;167:717e24. [DOI] [PubMed] [Google Scholar]
- Gordon K, Kochkodan JJ, Blatt H, Lin SY, Kaplan N, Johnston A, et al. Alter- ation of the EphA2/Ephrin-A signaling axis in psoriatic epidermis. J Invest Dermatol 2013;133:712e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers 2016;2:16082. [DOI] [PubMed] [Google Scholar]
- Hawkes JE, Gudjonsson JE, Ward NL. The snowballing literature on imiquimod-induced skin inflammation in mice: a critical appraisal. J Invest Dermatol 2017;137:546e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheu- matoid arthritis, and uveitis. Sci Transl Med 2010;2:52ra72. [DOI] [PubMed] [Google Scholar]
- Kravvas G, Gholam K. Use of topical therapies for pediatric psoriasis: A systematic review. Pediatr Dermatol 2018;35:296e302. [DOI] [PubMed] [Google Scholar]
- Lenn JD, Neil J, Donahue C, Demock K, Tibbetts CV, Cote-Sierra J, et al. RNA aptamer delivery through intact human skin. J Invest Dermatol 2018;138: 282e90. [DOI] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012;366:1190e9. [DOI] [PubMed] [Google Scholar]
- Lewandowski KT, Thiede R, Guido N, Daniel WL, Kang R, Guerrero- Zayas MI, et al. Topically delivered tumor necrosis factor-alpha-targeted gene regulation for psoriasis. J Invest Dermatol 2017;137:2027e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Regulating immune response using polyvalent nucleic acid-gold nano- particle conjugates. Mol Pharm 2009;6:1934e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos S, Mohebiany AN, Waisman A, Kurschus FC. Imiquimod-induced psoriasis in mice depends on the IL-17 signaling of keratinocytes. J Invest Dermatol 2019;139:1110e7. [DOI] [PubMed] [Google Scholar]
- Oliver JC, Bland LA, Oettinger CW, Arduino MJ, McAllister SK, Aguero SM, et al. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res 1993;12:115e20. [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012a;366:1181e9. [DOI] [PubMed] [Google Scholar]
- Papp KA, Reid C, Foley P, Sinclair R, Salinger DH, Williams G, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol 2012b;132: 2466e9. [DOI] [PubMed] [Google Scholar]
- Rabeony H, Pohin M, Vasseur P, Petit-Paris I, Jégou J, et al. IMQ-induced skin inflammation in mice is dependent on IL-1R1 and MyD88 signaling but independent of the NLRP3 inflammasome. Eur J Immunol 2015;45: 2847e57. [DOI] [PubMed] [Google Scholar]
- Randeria PS, Seeger MA, Wang XQ, Wilson H, Shipp D, Mirkin CA, et al. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc Natl Acad Sci USA 2015;112:5573e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferos DS, Prigodich AE, Giljohann DA, Patel PC, Mirkin CA. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett 2009;9: 308e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SH, Peredo CE, Takeda Y, Bui T, Neil J, Rickard D, et al. Development of a topical treatment for psoriasis targeting RORg: from bench to skin. PLOS ONE 2016;11:e0147979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009;182:5836e45. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Lee S, Wilson H, Seeger M, Iordanov H, Gatla N, et al. Gangli- oside GM3 depletion reverses impaired wound healing in diabetic mice by activating IGF-1 and insulin receptors. J Invest Dermatol 2014;134: 1446e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 2006;36:1309e23. [DOI] [PubMed] [Google Scholar]
- Zahn G, Greischel A. Pharmacokinetics of tumor necrosis factor alpha after intravenous administration in rats. Dose dependence and influence of tu- mor necrosis factor beta. Arzneimittelforschung 1989;39:1180e2. [PubMed] [Google Scholar]
- Zheng D, Giljohann DA, Chen DL, Massich MD, Wang XQ, Iordanov H, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci USA 2012;109: 11975e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.