Abstract
Right ventricular dysfunction is a hallmark of advanced pulmonary vascular, lung parenchymal, and left heart disease, yet the underlying mechanisms that govern (mal)adaptation remain incompletely characterized. Owing to the knowledge gaps in our understanding of the right ventricle (RV) in health and disease, the National Heart, Lung, and Blood Institute (NHLBI) commissioned a working group to identify current challenges in the field. These included a need to define and standardize normal RV structure and function in populations; access to RV tissue for research purposes and the development of complex experimental platforms that recapitulate the in vivo environment; and the advancement of imaging and invasive methodologies to study the RV within basic, translational, and clinical research programs. Specific recommendations were provided, including a call to incorporate precision medicine and innovations in prognosis, diagnosis, and novel RV therapeutics for patients with pulmonary vascular disease.
In 1929, Werner Forssmann performed the first right heart catheterization on himself and ushered in the era of using hemodynamics to characterize cardiopulmonary function. Early investigators who utilized this technique in patients with chronic heart and lung diseases recognized the critical contribution of the right ventricle (RV) to clinical outcomes. Over the past 20 years, studies have established RV dysfunction as a key determinant of morbidity and mortality in patients with pulmonary vascular disease (PVD), parenchymal lung disease, and left heart disorders. Despite recognition of this pathophysiology, there is limited mechanistic insight into the timing of, and contributors to, the transition from normal RV structure and function to the (mal)adapted RV and RV failure.
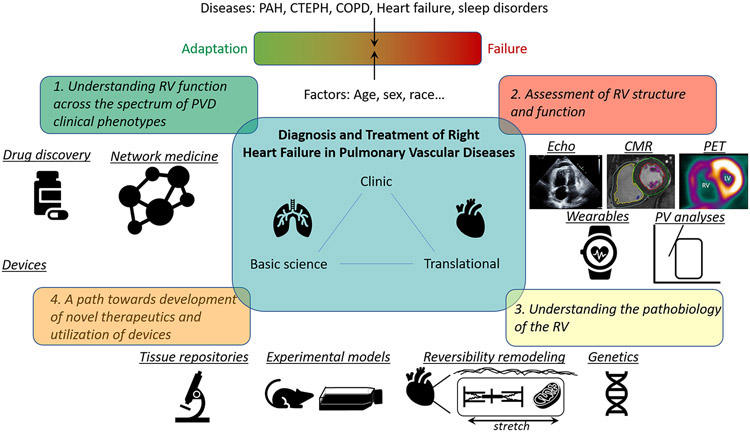
To address limitations in our understanding of the RV in health and disease, the National Heart, Lung, and Blood Institute (NHLBI) Division of Lung Diseases sponsored a workshop to identify knowledge gaps and research priorities to improve the prevention, prediction, diagnosis, and treatment of RV failure, with a focus on patients with PVD. Various stakeholders attended the workshop, including basic and translational scientists; biomedical engineers; clinical epidemiologists and trialists; and clinician-scientists with interests in hemodynamics, imaging, sleep, directed therapeutics, and network medicine (see Appendix for other contributors). The goal was a research agenda that would lead to improvements in the health and outcomes of patients with PVD with, or at high-risk for, RV failure (Figure).
Figure.
Right Ventricular Investigation: From Phenotype to Therapy
SPECIFIC AREAS OF INVESTIGATION AND RECOMMENDATIONS
1. Understanding RV function across the spectrum of PVD clinical phenotypes
Even in adults without clinical cardiovascular disease, RV structure and function vary by age, sex, race/ethnicity, timing of birth, and prenatal, childhood, and adult exposures (1-4). Considering this variability even amongst apparently healthy individuals, differences in RV morphology and function between and within the traditional WHO PH groups are not surprising. These distinctions might be attributable to the effects of the disease states themselves directly on the RV or manifestations of pulmonary vascular endotypes of afterload. The hydraulic load on the RV in a pulsatile system may be influenced by the location of PVD (proximal versus distal or both), heterogeneity of vascular and pulmonary structural disease, temporospatial variability, and other disease-specific features.
For example, patients with pulmonary arterial hypertension (PAH) attributable to systemic sclerosis (SSc) have reduced intrinsic myocardial contractility (both decreased end-systolic elastance and cardiomyocyte contractility) and increased RV fibrosis compared to patients with idiopathic PAH (IPAH), despite having lower pulmonary vascular resistance and higher cardiac index (5, 6). When challenged with submaximal exercise, SSc-PAH patients did not augment RV contractility and ventricular-vascular coupling declined, phenomena that were not seen in IPAH patients (7). In contrast to patients with SSc-PAH, patients with PAH secondary to congenital heart disease commonly have the benefit of RV adaptation to increased load beginning in utero. This results in a different natural history of RV failure owing to sustained exposure to volume/preload (e.g., from tetralogy of Fallot repair with resultant pulmonary valve insufficiency) or pressure/afterload (e.g., Eisenmenger’s syndrome) with outcomes related to the type of repair and ventricular function (8). RV remodeling in chronic thromboembolic PH is affected by variability in acute pulmonary embolism and the site of pulmonary vascular remodeling which extends from the proximal large vessels to the small distal vasculature (9). Following pulmonary thromboendarterectomy (which may be clinically curative), RV systolic function, volume, strain, and asynchrony may improve over time, but the RV does not completely normalize (10).
Notwithstanding the focus on rare diseases, most RV dysfunction occurs in the setting of common conditions which themselves have several phenotypes. For example, chronic obstructive pulmonary disease (COPD) has been linked both to smaller RV size, which may increase with bronchodilation, as well as to RV hypertrophy and dilation with concomitant abnormalities in RV function (11, 12). In patients with severe COPD, the greatest loss of pulmonary vasculature led to larger RV volumes, associated with impaired functional capacity and an increased risk of death (13).
RV dysfunction occurs frequently in patients with heart failure with reduced ejection fraction (HFrEF) and almost one-third of patients with heart failure with preserved ejection fraction (HFpEF) (14, 15). RV dysfunction causes increased venous impedance and renal congestion and impaired diuresis and natriuresis and leads to worse survival in patients with left ventricular disease (15-18). RV dysfunction in combined pre- and post-capillary PH is a strong prognostic marker of adverse outcomes (16, 19).
Finally, sleep disordered breathing (SDB) affects more than 25% of adults in the US (20). Approximately 17 to 53% of obstructive sleep apnea (OSA) patients and 83% of patients with central sleep apnea/Cheyne Stokes respiration and heart failure are estimated to have PH and are at risk of RV failure (21). OSA causes recurrent upper airway obstruction and swings in intrathoracic pressure, thereby cyclically and repetitively increasing cardiac preload and post-capillary pressure. Despite the frequency of these diagnoses, there is a paucity of data on the impact of SDB screening and treatment in PH and effects on RV dysfunction.
Recommendations for future research:
Understand how aging, sex, race, and ethnicity affect RV function across the lifespan. Study how these factors modulate the development of RV dysfunction.
Prenatal and early life events and lifetime exposures should be examined to determine how these affect RV development and maturation as well as determine long-term risk for RV failure.
Investigate the role of systemic comorbidities and modifiable risk factors, such as SDB, in mediating the transition from RV health to dysfunction.
Study differences between diseases (and types of RV afterload) to understand RV responses. Utilize these profiles to construct RV endophenotypes associated with RV adaptation or maladaptation to PVD and transition to failure.
2. Assessment of RV structure and function
While the pressure-volume relationship has been considered the gold standard for assessing ventricular function, these hemodynamic measurements are invasive and currently impractical for routine clinical practice or research in large numbers of patients (22). Derivative methodologies, such as single-beat pressure-volume loops, have limitations but may be useful (23, 24). Although the prevailing conventional thinking is that RV elastance and coupling predict clinical outcomes, data supporting this assumption are sparse (24). Measures of RV contractile reserve provide additional prognostic information in various types of RV dysfunction (7, 25-28).
Speckle tracking echocardiography to evaluate longitudinal and segmental strain in the RV can demonstrate early RV dysfunction and has prognostic implications (29). Magnetic resonance imaging (MRI) is still considered the gold standard for the measurement of RV ejection fraction, volumes, and mass and can also determine extracellular volume as well as RV metabolism (30, 31). 4D MRI can assess flow and biomechanical consequences of RV and pulmonary vascular remodeling (32).
Functional imaging modalities can give insight into metabolic reprogramming in the RV. Noninvasive 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) imaging has demonstrated increased RV glucose uptake in PVD as well as mechanical dyssynchrony (33, 34). Other novel methods, such as magnetic resonance spectroscopy, are being utilized to detect metabolic abnormalities and lipid accumulation in cardiomyocytes (35).
Traditional findings on electrocardiography are not useful for screening for RV hypertrophy in adults without known cardiovascular disease (36); enhanced electrophysiologic mapping or machine learning approaches could identify novel signatures of RV dysfunction.
Continuous monitoring using implantable devices or wearable activity trackers may provide a more comprehensive profile of RV performance over time while patients are engaged in their activities of daily living (37-39). These methods capture variation in physiological markers over rest, stress, circadian rhythms, and sleep-wake cycles, which may be more informative than simple resting or one-time exercise assessments. Technical advances and standards in image quality and data fidelity, transmission, storage, and security are required to incorporate these devices into large multicenter human studies. The Table summarizes some of the methods of assessment and measurements which provide insight into RV structure and function.
Table.
Methods and measures of right ventricular shape and function
| Method | Measures |
|---|---|
| Hemodynamic | Right atrial pressure, RV systolic and diastolic pressures, cardiac output, stroke volume, RV power, pulmonary vascular resistance |
| Pressure-volume loops | Elastance, coupling |
| Echocardiographic (2-dimensional and 3-dimensional) | Right atrial volume, right atrial emptying fraction, RV basal, mid-cavity, and outflow tract dimensions, end-diastolic and systolic ventricular areas, RV/left ventricular ratio, RV wall thickness, RV end-diastolic and end-systolic volumes, RV ejection fraction, RV fractional area change, RV myocardial performance index, RV free wall strain, RV global longitudinal strain, tricuspid annular plane systolic excursion, transtricuspid E/A, early component (e’)/atrial component (a’) of tricuspid annular tissue Doppler velocity, e’, E/e’, tricuspid annular lateral systolic velocity (s’) |
| Magnetic resonance imaging | Right atrial and ventricular end-diastolic and -systolic volumes, RV ejection fraction, main pulmonary artery forward and reverse flows, RV cardiac output, RV stroke volume, main pulmonary artery peak velocity, RV strain, RV extracellular volume fraction, late gadolinium enhancement |
| Computed tomographic imaging | Right atrial and ventricular end-diastolic and -systolic volumes, RV ejection fraction, RV stroke volume, |
| Positron emission tomography | Metabolism, fatty acid uptake |
| Wearable/mobile health/implantable devices | Heart rate, heart rate variability, electrocardiogram, accelerometry, step count, minutes of physical activity, pulmonary artery pressure, stroke volume |
| Exercise testing | Six minute walk distance, oxygen consumption at peak exercise, pulmonary arterial and systemic blood pressure response to exercise |
| Biomarkers | Brain natriuretic peptide, N-terminal probrain natriuretic peptide, galectin-3, ST2, troponin |
Recommendations for future research:
Define normative values of RV structure and function for imaging modalities.
Studies of RV reserve and coupling should be pursued to determine the importance of these parameters in functional outcomes and survival in PVD.
Develop and advance hemodynamic challenges and exercise to assess RV response under stress.
Identify biomarkers for RV structure and function.
Determine the optimal analytics for implantable monitors and wearable devices that provide detail about RV phenotypes.
3. Understanding the pathobiology of the RV
The biomechanical milieu of the RV
Although classical pathologic examination of the RV has demonstrated gross structural changes at end-stage PVD, reliance on this approach alone to phenotype the RV has not been highly informative to date. Striking differences in cardiomyocyte contractile force have been demonstrated in patients with IPAH as compared to those with SSc-PAH. Using isolated RV cardiomyocytes, maximal Ca2+-activated force was 28% higher in patients with IPAH compared to controls but 37% lower in patients with SSc-PAH. Passive stiffness, however, was increased in both PAH populations (5-7). This difference has been attributed, in part, to reduced titin phosphorylation and increased collagen-mediated stiffness (40). Multiscale (cardiomyocyte, myofibril, and RV) computational modeling has provided additional insight into the contributions of RV cardiomyocyte dysfunction to overall hemodynamics. Simulations revealed that pressure overload and cardiomyocyte dysfunction predicted a decrease in RV ejection fraction and cardiac output (41).
Metabolic reprogramming of the RV
The RV typically reverts to the “fetal phenotype” under stress. This phenotypic switch is characterized by inhibition of pyruvate dehydrogenase and shunting to aerobic glycolysis, which generates less ATP and leads to RV hibernation. Pyruvate dehydrogenase can be targeted therapeutically either directly (pyruvate dehydrogenase kinase inhibitors) or indirectly (glutaminolysis) to improve RV contractility (42). Mitochondria themselves remodel in RV cardiomyocytes with changes in their transcriptome that affect metabolism (43, 44). Mitochondrial fission increases reactive oxygen species generation impairing RV diastolic function (45).
Fatty acid oxidation is also downregulated in RV cardiomyocytes in PVD (46). RV lipotoxicity has been demonstrated in PAH patients using proton magnetic resonance spectroscopy, which revealed an increase in RV triglyceride content compared to controls (35). The RV may be affected by obesity and the Western diet, suggesting a susceptibility to micro- and macro-nutrients (47, 48). Metabolic or nutritional interventions for the RV in PVD are just starting to be tested (49).
Sex, sex steroid hormones, and RV function
Women have better RV function compared to men both in health and disease (2, 50, 51). Plausible mechanisms for these observations include direct effects of sex steroid hormones and their metabolites on RV adaptation as well as interactions between sex and the sex steroid hormone milieu with treatment (52-54). Experimental models and human studies have shown mixed results for the role of female sex and estrogen (resulting in the term “estrogen paradox”) (55).
Evidence suggests that sex hormones affect the RV in patients with PAH. The cytochrome superfamily, which includes CYP1B1 and aromatase (CYP19A1), is preferentially expressed in the RV as compared to the left ventricle (56). Higher estradiol and lower dehydroepiandrosterone-sulfate levels in PAH are associated with worse heart function (57, 58). Response to PAH treatment also varies by sex (52, 53). One study showed that RV ejection fraction improved over time with PAH treatment in women but not in men, explaining the better survival for women (54). In a pilot study, the aromatase inhibitor anastrozole lowered estradiol levels and improved six-minute walk distance, but had no effect on RV function (59). A larger, longer Phase II trial of anastrozole in post-menopausal women and men with PAH is underway (NCT03229499), as are trials of tamoxifen (NCT03528902) and dehydroepiandrosterone supplementation (NCT 03648385).
Experimental models in preclinical studies
Animal models of precapillary PH are well-established. Models which aim to recapitulate WHO Group 2 disease incorporate aortic banding or limitation of coronary flow or infarction. Recently, the normoxic ZSF1 rat model treated with a single high dose of Sugen was shown to develop pulmonary vascular remodeling, characteristics of HFpEF, and increased RV mass (60). The mouse myocardial infarction model of HFrEF also has RV contractile dysfunction, increased RV stiffness, and evidence of RV-pulmonary artery uncoupling (61). Models with systemic co-morbidities, including mild renal insufficiency, hypertension, insulin resistance and obesity, mimic human disease. Sub-type specific induced pluripotent stem cell-derived cardiomyocytes have been generated and characterized, but, to date, this has not been done for the RV in PH (62).
Recommendations for future research:
Define the normal molecular (genomic, metabolomic, and proteomic) and cellular RV profile as well as the effect of perturbations of these factors.
Develop tissue repositories of RV myocardial samples. Further develop RV induced pluripotent stem cells as a relevant model for mechanistic studies and preclinical testing of therapies. Utilize advanced cell culture methodologies, such as 3D matrix and organ-on-a-chip.
Determine if decreased contractility, increased diastolic stiffness, and fibrosis are reversible and correctable at a cellular level.
Determine if targeting metabolic and mitochondrial dysfunction improves RV function.
Understand the effects of specific sex hormones, age, and sex-based lifecycle changes (menarche and menopause) on the RV response to stress.
Develop animal models of RV dysfunction that incorporate the full range of ages, sexes, human health, resilience, and PVD. Novel ways to interrogate such models of RV dysfunction and isolate therapeutic effects on PVD from those on RV dysfunction are needed.
4. A path towards development of novel therapeutics and utilization of devices
Challenges associated with RV drug discovery and implementation
Currently approved pharmacotherapeutics are not known to target the RV directly. The pursuit of therapeutics which have shown efficacy in left ventricular disease has not yet borne fruit in treating RV dysfunction (49, 63-66). The development of an effective therapy for RV remodeling and dysfunction will face distinct challenges, including: 1) inability to use short-term exercise capacity as a primary endpoint in trials of beta-blockers and possibly other drug classes (67); 2) lack of endpoints focused on remodeling in left-sided heart disease (including improved ventricular function) that are accepted by the Food and Drug Administration and other regulatory agencies; 3) potential proarrhythmic properties of therapies that either a) initially depress ventricular function prior to improving it, or b) are inotropes; and 4) the requirement that investigators have equipoise.
Many decisions need to be made before designing a development program of RV therapy, including whether to target patients with advanced heart failure or with no or subclinical disease (primary or secondary prevention), the optimal duration of Phase II and III trials, and the role of intermediate or ultimate end points in the absence of validated surrogates.
The success of drug and device development for left heart failure has been predicated on targeting adverse remodeling (i.e., contractile dysfunction), which has translated into improvement in hard clinical endpoints, like survival, in a much more common syndrome than RV failure. This traditional pathway may hold promise for treatment of RV failure.
Novel therapeutics require equally innovative approaches to study and regulatory approval. Gene transfer for PH and RV dysfunction has been trialed in preclinical models showing safety and efficacy, but questions remain with respect to the method of gene transfer, target selection, target cell type, and delivery technique as this therapeutic is translated to the clinic (68-70). The optimism for cell-based therapy as a therapeutic for the RV has been tempered by the track record to date in other cardiovascular diseases despite beneficial results in preclinical studies (71).
Network medicine to identify RV clinical endpoints, treatment targets, and therapeutics
Network medicine could identify treatment targets from RV disease modules from the (incomplete) human interactome or by using systems pharmacology for drug repurposing (72, 73). Unbiased strategies for drug discovery involve screening millions of small-molecule compounds against all of the human proteins with known tertiary structures and identifying relevant RV targets from a network analysis (73). Networks of clinical variables may also have utility for RV endophenotyping. A module of ten variables from a network of all variables measured during invasive cardiopulmonary exercise testing identified distinct exercise phenotypes in PVD and predicted outcomes (74). This unbiased approach identified clinical variables relevant to RV pathophysiology that could be used to identify specific intermediate phenotypes and mechanism(s) of RV dysfunction.
Advancing mechanical support and interventions for RV dysfunction
The use of mechanical devices and surgical or percutaneous interventions for RV support and rescue in PVD is not well studied. RV assist devices, intra-pulmonary artery balloon pumps, RV volume adjustments, and extracorporeal life support are all under evaluation for the management of RV failure. The benefits of mechanical strategies to support the failing RV are suggested by atrial septostomy, where decreasing right atrial pressure and RV filling improves cardiac output, functional capacity, and potentially survival, all without targeting PVD directly (75). The role of surgical approaches to correct functional tricuspid regurgitation is undefined. Percutaneous RV assist devices, which pump blood from the right atrium to the pulmonary artery to unload the RV, have shown promise in acute RV failure (76). Isolated implantable RV assist devices for longer duration support of chronic RV failure in PVD are not currently available; however, in some instances, left ventricular assist devices can support the RV in the setting of biventricular failure (77, 78). There is an unmet need for durable mechanical support of the failing RV in patients with PH who are not candidates for (or are awaiting) lung- or heart-lung transplantation. The potential of RV assist devices as destination therapy or bridge-to-recovery requires further study.
Recommendations for future research:
New therapeutics that selectively target the RV should continue to be developed and tested.
New analytical methodologies, such as systems biology and network medicine, should be used to endophenotype RV dysfunction in PVD and identify new RV treatment targets, new drugs, or drugs that can be repurposed for patients with RV dysfunction.
Design trials to assess existing techniques while fostering innovation in mechanical support for RV dysfunction.
Conclusion
RV dysfunction contributes to adverse clinical outcomes in patients with PVD. Despite the recognition of the clinical importance of RV dysfunction, the parameters that define normal RV structural and functional adaptation under (patho)physiological conditions have not been defined. To improve health in patients with (or destined for) RV failure, the workshop has identified basic, translational, and clinical areas for study. Advances in these areas will provide novel mechanistic understanding into RV dysfunction as well as improve, preventative, diagnostic, therapeutic, and prognostic RV-specific modalities.
Appendix. Other Workshop Participants
Hua Linda Cai, MD
Lu Cai, MD, PhD
Xiongwen Chen, PhD
Jason M. Elinoff, MD
Benjamin H. Freed, MD
Andrea L. Frump, PhD
Kara N. Goss, MD
Michael P. Gray, MPH
Wei Huang, MD, PhD
Todd M. Kolb, MD, PhD.
Marc A. Simon, MD, MS
Michael A. Solomon, MD
Edda Spiekerkoetter, MD
Rebecca R. Vanderpool, MD
Ming-Hui Zou, MD, PhD
Yingjie Chen, MD, PhD
NHLBI DLD & DCVS Program Staff:
Adhikari Bishow, PhD (Bethesda, MD)
Scarlet Shi, PhD (Bethesda, MD)
Gail G. Weinmann, MD (Bethesda, MD)
Renee Wong, PhD (Bethesda, MD)
Footnotes
Conflict of Interest Statement
Dr. Aggarwal has nothing to disclose.
Dr. Aldred reports grants from NHLBI during the conduct of the study.
Dr. Archer has nothing to disclose.
Dr. Benza reports grants from Abbott during the conduct of the study; grants from Actelion, grants from United Therapeutics, grants from Bayer, grants from NIH/NHLBI outside the submitted work.
Dr. Bristow has nothing to disclose.
Dr. Brittain has nothing to disclose.
Dr. Chesler reports personal fees from Endotronix, Inc. and personal fees from Aria CV outside the submitted work.
Dr. de Man has nothing to disclose.
Dr. Erzurum has nothing to disclose.
Dr. Gladwin is a co-inventor of patents and patent applications directed to the use of recombinant neuroglobin and heme-based molecules as antidotes for CO poisoning, which have been licensed by Globin Solutions, Inc. Dr. Gladwin is a shareholder, advisor, and director in Globin Solutions, Inc. Dr. Gladwin is also co-inventor on patents directed to the use of nitrite salts in cardiovascular diseases, which were previously licensed to United Therapeutics, and is now licensed to Globin Solutions and Hope Pharmaceuticals. Dr. Gladwin is a principal investigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguat as a treatment for patients with SCD. Dr. Gladwin has served as a consultant for Epizyme, Inc., Actelion Clinical Research, Inc., Acceleron Pharma, Inc., Catalyst Biosciences, Inc., Modus Therapeutics, Sujana Biotech, LLC, Complexa Inc., Pfizer Inc., and United Therapeutics Corporation. Dr. Gladwin is also on Bayer HealthCare LLC’s Heart and Vascular Disease Research Advisory Board.
Dr. Hemnes reports personal fees from Actelion, personal fees from Bayer, personal fees from Complexa, personal fees from United Therapeutics, other from PHPrecisionMed, outside the submitted work.
Dr. Hassoun has served on an advisory board for Merck in 2019.
Dr. Kawut reports grants from NIH, non-financial support from the ATS, and grants from Actelion, United Therapeutics, Gilead, Lung Biotech, Bayer, and Mallinkrodt to the Perelman School of Medicine for CME courses. Dr. Kawut reports grants and non-financial support from Cardiovascular Medical Research and Education Fund and non-financial support from Pulmonary Hypertension Association. Dr. Kawut has served in an advisory capacity (for grant review and other purposes) for United Therapeutics, Glaxo SmithKline, and Complexa, Inc. without financial support or in-kind benefits.
Dr. Lahm reports personal fees from Bayer, personal fees from Gilead, personal fees from Actelion, other from Eli Lilly outside the submitted work.
Dr. Leopold has nothing to disclose.
Dr. Lima has nothing to disclose.
Dr. Loscalzo is a scientific co-founder of Scipher, a startup company that uses network concepts to explore human disease treatment strategies.
Dr. Maron reports other from Actelion Pharmaceuticals Inc., outside the submitted work. In addition, Dr. Maron has a patent U.S. Patent #9,605,047 issued, a patent U.S. Provisional Application ID: 62475955 pending, a patent U.S. Provisional Application Cover Sheet ID: 24624 pending, and a patent U.S. Patent application PCT/US2019/059890 pending.
Dr. Mercer-Rosa has nothing to disclose.
Dr. Newman has nothing to disclose.
Dr. Redline reports grants and personal fees from Jazz Pharmaceuticals, personal fees from RespirCardia Inc. outside the submitted work.
Dr. Rich has nothing to disclose.
Dr. Rischard has nothing to disclose.
Dr. Sugeng has nothing to disclose.
Dr. Tang reports grants from National Institutes of Health, personal fees from Sequana Medical Inc, personal fees from Springer, personal fees from MyoKardia Inc outside the submitted work.
Dr Tedford reports other from Actelion, other from Merck, personal fees from United Therapeutics, personal fees from Aria CV, personal fees from Arena pharmaceuticals, personal fees from Gradient, personal fees from Eidos Therapeutics, personal fees and other from Abbott, personal fees and other from Medtronic, personal fees from Itamar, other from Abiomed, and personal fees and other from Acceleron outside the submitted work.
Dr. Tsai reports grants from National Heart, Lung, and Blood Institute (NHLBI), grants from American College of Cardiology, grants from The Rachel and Drew Katz Foundation outside the submitted work. In addition, Dr. Tsai has a patent Pharmacologic Treatment for Right Ventricular Failure (USSN 62/836,315) issued to The Trustees of Columbia University in the City of New York.
Dr. Ventetuolo reports grants from NHLBI during the conduct of the study; grants from United Therapeutics, grants from American Thoracic Society, personal fees from Acceleron Pharma, personal fees from Bayer outside the submitted work.
Dr. Lei Xiao has no conflict of interest to disclose.
Dr. Zhao has nothing to disclose.
References
- 1.Mulchrone A, Bellofiore A, Douwes JM, Duong N, Beshish AG, Barton GP, Francois CJ, Eldridge MW, Goss KN and Chesler NC. Impaired right ventricular-vascular coupling in young adults born preterm. Am J Respir Crit Care Med. 2020;201:615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewandowski AJ, Lamata P, Francis JM, Piechnik SK, Ferreira VM, Boardman H, Neubauer S, Singhal A, Leeson P and Lucas A. Breast milk consumption in preterm neonates and cardiac shape in adulthood. Pediatrics. 2016;138:e20160050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leary PJ, Kaufman JD, Barr RG, Bluemke DA, Curl CL, Hough CL, Lima JA, Szpiro AA, Van Hee VC and Kawut SM. Traffic-related air pollution and the right ventricle. The Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2014;189:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, Boyce D, Kelemen BW, Bacher AC, Shah AA, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu S, Kokkonen-Simon KM, Kirk JA, Kolb TM, Damico RL, Mathai SC, Mukherjee M, Shah AA, Wigley FM, Margulies KB, et al. Right Ventricular Myofilament Functional Differences in Humans With Systemic Sclerosis-Associated Versus Idiopathic Pulmonary Arterial Hypertension. Circulation. 2018;137:2360–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu S, Houston BA, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, Damico RL, Kolb TM, Hummers LK, Shah AA, et al. Right Ventricular Functional Reserve in Pulmonary Arterial Hypertension. Circulation. 2016;133:2413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budts W, Roos-Hesselink J, Radle-Hurst T, Eicken A, McDonagh TA, Lambrinou E, Crespo-Leiro MG, Walker F and Frogoudaki AA. Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2016;37:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delcroix M, Vonk Noordegraaf A, Fadel E, Lang I, Simonneau G and Naeije R. Vascular and right ventricular remodelling in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2013;41:224–32. [DOI] [PubMed] [Google Scholar]
- 10.Bonderman D, Martischnig AM, Vonbank K, Nikfardjam M, Meyer B, Heinz G, Klepetko W, Naeije R and Lang IM. Right ventricular load at exercise is a cause of persistent exercise limitation in patients with normal resting pulmonary vascular resistance after pulmonary endarterectomy. Chest. 2011;139:122–7. [DOI] [PubMed] [Google Scholar]
- 11.Hilde JM, Skjorten I, Grotta OJ, Hansteen V, Melsom MN, Hisdal J, Humerfelt S and Steine K. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol. 2013;62:1103–1111. [DOI] [PubMed] [Google Scholar]
- 12.Kawut SM, Poor HD, Parikh MA, Hueper K, Smith BM, Bluemke DA, Lima JA, Prince MR, Hoffman EA, Austin JH, et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: the MESA COPD study. J Am Coll Cardiol. 2014;64:2000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washko GR, Nardelli P, Ash SY, Vegas Sanchez-Ferrero G, Rahaghi FN, Come CE, Dransfield MT, Kalhan R, Han MK, Bhatt SP, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease. A longitudinal observational study. Am J Respir Crit Care Med. 2019;200:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenkranz S, Kramer T, Gerhardt F, Opitz C, Olsson KM and Hoeper MM. Pulmonary hypertension in HFpEF and HFrEF: Pathophysiology, diagnosis, treatment approaches. Herz. 2019;44:483–490. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL and Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderpool RR, Saul M, Nouraie M, Gladwin MT and Simon MA. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2018;3:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melenovsky V, Hwang SJ, Lin G, Redfield MM and Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nijst P, Martens P, Dupont M, Tang WHW and Mullens W. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail. 2017;5:672–681. [DOI] [PubMed] [Google Scholar]
- 19.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, Farber-Eger EH, Sheng Q, Shyr Y, Harrell FE, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcantara C, Jackson CL, Williams MA and Redline S. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis. Sleep. 2015;38:877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail K, Roberts K, Manning P, Manley C and Hill NS. OSA and pulmonary hypertension: time for a new look. Chest. 2015;147:847–861. [DOI] [PubMed] [Google Scholar]
- 22.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198:e15–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philip JL and Chesler NC. Know your limitations: assumptions in the single-beat method for estimating right ventricular-pulmonary vascular coupling. Am J Respir Crit Care Med. 2018;198:707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter MJ, Peters D, Ghofrani HA, Naeije R, Roller F, Sommer N, Gall H, Grimminger F, Seeger W and Tello K. Evaluation and Prognostic Relevance of Right Ventricular-Arterial Coupling in Pulmonary Hypertension. Am J Respir Crit Care Med. 2020;201:116–119. [DOI] [PubMed] [Google Scholar]
- 25.Guihaire J, Haddad F, Noly PE, Boulate D, Decante B, Dartevelle P, Humbert M, Verhoye JP, Mercier O and Fadel E. Right ventricular reserve in a piglet model of chronic pulmonary hypertension. Eur Respir J. 2015;45:709–17. [DOI] [PubMed] [Google Scholar]
- 26.Guazzi M, Villani S, Generati G, Ferraro OE, Pellegrino M, Alfonzetti E, Labate V, Gaeta M, Sugimoto T and Bandera F. Right ventricular contractile reserve and pulmonary circulation uncoupling during exercise challenge in heart failure: pathophysiology and clinical phenotypes. JACC Heart Fail. 2016;4:625–35. [DOI] [PubMed] [Google Scholar]
- 27.Sharma T, Lau EM, Choudhary P, Torzillo PJ, Munoz PA, Simmons LR, Naeije R and Celermajer DS. Dobutamine stress for evaluation of right ventricular reserve in pulmonary arterial hypertension. Eur Respir J. 2015;45:700–8. [DOI] [PubMed] [Google Scholar]
- 28.DeFaria Yeh D, Stefanescu Schmidt AC, Eisman AS, Serfas JD, Naqvi M, Youniss MA, Ryfa AD, Khan AA, Safi L, Tabtabai SR, et al. Impaired right ventricular reserve predicts adverse cardiac outcomes in adults with congenital right heart disease. Heart. 2018;104:2044–2050. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee M, Mercurio V, Tedford RJ, Shah AA, Hsu S, Mullin CJ, Sato T, Damico R, Kolb TM, Mathai SC, et al. Right ventricular longitudinal strain is diminished in systemic sclerosis compared with idiopathic pulmonary arterial hypertension. Eur Respir J. 2017;50:1701436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta BB, Auger DA, Gonzalez JA, Workman V, Chen X, Chow K, Stump CJ, Mazimba S, Kennedy JL, Gay E, et al. Detection of elevated right ventricular extracellular volume in pulmonary hypertension using Accelerated and Navigator-Gated Look-Locker Imaging for Cardiac T1 Estimation (ANGIE) cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sree Raman K, Stokes M, Walls A, Perry R, Steele PM, Burdeniuk C, De Pasquale CG, Celermajer DS and Selvanayagam JB. Feasibility of oxygen sensitive cardiac magnetic resonance of the right ventricle in pulmonary artery hypertension. Cardiovasc Diagn Ther. 2019;9:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng AL, Wee CP, Pahlevan NM and Wood JC. A 4D flow MRI evaluation of the impact of shear-dependent fluid viscosity on in vitro Fontan circulation flow. Am J Physiol Heart Circ Physiol. 2019;317:H1243–H1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saygin D, Highland KB, Farha S, Park M, Sharp J, Roach EC, Tang WHW, Thomas JD, Erzurum SC, Neumann DR, et al. Metabolic and functional evaluation of the heart and lungs in pulmonary hypertension by gated 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Pulmonary circulation. 2017;7:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Zhou W, Liang Y, Yang Y, Garcia EV, Chen J and Fang W. Right ventricular dyssynchrony in pulmonary hypertension: Phase analysis using FDG-PET imaging. J Nucl Cardiol. 2017;24:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brittain EL, Talati M, Fessel JP, Zhu H, Penner N, Calcutt MW, West JD, Funke M, Lewis GD, Gerszten RE, et al. Fatty Acid Metabolic Defects and Right Ventricular Lipotoxicity in Human Pulmonary Arterial Hypertension. Circulation. 2016;133:1936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitman IR, Patel VV, Soliman EZ, Bluemke DA, Praestgaard A, Jain A, Herrington D, Lima JAC and Kawut SM. Validity of the surface electrocardiogram criteria for right ventricular hypertrophy: the MESA-RV Study (Multi-Ethnic Study of Atherosclerosis-Right Ventricle). J Am Coll Cardiol. 2014;63:672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biederman RWW, Doyle M, Correa-Jaque P, Rayarao G and Benza RL. Integrated use of cardiac MRI and the CardioMEMS HF system in PAH: the utility of coincident pressure and volume in RV failure-the NHLBI-VITA trial. Cardiovasc Diagn Ther. 2019;9:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxer S, Lichtblau M, Berlier C, Hasler ED, Schwarz EI and Ulrich S. Physical activity in incident patients with pulmonary arterial and chronic thromboembolic hypertension. Lung. 2019;197:617–625. [DOI] [PubMed] [Google Scholar]
- 39.Lachant DJ, Light AN, Mackin ML, Schwartz RG and White RJ. Heart rate expenditure correlates with right ventricular function. Ann Am Thorac Soc. 2020;17:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rain S, Andersen S, Najafi A, Gammelgaard Schultz J, da Silva Goncalves Bos D, Handoko ML, Bogaard HJ, Vonk-Noordegraaf A, Andersen A, van der Velden J, et al. Right ventricular myocardial stiffness in experimental pulmonary arterial hypertension: Relative contribution of fibrosis and myofibril stiffness. Circ Heart Fail. 2016;9: e002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philip JL, Pewowaruk RJ, Chen CS, Tabima DM, Beard DA, Baker AJ and Chesler NC. Impaired myofilament contraction drives right ventricular failure secondary to pressure overload: model simulations, experimental validation, and treatment predictions. Front Physiol. 2018;9:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan JJ and Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circulation research. 2014;115:176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potus F, Hindmarch CCT, Dunham-Snary KJ, Stafford J and Archer SL. Transcriptomic signature of right ventricular failure in experimental pulmonary arterial hypertension: deep sequencing demonstrates mitochondrial, fibrotic, inflammatory and angiogenic abnormalities. International journal of molecular sciences. 2018;19:2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piao L, Fang YH, Parikh K, Ryan JJ, Toth PT and Archer SL. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl). 2013;91:1185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian L, Neuber-Hess M, Mewburn J, Dasgupta A, Dunham-Snary K, Wu D, Chen KH, Hong Z, Sharp WW, Kutty S, et al. Ischemia-induced Drp1 and Fis1-mediated mitochondrial fission and right ventricular dysfunction in pulmonary hypertension. J Mol Med (Berl). 2017;95:381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L, Kraskauskas D, Wijesinghe DS, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail. 2013;6:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brittain EL, Talati M, Fortune N, Agrawal V, Meoli DF, West J and Hemnes AR. Adverse physiologic effects of Western diet on right ventricular structure and function: role of lipid accumulation and metabolic therapy. Pulmonary circulation. 2019;9:2045894018817741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V and Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure With preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brittain EL, Niswender K, Agrawal V, Chen X, Fan R, Pugh ME, Rice TW, Robbins IM, Song H, Thompson C, et al. Mechanistic phase II clinical trial of metformin in pulmonary arterial hypertension. J Am Heart Assoc. 2020;9:e018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swift AJ, Capener D, Hammerton C, Thomas SM, Elliot C, Condliffe R, Wild JM and Kiely DG. Right ventricular sex differences in patients with idiopathic pulmonary arterial hypertension characterised by magnetic resonance imaging: pair-matched case controlled study. PLoS One. 2015;10:e0127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foppa M, Arora G, Gona P, Ashrafi A, Salton CJ, Yeon SB, Blease SJ, Levy D, O'Donnell CJ, Manning WJ, et al. Right ventricular volumes and systolic function by cardiac magnetic resonance and the impact of sex, age, and obesity in a longitudinally followed cohort free of pulmonary and cardiovascular disease: The Framingham Heart Study. Circulation Cardiovascular imaging. 2016;9:e003810. [DOI] [PubMed] [Google Scholar]
- 52.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, Kawut SM and Halpern SD. Race and Sex Differences in Response to Endothelin Receptor Antagonists for Pulmonary Arterial Hypertension. Chest. 2012;141:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathai SC, Hassoun PM, Puhan MA, Zhou Y and Wise RA. Sex differences in response to tadalafil in pulmonary arterial hypertension. Chest. 2015;147:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard HJ, Boonstra A and Vonk Noordegraaf A. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145:1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, et al. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol. 2015;308:L873–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thum T and Borlak J. Gene expression in distinct regions of the heart. Lancet (London, England). 2000;355:979–83. [DOI] [PubMed] [Google Scholar]
- 57.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193:1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baird GL, Archer-Chicko C, Barr RG, Bluemke DA, Foderaro AE, Fritz JS, Hill NS, Kawut SM, Klinger JR, Lima JAC, et al. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur Respir J. 2018;51:1800467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, Palevsky HI, Palmisciano AJ, Patel M, Pinder D, et al. Anastrozole in pulmonary arterial hypertension. A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2017;195:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia-Ocana A, Goncharova EA, Tofovic SP, et al. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133:717–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Philip JL, Murphy TM, Schreier DA, Stevens S, Tabima DM, Albrecht M, Frump AL, Hacker TA, Lahm T and Chesler NC. Pulmonary vascular mechanical consequences of ischemic heart failure and implications for right ventricular function. Am J Physiol Heart Circ Physiol. 2019;316:H1167–H1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, Henze S, Stauske M, Salinas G, Zimmermann WH, et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3:e99941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grinnan D, Bogaard HJ, Grizzard J, Van Tassell B, Abbate A, DeWilde C, Priday A and Voelkel NF. Treatment of group I pulmonary arterial hypertension with carvedilol is safe. Am J Respir Crit Care Med. 2014;189:1562–4. [DOI] [PubMed] [Google Scholar]
- 64.Han Y, Forfia P, Vaidya A, Mazurek JA, Park MH, Ramani G, Chan SY and Waxman AB. Ranolazine improves right ventricular function in patients with precapillary pulmonary hypertension: results from a double-blind, randomized, placebo-controlled trial. J Card Fail. 2021;27:253–257. [DOI] [PubMed] [Google Scholar]
- 65.Rijnierse MT, Groeneveldt JA, van Campen J, de Boer K, van der Bruggen CEE, Harms HJ, Raijmakers PG, Lammertsma AA, Knaapen P, Bogaard HJ, et al. Bisoprolol therapy does not reduce right ventricular sympathetic activity in pulmonary arterial hypertension patients. Pulmonary circulation. 2020;10:2045894019873548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safdar Z, Frost A, Basant A, Deswal A, O'Brian Smith E and Entman M. Spironolactone in pulmonary arterial hypertension: results of a cross-over study. Pulmonary circulation. 2020;10:2045894019898030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farha S, Saygin D, Park MM, Cheong HI, Asosingh K, Comhair SA, Stephens OR, Roach EC, Sharp J, Highland KB, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight. 2017;2:e95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguero J, Ishikawa K, Hadri L, Santos-Gallego CG, Fish KM, Kohlbrenner E, Hammoudi N, Kho C, Lee A, Ibanez B, et al. Intratracheal gene delivery of SERCA2a ameliorates chronic post-capillary pulmonary hypertension: A large animal model. J Am Coll Cardiol. 2016;67:2032–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hadri L, Kratlian RG, Benard L, Maron BA, Dorfmuller P, Ladage D, Guignabert C, Ishikawa K, Aguero J, Ibanez B, et al. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation. 2013;128:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe S, Ishikawa K, Plataki M, Bikou O, Kohlbrenner E, Aguero J, Hadri L, Zarragoikoetxea I, Fish K, Leopold JA, et al. Safety and long-term efficacy of AAV1.SERCA2a using nebulizer delivery in a pig model of pulmonary hypertension. Pulmonary circulation. 2018;8:2045894018799738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asosingh K, Farha S, Lichtin A, Graham B, George D, Aldred M, Hazen SL, Loyd J, Tuder R and Erzurum SC. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension. Blood. 2012;120:1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menche J, Sharma A, Kitsak M, Ghiassian SD, Vidal M, Loscalzo J and Barabasi AL. Disease networks. Uncovering disease-disease relationships through the incomplete interactome. Science. 2015;347:1257601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng F, Lu W, Liu C, Fang J, Hou Y, Handy DE, Wang R, Zhao Y, Yang Y, Huang J, et al. A genome-wide positioning systems network algorithm for in silico drug repurposing. Nat Commun. 2019;10:3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oldham WM, Oliveira RKF, Wang RS, Opotowsky AR, Rubins DM, Hainer J, Wertheim BM, Alba GA, Choudhary G, Tornyos A, et al. Network analysis to risk stratify patients with exercise intolerance. Circulation research. 2018;122:864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan MS, Memon MM, Amin E, Yamani N, Khan SU, Figueredo VM, Deo S, Rich JD, Benza RL and Krasuski RA. Use of balloon atrial septostomy in patients with advanced pulmonary arterial hypertension: A systematic review and meta-analysis. Chest. 2019;156:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machuca TN and de Perrot M. Mechanical support for the failing right ventricle in patients with precapillary pulmonary hypertension. Circulation. 2015;132:526–36. [DOI] [PubMed] [Google Scholar]
- 77.Kapur NK, Paruchuri V, Jagannathan A, Steinberg D, Chakrabarti AK, Pinto D, Aghili N, Najjar S, Finley J, Orr NM, et al. Mechanical circulatory support for right ventricular failure. JACC Heart Fail. 2013;1:127–34. [DOI] [PubMed] [Google Scholar]
- 78.Anderson MB, Goldstein J, Milano C, Morris LD, Kormos RL, Bhama J, Kapur NK, Bansal A, Garcia J, Baker JN, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015;34:1549–60. [DOI] [PubMed] [Google Scholar]