Abstract
Preclinical studies of nicotine self-administration provide important value for the field as they are highly rigorous, controlled, can be conducted quickly, and are generalizable to humans. Given the translational value of the nicotine self-administration model, and the relatively new guildelines of the National Institutes of Health to include sex as a biological variable, strain and sex differences in nicotine acquisition were examined here in two outbred rat strains. Sprague-Dawley (SD) and Long Evans (LE; wild-type and ChAT::cre transgenic) rats of each sex were implanted with indwelling intravenous jugular catheters. Rats were trained to self-administer nicotine (0.02 mg/kg/infusion, paired with contingent light+tone stimuli). Acquisition criteria were set at a minimum active/inactive response ratio of 2:1 and a minimum of 10 infusions per session, both of which had to be met for a minimum of 10 sessions. Across 10 sessions, male SD rats self-administered significantly more nicotine than female SD rats (p<0.05), indicating a sex difference in this strain. LE females self-administered more nicotine than SD females indicative of a strain difference between females (p<0.05). SD males increased nicotine infusions across sessions compared to LE males and SD females (p<0.05). No strain or sex differences were observed in the number of sessions to reach criteria. No differences between wild-type and transgenic LE rats were observed. These results demonstrate sex and strain differences in nicotine self-administration between SD and LE rats and may lend insight into development of other nicotine self-administration models, where sex and strain may impact acquisition.
Keywords: Nicotine, Self-administration, Sprague-Dawley, Long Evans, Strain differences, Sex Differences, Transgenic rats
1. Introduction
Nicotine, the primary addictive substance in tobacco products, plays a large role in maintenance of smoking behavior and dependence (Stolerman & Jarvis, 1995). While a recent decrease in use of combustible cigarettes has occurred, it has been contrasted by a substantial increase in use of electronic nicotine delivery systems (ENDS), especially among adolescents (Miech et al., 2019; Wang et al., 2018). In general, nicotine addiction is characterized by behavioral phenotypes such as drug craving, seeking, taking, intermittent phases of withdrawal and in many cases relapse (Benowitz, 2010; Kreek & Koob, 1998). However, nicotine addiction is multifaceted where genetic, epigenetic and environmental factors often contribute to the disorder (Benowitz, 2010; Butelman et al., 2012; Nestler, 2014). Given the findings indicating a role of genetic and epigenetic factors in drug addiction, identifying critical mechanisms that may increase drug addiction vulnerability through preclinical models has become a critical goal within the field.
To date, a handful of studies have identified strain-specific differences in drug self-administration, which are likely driven by differences in genetic background. However, a majority of these studies have focused on Lewis, Fischer, and Wistar rat strains, and do not include the commonly used Sprague-Dawley and Long Evans strains. Some studies have shown strain differences in nicotine self-administration (Shoaib et al., 1997). Specifically, Lewis rats more reliably acquire nicotine self-administration and have higher breakpoints (i.e. the point in which a subject ceases engagement in self-administration due to the demand of the task) than outbred Holtzman rats (Brower et al., 2002). Subcutaneous exposure to nicotine has also been shown to preferentially alter mesolimbic circuitry within Lewis but not Fischer rats following 30 days of abstinence (Cadoni et al., 2019), further suggesting that differences in genetic background (i.e. different strains) could contribute to differential phenotypes observed during nicotine self-administration paradigms. Thus, differences in genetic background may alter self-administration in preclinical animal models, which may be an important factor to consider when choosing a rat strain for models of tobacco use disorder (TUD).
Clinically, numerous differences in characteristics of substance use disorders (SUDs) have been reported between men and women. Most commonly, differences in patterns of use, progression of abuse patterns, and biological responses have been reported. Interestingly, adult men are more likely than women to be current illicit substance users, alcohol users and tobacco users, where men are 2–3 times more likely to abuse drugs or develop a dependence disorder compared to women (Perkins, 1999; Perkins et al., 2001). While sex differences in use patterns are less robust for cocaine and heroin use, nicotine use in humans has vastly different patterns of intake, reward sensitivity and continued drug use between sexes. For example, women smoke fewer cigarettes per day than men (Jensen, et al., 2016), yet women display heightened sensitivity to the rewarding effects of nicotine due to non-nicotine components, such as cues, compared to men (Perkins et al., 2001). Specifically, women are unable to distinguish between nicotine doses as readily as men (Perkins et al., 2002), and are more responsive to nicotine-associated cues (Doran, 2014), likely contributing to their perception of reward. Thus, the presence of cues alone are perceived as more rewarding in women than men. Additionally, women have been shown to have less sensitivity than males to discriminative stimulus effects of nicotine (for review see Perkins et al. 1999). Taken together, these studies suggest sex differences in nicotine use, and thus, warrant additional research to fully characterize these differences and the impact they may have on nicotine addiction and treatment options.
Sex is now a mandated biological variable to consider in National Institutes of Health-funded grants in the United States (Lee, 2019; Miller et al., 2017). Recent rodent studies indicate differential responding for nicotine as a function of sex (Chaudhri et al., 2005; Donny et al., 2000; Feltenstein et al., 2012; Flores et al., 2019; Pogun et al., 2017; Swalve et al., 2016), which underscores the importance of examining neurobehavioral consequences of nicotine use in both males and females. While sex differences have been primarily reported within Sprague-Dawley (SD) rats, available studies often report conflicting results. Within the current literature, some studies report that females have higher nicotine intake (Halder et al., 2013; Rezvani et al., 2008; Sanchez et al., 2014) and faster acquisition of nicotine self-administration than males (Donny et al., 2000), whereas others report that males acquire nicotine self-administration at a faster rate than females (Lynch, 2009). Further, others observed no difference between SD males and females (Feltenstein et al., 2012; Goenaga et al., 2019). In addition, one study using LE rats suggests no sex differences in nicotine self-administration during a short-access paradigm (Li et al., 2014). Together, these studies suggest that nicotine intake in females is higher than males at higher unit doses (specifically, 0.06 mg/kg/infusion (Chaudhri et al., 2005; Flores et al., 2016) and 0.15 mg/kg/infusion (Chaudhri et al., 2005)), while lower dosages reveal no difference between sex (0.015 mg/kg/infusion (Li et al., 2014); 0.02 mg/kg/infusion (Goenaga et al., 2019), 0.03 mg/kg/infusion (Feltenstein et al., 2012; Flores et al., 2016; Li et al., 2014; Swalve et al., 2016), or 0.05 mg/kg/infusion (Feltenstein et al., 2012)) and thus, suggest dose-dependent sex differences in nicotine intake. For an extensive table highlighting findings from these studies and others, we direct the reader to the review by Pogun and colleagues (2017). Given the differential results of these studies, addressing sex differences in nicotine self-administration as a function of strain remains an important question and has translational importance.
To date, preclinical intravenous self-administration studies which model aspects of TUD have primarily used the outbred SD strain, and males have been predominantly used. With the recent focus on transgenic (Tg) animals to enhance understanding of the neurobiology of addiction (for example, use of recombinase-driver rat and mouse lines that allow for cell-type specific manipulations (Chen et al., 2018; Witten et al., 2011)), phenotypical differences in nicotine self-administration due genetic manipulation have been found (specifically in mice (Chen et al., 2018)). These are genetically restricted recombinase-driver rodent lines, which drive gene expression in certain cell types which are modified to express Cre recombinase (Witten et al., 2011). In rats, many commonly used Tg strains are generated using the LE background strain, which has been relatively underused for nicotine self-administration studies. In a literature search, we only found 3 studies using the LE strain in nicotine self-administration models (Li et al., 2014; Shoaib et al., 1997; Shram et al., 2008). Consequently, strain-specific phenotypic differences may be overlooked without proper comparisons. Thus, due to the lack of current research exploring sex and strain differences in nicotine intravenous self-administration and the recent surge in Tg animal lines, the current study aimed to address this gap and examine potential self-administration differences between SD and LE strains. Additionally, the current study explored sex differences within and across the two strains as well as in the choline acetyltransferase (ChAT)::cre Tg rat line. Given that nicotine is an agonist at nicotinic acetylcholine receptors (nAChRs) and alters cholinergic signaling (Armitage et al., 1969; Mansvelder et al., 2003; Quirion et al., 1994), examining this particular rat strain in the context of nicotine self-administration is important.
2. Materials and Methods
2.1. Subjects
Sixty to ninety day old adult SD male (n=25) and female (n=20) and LE male (N=28; 10 wildtype and 18 ChAT::cre) and female (N=28; 7 wildtype and 21 ChAT::cre) rats were housed on a 12-hour reverse-light cycle and had ad libitum access to food and water prior to surgery and self-administration. All animals were kept in a humidity and temperature-controlled animal facility, and were handled daily. All procedures used were approved by the Institutional Animal Care and Use Committee (IACUC) of Arizona State University (Protocol number 18-1642R). Animals used in the study were obtained from Charles River (SD rats) or bred in house (LE rats). In-house bred LE rats were composed of either wild-type males and females or ChAT::cre positive males and females, where cholinergic interneurons (ChIs) expressed the cre-recombinase transgene. ChAT::cre positive males (Long Evans-Tg(ChAT-Cre5)5.1 Deis) used for breeding were purchased from RRRC (RRRC #658; Columbia, MO), and bred in-house with LE wild-type females purchased from Envigo (Indianapolis, IN). Wild-type animals from the in-house breeding colony were used for this study consistent with the guiding principles for ethical use of animals in research including reduction, replacement and refinement (the three “R”s). All rats used within the present study were re-purposed from other studies, which spanned the course of 15 months.. Of these animals, 39 of the 101 (56 LE and 45 SD) were positive for the cre-recombinase transgene. All other studies in which these animals were used explored post-nicotine self-administration behavioral endpoints and cellular modifications, such as reinstatement and electrophysiological measures. However, the self-administration phase was identical across all studies in which these animals were used, and thus, group treatments were considered identical for the data collected and reported here, unless otherwise specified (i.e., surgical procedures).
2.2. Genotyping
At post-natal day 10, tails of all Long-Evans rats used within the current study were clipped for genetic analysis. Subjects were genotyped with the following primers: 5’-AGA GTA CAC TGT GGG CAG GA-3’ (R658.F2 located within the promotor region of Chat; forward primer) and 5’-GCA AAC GGA CAG AAG CAT TT-3’ (Cre.R located in cre-recombinase reverse primer). All Long-Evans rats were confirmed transgenic or wild type using standard PCR-based genotyping.
2.3. Surgical Procedures
Rats were anesthetized using ketamine hydrochloride (80–100 mg/kg, i.m.) and xylazine (8 mg/kg, i.m.) and underwent surgical implantation of intravenous jugular catheters [made from polyurethane tubing (BTPU-040; Instech, Plymouth Meeting, PA, USA)]. Catheters were inserted 2.5–3 cm into the right jugular vein and were threaded subcutaneously to the posterior side and connected to an indwelling back port [Vascular Access Button (Instech, Plymouth Meeting, PA, USA)], where dental cement [SNAP (Parkell, NY, USA) or Ortho-Jet (Lang Dental, IL, USA)] was used to adhere the catheter to the port. The indwelling port was sutured subcutaneously approximately 2 cm caudal from the shoulder blades. A subset of animals (SD Males and Tg LE male and females) underwent stereotaxic surgery, where NAcore guide cannulae were bilaterally inserted into the NAcore of the nucleus accumbens (+1.5 mm anterior/posterior (A/P), +2.0 mm medial/lateral (M/L), and −5.5 mm dorsal/ventral (D/V), as derived from the rat stereotaxic atlas (Paxinos et al. 1985)). Comparisons with our previously published study (Powell et al., 2019) showed no differences in nicotine self-administration between animals with and without cannulae implantation, thus data are collapsed. Additionally, Tg LE ChAT::cre positive rats received Designer Receptors Exclusively Activated by Designer Drugs (DREADD) viral infusions of AAV5-hsyn-DIO-mcherry (control; titer: 1.5×1013), AAV5-hsyn-DIO-HM4D(Gi)-mcherry (inhibitory; titer: 1.2×1013) or AAV5-hsyn-DIO-rM3D(Gs)-mcherry (excitatory; titer: 1.3×1013 vg/mL) directly into the NAcore at a volume of 0.5 μL, immediately following cannula implantation. Importantly, rats did not receive injections of clozapine-N-oxide (CNO) during nicotine self-administration or at any point during the present study. It is important to note that although animals were used from multiple different studies as described above, all animals received the same handling and self-administration procedures while collecting the data reported here. The experimental manipulations that differed between groups were conducted after self-administration phases were completed. Thus, aside from the surgical procedures outlined here, all animals within the current study were considered identical in regards to the self-administration paradigm described. Rats were given cefazolin (100 mg/kg, i.v.) and heparin (10 usp, i.v.) on the day of surgery and for 7 days during the post-surgical recovery period. Meloxicam (1 mg/kg, s.c.) was given on the day of surgery and 3 days during the post-surgical recovery period. Daily heparin was also administered throughout self-administration of nicotine to maintain patency (10 usp, i.v.).
2.4. Self-Administration Operant Chambers
Nicotine self-administration was conducted in 28 modular test chambers (13 ENV-008, 15 ENV-007; Med Associates, St. Albans, VT). All chambers were enclosed in sound-attenuating cubicles each equipped with ventilation fans. Each chamber was composed of two aluminum side walls and clear Plexiglas, which composed the front and back walls as well as the ceiling. The floors of all chambers consisted of thin metal bars positioned above a catch pan. Chambers were equipped with intelligence panels that include two stimulus lights, a food hopper, two levers and a house light. In each chamber, two levers were located 4.3 cm from the chamber base. Separating the two levers was a flanked pellet receptacle, used for food-training. A jewel light was located above both levers and a house light was located on the opposite wall. The house light was only used for food-training purposes. One lever was designated as an “active” lever, which yielded one intravenous nicotine infusion or one food pellet and initiation of nicotine-paired cues (i.e. light+tone) when pressed, whereas the other was designated as “inactive”, and had no programmed consequence. A circular 3-cm diameter hole is present at the top of each chamber to allow a drug delivery tether to pass into the chamber. The drug delivery tether was connected to a syringe containing the nicotine solution, and attached to a single-channel liquid swivel that was mounted on the top of the chamber enclosure. Each chamber was outfitted with a single-speed automated drug infusion pump (PHM-100) for drug delivery. These chambers have also been described in our previous study (Overby et al., 2018).
2.5. Food Training Procedures
Following 6 days of post-operative care, rats were food restricted (20 g of chow/day for both male and female rats) a minimum of 2 hours before food training. Rats underwent overnight food training (15 hrs) in which one lever press (fixed-ratio-1, or FR-1, schedule of reinforcement) resulted in the delivery of one food pellet [Bio-Serv (Flemington, NJ), 45 mg/pellet]. Concurrently, one food pellet was delivered every 20 minutes, regardless of response. Pellet administration was not paired with light or tone stimuli. Rats were required to achieve a 2:1 ratio of active to inactive lever presses throughout food training and were required to produce 200 active lever presses throughout the session. If criteria were not met, animals were re-food trained two days following the first food training session. Rats had a maximum of two food training sessions. The side of the chamber associated with the active lever was the same for all subjects (right side was active) and was constant throughout food training and self-administration.
2.6. Intravenous Nicotine Self-Administration
All rats were trained to self-administer nicotine (0.02 mg/kg/infusion). This dose was chosen because it is on the ascending limb and near the peak of the dose-response curve (Matta et al., 2007). One week after catheterization, rats were placed into a self-administration paradigm on an FR-1 schedule of reinforcement, where one lever press resulted in the delivery of one nicotine infusion. Nicotine was delivered across a 5.9-s infusion (0.1 mL/infusion) after a lever press on the designated active (right) lever. An inactive lever was extended at all times and presses were recorded, but produced no programmed consequences. Simultaneous with infusion, lights located above both levers were illuminated and a tone (2900 Hz) was presented, followed by a 20-s timeout period. Active lever presses were recorded throughout the timeout period, but did not result in additional light/tone presentations or nicotine infusion (Gipson et al., 2013; Goenaga et al., 2019; Powell et al., 2019). Sessions were 2 hrs in duration, with the first two sessions capped at 25 infusions to prevent high nicotine intake from potentially producing aversive effects. All animals were required to complete at least 10 sessions that met the following criteria: > 10 infusions and ≥ 2:1 active/inactive lever press ratio, both of which needed to be met for 10 non-consecutive sessions.
2.7. Drugs
(−)Nicotine tartrate salt (MP Biomedicals, Solon, OH) was dissolved in 0.9% sterile saline and adjusted to pH 7.2–7.4 with 1 M NaOH. The final stock concentration was 0.2 mg/mL free base, which was diluted to 0.02 mg/kg/mL based on body weight. DREADD Viral vectors AAV5-hsyn-DIO-mcherry (Catalog # 50459) and AAV5-hsyn-DIO-HM4D(Gi)-mcherry (Catalog # 44362) were obtained from Addgene (Watertown, MA, USA). The AAV5-hsyn-DIO-rM3D(Gs)-mcherry plasmid (Catalog # 50458) was obtained from Addgene and packaged using Penn Vector Core (Philadelphia, PA, USA). Heparin was purchased from Sagent Pharmaceuticals (Schaumburg, IL). Xylazine (100 mg/mL) was purchased from Biomeda (Cambridge, ON, Canada) and diluted to 8 mg/kg/mL with sterile saline prior to use. Ketamine was purchased from Dechra Veterinary Products (Overland Park, KS). Meloxicam and Cefazolin were both purchased through Henry Schein (Melville, NY) and diluted to 1 mg/kg and 100 mg/kg in sterile saline, respectively.
2.8. Data Analysis
Nicotine self-administration data were analyzed using the 10 sessions in which animals met criteria (non-consecutively), or pooled across all sessions (criteria-making sessions only). All data were analyzed using analyses of variance (ANOVAs) or linear regressions. The three-way repeated measures ANOVA used to explore differences in nicotine intake across criteria-making sessions considered sex and strain as between-subject factors and session as the repeated measure. Two-way ANOVAs used sex and strain as between-subject factors. Tukey post hoc tests (α=0.05) were used to determine differences between means as appropriate. Linear regression analyses were used to compare the relationship of infusions, active lever pressing or inactive lever pressing across self-administration sessions, and included session as a variable. The slopes of the lines across sessions were compared between groups. Statistical analyses were performed in GraphPad Prism 8.0, and p<0.05 was considered statistically significant. Values presented are represented as mean ± standard error of the mean (SEM). Specific analyses used are presented within each figure legend.
3. Results
3.1. Nicotine Intake Differs across Sessions as a Function of Sex and Strain
All groups readily distinguished between active and inactive levers, with greater responding on the active lever (data not shown). Differences in nicotine intake across criteria-making sessions were examined using a 3-way mixed model ANOVA. Main effects of sex (F1,968 = 7.5; p<0.05), strain (F1,968 = 26.5; p<0.05), and session (F9,968 = 8.4; p<0.05) were found as well as an interaction between sex and strain (F1,968 = 75.9; p<0.05) (Figure 1). However, no interaction between sex, strain and session was observed (p>0.05). Using a linear regression analysis, the slope of infusion curves across the 10 sessions that met criteria were compared between experimental groups to examine whether an increase in nicotine infusions across sessions was different across groups. Interestingly, SD males had a greater slope relative to LE males (linear regression; F1,525 = 6.87; p<0.01) and SD females (linear regression; F1,445 = 21.0; p<0.0001), indicating that SD males show a greater increase in nicotine intake across sessions relative to the compared groups (Figure 1). However, no differences in slope were observed between LE females and SD females (p>0.05) nor LE males and LE females (p>0.05). Further, when sex was collapsed across strain, no differences in slope were observed between SD and LE rats (main effect of strain; p>0.05).
Figure 1: Differences in Infusions across Sessions is Sex and Strain Specific.
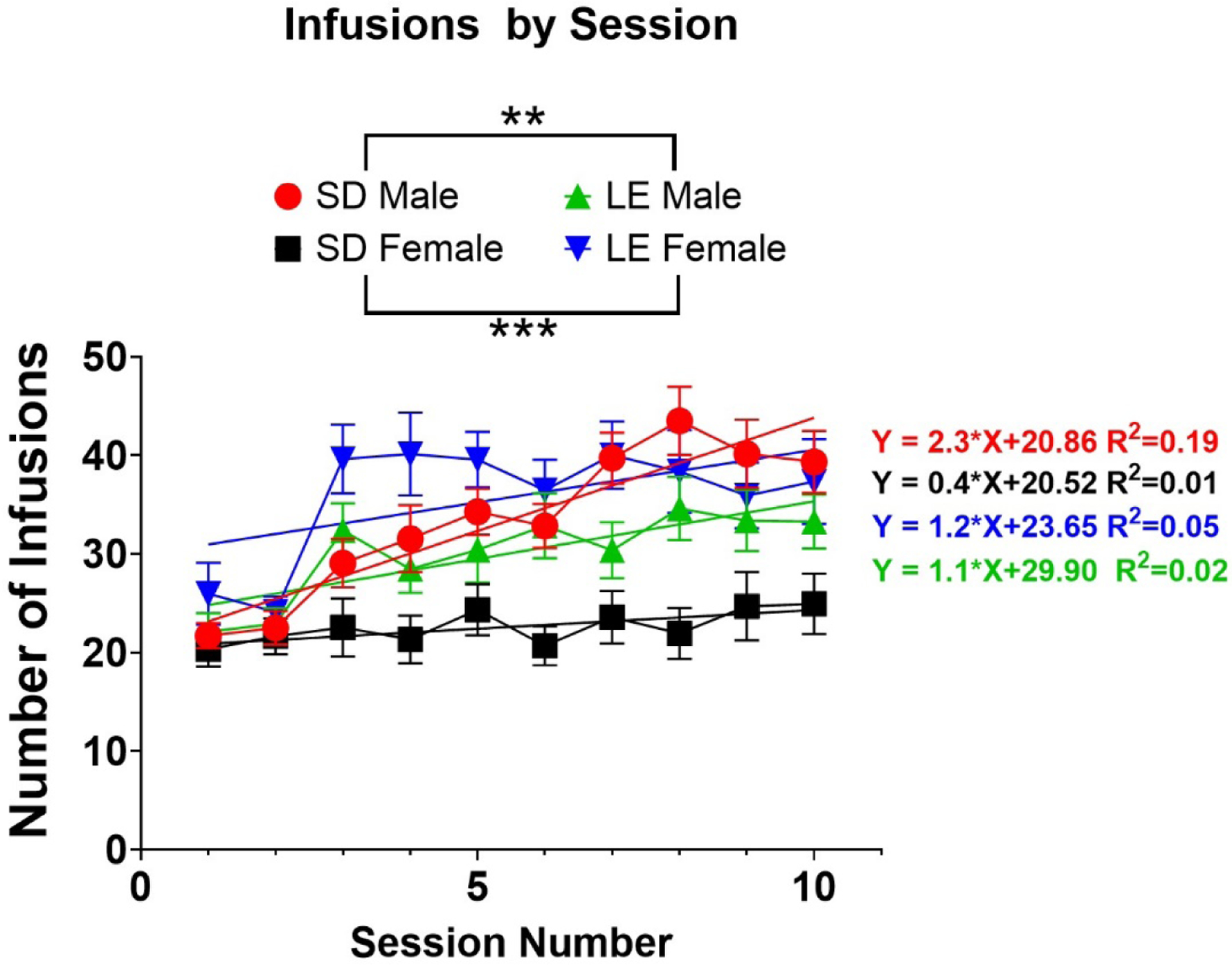
A 3-way mixed model ANOVA revealed a main effect of sex, strain and interaction. Significant effects were observed between SD males (red) and LE males (green) as well as between SD females (black) and LE females (blue). These effects are indicated by asterisks within the graph legend. Fitted linear regressions are shown for each group within the graph and respective line equations and R2 values are shown. **p<0.01; ***p<0.001. Error bars indicate ± SEM.
3.2. Strain and Sex Influence Total Nicotine Infusions across Self-Administration Sessions
The total number of nicotine infusions earned across sessions in which criteria were met was analyzed for all animals. Thus, the total number of nicotine infusions is the sum of infusions across the 10 sessions which were highlighted in Figure 2A. Notably, the sessions in which animals met criteria were not always consecutive. The average number of sessions to achieve 10 criteria-meeting sessions ranged between 11–13 (Figure 2A). A two-way between-subjects ANOVA revealed a main effect of strain (F1,97 = 5.5, p<0.05) but not sex (p>0.05). A significant interaction (sex × strain) was observed (F1,97 = 15.2; p<0.001). An interaction plot for the described data is shown in figure 2B. A multiple-comparisons analysis revealed that SD males (n=25) earned significantly more infusions of nicotine than SD females (n=20) (t97 = 3.4; p<0.05), indicating a strain-specific sex difference. The total number of infusions earned did not differ between SD males and LE males (p>0.05) or SD males and LE females (p>0.05). Further, LE females self-administered more nicotine than SD females, indicating a strain difference between female groups (t97 = 4.3; p<0.001), but did not differ from LE males (p>0.05). A two-way sex × type (Wildtype/Transgenic) ANOVA revealed no main effect of sex or type on total infusions earned, demonstrating that wild-type and transgenic rats do not differ in total infusions earned across the 10 criteria-making sessions (p>0.05; data not shown).
Figure 2: Sex and Strain Differences in Total Number of Nicotine Infusions.
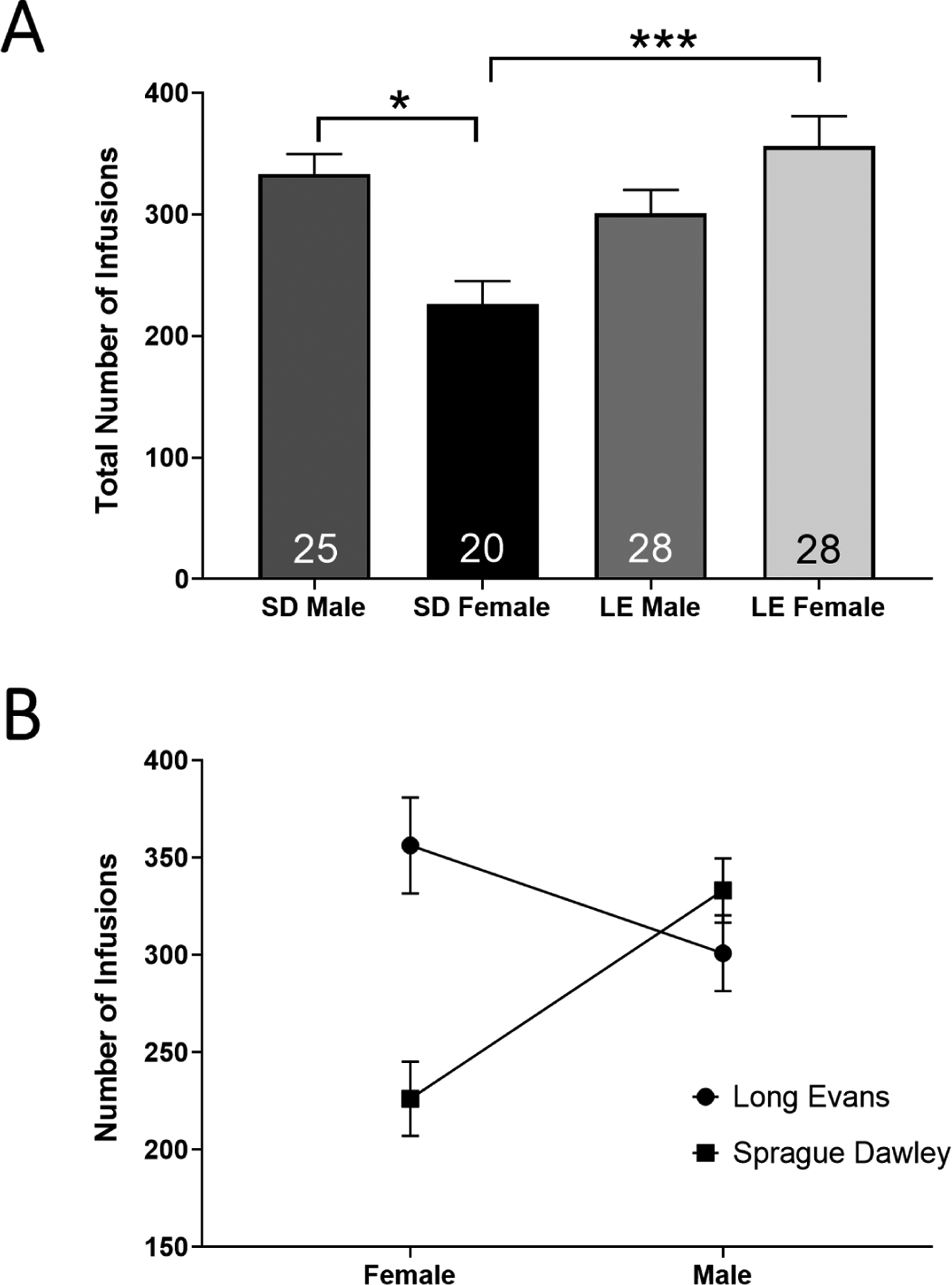
(A) Mean total number of infusions earned across the 10 sessions in which rats met criteria for SD males, SD females, LE males and LE females. (B) An interaction plot, where the lines depict strain and sex is located on the x-axis, is displayed. *p<0.05; ***p<0.001. Numbers within bars of panel A represent number of animals. Error bars indicate ± SEM.
3.3. Acquisition of Nicotine Self-Administration Did Not Differ as a Function of Sex or Strain
Acquisition of nicotine self-administration was defined as the total number of days to reach 10 criteria-meeting sessions. Acquisition data were examined using a two-way sex by strain ANOVA. No effect of sex, strain or interaction between sex and strain was observed (p>0.05). A multiple comparisons analysis revealed no statistical difference in sessions to reach criteria between SD males, SD females, LE males or LE females (p>0.05; Figure 3A). Further, a two-way sex × type (Wildtype/Transgenic) ANOVA revealed no main effects of sex or type on sessions to reach criteria, demonstrating that wild-type and transgenic rats do not differ in the number of sessions needed to meet 10 criteria-making sessions (p>0.05; data not shown). The percentage of animals reaching criteria within 10, 11–12, 13–14, and >15 days is illustrated in Figure 3B.
Figure 3: Sessions to Reach Criteria is Unaffected by Sex or Strain.
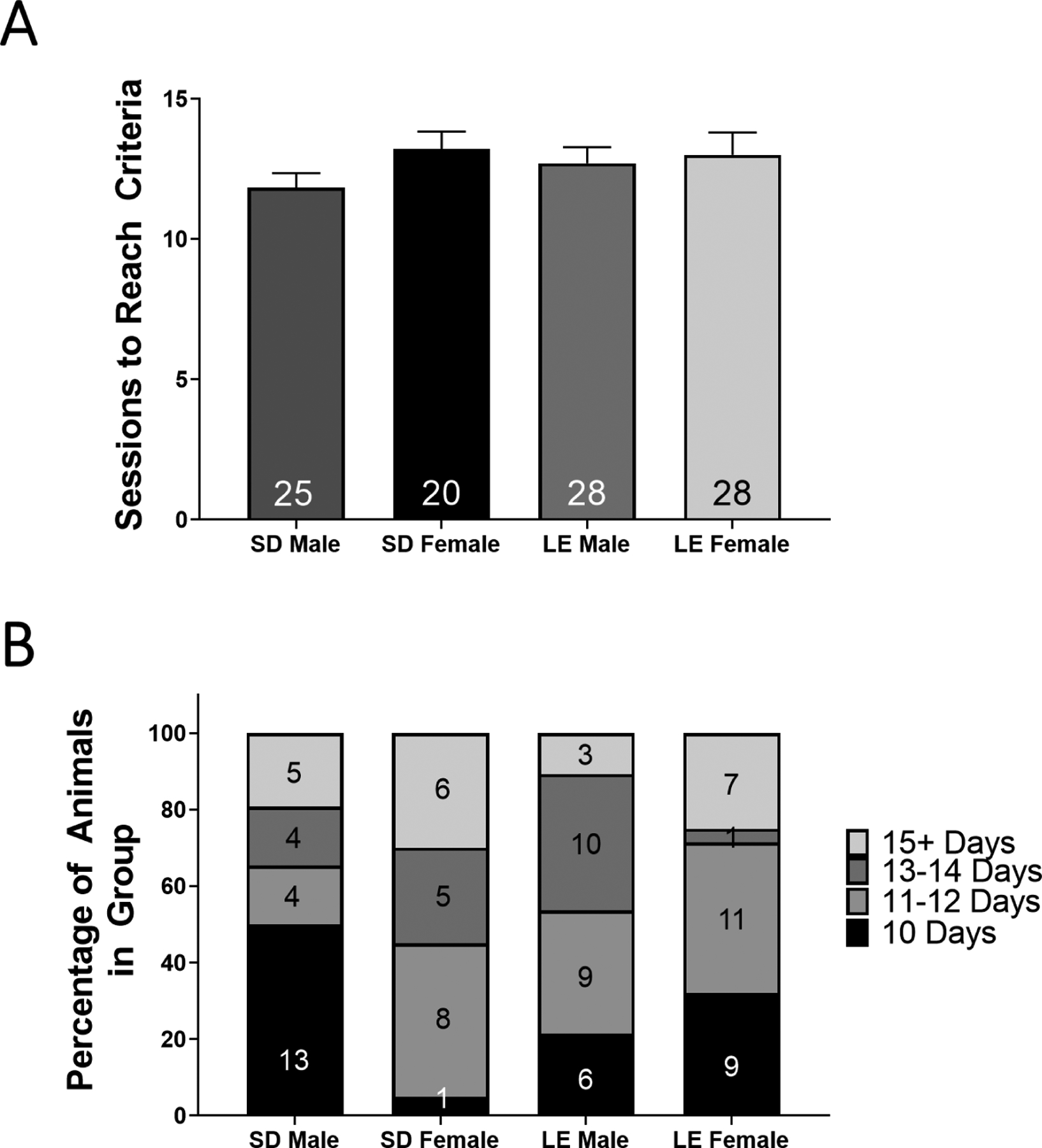
(A) Mean total number of sessions needed to achieve 10 sessions of criteria is shown for SD males, SD females, LE males and LE females. Numbers within bars represent number of animals. Error bars indicate ± SEM. (B) A breakdown of the percentage of animals meeting 10 criteria-making sessions within specified bins are shown. Numbers within columns represent the number of animals in each bin.
4. Discussion
In the present study, differences in nicotine self-administration as a function of sex in commonly utilized outbred strains as well as differences in nicotine intake between wildtype and a cre-recombinase-driver Tg rat line (ChAT::cre) generated on a LE background were explored. The results show that SD males earn significantly more nicotine infusions than SD females, contrary to previously published results (Goenaga et al., 2019). Potential reasons for these discordant findings are presented in the following paragraph. Further, LE females take significantly more total nicotine infusions than SD females. Compared to SD females and LE males, SD males increase the number of infusions across sessions at a faster rate, indicating that SD males have more rapid acquisition of self-administration than the other groups. Although intake differed, the number of sessions to reach criteria was not significantly different between sex or strain, indicating that males and females from both strains acquire nicotine self-administration at similar rates. Finally, the current results show that wild-type and ChAT::cre rats generated from a LE background show no differences in total infusions, sessions to reach criteria, or number of infusions across session for either sex. This study is the first to highlight strain and sex differences in nicotine self-administration between SD and LE rats. Further, this is the first study to our knowledge that examines potential self-administration differences between wild-type and ChAT::cre LE rats infused with DREADD viral vectors within the nucleus accumbens core, which is a key region involved in nicotine addiction processes (Gipson et al., 2013; Mansvelder et al., 2003; Mansvelder et al., 2009).
4.1. Clinical Finding of Sex Differences in Drug Consumption
As discussed above, sex differences in nicotine intake, subjective rewarding sensitivity and stimuli sensitivity have been reported. Interestingly, men begin using nicotine earlier and self-report greater nicotine use than women (SAMHSA, 2017). However, women escalate use and achieve criteria for TUD faster than men (DiFranza et al., 2002). Interestingly, women self-report heightened levels of reward following smoking compared to men at the same dosage, suggesting enhanced nicotine sensitivity (Perkins et al., 2001). Men also perceive differences in nicotine doses when administered intravenously and women do not, suggesting baseline differences in subjective rewarding sensitivity. Specifically, low doses of intravenous nicotine are preferentially chosen by men over high doses of nicotine, whereas women display no choice preference (Jensen et al., 2016). Further, women exhibit a greater difficulty quitting smoking and show greater negative affect and more cue-induced craving than men during nicotine withdrawal (Doran, 2014; Xu et al., 2008). While women smokers show to have less sensitivity to discriminative stimulus cues for nicotine than men (see Perkins et al. 1999 for review), it is hypothesized that differences in nicotine sensitivity and menstrual cycle effects are thought to contribute to sex differences in nicotine acquisition and cessation success. In the current study, we have shown that male SD rats self-administer more nicotine than SD female rats, which may reflect clinical findings that men self-administer more nicotine due to lower sensitivity than women. Given this possibility, future studies could utilize this strain when modeling aspects of nicotine use with the goal of achieving sex-specificity. However, additional studies would be necessary to properly address this question. Below we have included limitations of the current study that could be used to explore this difference further. However, the results reported here provide a baseline for the field to further determine if specific strains may enhance translational value when examining sex differences in nicotine intake.
4.2. Sex Differences within Strain
Current literature regarding SD rats has yielded opposing results regarding sex differences in nicotine self-administration. For example, on an FR-1 schedule of reinforcement and short-access paradigm (2 hr), similar to the paradigm used here, Donny and colleagues report that adult females acquire nicotine self-administration faster than male counterparts at a 0.02 mg/kg/infusion dose (Donny et al., 2000). Additionally, adult female rats self-administer more nicotine than male counterparts in a short-access (1 hr) paradigm, under a FR-1 schedule of reinforcement with a 0.03 mg/kg/infusion dose (Rezvani et al., 2008). Others, however, report that a greater percentage of SD males acquire nicotine self-administration within the first 2 sessions than females during 1 hr sessions at 0.01 mg/kg/infusion. Further, others report a lack of sex differences in self-administration at varying session lengths with 0.015 mg/kg/infusion (Li et al., 2014); 0.02 mg/kg/infusion (Goenaga et al., 2019), 0.03 mg/kg/infusion (Feltenstein et al., 2012; Flores et al., 2016; Li et al., 2014; Swalve et al., 2016), and 0.05 mg/kg/infusion nicotine (Feltenstein et al., 2012). Although it is unclear why the current study did not replicate our previously published findings which showed a lack of sex difference in nicotine self-administration of SD males and females (Goenaga et al., 2019) using the same parameters (e.g., session length and dose), it is possible that differences in criteria between the two studies account for this difference. Specifically, the current study utilized a 10-session minimum with ≥ 10 infusions and a ≥ 2:1 active:inactive lever press ratio, whereas Goenaga et al. only utilized the infusion criterion. In the current study, 25% of all females (16/64) did not make criteria and were thus removed from analysis, whereas only 7% (1/15) were removed in our prior study for not meeting criteria. Thus, the stricter criteria used in the current study may lead to inclusion of only higher nicotine responders, which may represent a subpopulation in which sex differences in nicotine self-administration emerge.
To our knowledge, the only study to explore sex differences within LE rats was conducted by Li and colleagues (Li et al., 2014), where no sex differences in nicotine self-administration characteristics were reported using 0.0075 mg/kg/infusion, 0.015 mg/kg/infusion or 0.03 mg/kg/infusion unit doses. However, we ascertain that moderate doses of nicotine, as used in the current study, produce differential effects that may be reliant on session duration and are likely strain specific. Given that only one other study has explored nicotine self-administration in LE rats, it is difficult to make any definitive conclusions in the context of these varying methods. Thus, additional research examining differences in sex and strain across dose and session duration for both strains is warranted.
4.3. Strain Differences in Nicotine Self-Administration
Regarding nicotine self-administration, many studies have identified strain differences between Lewis, Wistar and Fischer strains. However, the current study is the first to address differences between SD and LE strains. Results of the current study indicate that female LE rats have higher overall nicotine intake across the 10 sessions that met criteria compared to female SD rats, indicative of a possible genotype-driven difference in nicotine reinforcement. Here we also report that SD males have enhanced nicotine intake across 10 sessions compared to LE male counterparts. Interestingly, strain differences were only observed when separated by sex. When data were collapsed across sex, SD and LE strains show no significant difference between total infusions over 10 criteria-making sessions, number of days to meet criteria, or number of infusions across sessions. Thus, in order to fully characterize strain differences, future studies must include sex as an influencing factor.
4.4. Nicotine Self-Administration in Tg Animal Lines
As stated above, ChAT::cre mouse lines have been shown to have altered baseline nicotine self-administration characteristics (Chen et al., 2018). Specifically, Chen and colleagues examined differences in nicotine self-administration between two Tg ChAT::cre mouse lines depicted as ChAT(BAC)-cre-Tg, generated using the bacterial artificial chromosome method, and ChAT(IRES)-cre-Tg, generated using the internal ribosome entry site (IRES) method. All controls were littermate wild-type (WT) mice, generated within each respective mouse line (i.e. ChAT(BAC)-cre-WT and ChAT(IRES)-cre-WT). Compared to ChAT(BAC)-cre-WT, ChAT(BAC)-cre-Tg male mice displayed attenuated responding for nicotine across sessions at the 0.03 mg/kg/infusion dose. Further, ChAT(BAC)-cre-WT readily distinguished between active and inactive lever pressing whereas their Tg littermates did not, overall indicating an altered baseline in nicotine self-administration of Tg mice. However, ChAT(IRES)-cre-WT and ChAT(IRES)-cre-Tg mice displayed stable preference for the active (nicotine-infusion associated) lever, where active lever responding increased across acquisition and no differences between the two groups were reported differences. Additionally, Chen and colleagues report ChAT(BAC)-cre-WT mice exhibited a dose-response curve similar to their WT C57BL/6J counterparts (compared from previous data in (Fowler & Kenny, 2011)), whereas ChAT(BAC)-cre-Tg exhibited a flattened and lowered dose-response curve relative to both groups. These results suggest a genotypic difference in nicotine self-administration of ChAT(BAC)-cre-Tg mice. ChAT(IRES)-cre-WT and Tg mice, however, display similar dose-response curves and thus, no difference in nicotine self-administration baseline characteristics all together.
Interestingly, ChAT(BAC)-cre-Tg mice showed enhanced vesicular acetylcholine transporter VAChT and ChAT expression compared to ChAT(BAC)-cre-WT mice, suggesting an enhancement in cholinergic tone within these animals, which can lead to nicotine aversion and/or decreased nicotine reinforcement (Chen et al., 2018). Because the LE ChAT::cre strain used in the current study was also generated using the same BAC method, we aimed to address whether rats in the present study showed differences in baseline nicotine self-administration. Unlike Chen and colleagues, we did not observe significant differences between LE WT and ChAT::cre animals in any measure. While we did not address whether the LE ChAT::cre strain used in the current study had altered VAChT or ChAT, our results support that they are not altered in the Tg animals. Further, the ChAT(BAC)-cre-Tg mouse line used by Chen et al. has a known transgene insertion point within chromosome 17, whereas the LE ChAT::cre rat strain has six separate transgene copies at the insertion site, making it difficult to decipher where the transgene gets inserted. Thus, it is difficult to make inferences between the two species, and difficult to determine whether alterations in protein levels occurred due to strain generation alone. Due to the lack of differences between LE WT and ChAT::cre nicotine self-administration observed in the current study, we hypothesize that cholinergic protein expression within this Tg line is unaltered; however, additional experiments are necessary to address this question.
4.5. Limitations of the Current Study
Limitations of the current study include use of a short-access session lasting 2h in duration and use of a low effort schedule of reinforcement (FR-1). While use of short-access is common for nicotine self-administration studies (Chaudhri et al., 2005; Donny et al., 2000; Feltenstein et al., 2012; Levin et al., 2011; Powell et al., 2019), future studies would benefit from exploring long-access nicotine self-administration paradigms. Interestingly, escalation has not been observed by our lab with 6h access (unpublished data) or in other labs using extended 21h access to nicotine without an intermittent access schedule (Cohen et al., 2012). However, these studies were conducted using male SD and Wistar rats, respectively. Thus, it remains unknown whether escalation of nicotine intake occurs in other strains such as Long Evans, and whether sex differences in escalation would be detected. Of note, it is unclear if escalation in the long access rodent paradigm reflects the human experience of dysregulation. One study showed that escalation may be regulated acquisition of self-administration, as it can come under stimulus control and occurs in short access (60 min) sessions when animals are initially trained on 10 min sessions of cocaine (Beckmann et al., 2012). As noted above, nicotine escalation models require an intermittent access schedule, with animals receiving interpolated withdrawal days. As NIDA has recently issued a request for information (RFI) on the utility of established preclinical models of substance use disorders, it is important to consider if these models reflect the human experience (see NOT-DA-19-036).
The current study also used a FR-1 schedule of reinforcement, which also poses a potential limitation as it is low effort and as such, reflects a low unit price. While many studies utilize this schedule, intermittent schedules of reinforcement have shown that females have higher baseline nicotine intake with an FR-3 (Grebenstein et al., 2013), higher responding with an FR-5 (Chaudhri et al., 2005) and greater nicotine intake with an FR-10 (Wang et al., 2014) than males. Thus, future studies exploring sex- and strain-dependent effects on nicotine self-administration on intermittent schedules of reinforcement would enhance the field’s understanding of nicotine self-administration. Additionally, assessing the essential value of nicotine via a threshold procedure in which unit price for nicotine is increased may prove beneficial for fully exploring and understanding the impact of sex and strain on nicotine intake. Using behavioral economic demand curves to measure nicotine value allows for more accurate measurement of the value of nicotine, and is generalizable across many species including rats and humans (Hofford, et al., 2016; Koffarnus et al., 2015; Powell et al., 2019; Yates et al., 2019). Evaluating the impact of sex and strain on nicotine value may further reveal which animal model more accurately reflects the human experience. The use of a variability/stability criterion should also be considered in future studies. While the current study required that animals meet criteria of both a 2:1 active to inactive lever pressing and achieve greater than 10 infusions per session, a variability criterion was not utilized here (e.g., responding within a certain percentage of variability over a specified number of sessions). Thus, future studies using variability criteria are warranted.
Ovarian hormones and estrous cycle were not examined throughout the current study. However, changes in estrogen and circulating ovarian hormones have been shown to contribute to sensitivity in the reinforcing effects of nicotine (Lynch, 2009). Studies on nicotine self-administration have reported a lack of estrous cycle-effects but this may be strain-dependent. For example, we and others have shown a lack in estrous cycle-dependent effects on nicotine self-administration (Donny et al., 2000; Goenaga et al., 2019) using SD females. Thus, future studies would benefit from exploring both hormone and estrous cycle dependent effects across strains in relevance to nicotine self-administration.
Lastly, nicotine is known to alter locomotor activity. Specifically, single high doses of nicotine have been shown to decrease locomotor behavior, and repeated subcutaneous nicotine administration induces tolerance to its depressive locomotor effects and sensitizes rats to its stimulatory effects (Belluzzi et al., 2004; Domino, 2001). While alterations in nicotine-induced locomotor activity can lend insight into the perceived stimulatory effects of nicotine, most nicotine-induced locomotor studies have been conducted in SD rats. Thus, there may be potential strain differences in nicotine-induced locomotor activity across strain. To fully address strain and sex differences in regard to nicotine self-administration and its stimulatory effects, future studies would also benefit from exploring potential strain differences in nicotine-induced locomotor activity.
5. Conclusion
The results of the current study provide insight into genotypic and sex differences underlying preclinical modeling of TUD. Our results provide evidence that sex and strain can influence nicotine self-administration, and when unaccounted for, can lead to inaccurate conclusions regarding strain and sex influences on nicotine self-administration. While sex is now a mandated biological variable to consider in National Institutes of Health-funded grants, these results further validate the importance of examining neurobehavioral consequences of nicotine use in both male and female animal models (Mennenga & Bimonte-Nelson, 2014). Additionally, while we have previously reported that estrous cycle phase does not affect nicotine self-administration (Goenaga et al., 2019), these findings were characterized in SD females and thus, should be addressed in LE females where strain differences may be present. Given the differences in nicotine self-administration between female SD and LE strains, cautionary measures should be taken when choosing a strain to model aspects of nicotine addiction. Lastly, while we report no differences in WT and Tg ChAT::cre rats, further validation that genetic modifications do not alter behavioral phenomena should be established.
Public Significance Statement:
It is important for preclinical animal models of nicotine addiction to approximate patterns of nicotine use in humans, taking sex into account. Further, choice of strain may directly influence behavioral outcomes in preclinical nicotine models, thus it is important to characterize strain differences in nicotine intake. This study highlights sex and strain differences that provide beneficial insight into acquisition of nicotine self-administration behavior in preclinical models.
Acknowledgments
This work was supported by the National Institutes of Health Grant DA036569, DA036569-S1, DA044479, and DA045881 (to CDG) as well as F32AA027962-01 to JMLJ, and the Arizona Alzheimer’s Consortium (to CDG).
The authors would like to acknowledge Dr. Jason Newbern for his technical insight as well as Hanaa Ulangkaya, Ngoc Van Do, Jose Piña and Vincent Carfagno for technical assistance.
Abbreviations:
- LE
Long Evans
- SD
Sprague-Dawley
- ChAT::cre
cholinergic acetyltransferase cre-recombinase
Footnotes
All authors have no conflicts of interest to declare.
References
- Armitage AK, Hall GH, & Sellers CM (1969). Effects of nicotine on electrocortical activity and acetylcholine release from the cat cerebral cortex. Br J Pharmacol, 35(1), 152–160. doi: 10.1111/j.1476-5381.1969.tb07976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich JA, & Bardo MT (2012). Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl), 222(2), 257–267. doi: 10.1007/s00213-012-2641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, & Leslie FM (2004). Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl), 174(3), 389–395. doi: 10.1007/s00213-003-1758-6 [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2010). Nicotine addiction. N Engl J Med, 362(24), 2295–2303. doi: 10.1056/NEJMra0809890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, & Sharp BM (2002). Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res, 930(1–2), 12–20. doi: 10.1016/s0006-8993(01)03375-3 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, & Kreek MJ (2012). κ-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci, 35(10), 587–596. doi: 10.1016/j.tins.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, De Felice M, Corongiu S, Dessì C, Espa E, Melis M, & Fenu S (2019). Role of genetic background in the effects of adolescent nicotine exposure on mesolimbic dopamine transmission. Addict Biol, e12803. doi: 10.1111/adb.12803 [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, … Perkins KA (2005). Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl), 180(2), 258–266. doi: 10.1007/s00213-005-2152-3 [DOI] [PubMed] [Google Scholar]
- Chen E, Lallai V, Sherafat Y, Grimes NP, Pushkin AN, Fowler JP, & Fowler CD (2018). Altered Baseline and Nicotine-Mediated Behavioral and Cholinergic Profiles in ChAT-Cre Mouse Lines. J Neurosci, 38(9), 2177–2188. doi: 10.1523/JNEUROSCI.1433-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Koob GF, & George O (2012). Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology, 37(9), 2153–2160. doi: 10.1038/npp.2012.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, … Wood C (2002). Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control, 11(3), 228–235. doi: 10.1136/tc.11.3.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF (2001). Nicotine induced behavioral locomotor sensitization. Prog Neuropsychopharmacol Biol Psychiatry, 25(1), 59–71. doi: 10.1016/s0278-5846(00)00148-2 [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, … McCallum S (2000). Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl), 151(4), 392–405. [DOI] [PubMed] [Google Scholar]
- Doran N (2014). Sex differences in smoking cue reactivity: craving, negative affect, and preference for immediate smoking. Am J Addict, 23(3), 211–217. doi: 10.1111/j.1521-0391.2014.12094.x [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, & See RE (2012). Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend, 121(3), 240–246. doi: 10.1016/j.drugalcdep.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RJ, Pipkin JA, Uribe KP, Perez A, & O’Dell LE (2016). Estradiol promotes the rewarding effects of nicotine in female rats. Behav Brain Res, 307, 258–263. doi: 10.1016/j.bbr.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RJ, Uribe KP, Swalve N, & O’Dell LE (2019). Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiol Behav, 203, 42–50. doi: 10.1016/j.physbeh.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, & Kenny PJ (2011). Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology, 61(4), 687–698. doi: 10.1016/j.neuropharm.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, & Kalivas PW (2013). Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A, 110(22), 9124–9129. doi: 10.1073/pnas.1220591110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenaga J, Powell GL, Leyrer-Jackson JM, Pina J, Phan S, Prakapenka AV, … Gipson CD (2019). N-acetylcysteine yields sex-specific efficacy for cue-induced reinstatement of nicotine seeking. Addict Biol. doi: 10.1111/adb.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, & LeSage MG (2013). Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav, 114–115, 70–81. doi: 10.1016/j.pbb.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S, Lynch JM, & Pearce AR (2013). The multiple bottle effect is overridden in male and female rats by simultaneous presentation of two oral nicotine solutions. Am J Drug Alcohol Abuse, 39(3), 161–167. doi: 10.3109/00952990.2013.776065 [DOI] [PubMed] [Google Scholar]
- Hofford RS, Beckmann JS, & Bardo MT (2016). Rearing environment differentially modulates cocaine self-administration after opioid pretreatment: A behavioral economic analysis. Drug Alcohol Depend, 167, 89–94. doi: 10.1016/j.drugalcdep.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Valentine G, Gueorguieva R, & Sofuoglu M (2016). Intravenous Nicotine Self-Administration in Smokers: Dose-Response Function and Sex Differences. Neuropsychopharmacology, 41(8), 2034–2040. doi: 10.1038/npp.2015.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Franck CT, Stein JS, & Bickel WK (2015). A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol, 23(6), 504–512. doi: 10.1037/pha0000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, & Koob GF (1998). Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend, 51(1–2), 23–47. [DOI] [PubMed] [Google Scholar]
- Lee SK (2019). Sex as an important biological variable in biomedical research. BMB Reports, 4(51), 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, … Rezvani AH (2011). Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behav Brain Res, 225(2), 473–481. doi: 10.1016/j.bbr.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, & Lê AD (2014). Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict Biol, 19(2), 156–164. doi: 10.1111/j.1369-1600.2012.00473.x [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2009). Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav, 94(1), 43–50. doi: 10.1016/j.pbb.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, & Brussaard AB (2003). Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol, 480(1–3), 117–123. doi: 10.1016/j.ejphar.2003.08.099 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Mertz M, & Role LW (2009). Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin Cell Dev Biol, 20(4), 432–440. doi: 10.1016/j.semcdb.2009.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, … Zirger JM (2007). Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl), 190(3), 269–319. doi: 10.1007/s00213-006-0441-0 [DOI] [PubMed] [Google Scholar]
- Mennenga SE, & Bimonte-Nelson HA (2014). The Importance of Incorporating Both Sexes and Embracing Hormonal Diversity When Conducting Rodent Behavioral Assays. In Bimonte-Nelson H (Ed.), The Maze Book (Vol. 94, pp. 299–321). New York, NY: Humana Press. [Google Scholar]
- Miech R, Johnston L, O’Malley PM, Bachman JG, & Patrick ME (2019). Adolescent Vaping and Nicotine Use in 2017–2018 - U.S. National Estimates. N Engl J Med, 380(2), 192–193. doi: 10.1056/NEJMc1814130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, … Clayton JA (2017). Considering sex as a biological variable in preclinical research. FASEB J, 31(1), 29–34. doi: 10.1096/fj.201600781R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2014). Epigenetic mechanisms of drug addiction. Neuropharmacology, 76 Pt B, 259–268. doi: 10.1016/j.neuropharm.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby PF, Daniels CW, Del Franco A, Goenaga J, Powell GL, Gipson CD, & Sanabria F (2018). Effects of nicotine self-administration on incentive salience in male Sprague Dawley rats. Psychopharmacology (Berl), 235(4), 1121–1130. doi: 10.1007/s00213-018-4829-4 [DOI] [PubMed] [Google Scholar]
- Perkins KA (1999). Nicotine Discrimination in Men and Women. Pharmacology Biochemistry and Behavior, 64(2), 295–299. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, & Hutchison S (2001). Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res, 3(2), 141–150. doi: 10.1080/14622200110043059 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, & Caggiula AR (2002). Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl), 163(2), 194–201. doi: 10.1007/s00213-002-1168-1 [DOI] [PubMed] [Google Scholar]
- Pogun S, Yararbas G, Nesil T, & Kanit L (2017). Sex differences in nicotine preference. J Neurosci Res, 95(1–2), 148–162. doi: 10.1002/jnr.23858 [DOI] [PubMed] [Google Scholar]
- Powell GL, Cabrera-Brown G, Namba MD, Neisewander JL, Marusich JA, Beckmann JS, & Gipson CD (2019). Economic demand analysis of within-session dose-reduction during nicotine self-administration. Drug Alcohol Depend, 201, 188–196. doi: 10.1016/j.drugalcdep.2019.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell GL, Leyrer-Jackson JM, Goenaga J, Namba MD, Piña J, Spencer S, … Gipson CD (2019). Chronic treatment with N-acetylcysteine decreases extinction responding and reduces cue-induced nicotine-seeking. Physiol Rep, 7(1), e13958. doi: 10.14814/phy2.13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirion R, Richard J, & Wilson A (1994). Muscarinic and nicotinic modulation of cortical acetylcholine release monitored by in vivo microdialysis in freely moving adult rats. Synapse, 17(2), 92–100. doi: 10.1002/syn.890170205 [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, … Levin ED (2008). Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience, 154(3), 885–897. doi: 10.1016/j.neuroscience.2008.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, & Lynch WJ (2014). Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology (Berl), 231(8), 1753–1762. doi: 10.1007/s00213-013-3359-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, & Goldberg SR (1997). Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl), 129(1), 35–43. doi: 10.1007/s002130050159 [DOI] [PubMed] [Google Scholar]
- Shram MJ, Li Z, & Lê AD (2008). Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl), 197(1), 45–58. doi: 10.1007/s00213-007-1003-9 [DOI] [PubMed] [Google Scholar]
- Stolerman IP, & Jarvis MJ (1995). The scientific case that nicotine is addictive. Psychopharmacology (Berl), 117(1), 2–10; discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, & Carroll ME (2016). Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology (Berl), 233(6), 1005–1013. doi: 10.1007/s00213-015-4183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Han W, Wang B, Jiang Q, Solberg-Woods L, Palmer A, & Chen H (2014). Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genes, Brain and Behavior, 13, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, & Jamal A (2018). Tobacco Product Use Among Middle and High School Students - United States, 2011–2017. MMWR Morb Mortal Wkly Rep, 67(22), 629–633. doi: 10.15585/mmwr.mm6722a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, … Deisseroth K (2011). Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron, 72(5), 721–733. doi: 10.1016/j.neuron.2011.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, & London ED (2008). Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res, 10(11), 1653–1661. doi: 10.1080/14622200802412929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Bardo MT, & Beckmann JS (2019). Environmental enrichment and drug value: a behavioral economic analysis in male rats. Addict Biol, 24(1), 65–75. doi: 10.1111/adb.12581 [DOI] [PMC free article] [PubMed] [Google Scholar]


