Abstract
Background
Recent studies have reported conflicting data on the risk of postoperative complications in patients with Crohn’s disease (CD) exposed to ustekinumab (UST) preoperatively. We performed a systematic review and meta-analysis to better assess and quantify the risk of postoperative complications in this population undergoing major abdomino-pelvic surgery.
Methods
We conducted a comprehensive search of multiple electronic databases and conference proceedings (earliest inception through October 2020) to identify studies that reported the postoperative outcomes in CD patients with preoperative UST exposure. We estimated and compared the pooled rates of postoperative complications, including intra-abdominal sepsis, surgical site infection, any infection, any adverse event, readmission, and reoperation.
Results
A total of 5 studies were included in the analysis. The last dose of the drug was at most 16 weeks prior to abdomino-pelvic surgery. A total of 172 CD patients (61% female; median age 35 years) were included. The pooled rate of any complication and any infectious complications was 23.5% (95% confidence interval [CI] 16-33.1) and 20.2% (95%CI 10.3-35), respectively. There was no difference in rates of intra-abdominal sepsis between the UST group (7.2%, 95%CI 3-16.4) and the anti-tumor necrosis factor (TNF) group (11.9%, 95%CI 5.9-22.5; P = 0.4). The rates of readmission and reoperation in the UST group were 17.4% (95%CI 7.9-34) and 14.6% (95%CI 9-22.7), respectively.
Conclusions
The postoperative complication rate in patients with preoperative UST exposure may be similar to that for anti-TNF medication. Preoperative exposure to UST does influence postoperative complication risk. Future prospective studies are needed to validate these findings.
Keywords: Ustekinumab, postoperative, complications, Crohn’s disease
Introduction
Crohn’s disease (CD) is chronic inflammatory condition affecting the gastrointestinal tract [1,2]. There has been significant expansion of medical therapy in last 3 decades following the initial approval of tumor necrosis factor (TNF) inhibitors in 1998 [3,4]. There are now multiple monoclonal antibody mechanisms approved for CD, including TNF inhibitors (infliximab, adalimumab, certolizumab pegol), integrin inhibitors (natalizumab, vedolizumab), and an interleukin 12/23 inhibitor, ustekinumab (UST). UST has shown good efficacy and safety similar to anti-TNF agents and vedolizumab in the treatment of CD [5-8].
Despite the availability of multiple medical therapies, as many as 75% of CD patients will undergo major abdominal surgery in their lifetime [9]. Furthermore, approximately 30-50% of patients are on biologic therapy at the time of surgery [10]. There has been much interest in the impact of biologic medications on postoperative outcomes, given the theoretical risks of immune modulation throughout the perioperative period. Initially anti-TNFs were thought to increase the risk of anastomotic leaks, but later studies refuted these findings and the literature remains controversial [11,12]. Subsequently, retrospective studies assessing the impact of vedolizumab on postoperative outcomes suggested vedolizumab exposure was also a potential risk factor for adverse postoperative outcomes; however, further data has contradicted these early findings [13-15].
Most recently, studies have reported conflicting data on the risk of postoperative complications in patients receiving UST for CD [16-18]. Therefore, we performed a systematic review and meta-analysis to better assess and quantify the risk of postoperative complications in CD patients exposed to UST prior to abdomino-pelvic surgery.
Materials and methods
Search strategy
A comprehensive search of multiple databases was conducted from inception to October 2020. These databases were Ovid MEDLINE®, Scopus, Embase, Cochrane Central Register of Controlled trials, and Cochrane Database of Systematic Reviews. The keywords used were: “ustekinumab”, “complications”, “postoperative”, “infections”, “crohn’s disease”. The MOOSE checklist was followed and is provided in Supplementary Table 1 (163.7KB, pdf) [19,20].
Study selection
In this meta-analysis, we included studies that evaluated postoperative complications in the setting of preoperative UST exposure in adult patients. We included studies irrespective of the sample size, geography and clinical setting, as long as data were provided for analysis.
Our exclusion criteria were pediatric population (age <18 years), and studies published in a language other than English.
Data abstraction and quality assessment
All data from individual studies on outcomes were abstracted on a standardized form by 2 authors (RG, ALL). In addition, 2 authors (RG, BPM) did the quality scoring independently. We contacted primary study authors via email for further information or clarification of data if needed.
In the case of data from the same author or institution(s), we contacted the study authors to ascertain the potential for data duplication. If overlap existed, we included overlapping studies if there were differences in outcomes or data reported. To limit potential bias and assess the influence of this approach, we excluded overlapping outcomes when possible, and performed sensitivity analyses excluding the potentially overlapping data.
We used the Newcastle-Ottawa scale for cohort studies to assess the quality of studies [21]. It consists of 8 questions; details are provided in Supplementary Table 2 (163.7KB, pdf) .
Outcomes
The following outcomes and definitions were included in CD patients who underwent abdomino-pelvic surgery with preoperative UST use: pooled rates of any postoperative complication, any infectious complication, intra-abdominal sepsis, readmission, and reoperation. The term “infectious complication” comprised any infections, including surgical site infections, urinary tract infections or non-surgical site infections such as pneumonia. The definition was consistent across all studies. Because of study variability and limited data, further subgrouping of infectious complications was not possible. Intra-abdominal sepsis was defined as the combination of anastomotic leak and intra-abdominal abscess, as previously reported [16]. For studies that reported comparator populations, these were included in the subgroup analysis if at least 3 studies presented similar comparison groups. All definitions were assigned by individual study authors.
Other data variables
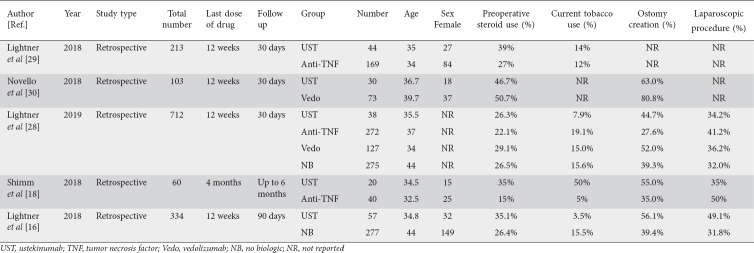
We extracted baseline data on study type, last dose of drug prior to surgery, sex, age, tobacco use and preoperative steroid use. In addition, procedural data including ostomy creation and type of procedure, either laparoscopic or open, were also collected. All the data abstracted are shown in Tables 1 and 2 as population characteristics.
Table 1.
Baseline and operative characteristics of studies included in the analysis
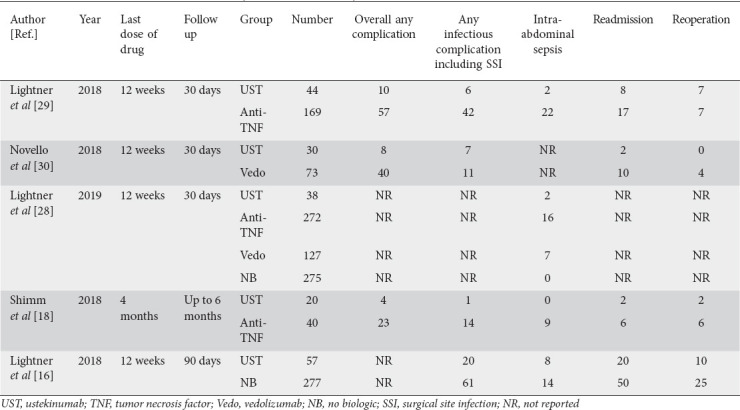
Table 2.
Data on assessed outcomes of each study included in the analysis
Statistical analysis
Meta-analysis techniques were used to calculate the pooled estimates for each outcome, using the logit transformed proportion and random-effects model suggested by DerSimonian and Laird [22]. A continuity correction of 0.5 was added if the incidence of an outcome was zero before statistical analysis [23]. Heterogeneity was assessed between study-specific estimates using the Cochran Q statistical test for heterogeneity and the I2 statistics along with 95% confidence intervals (CI) [24,25]. The I2 value signifies what proportion of the dispersion is true vs. chance [26]: low, moderate, substantial, and considerable heterogeneity were suggested by values of <30%, 30-60%, 61-75%, and >75%, respectively [27]. Publication bias was ascertained qualitatively, by visual inspection of funnel plots, and quantitatively, by the Egger test [28]. A P value of <0.05 was used to define statistically significant difference. For outcomes and variables of interest, further meta-regression analyses were also performed to identify predictors. The choice of variables was based on data availability and all variables were considered for inclusion. Subgroup analysis was performed if the outcome of interest and the variables were reported in at least 3 studies. Comprehensive Meta-Analysis (CMA) software, version 3 (BioStat, Englewood, NJ) was used to perform all analysis.
Results
Search results and population characteristics
The initial search resulted in 94 studies. Sixty records were screened after removing duplicates and 11 full-length articles were assessed after initial screening. Six studies were excluded as the outcome of interest was not present, leaving 5 studies in the final analysis that reported postoperative outcomes in CD patients with preoperative UST use [16,18,29-31]. Fig. 1 shows the schematic diagram of study selection.
Figure 1.
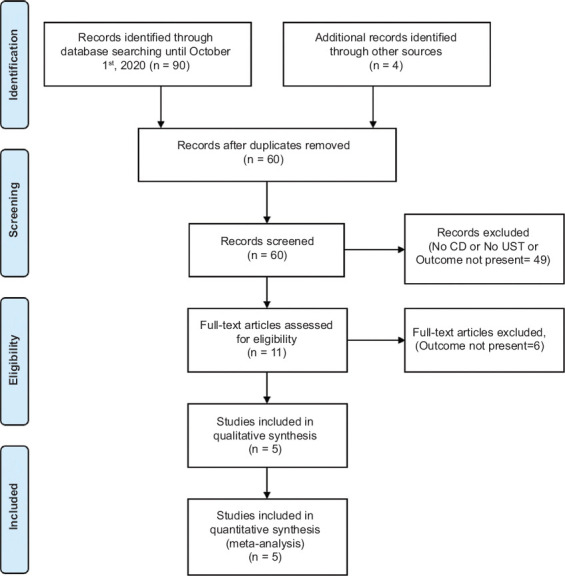
PRISMA flow diagram showing search strategy for meta-analysis
CD, Crohn’s disease; UST, ustekinumab
A total of 1422 patients were included; 189 (13.2%) received preoperative UST, 481 (33.8%) anti-TNF, 200 (14.0%) vedolizumab, and 552 (38.8%) no biologics. Sex was reported in 4 studies in the UST group and the majority of patients were female (61%). The median age was 35 years, with a general age range of 34.5-36.7 years. Two studies compared UST and anti-TNF, one study compared UST and no biologic, one study compared UST and vedolizumab, and one study compared UST, anti-TNF, vedolizumab and no biologic to each other for rates of intra-abdominal sepsis. Patients received UST within 12 weeks of surgery in 4 studies, and within 16 weeks in one study. The follow-up period was 30 days in 3 studies, up to 6 months in one, and 90 days in one study. Among patients with UST exposure, 36.4% of patients were on preoperative steroids and 18.8% were smokers. The surgical procedure ranged from total abdominal colectomy to strictureplasty, depending on the individual patient and operating surgeon. A majority (63.8%) of patients had ostomy creation and only 39.4% had laparoscopic procedures. The baseline and operative characteristics are described in Table 1.
Characteristics and quality of included studies
All the included studies were retrospective in nature. Three studies were multicenter and the other 2 were single-center studies. Based on the Newcastle-Ottawa scale, all studies were of high quality.
Meta-analysis outcomes
A total of 189 patients exposed to UST preoperatively were included in the analysis from 5 studies. Data on assessed outcomes are described in Table 2.
There were 3 studies reporting adverse events in the UST group. The pooled rate of any postoperative complication was 23.5% (95%CI 16-33.1), I2 = 0% (95%CI 0-34.4) (Fig. 2A). The pooled rate of any infectious complications was 20.2% (95%CI 10.3-35.9), I2 = 67 (95%CI 5.1-88.8) from 4 studies (Fig. 2B). Because of the study variability and limited data, further subgrouping of infectious complications was not possible.
Figure 2.
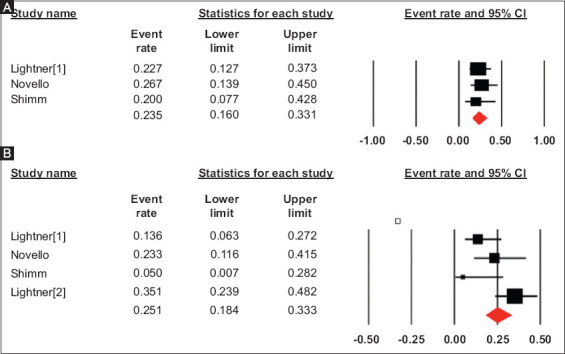
Pooled rate of any postoperative complication (a) and any infectious complication (b) in patients with preoperative ustekinumab use
CI, confidence interval
The pooled rate of intra-abdominal sepsis in UST group was 7.2% (95%CI 3.0-16.4), I2 = 15 (95%CI 0-86.9) from 4 studies, whereas the pooled rate of intra-abdominal sepsis in the anti-TNF group was 11.9% (95%CI 5.9-22.5), I2 = 71 (95%CI 53.3-94.8) from 3 studies (Fig. 3). There was no significant difference between the 2 groups (P = 0.4). We also performed a second analysis including 3 studies that directly compared UST and anti-TNF. The odds ratio of intra-abdominal sepsis comparing preoperative UST use to anti-TNF use was 0.41 (95%CI 0.12-1.23), I2 = 14 (95%CI 0-91; P = 0.11) (Supplementary Fig. 1 (163.7KB, pdf) ).
Figure 3.
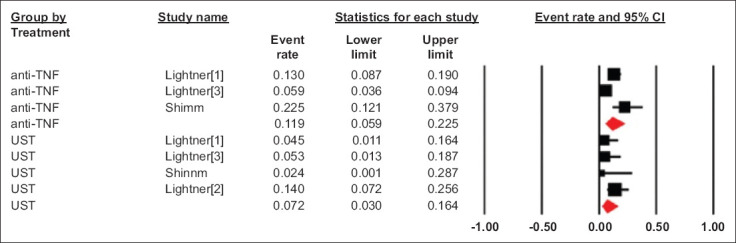
Pooled rate of postoperative intra-abdominal sepsis in patients who received preoperative anti- TNF and UST
TNF, tumor necrosis factor; UST, ustekinumab; CI, confidence interval
The pooled rate of readmission and reoperation in the UST group was 17.4% (95%CI 7.9-34), I2 = 72 (95%CI 20-90) (Fig. 4A) and 14.6% (95%CI 9-22.7), I2 = 12 (95%CI 0-86.8), (Fig. 4B) respectively, from 4 studies.
Figure 4.
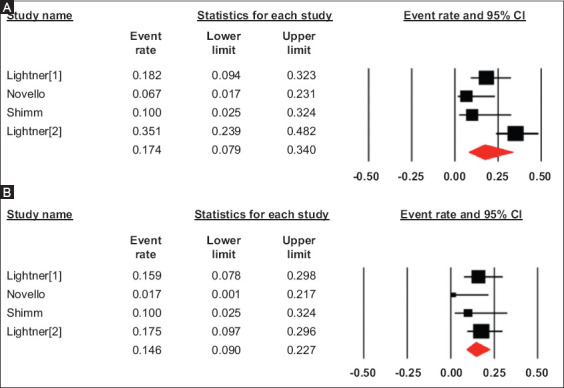
Pooled rates of readmission (A) and reoperation (B) in patients with preoperative ustekinumab use
CI, confidence interval
Meta-regression
Meta-regression was performed for any adverse outcomes, infectious complications, intra-abdominal sepsis and reoperation. The variables included were preoperative steroid use and stoma creation. Preoperative steroid use and ostomy creation were not significant predictors for any of the outcomes. The results of meta-regression with their coefficient and 95%CI are summarized in Supplementary Table 3 (163.7KB, pdf) .
Validation of meta-analysis results
Sensitivity analysis
Sensitivity analysis was performed by excluding one study at a time and analyzing its effect on the main summary estimate. On this analysis, no single study significantly affected the outcome or the heterogeneity.
To assess for potential data duplication from overlapping authors or institution(s), we contacted the study authors and determined that there was patient overlap in 3 studies included in the meta-analysis, but differences in outcomes were reported. One study [30] had only rates of intra-abdominal sepsis whereas another 2 [16,29] contributed to other outcomes. When the first overlapping study was excluded [29], the rates of infectious complications, readmissions and reoperations were 23.4% (95%CI 10.9-43.2), 15.6% (95%CI 4.2-43.4) and 11.4% (95%CI 4.3-26.9), respectively, and when the second overlapping study was excluded [16], the respective rates were 15.6% (95%CI 8.0-28.0), 13.5% (95%CI 7.5-23.1) and 10.9% (95%CI 4.5-24.6). There were no studies with overlap in the outcomes regarding overall complications.
Heterogeneity
We assessed the dispersion of the calculated rates using the I2 percentage values. The calculated I2 values are reported with the pooled results. There was low heterogeneity in overall complication, intra-abdominal sepsis and reoperation outcomes; however, in view of the wide 95%CIs, high heterogeneity cannot be excluded. Heterogeneity was substantial in the infectious complications and readmissions outcomes. This was probably due to baseline patient characteristics, as we were unable to do a subgroup analysis of patients on steroids and other CD medications preoperatively. In addition, the number of procedures and types of surgery also contributed to heterogeneity in the study population. We also acknowledge that I2 values have limited utility to detect heterogeneity when the number of studies is small.
Publication bias
Publication bias was not assessed as fewer than 10 studies were included in the analysis.
Discussion
Our study demonstrated that the surgical complication rate in patients with preoperative UST exposure is similar to that for anti-TNFs. Even though we were not able to directly compare UST with vedolizumab and other biologics, the reported rate of postoperative complications is comparable to that of other biologics, including vedolizumab. Preoperative exposure to ustekinumab does not seem to influence postoperative complication risk. Identifying risk factors for postoperative complications is of paramount importance to optimize surgical and disease-related outcomes. We are likely to see a higher number of patients with medically refractory disease who received UST preoperatively.
The current literature on postoperative complications and biologic use is controversial, limited by observational studies and significant heterogeneity. The reported rates of overall postoperative complications and infectious complications after preoperative anti-TNF exposure are 42.3% and 27.2%, respectively, and the respective rates after preoperative vedolizumab exposure are 30.4% and 22.4% [15,32]. Based on our study, we reported that preoperative UST exposure was associated with similar rates of any adverse event (23.5%, 95%CI 16-33.1), and any infectious complications (20.2%, 95%CI 10.3-35.9). Two systematic reviews did not find any significant risk of postoperative infection and complications in patients exposed to vedolizumab preoperatively, compared to anti-TNF and no biologic therapy [15,32]. On the other hand, the data on anti-TNF are much more conflicting, with some studies reporting higher rates of postoperative complications and others reporting no difference compared to no biologic therapy [12,33-36].
While the current study could only make limited comparator assessments, overall, the data on preoperative UST exposure in CD are reassuring. One study reported a higher rate of intra-abdominal sepsis in UST exposed patient compared to no biologic therapy [16]. In that study, there was significantly more use of preoperative immunomodulators in the UST group, while a greater number of patients underwent laparoscopic procedures and primary anastomoses in the no biologic group, which may have confounded the results. These results were not consistent in a large multicenter study that compared preoperative no biologic, anti-TNF, vedolizumab and UST exposure and did not report an increased risk of intra-abdominal sepsis in the UST-exposed group. On multivariate analysis, preoperative steroid use and combination immunosuppression with steroids remained an independent predictor of intra-abdominal sepsis, suggesting steroids as a likely confounding agent [29,37]. Similarly, Novello et al in a case-matched analysis, did not find a higher risk of postoperative complications (any postoperative complications, infectious complications, readmission and reoperation) in the UST group as compared to preoperative vedolizumab use [31]. Another multicenter study from Canada also did not show any higher risk of postoperative complications in patients who received UST, compared to preoperative anti-TNF use [18]. A similar trend is seen for vedolizumab, with earlier studies reporting a higher risk of adverse events and later studies with larger sample size reporting no greater risk of postoperative complications after preoperative vedolizumab use [13,15]. Taken together, these studies suggest the relative safety of UST in a perioperative setting.
The limitations of this study include the fact that it evaluated only small, tertiary-care referral center studies, potentially restricting the generalizability of the results. Studies were retrospective in nature, potentially contributing to selection bias and confounding. The time period of preoperative UST administration was not consistent throughout the study period. In addition, there was no standardization of the surgical procedure in the studies evaluated. UST patients were more likely to undergo ostomy creation rather than primary anastomosis, given their severe disease and complex phenotype. Small sample sizes and studies with overlapping cohorts further contributed to the low power and low impact of meta-regression results in our study. Because of limited data availability, further analysis with subgroups, such as the number of surgeries already performed, was not possible. Data limitations also did not allow for adequate assessment of confounders, comparator populations, or additional subgroup analysis. We also could not control for other factors such as nutrition, disease severity, prior biologic use, and type of surgery.
The strengths of this review include the systematic literature search with well-defined inclusion criteria, careful exclusion of redundant studies, inclusion of good quality studies, detailed extraction of data and rigorous evaluation of study quality. This is the first meta-analysis reporting the effect of preoperative UST use on postoperative complications in CD.
In conclusion, our meta-analysis demonstrates that the rate of postoperative complications in UST-exposed CD surgical patients may be similar to that of other biologics. Together, perioperative biologics may appear safe. Future prospective studies are needed to validate these findings and determine their influence on surgical decision-making.
Summary Box.
What is already known:
Current data on the risk of postoperative complications in patients receiving ustekinumab for Crohn’s disease undergoing surgery are conflicting
What the new findings are:
In a meta-analysis of 189 patients exposed to ustekinumab preoperatively, the surgical complication rate was similar to that of anti-tumor necrosis factor
The reported rate of postoperative complications after preoperative ustekinumab exposure may be comparable to other biologics, including vedolizumab
Biography
Cleveland Clinic Foundation, Cleveland; Banner University Medical Center/University of Arizona, Tucson; Carilion Roanoke Medical Center, Roanoke, Virginia, USA
Footnotes
Conflict of Interest: Miguel Regueiro: Research support from AbbVie, Janssen, Takeda, Pfizer Unrestricted Educational Grants from AbbVie, Janssen, UCB, Pfizer, Takeda, Salix, Shire Advisory Boards and Consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, and Prometheus.
Amy L. Lightner: Takeda, consultant. Benjamin Click: Consultant for Takeda and TARGET PharmaSolutions along with speakers’ bureau for Takeda
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF therapy in Crohn's disease. Int J Molec Sci. 2018;19:2244. doi: 10.3390/ijms19082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akobeng AK, Zachos M. Tumor necrosis factor-alpha antibody for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2004;1:CD003574. doi: 10.1002/14651858.CD003574.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66:839–851. doi: 10.1136/gutjnl-2015-311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deepak P, Sandborn WJ. Ustekinumab and anti-interleukin-23 agents in Crohn's disease. Gastroenterol Clin North Am. 2017;46:603–626. doi: 10.1016/j.gtc.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Feagan BG, Sandborn WJ, Gasink C, et al. UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 8.Khanna R, Mosli MH, Feagan BG. Anti-integrins in ulcerative colitis and Crohn's disease:what is their place? Dig Dis. 2016;34:153–159. doi: 10.1159/000443132. [DOI] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn's disease. Gut. 2011;60:930–936. doi: 10.1136/gut.2010.227884. [DOI] [PubMed] [Google Scholar]
- 10.Holubar SD, Dozois EJ, Privitera A, Pemberton JH, Cima RR, Larson DW. Minimally invasive colectomy for Crohn's colitis:a single institution experience. Inflamm Bowel Dis. 2010;16:1940–1946. doi: 10.1002/ibd.21265. [DOI] [PubMed] [Google Scholar]
- 11.Lau C, Dubinsky M, Melmed G, et al. The impact of preoperative serum anti-TNFa therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg. 2015;261:487–496. doi: 10.1097/SLA.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narula N, Charleton D, Marshall JK. Meta-analysis:peri-operative anti-TNFa treatment and post-operative complications in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:1057–1064. doi: 10.1111/apt.12313. [DOI] [PubMed] [Google Scholar]
- 13.Lightner AL, Raffals LE, Mathis KL, et al. Postoperative outcomes in vedolizumab-treated patients undergoing abdominal operations for inflammatory bowel disease. J Crohns Colitis. 2017;11:185–190. doi: 10.1093/ecco-jcc/jjw147. [DOI] [PubMed] [Google Scholar]
- 14.Yamada A, Komaki Y, Patel N, et al. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. Am J Gastroenterol. 2017;112:1423–1429. doi: 10.1038/ajg.2017.201. [DOI] [PubMed] [Google Scholar]
- 15.Yung DE, Horesh N, Lightner AL, et al. Systematic review and meta-analysis:vedolizumab and postoperative complications in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:2327–2338. doi: 10.1093/ibd/izy156. [DOI] [PubMed] [Google Scholar]
- 16.Lightner A, Grass F, Alsughayer A, Petersen M, Raffals L, Loftus E. Postoperative outcomes in ustekinumab-treated patients undergoing abdominal operations for Crohn's disease:single-center series. Crohns Colitis 360. 2019;1:otz018. doi:10.1093/crocol/otz018. [Google Scholar]
- 17.Shim HH, Ma C, Kotze PG, Panaccione R. Pre-operative exposure to ustekinumab:a risk factor for postoperative complications in Crohn's disease (CD)? Curr Drug Targets. 2019;20:1369–1372. doi: 10.2174/1389450120666190515094435. [DOI] [PubMed] [Google Scholar]
- 18.Shim HH, Ma C, Kotze PG, et al. Preoperative ustekinumab treatment is not associated with increased postoperative complications in Crohn's disease:a Canadian multi-centre observational cohort study. J Can Assoc Gastroenterol. 2018;1:115–123. doi: 10.1093/jcag/gwy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology:a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Sutton AJ, Abrams KR, Jones DR, et al. Methods for meta-analysis in medical research. John Wiley and Sons Ltd.:New York. 2000:205–228. [Google Scholar]
- 24.Kanwal F, White D. “Systematic reviews and meta-analyses”in Clinical Gastroenterology and Hepatology. Clin Gastroenterol Hepatol. 2012;10:1184–1186. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan BP, Adler DG. Heterogeneity in systematic review and meta-analysis:how to read between the numbers. Gastrointest Endosc. 2019;89:902–903. doi: 10.1016/j.gie.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines:7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 29.Lightner AL, McKenna NP, Alsughayer A, et al. Biologics and 30-day postoperative complications after abdominal operations for Crohn's disease:are there differences in the safety profiles? Dis Colon Rectum. 2019;62:1352–1362. doi: 10.1097/DCR.0000000000001482. [DOI] [PubMed] [Google Scholar]
- 30.Lightner AL, McKenna NP, Tse CS, et al. Postoperative outcomes in ustekinumab-treated patients undergoing abdominal operations for Crohn's disease. J Crohns Colitis. 2018;12:402–407. doi: 10.1093/ecco-jcc/jjx163. [DOI] [PubMed] [Google Scholar]
- 31.Novello M, Stocchi L, Holubar S, et al. Surgical outcomes of patients treated with ustekinumab vs vedolizumab in inflammatory bowel disease:a matched case analysis. Int J Colorectal Dis. 2019;34:451–457. doi: 10.1007/s00384-018-3212-6. [DOI] [PubMed] [Google Scholar]
- 32.Law CCY, Narula A, Lightner AL, McKenna NP, Colombel JF, Narula N. Systematic review and meta-analysis:preoperative vedolizumab treatment and postoperative complications in patients with inflammatory bowel disease. J Crohns Colitis. 2018;12:538–545. doi: 10.1093/ecco-jcc/jjy022. [DOI] [PubMed] [Google Scholar]
- 33.Kopylov U, Ben-Horin S, Zmora O, Eliakim R, Katz LH. Anti-tumor necrosis factor and postoperative complications in Crohn's disease:systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18:2404–2413. doi: 10.1002/ibd.22954. [DOI] [PubMed] [Google Scholar]
- 34.Fumery M, Seksik P, Auzolle C, et al. REMIND study group investigators. Postoperative complications after ileocecal resection in Crohn's disease:a prospective study from the REMIND group. Am J Gastroenterol. 2017;112:337–345. doi: 10.1038/ajg.2016.541. [DOI] [PubMed] [Google Scholar]
- 35.Kotze PG, Saab MP, Saab B, et al. Tumor necrosis factor alpha inhibitors did not influence postoperative morbidity after elective surgical resections in Crohn's disease. Dig Dis Sci. 2017;62:456–464. doi: 10.1007/s10620-016-4400-2. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld G, Qian H, Bressler B. The risks of post-operative complications following pre-operative infliximab therapy for Crohn's disease in patients undergoing abdominal surgery:a systematic review and meta-analysis. J Crohns Colitis. 2013;7:868–877. doi: 10.1016/j.crohns.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen GC, Elnahas A, Jackson TD. The impact of preoperative steroid use on short-term outcomes following surgery for inflammatory bowel disease. J Crohns Colitis. 2014;8:1661–1667. doi: 10.1016/j.crohns.2014.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.