Abstract
Opioid use disorder (OUD) is a national public health concern. Craving, stress, and exposure to conditioned drug cues are implicated in risk of relapse to opioids. Although impaired sleep has been implicated in risk of relapse to other substances of misuse, little research to date has examined the relationship between sleep and craving in individuals with OUD. The present study examined sleep as a moderator of the relationship between craving and stress in a randomized controlled human laboratory study. Individuals with current OUD (N=39) completed a one-night hospital stay to control for factors that may affect craving, stress and sleep. Sleep was monitored via an actigraphy watch and the Pittsburgh Sleep Quality Index (PSQI). The next morning, participants were randomized to a 15-minute laboratory stress task or a no-stress condition. All participants were then exposed to a 15-minute opioid cue paradigm, and craving was measured via self-report. Moderation models were conducted to evaluate whether the sleep indices moderated the relationship between stress condition (independent variable) and craving (dependent variable). Average self-reported nightly sleep duration moderated the relationship between stress condition and craving for participants in the no-stress condition (b = 0.95, p < .05). Specifically, participants in the no-stress condition with lower average nightly sleep duration exhibited significantly greater craving following the opioid cue paradigm. Although preliminary, the findings add to the literature on craving, stress, and sleep among individuals with OUD. Sleep impairment may be an important target of a comprehensive, long-term treatment plan for some patients with OUD.
Keywords: opioids, opioid use disorder, craving, sleep, drug cues, stress
Opioid use disorder (OUD) is a rapidly escalating public health problem in the United States. Compared to other substances of misuse, opioids are responsible for the highest rates of unintentional fatal overdose (Scholl et al., 2018). In 2017, 47,600 overdose deaths (an average of 1 death every 12 minutes) involved opioids (Scholl et al., 2018). Despite the use of pharmacotherapies (i.e., methadone, buprenorphine, naltrexone), the majority of individuals with OUD do not maintain abstinence (Brewer et al., 1998; Comer et al., 2006; Soeffing et al., 2009; Soyka et al., 2008). Given the high rates of mortality and socioeconomic burden associated with OUD, it is critical to identify factors that may increase the risk of relapse.
Craving is thought to be a critical factor in relapse following a period of abstinence (Huhn et al., 2016). A systematic review of 93 studies using ecological momentary assessment (EMA) to evaluate craving and substance use showed a strong link between craving and subsequent substance use, both pre- and post-quit attempts (Serre et al., 2015). While the majority (73%) of these studies evaluated craving in tobacco use disorder, this relationship appears to hold true for OUD as well (Breese et al., 2011). Both stress and exposure to conditioned drug cues (e.g., people, places, and stimuli that remind an individual of using) are robust predictors of craving. In experimental and person-level studies, stress and drug cue exposure have been associated with increased craving for alcohol, tobacco, and cocaine (Bergquist et al., 2010; Fox et al., 2012; Hyman et al., 2007; Preston & Epstein, 2011). Thus, both stress and drug cue exposure may serve to further increase risk of relapse via increased craving.
Recent research has focused on the interaction of these factors, as craving, stress, and exposure to conditioned drug cues in the ‘real world’ are often experienced simultaneously by substance users. Animal research shows that stress via a footshock interacts with ethanol conditioned cues and leads to reinstatement of ethanol-seeking behaviors after extinction (Liu & Weiss, 2002). Stress via yohimbine administration has also been found to potentiate cue-induced reinstatement to heroin among rats (Banna et al., 2010). In contrast to the animal literature, several human laboratory studies using stress provocation tasks find no potentiation of craving following exposure to condition cues for alcohol, marijuana, or prescription opioids (Back et al., 2015; McRae-Clark et al., 2011; Thomas et al., 2010). However, it remains unclear whether and how stress and drug cues interact to trigger craving or relapse.
Sleep is one factor that may influence the associations between craving, stress, and drug cues (Åkerstedt et al., 2012; Benham, 2010; Brower, 2003; Kashani et al., 2012; Lydon-Staley et al., 2017; Sharkey et al., 2011). An extensive body of evidence documents that substance use, in general, has a detrimental impact on sleep (Angarita et al., 2016; Conroy & Arnedt, 2014). However, the relationship between substance use and sleep is bi-directional. Thus, substance use leads to worsened sleep, and worsened sleep increases the risk of subsequent substance use (Ara et al., 2016; Babson et al., 2013; Brower, 2003). Sleep is now recognized as an important factor in stress-health models, and daily stress levels have been shown to predict poorer sleep quality (Drake et al., 2014). Sleep quantity and quality are also associated with drug and alcohol craving, such that patients who report better quality and less fragmented sleep also report less craving (Lydon-Staley et al., 2017).
While opioids have somnolent properties and may induce sleep, some studies show abnormalities in both objective and subjective parameters of sleep following the use of opioids. For example, polysomnography studies of individuals using opioids for the treatment of chronic pain demonstrate decreased total sleep, Rapid Eye Movement (REM) sleep, slow-wave sleep, sleep efficiency, and sleep duration (Dimsdale et al., 2007; Robertson et al., 2016). Acute detoxification from opioids is associated with increased sleep latency, less sleep time, and more sleep disturbance (Beswick et al., 2003). Furthermore, opioids are associated with increased risk of central sleep apnea, which is one mechanism by which fatal respiratory depression occurs (Cao & Javaheri, 2018). Subjective reports of sleep impairment are consistent with objective measures of sleep deficits (Hartwell et al., 2014; Sharkey et al., 2011). While sleep abnormalities in individuals with OUD generally improve over time with the use of buprenorphine or methadone, sleep remains highly impaired among these individuals as compared to healthy controls (Baykara & Alban, 2019; Sarram et al., 2013).
In the current study, we investigated the relationship between sleep, stress, and craving among individuals with OUD. Specifically, we examined the moderating effect of several important sleep indices (i.e., self-reported nightly sleep duration and sleep quality and actigraphy measured minutes to fall asleep, sleep efficiency, wake after sleep onset (WASO) and total time asleep) on the association between stress and craving during a human laboratory task. We hypothesized that decreased sleep quantity and quality would be associated with increased craving in response to the opioid cue. Given previous literature showing that stress is associated with lack of sleep and greater craving, we also expected that the magnitude of this effect would be greater for participants exposed to the stress task versus those in the no-stress condition.
Method
Participants
Participants were 39 individuals with OUD (46.2% female) participating in a larger study on stress, craving, and prescription opioids (Back et al., 2015). Participants were recruited using media advertisements and met Diagnostic and Statistical Manual-Fourth Edition (DSM-IV) criteria for substance dependence on prescription opioid (PO) analgesics. Participants identified as Caucasian (82.1%), African American (5.1%), Hispanic or Latino (5.1%), and American Indian or Alaska Native (7.7%). Exclusion criteria included: 1) past month use of any medication that could interfere with the stress response (e.g., antihypertensive medications, beta-blockers, synthetic glucocorticoid therapy); 2) pregnant or nursing; 3) body mass index (BMI) ≥ 39; 4) major medical problems or comorbid psychiatric conditions that could affect the stress response (e.g., HIV, posttraumatic stress disorder); and 5) use of methadone, naltrexone, or buprenorphine in the past 3 months. Prescription opioids had to be identified as the primary drug of choice for individuals reporting misuse of other substances.
Procedure
The local institutional review board (IRB) approved all study procedures and written informed consent was obtained from participants prior to beginning the study. After an initial screening over the phone, participants came into the office to complete a series of structured clinical interviews, self-report measures, urine drug screen, and breathalyzer tests to determine eligibility. A total of 220 participants were invited to the in-person baseline assessment. Of these participants, 75 were deemed eligible and continued in the study (39 with current OUD and 36 control participants). Because the purpose of the current study was to examine sleep, stress, and craving among individuals with OUD, only participants with OUD were included in analyses. Eligible participants were then scheduled for a one-night hospital visit at the Medical University of South Carolina. Participants were admitted to the hospital at 8:00PM the evening before testing. Opioid withdrawal symptoms were assessed using the Short Opioid Withdrawal Scale (Gossop, 1990) and those who demonstrated active opioid withdrawal were rescheduled. Participants were required to demonstrate three days of abstinence from all substances (other than caffeine and nicotine) via urine drug screen, breathalyzer, and self-report. Nicotine patches were provided to all individuals who smoked cigarettes upon admission to the hospital. For more details on procedures, refer to Back et al., 2015.
Participants completed laboratory testing the next morning and were randomized to either 1) a 15-minute Trier Social Stress Task (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) in which participants were asked to give a speech and perform serial subtractions in front of three individuals unknown to the participant, or 2) a no-stress condition in which participants sat quietly and relaxed for 15 minutes. Following the stress or no-stress task, all participants were exposed to a 15-minute prescription opioid cue paradigm that has been shown in previous studies to elicit craving (Back et al., 2010). The opioid cue paradigm consisted of a 5-minute induction script (participants were guided to think about the last time they used opioids), 5 minutes of exposure to prescription opioid-related paraphernalia, and a 5-minute video of people using prescription opioids. Participants were compensated $150 for completing the study.
Measures
Demographics.
Demographic information was collected via self-report using a form created for this study.
Substance use.
The Timeline Follow-Back (TLFB; Sobell & Sobell, 1992) was used to assess substance use during the month prior to the hospital stay. The Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002) was used to assess for substance use disorders. To screen for substances (opioids, marijuana, oxycodone, cocaine, methamphetamine, methadone, and amphetamines) the On Track Test Cup (Roche Diagnostics) multi-panel urine drug screen (UDS) test was utilized. Breathalyzer tests were administered to determine blood alcohol concentration.
Craving.
Participants rated their level of craving for opioids on a 0–100 visual analogue scale (0 = no craving to 100 = highest craving) immediately before and 5-min, 10-min, 15-min, 30-min, and 60-min after the stress and opioid cue tasks. Peak craving was the maximum craving rating reported post opioid cue task.
Sleep.
Self-report.
The Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) was used to measure nightly sleep duration, subjective sleep quality, latency, sleep efficiency, sleep disturbances, sleep medication, and daytime dysfunction over the past month. Participants rated each of these seven areas of sleep on a 0 to 3 Likert scale (3 reflects the negative extreme on the scale; items are summed to produce a total score). A total score of 5 or greater indicates poor sleep. In addition to calculating a total score, the following items from the PSQI were used independently to assess nightly sleep duration and quality: “How many hours did you get of sleep each night” was used to determine average nightly sleep duration during the past month, and “During the past month, how would you rate your sleep quality overall” was used to assess subjective sleep quality during the past month. Sharkey and colleagues (Sharkey et al., 2011) demonstrated that patients self-reported sleep duration on the PSQI was accurate and reliable when compared to the gold standard of polysomnography (PSG).
Actigraphy.
Participants’ sleep was also monitored during their overnight hospital stay using a Respironics® Actiwatch (a wristwatch sized actigraphy device that collects objective sleep data during the night). The following variables were calculated via the Respironics software: total time to fall asleep (sleep latency), wake after sleep onset (WASO; duration in minutes of nighttime awakenings), total time asleep, and sleep efficiency (number of hours spent in bed divided by the total time asleep).
Data Analysis
In order to determine whether to include control variables in all models analyzed, t-tests and chi-squared analyses were conducted to determine if the stress vs. no-stress conditions varied significantly on demographic variables (gender, ethnicity, and age), days of past month opioid use, and self-reported and actigraphy sleep data. No significant differences were found between groups on any of these variables (see Table 1). Due to lack of differences between conditions, only the IV, DV, and moderator variables were included in the final models (reported in Table 3).
Table 1.
Descriptive Data by Stress vs. No-Stress Condition (N=39)
| Stress (n = 19) | No Stress (n = 20) | Statistical Test | ||
|---|---|---|---|---|
| Gender | χ2 | Φ | ||
| Male | n = 11 (57.9%) | n = 10 (50.0%) | 0.62 | 0.08 |
| Female | n = 8 (42.1%) | n = 10 (50.0%) | ||
| Ethnicity* | 0.17 | 0.35 | ||
| White | n = 16 (84.2%) | n = 16 (80.0%) | ||
| Non-White | n = 3 (15.8%) | n = 4 (20.0%) | ||
| t | df | |||
| Age (years) | 36.47 (12.58) | 33.40 (12.70) | −0.76 | 37 |
| Days of Use (Past Month) | 17.74 (9.27) | 18.65 (8.02) | 0.33 | 37 |
| Average sleep duration (hours) | 6.64 (1.93) | 6.25 (1.54) | −0.70 | 37 |
| Total sleep during hospital stay (minutes) | 353.18 (68.35) | 366.22 (50.99) | 0.17 | 30 |
Note: Ethnicity was dichotomized in these analyses because all but 7 individuals identified as Caucasian
Table 3.
Sleep Indices as Moderators of Stress Condition and Opioid Craving
| Predictor | R2 | β | SE | t | 95% CI | |
|---|---|---|---|---|---|---|
| 1) Average Sleep Duration (PSQI#4) | 0.15 | −0.38 | 0.23 | −1.64 | −0.85 | 0.09 |
| Stress condition | 0.44 | 0.74 | 0.59 | −1.08 | 1.95 | |
| Sleep duration (PSQI#4) × Condition | 0.95* | 0.46 | 2.04 | 0.01 | 1.88 | |
| 2) Sleep quality (PSQI#9) | 0.40 | 0.47 | 0.85 | 0.39 | −0.55 | 1.35 |
| Stress condition | 0.30 | 0.77 | 0.39 | −1.26 | 1.87 | |
| Sleep quality (PSQI#9) × Condition | −1.61 | 0.94 | −1.72 | −3.52 | 0.29 | |
| 3) Actigraphy: Total time asleep | 0.09 | −0.00 | 0.01 | −0.35 | −0.02 | 0.02 |
| Stress condition | −0.07 | 0.98 | −0.08 | −2.09 | 1.94 | |
| Total time asleep × Condition | −0.00 | 0.02 | −0.22 | −0.04 | 0.03 | |
| 4) Actigraphy: Sleep efficiency | 0.01 | −0.02 | 0.04 | −0.57 | −0.12 | .067 |
| Stress condition | −0.10 | 0.97 | −0.10 | −2.10 | 1.90 | |
| Sleep efficiency × Condition | 0.00 | 0.09 | 0.02 | −0.18 | 0.18 | |
| 5) Actigraphy: WASO | 0.16 | 0.05 | 0.03 | 1.96 | −0.00 | 0.11 |
| Stress Condition | 1.26 | 1.79 | 0.70 | −2.40 | 4.93 | |
| WASO × Condition | −0.03 | 0.03 | −0.93 | −0.10 | 0.04 | |
| Actigraphy: Minutes to fall asleep | 0.08 | 0.02 | 0.01 | 1.40 | −0.05 | 0.02 |
| Stress Condition | 0.26 | 1.29 | 0.20 | −2.40 | 2.93 | |
| Minutes to fall asleep × Condition | −0.01 | 0.02 | −0.63 | −0.05 | 0.02 | |
p < .05.
Bivariate correlations were examined between self-report and actigraphy sleep data, stress condition, peak craving post drug cue, and level of opioid use for the stress vs. the no-stress group.
Moderation models were conducted to evaluate whether self-reported and actigraphy sleep indices moderated the relationship between stress condition (stress vs. no-stress) and craving. Following procedures outlined by Hayes (Hayes, 2013) using the PROCESS Macro model 1, analyses were conducted to determine whether sleep quality, average nightly sleep duration, minutes to fall asleep, sleep efficiency, WASO, and total time asleep were moderators of the relation between stress condition and peak post-cue craving following the opioid cue paradigm. Statistical significance was determined by 95% confidence intervals that do not contain zero. Significant interactions were probed according to Johnson-Neyman procedures via the PROCESS script for SPSS (Hayes, 2013). This technique isolates points of significance of the moderator for the conditional effect of the predictor on the dependent variable allowing for a more precise examination of moderator effects (Hayes, 2013).
Results
Descriptive Statistics
Descriptive data for participants as a function of condition (stress vs. no-stress) are included in Table 1. Overall, participants reported considerable sleep difficulties. According to the PSQI diagnostic criteria, 100% of participants reported global sleep quality scores suggestive of poor sleep quality (≥5 on the PSQI). On average, participants reported sleeping 6.4 (SD = 1.7) hours per night and slept an average of 6.0 (SD = 1.0) hours during their hospital stay the night prior to the laboratory procedures.
With regard to baseline opioid use, participants reported using opioids an average of 18.1 (SD = 8.5) days per month, using approximately 2.8 (SD = 2.6) prescription opioid pills per day, and using 4.2 pills on average per using day. No significant differences in opioid use characteristics were observed between participants in the stress and the no-stress group.
Bivariate Analyses
Bivariate correlations were examined between self-report and actigraphy sleep data, stress condition, peak craving post drug cue, and level of opioid use. Full sample correlations were assessed first followed by correlations in the stress vs. no-stress group. No self-report or actigraphy sleep variables were significantly associated with peak craving post drug cue in the full sample or in the stress condition. In the no-stress condition (see Table 2), average nightly sleep duration was associated with peak craving post drug cue (r = −0.46, p = .051).
Table 2.
Correlations between self-report and actigraphy sleep variables, peak craving post drug-cue, and days of opioid use in the no-stress condition
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. PSQI #4 (Total sleep duration) | 1 | |||||||
| 2. PSQI #9 (Sleep quality) | −.58** | 1 | ||||||
| 3. Actigraphy: Minutes to fall asleep | .17 | −.35 | 1 | |||||
| 4. Actigraphy: Sleep efficiency | −.31 | .28 | −.80** | 1 | ||||
| 5. Actigraphy: Total time asleep | −.31 | .40 | −.80** | .96** | 1 | |||
| 6. Actigraphy: Wake after sleep onset | −.29 | .25 | −.12 | .36 | −.24 | 1 | ||
| 7. Peak craving post drug-cue | −.46t | .35 | .31 | −.09 | −.02 | .42 | 1 | |
| 8. Past month opioid use days | −.42 | .00 | −.16 | .39 | .27 | −.03 | .07 | 1 |
Note.
p < .05.
p < .01.
.051.
Moderation Analyses
PROCESS model 1 (Hayes, 2013) was utilized to test two-way interactions. The first model examined average nightly sleep duration and the moderating variable. Stress condition (b = −0.38, p > .05) and average nightly sleep duration (b = 0.45, p > .05) were not significantly associated with peak post-cue craving. The interaction between stress condition × average nightly sleep duration (b = 0.95, p < .05) was significantly associated with peak post-cue craving (see Table 3). The results indicate that average nightly sleep duration moderated the relation between stress condition and peak post-cue craving for the no-stress group, but not the stress group. Among participants in the no-stress condition, less average nightly sleep duration was associated with increased craving following the opioid cue paradigm (see Figure 1). No other sleep variables examined (i.e., self-reported sleep quality and actigraphy measured total sleep, sleep onset, sleep efficiency, and WASO) were significant moderators of the relation between stress condition and peak post-cue craving (see Table 3).
Figure 1.
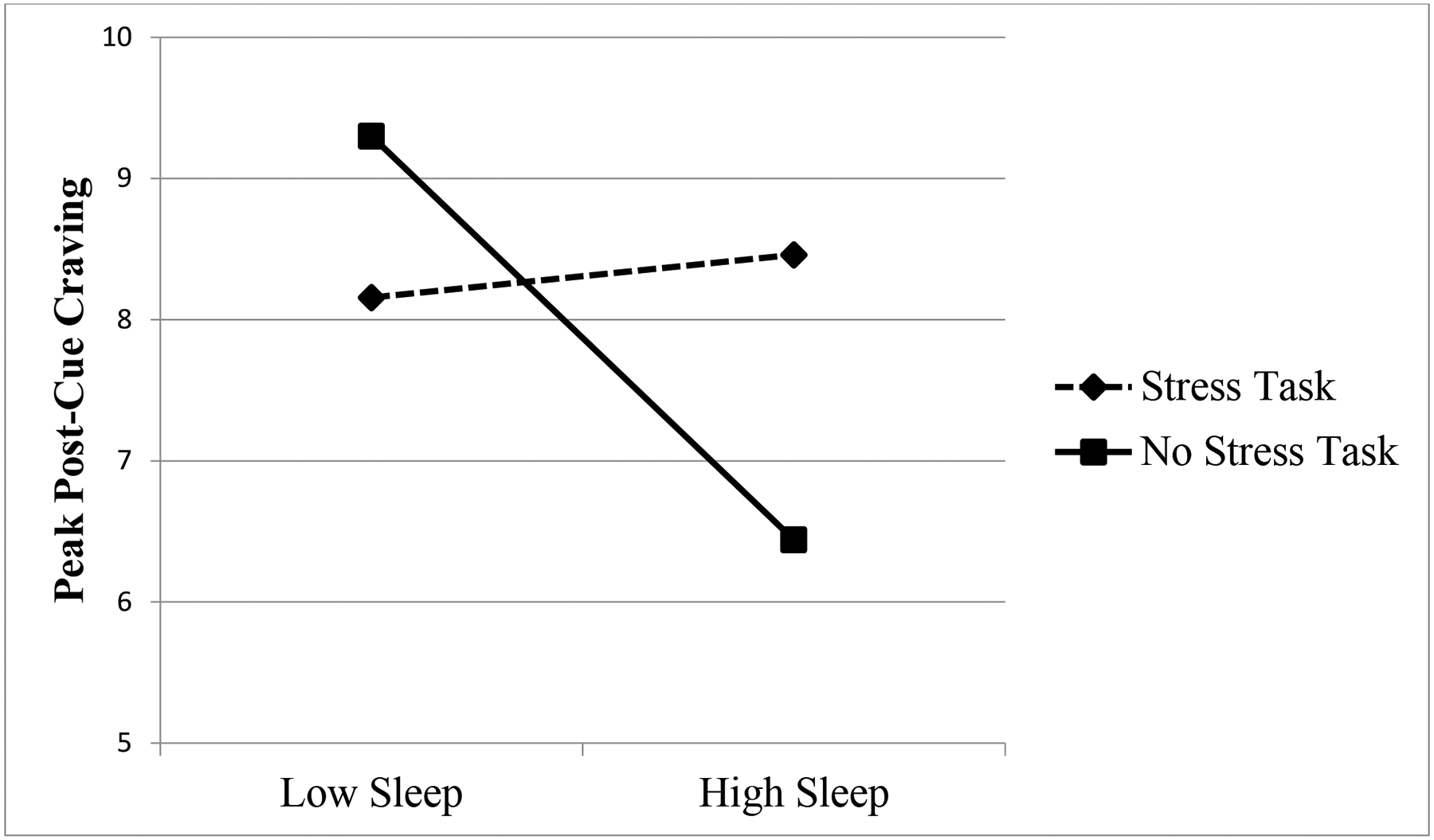
Interaction Between Stress Condition and Sleep on Post-Cue Opioid Craving.
*Note: Low sleep and high sleep represent 1 SD above and below the mean, respectively.
Discussion
This study examined the associations between several factors linked to increased risk for opioid relapse, namely craving, stress, and sleep. While previous studies have examined these factors in other substances of misuse, this is one of the first to focus on prescription opioids. Given the significant morbidity and mortality associated with OUD, a greater understanding of how these factors interact is of critical importance.
Consistent with the literature, the current findings revealed significant sleep deficits in individuals with current prescription OUD (Beswick et al., 2003; Dimsdale et al., 2007; Robertson et al., 2016). Disrupted and fragmented sleep patterns may put individuals with OUD at greater risk of relapsing during a quit attempt (Brower, 2003; Lydon-Staley et al., 2017). Interestingly, only average nightly sleep duration (as measured by the PSQI item #4) was significantly associated with craving post drug cue in the present sample. Sleep variables measured via actigraphy the night before the laboratory task (total sleep, sleep latency, WASO, and sleep efficiency) and self-reported sleep quality were not associated with craving post drug cue.
Though self-report and actigraphy sleep data were not correlated in the present study, both subjective and objective data collected as part of this study indicate that participants with OUD have poor sleep quality and quantity. Previous research has documented discrepancies between self-reported and actigraphy assessed sleep measures (Carney et al., 2004; Lauderdale et al., 2008; Short et al., 2012; Silva et al., 2007; Zinkhan et al., 2014), and limitations of both methods data have been noted in previous studies (Hartwell et al., 2014). In the present study, discrepancies may have been due in part to differential settings and time frames assessed. The PSQI measured self-reported average nightly sleep duration over the past month, whereas the actigraphy data measured total time asleep, minutes to fall asleep, WASO, and sleep efficiency during an overnight hospital stay. Though the overnight stay helped control for outside factors that may affect craving, stress, and sleep, this data may not provide an accurate reflection of sleep indices in a person’s real-life environment. Conversely, self-report sleep measures may be subject to retrospective reporting biases (Lauderdale et al., 2008). Due to the inherent limitations of both types of measures, previous research has encouraged the collection of both self-report and objective sleep data (Hartwell et al., 2014).
The present study also examined self-reported and actigraphy sleep indices as moderators of the relationship between stress and craving following an opioid cue paradigm. Contrary to our hypothesis, average nightly sleep duration was not associated with post-cue craving in the full sample. However, the hypothesis that sleep quantity would moderate the association between stress condition and craving was partially supported. Average nightly sleep duration moderated the relationship between stress and craving among participants in the no-stress group, but not the stress group. That is, individuals in the no-stress condition who reported low nightly sleep duration exhibited significantly higher post-cue craving as compared to those with high nightly sleep duration (see Figure 1). Previous research shows that poor sleep exacerbates stress and craving in other substances of misuse. Thus, we expected that participants with low nightly sleep duration who were randomized to the stress condition would exhibit the highest levels of craving when exposed to the condition drug cues. However, the current findings revealed that nightly sleep duration did not moderate the effect of stress and craving for participants in the stress condition. It is possible that when high levels of stress are present, adequate sleep is insufficient to buffer the impact of stress on craving. That is, regardless of sleep time and quality, a robust stressor alone may be sufficient to induce high levels of craving among individuals with OUD.
Alternatively, the stressor task utilized in this study could explain this result. While the TSST has been shown to increase stress and craving among some studies of substance users, other studies fail to observe an association between stress response to the TSST and reactivity to drug cues (Back et al., 2010, 2015; Buchmann et al., 2010). It has been suggested that because the TSST tends to yield a robust craving and stress response, there may be a ceiling effect (Back et al., 2015). Due to this potential ceiling effect, level of sleep may have been unlikely to differentially affect craving. Future studies should utilize different stress tasks in order to investigate the relations between sleep, stress, and craving. For example, Sinha and colleagues (Sinha et al., 2009) compared a social stress task similar to the TSST (i.e., participants give a five-minute video-recorded speech) to a five minute-guided imagery task consisting of exposure to a recording of a recent stressful situation and found that the imagery task led to fear, sadness, and anger whereas the social stress task elicited only fear. Thus, the guided-imagery task may better approximate real-life stress encountered in the lives of OUD patients.
Among participants in the no-stress condition, average nightly sleep duration moderated the relation between stress condition and cue-induced craving. Participants in this condition who reported lower nightly sleep duration exhibited significantly greater opioid craving, while participants who reported higher nightly sleep exhibited significantly lower opioid craving. This finding is consistent with previous research showing that poor sleep increases craving among OUD patients. Lydon-Staley and colleagues (Lydon-Staley et al., 2017) used EMA to examine the relationship between sleep and craving among 68 opioid-dependent patients in residential treatment. They found that participants reported significantly less craving on days when they had better quality of sleep. Taken together, the findings suggest that getting a good night’s sleep serves as a protective factor for cravings among individuals with OUD, and that getting a poor night’s sleep may be a risk factor for increased craving levels, even in the absence of stress.
Although not all research supports the hypothesis that increased craving leads to subsequent substance use, a review by Serre and colleagues (Serre et al., 2015) found evidence for increased risk of use and relapse following craving. Therefore, decreasing craving following exposure to conditioned drug cues represents a worthy target of substance use interventions. One possible method of reducing craving and subsequent substance use and related problems may be to focus on improving average nightly sleep duration. Bootzin and Stevens (Bootzin & Stevens, 2005) developed a six-session sleep treatment in a group format to examine whether treating sleep would lower risk of relapse among adolescents in substance use treatment. Participants were 55 adolescents (ages 13 to 19) experiencing sleep difficulties who had recently completed outpatient substance use treatment. Results revealed that participants who completed at least four treatment sessions demonstrated significant improvements in sleep efficiency, sleep onset latency, total sleep time, number of awakenings, and soundness of sleep. Pilot data from this trial suggests that participants completing the sleep treatment demonstrated decreased substance-related problems at 12-month follow-up, whereas non-completers showed continued increases in substance-related problems. Craving was not measured as a part of the pilot trial, making it difficult to determine the mechanism underlying the relationship between sleep treatment and substance-related problems. Future research is needed to determine whether providing sleep treatment to individuals with OUD leads to decreased craving and decreased substance use and related problems.
Cognitive Behavioral Therapy for Insomnia (CBT-I) utilizes similar components to improve sleep quality and has been shown to improve sleep in a variety of populations including adolescents, adults, and older adults (for reviews see (Irwin et al., 2006; Okajima et al., 2011; Wang et al., 2018). CBT-I has been utilized as an approach to treat insomnia among patients with alcohol use disorder with promising results. Currie and colleagues (Currie et al., 2004) found that five sessions of CBT-I led to significantly improved sleep efficiency and quality with fewer awakenings at 3 and 6-month follow-up among patients with alcohol use disorder. However, no effect was found for subsequent alcohol relapse in this sample. Additionally, recent research has focused on adapting CBT-I for mobile phones so that the treatment can be delivered remotely, with preliminary results suggesting improvements in sleep difficulties among cannabis users after two weeks of treatment (Babson et al., 2013). Future research is needed to determine whether employing CBT-I or a similar treatment as an adjunct to opioid use treatment would improve outcomes for OUD patients.
Several limitations of this study should be noted. The sample size is small and may have prevented the detection of additional significant effects. Because the study used DSM-IV-TR criteria to assess OUD, OUD severity was not accounted for in the current analyses. Thus, future research may want to examine how results might vary if OUD severity is considered. As mentioned above, the use of the TSST may have produced a ceiling effect, making it more difficult to examine the differential effects of sleep on drug craving among individuals in the stress condition. Additionally, the TSST represents an acute, one-time stressor, which may not translate to the more chronic stressors often experienced in the daily lives of substance users. It would be worthwhile for future studies to include other stress tasks that may better approximate daily stressors or chronic stressors experienced by individuals with OUD and to include a more comprehensive assessment of participants overall daily (persistent) stress. Furthermore, actigraphy sleep data was collected during an overnight hospital stay. Sleeping in an unfamiliar environment, along with a change in evening routine, may have impacted sleeping patterns.
Despite these limitations, this is the first study to investigate sleep as a moderator of the relationship between stress and craving among individuals with OUD. The finding that average nightly sleep duration differentially effects levels of craving following exposure to an opioid cue paradigm for those in the no-stress condition has important clinical implications, as it suggests that nightly amount of sleep may serve as a protective or risk factor for craving. Because increased craving has been linked to future substance use and relapse, decreasing levels of craving and increasing sleep are important intervention targets. As such, it would be beneficial to assess sleep difficulties before, during, and after OUD treatment, and provide sleep education and treatment for those experiencing insomnia and other sleep-related problems.
Public Significance:
The results of this study suggest that sleep impairment may contribute to increased opioid craving among individuals with OUD. Improving sleep may be an important target of a comprehensive, long-term treatment plan for some patients with OUD.
Acknowledgements:
This research was supported by grants from the National Institute on Drug Abuse (K23 DA021228, NIAA, T32 DA007288), the National Institute on Alcohol Abuse and Alcoholism (T32 AA747430) and the National Center for Advancing Translational Sciences (UL1 TR000062).
This research was supported by grants from the National Institute on Drug Abuse (K23 DA021228, K02 DA039229, T32 DA007288), the National Institute on Alcohol Abuse and Alcoholism (T32 AA747430) and the National Center for Advancing Translational Sciences (UL1 TR000062). These funding sources had no other role other than financial support.
Footnotes
Part of the data in the manuscript were presented as a poster presentation by Dr. Teeters at the Association for Behavioral and Cognitive Therapies (ABCT) conference in Washington, DC in Fall 2018. The data and ideas in this manuscript were not presented, shared, or discussed in any other venue.
All authors declare no conflicts of interest.
References
- Åkerstedt T, Orsini N, Petersen H, Axelsson J, Lekander M, & Kecklund G (2012). Predicting sleep quality from stress and prior sleep–a study of day-to-day covariation across six weeks. Sleep Medicine, 13(6), 674–679. [DOI] [PubMed] [Google Scholar]
- Angarita GA, Emadi N, Hodges S, & Morgan PT (2016). Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: A comprehensive review. Addiction Science & Clinical Practice, 11(1), 9. 10.1186/s13722-016-0056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara A, Jacobs W, Bhat IA, & McCall WV (2016). Sleep Disturbances and Substance Use Disorders: A Bi-Directional Relationship. Psychiatric Annals, 46(7), 408–412. 10.3928/00485713-20160512-01 [DOI] [Google Scholar]
- Babson KA, Boden MT, Harris AH, Stickle TR, & Bonn-Miller MO (2013). Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. Journal of Substance Abuse Treatment, 44(4), 438–443. 10.1016/j.jsat.2012.08.224 [DOI] [PubMed] [Google Scholar]
- Back SE, Gros DF, Price M, LaRowe S, Flanagan J, Brady KT, Davis C, Jaconis M, & McCauley JL (2015). Laboratory-induced stress and craving among individuals with prescription opioid dependence. Drug and Alcohol Dependence, 155, 60–67. 10.1016/j.drugalcdep.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, & Brady KT (2010). Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug and Alcohol Dependence, 106(1), 21–27. 10.1016/j.drugalcdep.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, & See RE (2010). Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behavioural Brain Research, 208(1), 144–148. 10.1016/j.bbr.2009.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykara S, & Alban K (2019). The effects of buprenorphine/naloxone maintenance treatment on sexual dysfunction, sleep and weight in opioid use disorder patients. Psychiatry Research, 272, 450–453. 10.1016/j.psychres.2018.12.153 [DOI] [PubMed] [Google Scholar]
- Benham G (2010). Sleep: An important factor in stress-health models. Stress and Health, 26(3), 204–214. 10.1002/smi.1304 [DOI] [Google Scholar]
- Bergquist KL, Fox HC, & Sinha R (2010). Self-reports of interoceptive responses during stress and drug cue-related experiences in cocaine- and alcohol-dependent individuals. Experimental and Clinical Psychopharmacology, 18(3), 229–237. 10.1037/a0019451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick T, Best D, Rees S, Bearn J, Gossop M, & Strang J (2003). Major disruptions of sleep during treatment of the opiate withdrawal syndrome: Differences between methadone and lofexidine detoxification treatments. Addiction Biology, 8(1), 49–57. 10.1080/1355621031000069882 [DOI] [PubMed] [Google Scholar]
- Bootzin RR, & Stevens SJ (2005). Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical Psychology Review, 25(5), 629–644. 10.1016/j.cpr.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, & Heilig M (2011). Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & Therapeutics, 129(2), 149–171. 10.1016/j.pharmthera.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, & Fleming CB (1998). A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction (Abingdon, England), 93(1), 73–92. [PubMed] [Google Scholar]
- Brower KJ (2003). Insomnia, alcoholism and relapse. Sleep Medicine Reviews, 7(6), 523–539. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Laucht M, Schmid B, Wiedemann K, Mann K, & Zimmermann U (2010). Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. Journal of Psychopharmacology, 24(2), 247–255. 10.1177/0269881108095716 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Cao M, & Javaheri S (2018). Effects of Chronic Opioid Use on Sleep and Wake. Sleep Medicine Clinics, 13(2), 271–281. 10.1016/j.jsmc.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Carney CE, Lajos LE, & Waters WF (2004). Wrist Actigraph Versus Self-Report in Normal Sleepers: Sleep Schedule Adherence and Self-Report Validity. Behavioral Sleep Medicine, 2(3), 134–143. 10.1207/s15402010bsm0203_2 [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, & O’Brien CP (2006). Injectable, Sustained-Release Naltrexone for the Treatment of Opioid Dependence: A Randomized, Placebo-Controlled Trial. Archives of General Psychiatry, 63(2), 210. 10.1001/archpsyc.63.2.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DA, & Arnedt JT (2014). Sleep and Substance Use Disorders: An Update. Current Psychiatry Reports, 16(10), 487. 10.1007/s11920-014-0487-3 [DOI] [PubMed] [Google Scholar]
- Currie SR, Clark S, Hodgins DC, & el-Guebaly N (2004). Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction, 99(9), 1121–1132. 10.1111/j.1360-0443.2004.00835.x [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Norman D, DeJardin D, & Wallace MS (2007). The effect of opioids on sleep architecture. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 3(1), 33–36. [PubMed] [Google Scholar]
- Drake CL, Pillai V, & Roth T (2014). Stress and Sleep Reactivity: A Prospective Investigation of the Stress-Diathesis Model of Insomnia. Sleep, 37(8), 1295–1304. 10.5665/sleep.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition SCID-I/P; New York, NY. [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, & Sinha R (2012). Prazosin Effects on Stress- and Cue-Induced Craving and Stress Response in Alcohol-Dependent Individuals: Preliminary Findings: THE EFFICACY OF PRAZOSIN IN REDUCING ALCOHOL CRAVING. Alcoholism: Clinical and Experimental Research, 36(2), 351–360. 10.1111/j.1530-0277.2011.01628.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M (1990). The development of a short opiate withdrawal scale (SOWS). Addictive Behaviors, 15(5), 487–490. 10.1016/0306-4603(90)90036-W [DOI] [PubMed] [Google Scholar]
- Hartwell EE, Pfeifer JG, McCauley JL, Moran-Santa Maria M, & Back SE (2014). Sleep disturbances and pain among individuals with prescription opioid dependence. Addictive Behaviors, 39(10), 1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press. [Google Scholar]
- Huhn AS, Harris J, Cleveland HH, Lydon DM, Stankoski D, Cleveland MJ, Deneke E, & Bunce SC (2016). Ecological momentary assessment of affect and craving in patients in treatment for prescription opioid dependence. Brain Research Bulletin, 123, 94–101. 10.1016/j.brainresbull.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong K-IA, Doebrick C, & Sinha R (2007). Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Experimental and Clinical Psychopharmacology, 15(2), 134–143. 10.1037/1064-1297.15.2.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole JC, & Nicassio PM (2006). Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychology, 25(1), 3–14. 10.1037/0278-6133.25.1.3 [DOI] [PubMed] [Google Scholar]
- Kashani M, Eliasson A, & Vernalis M (2012). Perceived stress correlates with disturbed sleep: A link connecting stress and cardiovascular disease. Stress, 15(1), 45–51. 10.3109/10253890.2011.578266 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, & Rathouz PJ (2008). Self-reported and measured sleep duration: How similar are they? Epidemiology (Cambridge, Mass.), 19(6), 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, & Weiss F (2002). Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(18), 7856–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon-Staley DM, Cleveland HH, Huhn AS, Cleveland MJ, Harris J, Stankoski D, Deneke E, Meyer RE, & Bunce SC (2017). Daily sleep quality affects drug craving, partially through indirect associations with positive affect, in patients in treatment for nonmedical use of prescription drugs. Addictive Behaviors, 65, 275–282. 10.1016/j.addbeh.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima I, Komada Y, & Inoue Y (2011). A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia: CBT for insomnia: a meta-analysis. Sleep and Biological Rhythms, 9(1), 24–34. 10.1111/j.1479-8425.2010.00481.x [DOI] [Google Scholar]
- Preston KL, & Epstein DH (2011). Stress in the daily lives of cocaine and heroin users: Relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology, 218(1), 29–37. 10.1007/s00213-011-2183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JA, Purple RJ, Cole P, Zaiwalla Z, Wulff K, & Pattinson KTS (2016). Sleep disturbance in patients taking opioid medication for chronic back pain. Anaesthesia, 71(11), 1296–1307. 10.1111/anae.13601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarram S, Debrabant R, Fatseas M, Daulouède JP, Sagaspe P, Taillard J, Philip P, & Auriacombe M (2013). Changes in sleep quality and alertness in opiate-dependent subjects after stable methadone and buprenorphine maintenance treatment. A pilot exploratory report. A Pilot Exploratory Report. Heroin Addict Relat Clin Probl, 15, 39–44. [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, & Baldwin G (2018). Drug and Opioid-Involved Overdose Deaths—United States, 2013–2017. MMWR. Morbidity and Mortality Weekly Report, 67(5152). 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Swendsen J, & Auriacombe M (2015). Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug and Alcohol Dependence, 148, 1–20. 10.1016/j.drugalcdep.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, & Stein MD (2011). Assessing sleep in opioid dependence: A comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug and Alcohol Dependence, 113(2–3), 245–248. 10.1016/j.drugalcdep.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright H, & Carskadon MA (2012). The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Medicine, 13(4), 378–384. [DOI] [PubMed] [Google Scholar]
- Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, Walsleben JA, Baldwin CM, & Quan SF (2007). Relationship between reported and measured sleep times: The sleep heart health study (SHHS). Journal of Clinical Sleep Medicine, 3(06), 622–630. [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, & Siedlarz KM (2009). Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 34(5), 1198–1208. 10.1038/npp.2008.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline Follow-Back. In Litten RZ & Allen JP (Eds.), Measuring Alcohol Consumption (pp. 41–72). Humana Press. http://link.springer.com/10.1007/978-1-4612-0357-5_3 [Google Scholar]
- Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, & Rastegar DA (2009). Buprenorphine maintenance treatment in a primary care setting: Outcomes at 1 year. Journal of Substance Abuse Treatment, 37(4), 426–430. 10.1016/j.jsat.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, & Kuefner H (2008). Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: Results from a randomized study. The International Journal of Neuropsychopharmacology, 11(5), 641–653. 10.1017/S146114570700836X [DOI] [PubMed] [Google Scholar]
- Wang K, Burton CL, & Pachankis JE (2018). Depression and Substance Use: Towards the Development of an Emotion Regulation Model of Stigma Coping. Substance Use & Misuse, 53(5), 859–866. 10.1080/10826084.2017.1391011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkhan M, Berger K, Hense S, Nagel M, Obst A, Koch B, Penzel T, Fietze I, Ahrens W, & Young P (2014). Agreement of different methods for assessing sleep characteristics: A comparison of two actigraphs, wrist and hip placement, and self-report with polysomnography. Sleep Medicine, 15(9), 1107–1114. [DOI] [PubMed] [Google Scholar]


