Abstract
Introduction
Many patients demonstrate an insufficient endogenous luteinising hormone (LH) concentration during ovarian stimulation. With traditional fixed or flexible gonadotropin-releasing hormone (GnRH) antagonist protocols, antagonist administration may further reduce LH activity. Previously, we proved that LH can be used as an indicator for the timing and dosage of antagonist. Patients with a persistently low LH concentration during ovarian stimulation may not require antagonists, whereas antagonist administration can affect reproductive outcomes. To further explore this hypothesis, we designed a randomised clinical trial to compare the LH-based flexible GnRH antagonist protocol with traditional flexible GnRH antagonist protocol in women with normal ovarian response.
Methods and analysis
This study was a multicentre, parallel, prospective, randomised, non-inferiority study. The primary efficacy endpoint was cumulative ongoing pregnancy rate per cycle. The study aimed to prove the non-inferiority of cumulative ongoing pregnancy rate per cycle with an LH-based flexible GnRH antagonist protocol versus traditional flexible GnRH antagonist protocol. Secondary endpoints were the high-quality embryo rate, clinical pregnancy rate and cancellation rate. Differences in cost-effectiveness and adverse events were evaluated. The cumulative ongoing pregnancy rate per cycle in women with normal ovarian response was 70%. Considering that a non-inferiority threshold should retain 80% of the clinical effect of a control treatment, a minimal clinical difference of 14% (one-sided: α, 2.5%; β, 20%) and a total of 338 patients were needed. Anticipating a 10% drop-out rate, the total number of patients required was 372.
Ethics and dissemination
This trial has been approved by the Institutional Ethical Committee of Beijing Chao-Yang hospital. All participants in the trial will provide written informed consent. The study will be conducted according to the principles outlined in the Declaration of Helsinki and its amendments. Results of this study will be disseminated in peer-reviewed scientific journals.
Trial registration number
ChiCTR1800018077.
Keywords: sex steroids & HRT, change management, health economics
Strengths and limitations of this study.
This is the first randomised controlled trial to examine the efficacy and safety of a novel luteinising hormone-based protocol in women with normal ovarian response who are undergoing in vitro fertilisation treatment.
This study was a multicentre, parallel, prospective, randomised, non-inferiority study, all investigators are required to undertake mandatory training in the protocol.
The individual eligibility criteria used in our study also limit the bias of advanced age, which is associated with a higher risk of adverse outcomes.
The sample size calculation is based on a difference of 10% in cumulative ongoing pregnancy rate between the two groups, thus, a smaller difference in the ongoing pregnancy rate may not be detected.
Introduction
There is evidence that a sufficient concentration of luteinising hormone (LH) is necessary for normal follicular growth and oocyte maturation. A low LH concentration during ovarian stimulation can adversely affect follicular morphology, quality and maturation, determining meiotic status and fertilisation ability. Inversely, clinical evidence from multiple LH measurements revealed that low LH levels were associated with increased early pregnancy loss and decreased the CLBRs per oocyte retrieval cycle.1–5 Extensive clinical trials have shown that a serum LH concentration of ≥1.2 IU/L is necessary to provide adequate LH support to follicle-stimulating hormone (FSH)-induced follicular development.2 6 7 Some studies have demonstrated that for optimal cyclic follicular development, the serum LH concentration should be between 1.2 IU/L and 5.0 IU/L.4–6 Other studies suggest that the indications for use of LH with gonadotropin-releasing hormone (GnRH) antagonists during ovarian follicular development are the mid-follicular period (day 6), an estradiol (E2) concentration of <200 pg/mL, no follicles>10 mm in size, an endometrial thickness of <6 mm and a baseline serum LH concentration of <1.2 IU/mL on day 6.8–10 However, only a small number of studies have been performed, and there are no clear guidelines with regard to the optimal serum LH concentration or supplementation time; thus, these are areas worthy of further study.
Although FSH can induce follicular growth in the absence of LH, follicles may have developmental defects, such as abnormally reduced E2 production and a lack of lutealisation and rupture capacity on the trigger day.11 12 GnRH antagonist protocols have been widely used for ovarian stimulation. Use of GnRH antagonists during the late follicular phase can prevent premature LH surges.13–15 A significant proportion of patients demonstrate an insufficient endogenous LH concentration during ovarian stimulation. However, these patients cannot be distinguished before ovarian stimulation using baseline characteristics alone. For these patients, with either traditional fixed or flexible GnRH antagonist protocols, administration of an antagonist might reduce LH activity further and lead to poor reproductive outcomes.16 17 Hence, these patients may not require antagonist supplementation.18 19 However, there are no clear guidelines regarding the use of GnRH antagonists based on serum LH concentrations during ovarian stimulation.
Our previous proof-of-concept study proved that the LH concentration can be used as an indicator for the timing and dosage of antagonist supplementation, and have demonstrated that serum LH of 4 IU/L could be used as the cut-off value, according to our former experience and observations that patients with sustained low LH levels (LHmax <4 IU/L) during ovarian stimulation might not require antagonist administration.19 Among women who received GnRH antagonists during ovarian stimulation, reproductive outcomes were similar, irrespective of whether the highest LH concentration (LHmax) was ≥4 IU/L or <4 IU/L. Conversely, patients with a sustained low LH concentration (LHmax of <4 IU/L) during ovarian stimulation might not require antagonist administration. In fact, in these patients, antagonist administration can adversely affect reproductive outcomes.19 To further confirm our results, a randomised controlled trial was performed to prospectively compare the efficacy of a novel LH-based treatment regimen with a traditional flexible GnRH antagonist protocol during ovarian stimulation. These results provide clinicians with new information on when to introduce antagonists and the appropriate dosage of GnRH antagonist.
Methods and analysis
Study design
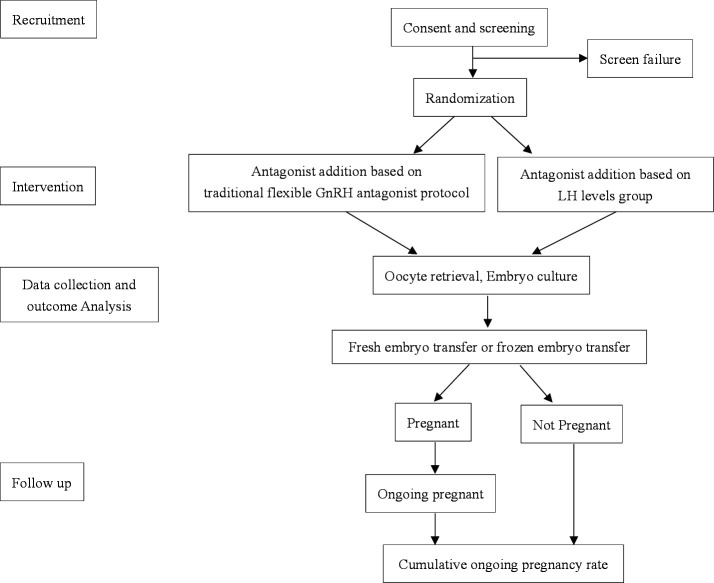
This study was a multicentre, randomised, controlled, non-inferiority trial that involved patients with normal ovarian responses undergoing in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI). Patients were randomly divided into two groups: the experimental group (stimulated with an LH-based flexible GnRH antagonist protocol) and the control group (stimulated with a traditional flexible GnRH antagonist protocol) at a 1:1 ratio on the first day of ovarian stimulation. Figure 1 shows a flow chart of the study design. Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist is given online as online supplemental file 1.
Figure 1.
Flow chart followed the checklist of Standard Protocol Items: Recommendations for Interventional Trials showing patient enrolment, allocation, treatment and follow-up of participants. GnRH, gonadotropin-releasing hormone; LH, luteinising hormone.
bmjopen-2020-047974supp001.pdf (72.5KB, pdf)
Study sites and recruitment procedures
This clinical trial involved eight hospitals in China. All patients undergoing IVF for the first time at the Centre for Reproductive Medicine were included in the study. Physicians will screen patients based on clinical data to assess whether they meet the inclusion criteria. Subjects who are eligible for and who agree to participate in the study are notified and recruited prior to the start of the IVF cycle.
Study population and inclusion/exclusion criteria
The trial enrolled women who were undergoing their first IVF cycle, and a GnRH antagonist regimen was used for ovarian stimulation. In addition, women must have an indication for IVF with or without ICSI treatment, such as tubal disease due to pelvic inflammatory disease and/or adhesions, unexplained infertility, etc.20–22 Eligible women met the following inclusion criteria: (1) 23–38 years of age; (2) a spontaneous cycle length of ≥21 days and ≤35 days; (3) a diagnosis of infertility for >1 year; (4) an antral follicle count (AFC) of 8–20 and (5) a body mass index (BMI) of ≥18 kg/m2 or ≤28 kg/m2.
The exclusion criteria were as follows: (1) a history of unilateral oophorectomy; (2) recurrent spontaneous abortion; (3) a diagnosis of polycystic ovarian syndrome; (4) uterine abnormalities (eg, submucosal myoma, adenomyosis, uterine scarring, intrauterine adhesion); (5) a chronic medical disease affecting pregnancy outcomes (eg, diabetes mellitus, hypertension, heart disease, liver dysfunction, renal disease) and (6) coagulation dysfunction or a history of deep venous thrombosis. All couples were screened by karyotyping, and those with an abnormal karyotype were excluded.
Randomisation
Randomisation was performed by a doctor on the initial day of ovarian stimulation from day 2 of the menstrual cycle. Participants were randomly divided into two groups in a 1:1 ratio and were stratified according to the study site. The randomisation scheme was entered into an online central randomisation database (www.medresman.org). After randomisation, the physicians will be informed about the allocation results by email. Embryologists, data assessors and the patients were all blinded in our study. Patients were randomly assigned to one of two groups: the control group (traditional flexible GnRH antagonist protocol) or the experimental group (LH-based flexible GnRH antagonist protocol).
Ovarian stimulation protocol
All patients underwent baseline transvaginal ultrasound and measurement of serum E2, FSH, LH, progesterone (P) and beta-human chorionic gonadotropin (β-hCG) on days 2–3 of the menstrual cycle. Recombinant FSH (Gonal-f, Merck, Germany) at a dose of 150–300 IU/day was administered according to age, BMI, Anti-Müllerian Hormone (AMH), AFC and basal serum FSH concentration. After 5 days of treatment, an ultrasound examination was performed. The gonadotropin dosage was adjusted according to follicle development and serum E2, P and LH concentrations.
Patients in the control group were administered a GnRH antagonist (cetrorelix acetate, Cetrotide, Merck, Germany) at a dose of 0.25 mg/day, which was initiated when at least one follicle was 14 mm in size or the E2 concentration was ≥300 pg/mL. Treatment was continued until the day of trigger. Blood samples will be collected for LH levels measured to determine the timing and dosage of rLH supplementation.
Patients in the experimental group were administered antagonist, and the dosage was based on the LH concentration from day 6 of ovarian stimulation. No antagonist was administered if the LH concentration was ≤4 IU/L. If the LH concentration was >4 IU/L or ≤6 IU/L, 0.125 mg of cetrorelix acetate was administered daily for 2 days until the next blood test. If the LH concentration was >6 IU/L or ≤10 IU/L, 0.25 mg of cetrorelix acetate was administered daily for 2 days. If the LH concentration was >10 IU/L or ≤15 IU/L, 0.375 mg of cetrorelix acetate was administered daily for 1 day. If the LH concentration was >15 IU/L, 0.5 mg of cetrorelix acetate was administered for 1 day. Whether or not antagonist cotreatment was administered depended on an LH concentration of >4 IU/L until the day of trigger.
Oocyte retrieval, embryo culture and luteal-phase support
Previous studies showed that dual trigger could increase the number of oocytes, mature oocytes and number of blastocysts as well as the percentage of top-quality blastocysts compared with triggering with hCG alone. On this basis, the dual trigger has been used in our clinic since 2018 in normal responder women.20–22 Final oocyte maturation is induced when at least three follicles reach ≥18 mm in mean diameter using 2000–3000 IU of hCG and 0.2 mg of triptorelin (Decapeptyl, Ipsen, France). Serum LH, E2 and P concentrations were measured, and transvaginal ultrasound-guided oocyte retrieval was performed 35–36 hours after the trigger injection. Embryo transfer (ET) is usually performed at the cleavage stage, 3 days after fertilisation, with two of the highest quality embryos. The remaining embryos are cultured for 2 or 3 more days, and good quality blastocysts are vitrified. Fresh ET was cancelled if patients were at risk of ovarian hyperstimulation syndrome (OHSS), had an unfavourable endometrium (endometrial thickness of ≤6 mm or ≥16 mm, cavity fluid or endometrial polyps), had a P concentration of ≥1.5 ng/mL on the day of hCG trigger, or if no embryo was present.
For fresh ET, luteal-phase support with vaginal progesterone gel (Crinone, Merck Serono) at a dose of 90 mg daily and oral dydrogesterone (Duphaston, Abbott) at a dose of 10 mg two times per day was started after oocyte retrieval and was continued until the day of hCG testing. For frozen ET, the endometrium was prepared using either a natural cycle regimen or an artificial cycle regimen based on the physician of decision. For the natural cycle regimen, luteal phase support is started from the ovulation day with oral dydrogesterone 10 mg two times daily; for the hormone replacement cycle regimen, the endometrium is prepared with oral estradiol valerate at a dose of 6–8 mg daily which started on day 3–5 of the menstrual cycle. Vaginal progesterone gel 90 mg daily and oral dydrogesterone 10 mg two times per day are added for endometrium translation. If pregnancy is achieved, luteal phase support will be continued until 10 weeks’ gestation.
Outcome measurement
The primary outcome measure was cumulative ongoing pregnancy rate per cycle. Ongoing pregnancy was defined as a gestational period of more than 12 weeks and fetal heart activity on ultrasound.
Secondary outcomes were high-quality embryo rate, clinical pregnancy rate and cancellation rate. Moreover, differences in cost-effectiveness and adverse events were evaluated. High-quality embryos were defined by two criteria: the number of cells in the embryo and their appearance under a high-power microscope. Typically, high-quality embryos on day 3 contained 7–9 cells with moderate or no fragmentation. Clinical pregnancy rate was defined as the presence of a gestational sac at 6–7 weeks of gestation when visualised by transvaginal ultrasound. Cancellation rate was defined as the number of cycles with no embryo for transfer divided by the number of ovum pick-up cycles. Moderate OHSS was diagnosed when ovarian enlargement of >5 cm and <12 cm was observed and when ultrasonographic ascites was present with or without nausea, vomiting and/or diarrhoea. Severe OHSS was diagnosed when ovarian enlargement of ≥12 cm was observed and when there was clinical evidence of ascites and/or hydrothorax or breathing difficulties with or without haemoconcentration, severe hypoproteinaemia, abnormal liver function, coagulation abnormalities or diminished renal function.
Study assessment
Screening and baseline assessments included an evaluation of the inclusion and exclusion criteria, study recruitment and informed consent processes. All patients were evaluated on the day of ovarian stimulation from day 2 of the menstrual cycle. Treatment phase assessments included blood and ultrasound monitoring of follicular development during ovarian stimulation. Hormone analyses were performed during ovarian stimulation as follows: (1) on the day of stimulation; (2) 4–5 days after stimulation initiation; (3) on the day of trigger and (4) 2 days after egg retrieval.
A pregnancy test was performed 12–14 days after ET to confirm pregnancy. In the case of biochemical pregnancy, vaginal ultrasound was performed 6–7 weeks after transplantation to confirm clinical pregnancy. Pregnancy that proceeded beyond 12 weeks of gestation was defined as an ongoing pregnancy. Pregnancy complications (eg, ectopic pregnancy, OHSS, miscarriage, gestational trophoblastic disease) will be evaluated by inspecting medical records.
Patient and public involvement
All aspects of this study (development of the research question, study design and conduct of the trial, interpretation of results and editing of the final manuscript for publication) are taking place independently of patients and public involvement. The results will be disseminated to participants by their physicians.
Data analysis
Sample size calculation
The sample size calculation was based on the cumulative ongoing pregnancy rate. The cumulative ongoing pregnancy rate per cycle in women with normal ovarian responses was approximately 70% in our retrospective clinical database. Considering that a non-inferiority threshold should retain 80% of the clinical effect of a control treatment, a minimum clinical difference of 14% (one-sided: α, 2.5%; β, 20%) and a total of 338 patients were needed. Anticipating a 10% drop-out rate, the total number of patients required was 372.
Data management
A clinical trial electronic case report form (http://www.clinicaltrialecrf.org) was used to record and deposit patient data to manage the data, monitor the process and promote research transparency. The study protocol (7 December 2018, V.1.0), operational and procedural manuals, case report forms, informational brochures, and informed consent forms were reviewed and approved by the eight participating hospitals.
Data analysis plan
Intergroup differences in demographic variables and baseline information were compared before the beginning of the study. Continuous data were analysed using a two-sample t-test or Wilcoxon’s rank-sum test, while categorical data were analysed using the χ2 test or Fisher’s exact test.
The primary analysis used an intention-to-treat analysis approach to examine differences in the cumulative ongoing pregnancy rate between the two groups using the χ2 test. The relative risk and 95% CI were calculated. The χ2 test or Fisher’s exact test was used to analyse secondary efficacy parameters and safety parameters (eg, clinical pregnancy rate, OHSS rate, cancellation rate).
An independent-samples t-test was used for continuous variables with a normal distribution, and the Mann-Whitney U test was used for data with a non-normal distribution. A p value of <0.05 (two-sided) was considered statistically significant.
Missing data and dropouts
Patients who dropped out of the study for whatever reason could still undergo IVF treatment without adversely affecting their cycle. Those who cannot use dual triggering will drop out of the study on account of an unexpected ovarian high response and a high risk of OHSS. Other outcome variables may have missing data due to missed patient visits.
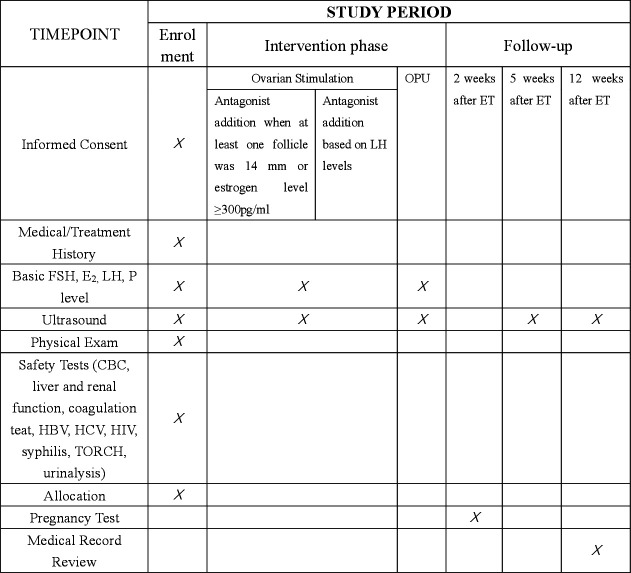
The flow chart of this study is presented in figure 1, and the Standard Protocol Items: Recommendations for Interventional Trials checklist is included as figure 2.
Figure 2.
Schedule of enrolment, interventions and assessments. ET, embryo transfer; FSH, follicle-stimulating hormone; HBV, hepatitis B virus; HCV, hepatitis C virus; LH, luteinising hormone; OPU, ovum pick up; TORCH, toxoplasmosis, others (Syphilis, Hepatitis B), rubella, Cytomegalovirus (CMV), and herpes simplex.
Discussion
This study aimed to evaluate the efficacy of an LH-based flexible GnRH antagonist protocol in women with a normal ovarian response. Moreover, we aimed to further determine whether LH could be used as an indicator for the timing and dose of antagonist administration with the GnRH antagonist protocol. We planned to enrol 372 subjects from eight academic IVF centres in China. Patient enrolment began on 29 August 2018. The results of this multicentre randomised trial will provide strong evidence for an LH-based flexible GnRH antagonist protocol during ovarian stimulation in patients with normal ovarian responses.
This study is a multicentre, randomised, prospective, parallel, non-inferiority study. A threshold concentration of 4 IU/L of serum LH was used as the cut-off value to determine whether a GnRH antagonist should be administered. This threshold is based on our previous study of frequent LH measurements during ovarian stimulation.19 Previously, we found that most patients with a low LH concentration (<4 IU/L) throughout ovarian stimulation had no LH surge. Considering that administration of a GnRH antagonist would further reduce the LH concentration, we decided to stimulate patients with LH at a concentration of <4 IU/L without antagonist cotreatment. If our hypothesis proved to be true, a new protocol could be established to control the LH concentration more effectively and potentially improve the effectiveness of IVF treatment, especially in patients with low LH concentrations during ovarian stimulation.
The majority of patients had sufficient endogenous LH to successfully maintain follicular development and oocyte maturation. However, a significant proportion of patients had an insufficient endogenous LH concentration. Poor pregnancy outcomes were observed in patients who had a continuously low LH concentration and in patients who experienced a sharp decrease in LH concentration during follicular development from baseline.6 7 23–25 A relative reduction in LH concentration in the mid-follicle during GnRH agonist cycles results in a lower live birth rate.26–29 Studies have shown that when the serum LH concentration on trigger day is lower than one-third of the baseline concentration, pregnancy and implantation rates are significantly reduced.30 For patients with endogenous LH deficiency, a regimen without antagonists would be more beneficial. Therefore, we implemented this randomised controlled trial to illustrate that LH can be used as an indicator for antagonist administration with a GnRH antagonist protocol among women undergoing ovarian stimulation for IVF/ICSI. This scheme can be applied to all patients, but is more beneficial for patients with insufficient endogenous LH concentrations. We hope to complete data collection and analysis in order to provide recommendations for the choice of protocol. The data will provide us with a new perspective on the administration of antagonist with a GnRH antagonist protocol.
Trial status
The protocol version number and date:V.1.0, 7 December 2018. The study was conceived and designed in 2017. Enrolment began in 2018 and is expected to end in December 2020. At the time of manuscript preparation, more than 200 subjects had been enrolled. Enrolment in this study was ongoing at the time of manuscript submission.
Supplementary Material
Acknowledgments
We thank all subjects for their voluntary participation in this study and physicians at all recruiting sites.
Footnotes
Y-sL and YL contributed equally.
Contributors: Y-sL was involved in the drafting of the manuscript. YL was involved in the study concept and design. SL was involved in the study concept and design and in the revision of the manuscript. All authors read and approved the final manuscript.
Funding: This study was supported by 1351 Talent training Programme of Beijing Chao-Yang Hospital (CYXX-2017-20, CYMY-2017-21); Capital Health Development Scientific Research Project (Independent Innovation,2020-1-2039); Beijing Health Promotion Foundation (2019-09-05); 2018 Fertility Research Programme of Young and Middle-aged Physicians-China Health Promotion Foundation. 2020 Fertility Research Programme of Young and Middle-aged Physicians-China Health Promotion Foundation; Beijing Hospitals Authority Youth Programme (QML20200301).
Disclaimer: The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Depalo R, Trerotoli P, Chincoli A, et al. Endogenous luteinizing hormone concentration and IVF outcome during ovarian stimulation in fixed versus flexible GnRH antagonist protocols: an RCT. Int J Reprod Biomed 2018;16:175–82. 10.29252/ijrm.16.3.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopkisson J. European practice in gynaecology and obstetrics: ovulation induction. The Obstetrician and Gynaecologist 2005;7:66. 10.1576/toag.7.1.066.27047 [DOI] [Google Scholar]
- 3.Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod 2000;15:1003-8. 10.1093/humrep/15.5.1003 [DOI] [PubMed] [Google Scholar]
- 4.Chen C-D, Chiang Y-T, Yang P-K, et al. Frequency of low serum LH is associated with increased early pregnancy loss in IVF/ICSI cycles. Reprod Biomed Online 2016;33:449–57. 10.1016/j.rbmo.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Luo Y, Liu S, Su H, et al. Low serum LH levels during ovarian stimulation with GnRH antagonist protocol decrease the live birth rate after fresh embryo transfers but have no impact in Freeze-All cycles. Front Endocrinol 2021;12:640047. 10.3389/fendo.2021.640047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemsey G, O’Brien F, O’Dea L. Objective evidence of LH-dependence in women with profound LH and FSH deficiency. Fertil Steril 2001;76:208. 10.1016/S0015-0282(01)02620-6 [DOI] [Google Scholar]
- 7.Balasch J, Miró F, Burzaco I, et al. The role of luteinizing hormone in human follicle development and oocyte fertility: evidence from in-vitro fertilization in a woman with long-standing hypogonadotrophic hypogonadism and using recombinant human follicle stimulating hormone. Hum Reprod 1995;10:1678–83. 10.1093/oxfordjournals.humrep.a136154 [DOI] [PubMed] [Google Scholar]
- 8.O'Dea L, O'Brien F, Currie K, et al. Follicular development induced by recombinant luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in anovulatory women with LH and FSH deficiency: evidence of a threshold effect. Curr Med Res Opin 2008;24:2785–93. 10.1185/03007990802374815 [DOI] [PubMed] [Google Scholar]
- 9.Raju GAR, Chavan R, Deenadayal M, et al. Luteinizing hormone and follicle stimulating hormone synergy: a review of role in controlled ovarian hyper-stimulation. J Hum Reprod Sci 2013;6:227–34. 10.4103/0974-1208.126285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raju GAR, Teng SC, Kavitha P, et al. Combination of recombinant follicle stimulating hormone with human menopausal gonadotrophin or recombinant luteinizing hormone in a long gonadotrophin-releasing hormone agonist protocol: a retrospective study. Reprod Med Biol 2012;11:129–33. 10.1007/s12522-012-0120-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pezzuto A, Ferrari B, Coppola F, et al. Lh supplementation in down-regulated women undergoing assisted reproduction with baseline low serum LH levels. Gynecol Endocrinol 2010;26:118–24. 10.3109/09513590903215516 [DOI] [PubMed] [Google Scholar]
- 12.Loumaye E, Engrand P, Shoham Z, et al. Clinical evidence for an LH 'ceiling' effect induced by administration of recombinant human LH during the late follicular phase of stimulated cycles in World Health Organization type I and type II anovulation. Hum Reprod 2003;18:314–22. 10.1093/humrep/deg066 [DOI] [PubMed] [Google Scholar]
- 13.Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update 2017;23:211–20. 10.1093/humupd/dmw047 [DOI] [PubMed] [Google Scholar]
- 14.Lahoud R, Al-Jefout M, Tyler J, et al. A relative reduction in mid-follicular LH concentrations during GnRH agonist IVF/ICSI cycles leads to lower live birth rates. Hum Reprod 2006;21:2645–9. 10.1093/humrep/del219 [DOI] [PubMed] [Google Scholar]
- 15.Ruvolo G, Bosco L, Pane A, et al. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril 2007;87:542–6. 10.1016/j.fertnstert.2006.06.059 [DOI] [PubMed] [Google Scholar]
- 16.Haas J, Ophir L, Barzilay E, et al. Standard human chorionic gonadotropin versus double trigger for final oocyte maturation results in different granulosa cells gene expressions: a pilot study. Fertil Steril 2016;106:653–9. 10.1016/j.fertnstert.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 17.Huang Q, Niu Y, Xu L, et al. Relationship between a low ratio of serum estradiol to follicle number and fertility treatment outcomes: a retrospective cohort study of 516 cases. Medicine 2018;97:e12017. 10.1097/MD.0000000000012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanaihara A, Yorimitsu T, Motoyama H, et al. The decrease of serum luteinizing hormone level by a gonadotropin-releasing hormone antagonist following the mild IVF stimulation protocol for IVF and its clinical outcome. J Assist Reprod Genet 2008;25:115–8. 10.1007/s10815-008-9205-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Liu S, Li L, et al. Lh levels may be used as an indicator for the time of antagonist administration in GnRH antagonist Protocols-A proof-of-concept study. Front Endocrinol 2019;10:67. 10.3389/fendo.2019.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas J, Bassil R, Samara N, et al. Gnrh agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod 2020;35:1648–54. 10.1093/humrep/deaa107 [DOI] [PubMed] [Google Scholar]
- 21.Eftekhar M, Mojtahedi MF, Miraj S, et al. Final follicular maturation by administration of GnRH agonist plus hCG versus hCG in normal responders in art cycles: an RCT. Int J Reprod Biomed 2017;15:429–34. 10.29252/ijrm.15.7.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Guo P, Chen X, et al. Comparison of dual trigger with combination GnRH agonist and hCG versus hCG alone trigger of oocyte maturation for normal ovarian responders. Int J Gynaecol Obstet 2018;141:327–31. 10.1002/ijgo.12457 [DOI] [PubMed] [Google Scholar]
- 23.Costello MF, Misso ML, Balen A, et al. Evidence summaries and recommendations from the International evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open 2019;2019:hoy021. 10.1093/hropen/hoy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Marca A, Grisendi V, Giulini S, et al. Individualization of the FSH starting dose in IVF/ICSI cycles using the antral follicle count. J Ovarian Res 2013;6:11. 10.1186/1757-2215-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benmachiche A, Benbouhedja S, Zoghmar A, et al. Low LH level on the day of GnRH agonist trigger is associated with reduced ongoing pregnancy and live birth rates and increased early miscarriage rates following IVF/ICSI treatment and fresh embryo transfer. Front Endocrinol 2019;10:639. 10.3389/fendo.2019.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boularak A, Humaidan P. Impact of mid-luteal phase GnRH agonist administration on reproductive outcomes in GnRH agonist-trigger: a randomized controlled trial. Front Endocrinol 2017;8:124. 10.3389/fendo.2017.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connell MT, Patounakis G, Healy MW, et al. Is the effect of premature elevated progesterone augmented by human chorionic gonadotropin versus gonadotropin-releasing hormone agonist trigger? Fertil Steril 2016;106:584–9. 10.1016/j.fertnstert.2016.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venetis CA, Kolibianakis EM, Bosdou JK, et al. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update 2013;19:433–57. 10.1093/humupd/dmt014 [DOI] [PubMed] [Google Scholar]
- 29.Doody KJ, Devroey P, Leader A, et al. No association between endogenous LH and pregnancy in a GnRH antagonist protocol: Part I, corifollitropin alfa. Reprod Biomed Online 2011;23:449–56. 10.1016/j.rbmo.2011.06.015 [DOI] [PubMed] [Google Scholar]
- 30.Bildik G, Akin N, Seyhan A, et al. Luteal granulosa cells from natural cycles are more capable of maintaining their viability, steroidogenic activity and LH receptor expression than those of stimulated IVF cycles. Hum Reprod 2019;34:345–55. 10.1093/humrep/dey353 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-047974supp001.pdf (72.5KB, pdf)