Abstract
Chronic intestinal pseudo-obstruction (CIPO) is a condition typified by the failure of the small bowel to propel contents in the absence of physical obstruction. CIPO is diagnosed after eliminating other causes, presenting a diagnostic challenge in emergency surgery. We report a case of a 32-year-old man with a rare mitochondrial disorder, Maternally inherited diabetes and deafness (MIDD), who presented to our hospital acutely unwell with peritonitis. Laparotomy revealed distended small bowel with no transition point, and turbid fluid with no macroscopic source. Postoperatively he had severe electrolyte and vitamin deficiencies. The diagnosis of CIPO leading to paralytic ileus and bacterial translocation was established and managed with aggressive electrolyte and vitamin replacement. He was discharged day 12 post operatively after a prolonged ileus with follow-up from a quaternary metabolic unit. We discuss here the challenges and gold standard in the emergency management of CIPO.
Keywords: metabolic disorders, gastroenterology, gastrointestinal surgery, neurogastroenterology
Background
Chronic intestinal pseudo-obstruction (CIPO) is a rare condition of the gastrointestinal (GI) tract which effects approximately 0.2 per 100 000 adults per year. These patients show severe dysfunction in GI motility in the absence of mechanical cause, which manifests as abdominal pain and distension (80%), nausea and vomiting (75%), constipation (40%) and/or diarrhoea (20%–30%). The causes of CIPO are multiple and can include metabolic, endocrine, neurological or paraneoplastic. Disease may be sporadic and lead to delayed diagnosis, unnecessary surgery and/or psychosocial issues.1
Mitochondrial disorders, such as maternally inherited diabetes and deafness (MIDD), are well recognised and significant cause of CIPO.2 3 MIDD affects approximately 1% of patients with diabetes, but often goes undiagnosed. MIDD results from an A to G substitution at position 3243 of the mitochondrial DNA and is a multisystem disease which can affect the brain, eyes, heart, muscles, kidneys and GI tract. The degree to which an end organ is affected is thought to be related to accumulation of these mutations in the end organ through heteroplasmy. GI manifestations are thought to be due to abnormally swollen mitochondria in the smooth muscle cells of the tract, supporting a myogenic rather than neurological aetiology, leading to impaired intestinal motility.4
Although the link between CIPO and mitochondrial diseases has occasionally been described, we present a rare case of MIDD as a cause of an acute abdomen.5–7
Case presentation
A 33-year-old man presented to the emergency department of our regional hospital in Victoria Australia with severe abdominal pain and 2 days of vomiting and obstipation. This followed 5 days of heavy alcohol consumption. He was diagnosed with MIDD 1 year prior and was currently treated with oral hypoglycaemics (sitagliptin and gliclazide) alone. The patient reported experiencing several similar episodes in the past, although with less severity.
On arrival he was febrile to 38.3°C, tachycardic (heart rate114) and hypotensive (blood pressure 90/60) with a distended peritonitic abdomen. Chest x-ray showed no evidence of pneumoperitoneum. The patient was fluid resuscitated with 2 L of compound sodium lactate and a nasogastric tube was inserted. Initial bloods were lipase 63 U/L (70–390), C-reactive protein 250 mg/L (<2.9), white cell count 3.3×109 (4–11), lactate 2.1 mmol/L (<1.5), pH 7.46, CO2 36 mm Hg (35–46), HCO3 26 mmol/L (21–28) and glucose 13.6 mmol/L. Liver function tests were unremarkable. Abdominal CT (figures 1 and 2) showed grossly distended proximal small bowel with collapsed distal ileum. No discrete transition point, pneumatosis coli or free gas were identified.
Figure 1.
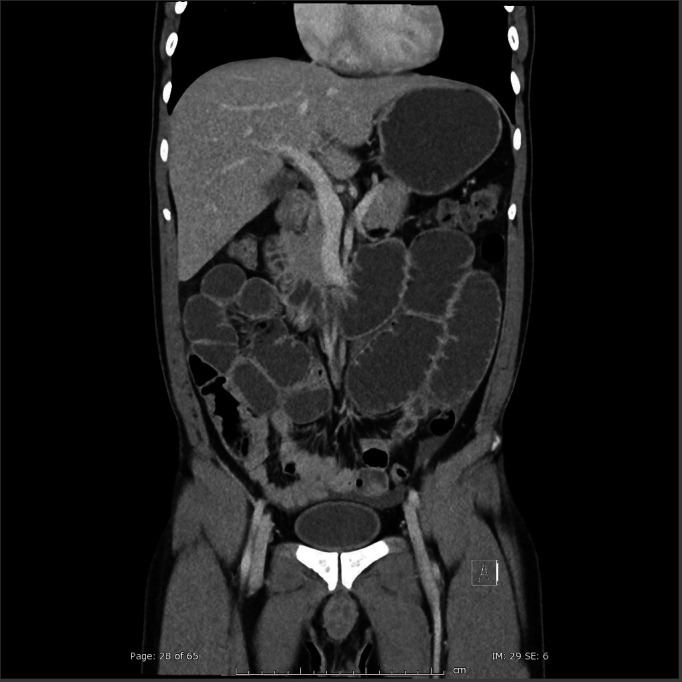
Coronal CT image showing diffusely dilated bowel. Images were reviewed by a consultant radiologist and were equivocal for the presence of a transition point.
Figure 2.
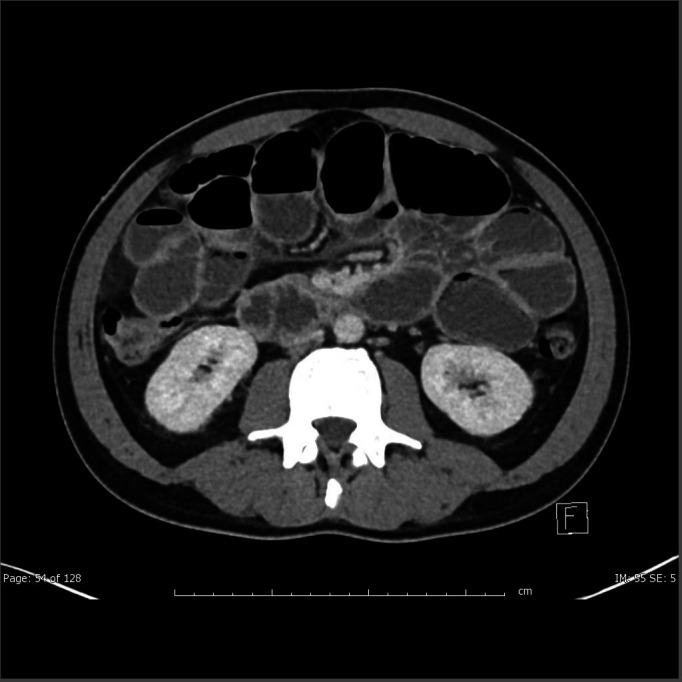
Axial images showing dilated small bowel.
Laparotomy revealed grossly distended small bowel with no obstruction and no transition point. There was a large amount of turbid/ purulent fluid (which would later show no leukocytes and no growth). Despite careful inspection of the peritoneal cavity (including lesser sac), no hollow viscus perforation or septic source was found as a cause of peritoneal soiling.
Treatment
The State-wide Service for Adults with Inborn Errors of Metabolism and Metabolic Disease Unit was contacted regarding the history, presentation and intraoperative findings to determine if this unusual presentation was related to the patient’s rare mitochondrial condition. In conjunction with their expert opinion on MIDD, a working diagnosis of CIPO was established. The patient had a history of abdominal pain and distension, and inability to gain weight (low-normal body mass index of 19) despite meeting 150% of estimated energy requirements and 180% of estimated protein requirements preoperatively. It was believed that this deterioration was acutely precipitated by the patient’s recent alcohol intake, which may have aggravated electrolyte imbalances leading to pseudo-obstruction and bacterial translocation.
Postoperatively, there were significant electrolyte disturbances which were corrected in intensive care unit (table 1). Vitamin and nutritional screens were performed to determine the chronicity of malabsorption, which confirmed specific deficiencies in fat soluble vitamins D, A and K. Persisting pancytopaenia lead to concerns of bone marrow failure, a known sequale of mitochondrial disease.8 Blood films, viral testing (Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus) and haematinic studies were performed all with negative results. Sepsis induced pancytopaenia was implicated, however, when counts returned to normal day 4 post laparotomy.
Table 1.
Electrolyte, vitamin and haematinics derangement
| Electrolytes | Patient value (range) | Vitamins | Patient value (range) | Blood count | Patient value (range) |
| Sodium | 132 mmol/L (135–145) | Vit D | 39 mmol/L (<50) | WCC | 1.1 (4.0–11.0) ×109/L |
| Potassium | 4.0 mmol/L (3.5–5.2) | Vit A | 0.44 umol/L (0.90–3.00) | Neutrophils | 0.8 (2.0–8.0) ×109/L |
| Calcium/adj calcium | 1.87 mmol/L (2.10–2.60) 2.06 mmol/L (2.10–2.60) |
Vit E | 15.0 umol/L (12.0–46.0) | Lymphocytes | 0.2 (1.0–4.0) ×109/L |
| Magnesium | 0.67 mmol/L (0.70–1.10) | INR | 1.3 (0.8–1.2) | Platelets | 120 (150–450) ×109/L |
| Phosphate | 0.49 mmol/L (0.75–1.50) | Active B12 | 74 pmol/L (30) | Haemoglobin | 118 (125–175) ×109/L |
| Folate | 27.5 nmol/L (>10) | Haematocrit | 0.34 (0.40–0.55) ×109/L | ||
| Creatinine | 109 (60–110) µmol/L |
Outcome and follow-up
The patient passed flatus 7 days postoperatively and was discharged home day 13. Follow-up required coordination between specialties including general surgery, gastroenterology, metabolic diseases and dietetics. After discharge the patient’s MIDD progressed to become insulin dependent. He continued to improve from a nutritional point of view with a gain of 3.1 kg post discharge and daily supplements under the guidance of dieticians from the metabolic unit. He has been scheduled for regular micronutrient screens as he is noted to be at risk of ongoing malabsorption.
Discussion
CIPO is a diagnostic challenge in surgery, especially when the patient presents acutely unwell. Sabbagh et al discuss these challenges in an analysis of 63 adult cases of CIPO requiring surgery, including 9 patients with mitochondrial disease.6 Their findings illustrate a wide variety of presentations, demographics (including paediatrics) and operation types. The underlying cause of CIPO can vary widely, or may be undiagnosed. These patients are vulnerable and have a high risk of morbidity and major complication, with the rate of Clavien-Dindo surgical complication score >3 at 20.7% (requiring radiological, surgical or endoscopic intervention).9 Early reoperation is common with approximately 17% of this cohort requiring unplanned return to theatre for prolonged ileus, anastomotic leakage, hemoperitoneum or evisceration. Morbidity was significantly linked with intraoperative bowel injury, idiopathic CIPO and emergency procedure. Late reoperation is also common, with 1, 3 and 5-year operation rates being 44%, 60% and 66%, respectively. These presentations often have the added complexity of adhesions, which can create a diagnostic complexity.
Although surgery is sometimes necessary, as this case describes, there are several steps which should be considered where a diagnosis of CIPO is suspected. The gold standard for diagnosis in CIPO is full thickness biopsies which may be taken to determine the underlying cause of the pseudo-obstruction. Unless selective gut segments are affected and requiring resection, biopsies should be taken from both dilated and non-dilated segments of the stomach, small bowel and colon, as CIPO is a diffuse motility disorder.10 These specimens require appropriate tissue handling including timely delivery to the laboratory, appropriate fixation (formalin), with a portion of the specimen frozen in the lab for molecular biology. Where possible, these specimens should be processed in a lab with a specific interest in this area.11 12 Studies show that these biopsies are rarely taken, leading to delayed diagnosis, and often further unnecessary surgery. The place for biopsies in the emergency setting needs careful consideration given the risk of full thickness biopsies in these patients. Although diagnostic surgery and elective biopsy in patients with a clinical diagnosis of CIPO may be impractical, where resection specimens are acquired in any operation on a patient with functional bowel symptoms, consideration of CIPO as a differential diagnosis and effective communication with the pathologist is essential.
Postoperative management of CIPO requires intense, multidisciplinary coordination. Malabsorption and inadequate nutritional intake typify this disease, and nutritional assessment and correction is crucial. Where gut motility exists, patients should be encouraged to take small, frequent liquid meals while avoiding high fat, residue and high lactose / fructose foods. Vitamins A, D, E, K, B1 and folic acid should be supplemented when needed. Where oral intake is inadequate, enteral nutrition may be required. Advanced feeding techniques must be tailored to the specific manifestations of the disease.1 Specific nutrients may also lead to benefits, and should be guided by knowledge of the underlying disease mechanism. As an example, coenzyme Q10 has been shown to be efficacious in treatment of CIPO in MIDD. Prokinetics such as erythromycin have been trialled with limited evidence, although may evoke symptomatic improvement and increase oral intake.1 13
Learning points.
Maternally inherited diabetes and deafness (MIDD) is a rare mitochondrial disorder that can cause chronic intestinal pseudo-obstruction (CIPO).
CIPO can present as an acute abdomen.
In patients with MIDD, CIPO should be kept in mind as a potential differential diagnosis for both acute and chronic abdominal presentations.
Treatment of CIPO requires a multidisciplinary approach with a focus on nutritional support and replacement.
In the operative setting, consideration should be given to full thickness biopsies of dilated and non-dilated segments gastrointestinal tract, balancing the risk of this intervention. Operative specimens should be flagged for appropriate histopathological assessment, including molecular biology, with fixed and frozen portions sent.
Acknowledgments
I would like to acknowledge the multidisciplinary team involved in this patients care including the intensive care specialists, dieticians and gastroenterologists at University Hospital Geelong. Furthermore I would like to thank the Metabolic Diseases Unit at the Royal Melbourne Hospital for their continuous support. Lastly I would like to thank the patient whom this case study is about. We are grateful for your patience and commitment to health education.
Footnotes
Contributors: KU: Conceptualisation; writing original draft; patient care. HD: Supervision; critical revision; patient care; validation GN: Research. SN: Conceptualisation; supervision; validation; accuracy and integrity.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Di Nardo G, Karunaratne TB, Frediani S, et al. Chronic intestinal pseudo-obstruction: progress in management? Neurogastroenterol Motil 2017;29:e13231. 10.1111/nmo.13231 [DOI] [PubMed] [Google Scholar]
- 2.Finsterer J, Frank M. Gastrointestinal manifestations of mitochondrial disorders: a systematic review. Therap Adv Gastroenterol 2017;10:142–54. 10.1177/1756283X16666806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergamin CS, Rolim LC, Dib SA, et al. Unusual occurrence of intestinal pseudo obstruction in a patient with maternally inherited diabetes and deafness (MIDD) and favorable outcome with coenzyme Q10. Arq Bras Endocrinol Metab 2008;52:1345–9. 10.1590/S0004-27302008000800023 [DOI] [PubMed] [Google Scholar]
- 4.Murphy R, Turnbull DM, Walker M, et al. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med 2008;25:383–99. 10.1111/j.1464-5491.2008.02359.x [DOI] [PubMed] [Google Scholar]
- 5.Dindyal S, Mistry K, Angamuthu N, et al. Melas syndrome presenting as an acute surgical abdomen. Annals 2014;96:1–3. 10.1308/003588414X13824511649733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabbagh C, Amiot A, Maggiori L, et al. Non-transplantation surgical approach for chronic intestinal pseudo-obstruction: analysis of 63 adult consecutive cases. Neurogastroenterol Motil 2013;25:n/a–6. 10.1111/nmo.12191 [DOI] [PubMed] [Google Scholar]
- 7.Granero Castro P, Fernández Arias S, Moreno Gijón M, et al. Emergency surgery in chronic intestinal pseudo-obstruction due to mitochondrial neurogastrointestinal encephalomyopathy: case reports. Int Arch Med 2010;3:35. 10.1186/1755-7682-3-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finsterer J, Frank M. Haematological abnormalities in mitochondrial disorders. Singapore Med J 2015;56:412–9. 10.11622/smedj.2015112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanghellini V, Cogliandro RF, de Giorgio R, et al. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil 2007;19:440–52. 10.1111/j.1365-2982.2007.00902.x [DOI] [PubMed] [Google Scholar]
- 11.Antonucci A, Fronzoni L, Cogliandro L, et al. Chronic intestinal pseudo-obstruction. World J Gastroenterol 2008;14:2953–61. 10.3748/wjg.14.2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Giorgio R, Camilleri M. Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol Motil 2004;16:515–31. 10.1111/j.1365-2982.2004.00538.x [DOI] [PubMed] [Google Scholar]
- 13.Emmanuel AV, Shand AG, Kamm MA. Erythromycin for the treatment of chronic intestinal pseudo-obstruction: description of six cases with a positive response. Aliment Pharmacol Ther 2004;19:687–94. 10.1111/j.1365-2036.2004.01900.x [DOI] [PubMed] [Google Scholar]


