Abstract
SARS-CoV-2 infection in hospital areas is of a particular concern, since the close interaction between health care personnel and patients diagnosed with COVID-19, which allows virus to be easily spread between them and subsequently to their families and communities. Preventing SARS-CoV-2 infection among healthcare personnel is essential to reduce the frequency of infections and outbreaks during the pandemic considering that they work in high-risk areas. In this research, silver nanoparticles (AgNPs) were tested in vitro and shown to have an inhibitory effect on SARS-CoV-2 infection in cultured cells. Subsequently, we assess the effects of mouthwash and nose rinse with ARGOVIT® silver nanoparticles (AgNPs), in the prevention of SARS-CoV-2 contagion in health workers consider as high-risk group of acquiring the infection in the General Tijuana Hospital, Mexico, a hospital for the exclusive recruitment of patients diagnosed with COVID-19. We present a prospective randomized study of 231 participants that was carried out for 9 weeks (during the declaration of a pandemic). The "experimental" group was instructed to do mouthwash and nose rinse with the AgNPs solution; the "control" group was instructed to do mouthwashes and nose rinse in a conventional way. The incidence of SARS-CoV-2 infection was significantly lower in the "experimental" group (two participants of 114, 1.8%) compared to the "control" group (thirty-three participants of 117, 28.2%), with an 84.8% efficiency. We conclude that the mouth and nasal rinse with AgNPs helps in the prevention of SARS-CoV-2 infection in health personnel who are exposed to patients diagnosed with COVID-19.
Introduction
On March 11, 2020, the World Health Organization declared the coronavirus disease 2019 (COVID-19) a worldwide public health problem; the responsible agent was named the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. An estimated of 191,148,056 infected cases and 4,109,303 deaths have being reported as of July 22nd, 2021 worldwide [2]. The infection triggered by SARS-CoV-2 can cause symptoms that appear from 2 to 14 days after exposure to the virus [3]. Caring for SARS-CoV-2 patients is a high risk exposition and causes complications due to the high mortality and morbidity [4]. In turn, there is a risk of nosocomial infections among patients with COVID-19 and patients without a diagnosis of atypical pneumonia [5]. To reduce the morbidity and morbidity rate of healthcare personal, a variety of public health interventions have been implemented, such as the adaptation of areas with negative air pressure, isolation areas for patients with COVID-19, and the mandatory use of personal protective equipment (gloves, goggles, overalls and N95/FFP2 respirators among others) to avoid contact with the respiratory and conjunctive tract [6]. International strategies focus on containment, diagnosis /monitoring, drug production and vaccines development against SARS-CoV-2 [7, 8]. Different candidate drugs have been identified as antivirals against SARS-CoV-2 [9], but there are only very few drugs approved by the US Food and Drug Administration (FDA||) [10]. Their efficacy and safety are still under evaluation. The main strategy to prevent SARS-CoV-2 infection would be vaccination. So far, a dozen vaccines have been approved by different international organizations [11–15], but global access to vaccines is one of the drawbacks, also vaccination strategies can take years to be an effective solution. On the other hand, no vaccine has been created so far against any coronavirus causing the last three pandemics such as SARS-CoV in 2002 [16], MERS-CoV in 2012 [17] and SARS-CoV-2 in 2019 [18]. Classic or traditional (attenuated or inactivated) vaccines have not worked because an Antibody-dependent enhancement (ADE) occurs [19]. Antibodies produced with one specific coronavirus subtype work, but they are not necessarily neutralizing, but rather opsonizing, leading to increased viral infectivity [20]. This leads to the fact that ADE-mediated secondary heterotypic infections caused by different coronavirus subtypes is the greatest risk factor [21, 22]. SARS-CoV-2 infection in hospital areas is especially problematic, since the close interaction between health care personnel and patients diagnosed with COVID-19 which allows virus to be easily spread between them and subsequently to their families and communities. On the other hand, in previous research, the in vitro antiviral effect of AgNPs has been demonstrated [23, 24]. Here, we confirmed the inhibitory effect of AgNPs in SARS-CoV-2 replication in cultured cells. For these reasons, a non-pharmaceutical public health intervention consisting of mouthwash and nose rinse with an AgNPs solution is proposed in order to reduce morbidity among healthcare personnel exposed to SARS-CoV-2 virus. We designed a prospective randomized controlled study of two-group (experimental vs. control) to evaluate the efficacy of mouthwash and nasal rinse with AgNPs solution for preventing SARS-CoV-2 infection in the health personnel at the General Tijuana Hospital in Mexico who works in high-risk areas with direct contact with patients infected and diagnosed with COVID-19.
Materials and methods
Virus and cells
All in vitro studies were performed using the SARS-CoV-2 NL/2020 strain (BetaCoV/Netherlands/01) provided by the European Virus Archive global (EVAg). VeroE6 cells (ATCC Cat.No. CRL-1586), obtained from the cell repository at Centro de Investigación en Sanidad Animal, (CISA-INIA, Valdeolmos, Spain) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and L-glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml), in a humid atmosphere of 5% CO2 at 37°C.
Cell viability assays
Vero E6 cells were seeded in 96-well plates, and 24 hours later, when 80% confluence was reached, AgNPs was added to the medium at serial two-fold dilutions, in triplicates. Vero E6 cells viability was tested in a long exposure experiment: AgNPs (starting from a 1/2 dilution) was kept in the culture medium for 24, 48 and 72 hours. The treated cells were maintained at 37°C in a 5% CO2 incubator and daily checked at the microscope. Viability of Vero E6 cells after treatment at the indicated times was checked using the MTS Cell Proliferation Assay (Promega) following manufacturer’s instructions. Viability percentages were calculated as the ratio OD450 nm treated wells/ OD450 nm AgNPs-free wells x 100. The ODs corresponded to the mean of three replicas and were corrected by the corresponding blank wells without cells.
In vitro infection experiments
Confluent monolayers of Vero E6 cells seeded on 12-well plates were infected with SARS-Cov2 at a multiplicity of infection (moi) of 0.001 plaque-forming units (pfu) per well in presence of metallic silver at serial two-fold dilutions from ½ to 1/2048 (concentrations of 0.5% to 0.0004%). Plates were maintained at 37°C for three days. At 72 hpi supernatants were collected and titrated in Vero E6 cells grown in 12-well plates. After 45 minutes of adsorption, the inoculum was removed and semi-solid medium with 1% carboxymethyl-cellulose in 1X DMEM 5% FBS was added. Plates were incubated for 72 hours, then fixed and crystal violet stained. Plaques were counted and the percentage of infectivity for each AgNPs dilution was calculated as follows: [1 –(number of plaques at the corresponding AgNPs dilution)/ number of plaques with medium alone] x 100. Controls corresponding to 100% infectivity (infection in the absence of AgNPs) as well as 100% viability (non-infected cells in medium containing AgNPs) were also included. IC50 values were calculated using GraphPad software (Prism).
Ethical considerations
The in vivo study was approved by the Research Ethics Committee of General Tijuana Hospital of the Institute of Public Health Services of the State of Baja California with favorable opinion number CONBIOETICA-02-CEI-001-20170 and was conducted in accordance with the Declaration of Helsinki. Signed consent was obtained from all participants. The objective of the study, the methodology and details of the experimental procedures and voluntary character of participation (including the right to withdraw from the study at any moment) were explained to all the participants. Participants were also informed on the anonymity and confidentiality of the data they reported during the study.
Materials
The hygiene product for mouthwash and nasal rinse ARGOVIT® AgNPs from the Investigation and Production Center Vector-Vita Ltd., made in Novosibirsk, Russia was applied. Metallic silver, polyvinylpyrrolidone, hydrolyzed collagen and distilled water concentrations in this solution are 0.06, 0.63, 0.31, and 99 wt.%, respectively. ARGOVIT® is registered in Russia as an oral and nasal hygiene product since 2015.
Study design
The in vivo study was carried out in the areas converted to care for patients diagnosed with atypical pneumonia and/or COVID-19 at General Tijuana Hospital belonging to the Mexican Ministry of Health. A 2-group randomized parallel study was conducted to compare the efficacy of mouthwash and nose rinse with the AgNPs 1 wt. % (0.6 mg of metallic Ag per mL) or direct spray to the oral cavity (experimental group); the second group was instructed to do mouthwash and nose rinse in a conventional way (control group). The investigation lasted 63 days (9 weeks) in the COVID-19 pandemic period, beginning in April 7 through June 9, 2020.
Participants and environment
For the in vivo study, 231 volunteers were selected from General Hospital Tijuana. Men and women were included among the participants, ranging from 18 to 65 years of age, and various occupations. Personnel excluded from the study included persons with history of hypersensitivity to silver (rashes and other contraindications), a history of SARS-CoV-2 infection in the three months prior to the start of the study, any respiratory distress, and refusal to sign the informed consent.
Instruments
The participants in the in vivo study were briefed on the objective of the study and were instructed on how to carry out the mouthwash and nose rinse. Subsequently, they were asked to complete a questionnaire where first identified the general data on the participants (Table 1) and the second part, the participants reported weekly data related to their activities pertaining the study and their state of health (Table 2). Experimental group were questioned about the presence of adverse effects by mouthwash and nose rinse. The diagnosis of COVID-19 was made by monitoring the signs and symptoms of the participants to finally confirm the infection status by RT-PCR (SuperScript III Platinum, Invitrogen, EEUU; Integrated DNA Technologies, Coralville, EEUU) and the kit 2019-nCoV ValuPanel Reagents (2019-nCoV_N1 Probe: FAM-ACC CCG CAT TAC GTT TGG TGG ACC-BHQ1, 2019-nCoV_N1 Forward Primer: GAC CCC AAA ATC AGC GAA AT, 2019-nCoV_N1 Reverse Primer: TCT GGT TAC TGC CAG TTG AAT CTG; 2019-nCoV_N2 Probe: FAM-ACA ATT TGC CCC CAG CGC TTC AG-BHQ1, 2019-nCoV_N2 Forward Primer: TTA CAA ACA TTG GCC GCA AA, 2019-nCoV_N2 Reverse Primer: GCG CGA CAT TCC GAA GAA; 2019-nCoV_N3 Probe: FAM-AYC ACA TTG GCA CCC GCA ATC CTG-BHQ1, 2019-nCoV_N3 Forward Primer: GGG AGC CTT GAA TAC ACC AAA A, 2019-nCoV_N3 Reverse Primer: TGT AGC ACG ATT GCA GCA TTG). Additionality, the two study groups were randomly selected for CT (Toshiba Aquilion 16, Japan) chest scan, RT-PCR analysis and clinical evaluation, to confirm the diagnosis of COVID-19. Participants with confirmed COVID-19 diagnosis were granted sick leave or were hospitalized.
Table 1. Characteristics of the experimental and control group.
| Basic characteristics | Experimental group (n = 114) | Control group (n = 117) | χ2 | P value |
|---|---|---|---|---|
| Gender | 41 / 73 | 40 / 77 | 0.80 | .785 |
| (Male/Female) (%) | 36% / 64% | 34% / 66% | ||
| Age (average/range) | (35) | (33) | 14.749 | .004 |
| (range 20–73) | (range 21–56) | |||
| Anti-influenza vaccination (number of individuals) (%) | (75) 66% | (101) 86% | 13.422 | .000 |
| Occupation | 9 / 91 / 14 | 4 / 103 / 10 | 3.294 | .210 |
| (Doctor/nurse/administrative) (%) | 8% / 80% / 12% | 3% / 88% / 9% | ||
| Smoking | 104 / 8 / 2 | 95 / 10 / 12 | 7.734 | .021 |
| (never/previous/current) (%) | 91% / 7% / 2% | 81% / 9% / 10% | ||
| Body Mass Index (mean) | 25.95 | 28.93 | 24.554 | .000 |
| Diseases | 5.187 | .273 | ||
| (number of individuals) (%) | ||||
| Nothing | 93(82.5%) | 91 (77.77%) | ||
| Diabetes | 4 (3.5%) | 6 (5.1%) | ||
| Hypertension | 7 (6.1%) | 5 (4.4%) | ||
| Asthma | 5 (4.4%) | 3 (3%) | ||
| Other | 4 (3.5%) | 12 (10.3%) | ||
| Annual frequency of RTIs in preceding year (mean) | (0.88) | (0.94) | 3.913 | .150 |
| (0/1-2/≥3) | (61 / 40 / 13) | (51 / 56 / 10) | ||
| Frequency of daily gargles before the study (mean) | (0.95) | (0.52) | 14.591 | .000 |
| (0/1-2/≥3) | (60 / 45 / 9) | (87 / 20 / 10) | ||
| Daily handwashing before the study (mean) | 6.3 | 8.9 | 20.818 | .000 |
| Daily handwashing in present study (mean) | 11.6 | 13.5 | 1.239 | .271 |
RTIs: Respiratory tract infections.
χ2: Chi-square test to determine the p-value of homogeneity or difference between groups.
Table 2. Prevention of SARS-CoV-2 infection in health workers.
| Variables | Experimental group (n = 114) | Control group (n = 117) | χ2 | P value |
|---|---|---|---|---|
| Infected cases SARS-CoV-2 (number of individuals) (%) | 2 (1.8%) | 33 (28.2%) | 31.423 | 0.000 |
| Participants that did mouthwashes by gargle (number of individuals) (%) | 28 (24.56%) | 28 (23.93%) | 0.013 | 0.838 |
| Participants that did mouthwashes by spray (number of individuals) (%) | 34 (29.82%) | - | - | - |
| Participants that did mouthwashes by gargle and spray (number of individuals) (%) | 52 (45.61%) | - | - | - |
| Participants that did nasal rinses (number of individuals) (%) | 64 (54.70%) | 21(17.95%) | 36.18 | 0.00 |
| Number of daily gargles (mean) | 2 | 2.14 | 0.1667 | 0.563 |
| Number of mouthwashes with AgNPs spray (mean) | 2 | - | - | - |
| Number of mouthwashes by gargles and spray (mean) | 2 and 4 | - | - | - |
| Number of nasal rinses (mean) | 0.70 | 0.25 | 5.031 | 0.00 |
| Adverse reactions reported from using mouthwash and nasal rinses with silver nanoparticles | 0 | - | - | - |
| Number of weekly contacts with patients COVID-19 (range) (mean) | (0–860) (169) | (0–729) (146) | - | - |
| Symptoms of RTIs (number of individuals) (%) | 21 (18.4%) | 42 (35.8%) | 8.891 | .003 |
| Occupation of infected individuals (doctor/nurse/administrative) | 0/2/0 | 2/29/2 | .515 | .773 |
AgNPs: silver nanoparticles. RTIs: Respiratory tract infections. χ2: Chi-square test to determine the p-value.
Randomization and experimental intervention
Eligible participants for the in vivo study were randomized using a computer generated block scheme and stratified according to duty position, work shifts and the area/department of the service at General Tijuana Hospital. Individuals from experimental group were provided with a 50 mL spray bottle containing AgNPs solution with 1 wt% concentration (0.6 mg/mL metallic silver). They were instructed to mix 4 to 6 spray shots (corresponding to volume ~ 0.5 mL) of this solution with 20 mL of water and to gargle with obtained solution for 15 to 30 seconds at least 3 times a day, also nasal lavages on the inner part of the nasal alae and nasal passage with the same solution using a cotton swap twice a day. As a second option, they were instructed to cover evenly the oral cavity with the direct 1 to 2 spray shots of solution without its previous dilution in water. Participants of the control group were instructed to do mouthwash and nose rinse with a conventional mouthwash the way they normally did before the study.
Data analysis
Data analysis was performed through the SPSS statistical program (version 26). Descriptive analysis was done with frequencies, percentages, means and ranges. To check the statistical difference between the results of the experimental and control groups, the Pearson’s "Chi-Square" tests, the "Fisher’s exact test" and the "likelihood ratio" were used. The symmetry measures for 2 x 2 contingency tables were determined by the Statistics of “Phi”, “Contingency Coefficient” and “Cramer’s V”. A logistic regression was performed to corroborate the efficacy of performing mouthwash and nose rinse with AgNPs and its impact on the incidence of SARS-CoV-2 infections among the participants under similar conditions. Likewise, the Chi-Square statistic was applied to determine the p-value in order to determine the homogeneity or heterogeneity between the experimental group and the control group.
Results
Antiviral activity of AgNPs in Vero E6 cell cultures
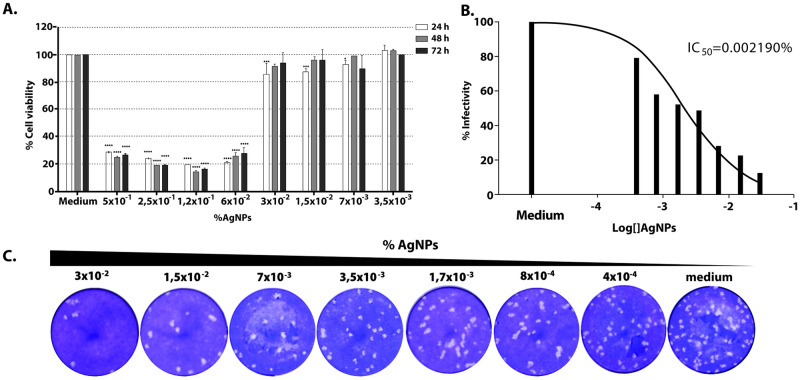
To determine the efficacy of AgNPs against SARS-CoV-2 in vitro, we first analyzed its cytotoxicity on cultured Vero E6 cells. Cells were seeded in 96-iwell plates and two-fold serial dilutions of AgNPs added to the medium, in triplicates. The cells were then incubated for 24, 48 or 72 hours. Cells grown in the presence of AgNPs over 24 hours showed the highest viability values over 1/32 dilution (or 0.03% of AgNPs) (Fig 1A). In order to ensure viral infection, further experiments were performed with [AgNPs] ≤ 0.03%. To analyze the effect of AgNPs on virus infectivity Vero E6 cells were infected with a fixed amount of virus and different concentrations of AgNPs, starting at 0.03%, were added to cells. At 72 hours post-infection (pi) supernatants were collected and titrated in order to determine virus yields normalized to those reached in medium alone (Fig 1B). Although AgNPs did not totally abolish viral production, infection was clearly controlled to some extent with a reduction of about 80% at a concentration of 0.03%. A 50% inhibitory concentration was determined by curve fitting (non-linear regression) (Fig 1B and 1C).
Fig 1. Antiviral activity of AgNPs in Vero E6 cell cultures.
A. Effect of AgNPs on viability of cultured Vero cells. Serial two-fold dilutions of AgNPs were added in triplicate to the medium of Vero cells seeded in 96-well plates. Viability at 24 (white bars), 48 (grey bars) and 72 h (black bars) was checked by the MTS Cell Proliferation Assay (Promega) and calculated as described in Methods. Statistically significant differences when compared to the corresponding 100% of cell viability (by 2 way ANOVA) are indicated. *, p < 0.05; ***, p < 0.001; ****, p < 0.0001. B. Percentages of infectivity values for each AgNPs concentration. The values were normalized to those in the absence of AgNPs (medium, 100%) and fitted using a non-linear regression algorithm. IC50: 50% inhibitory concentration. C. Representative SARS-CoV-2 plaque formation in presence of different dilutions of AgNPs.
Description of the groups
From the total of 231 participants, 114 were assigned to the experimental group, including 41 men (36%) and 73 women (64%), with an average age of 35 years. The control group included 117 participants, 40 men (34%) and 77 (66%) women with an average age of 33 years. 66% of the participants in the experimental group were vaccinated against influenza virus during the last year, while in the control group the vaccinated participants accounted for 86%. Regarding the profession of health personnel, there were 8% doctors, 80% nurses and 12% administrative in the experimental group and 3% doctors, 88% nurses and 9% administrative in the control group. A reduced number of participants reported being smokers (9% in the experimental group vs. 19% in the control group); as well as not having chronic diseases such as: diabetes (3% in the experimental group vs. 5% in the control group); hypertension (6% in the experimental group vs. 4% in the control group); and asthma (4% in the experimental group vs 3% in the control group). Most participants reported becoming ill with respiratory tract infections ≤ 2 times in the past year (89% in the experimental group and 91% in the control group). The frequency of daily handwashing and mouthwash prior to the study, in the experimental group were: 6.3 handwashing and 0.95 average daily mouthwash, and in the control group 8.9 handwashing and 0.52 average daily mouthwash. Studied groups characteristics and Chi Square tests results which determine the significance of differences between groups are presented in Table 1.
Results analysis
The incidence of SARS-CoV-2 infection (p = 0.000), was significantly lower in the experimental group vs the control group, where 1.8% (2 participants out of 114) and 28.2% (33 participants out of 117) were infected respectively (Table 2); frequency of mouthwashes (gargles and/or spray application) and nasal application daily with AgNPs, performed only by the experimental group, on average 2 mouthwashes made by 28 participants who only did it in the form of gargles, 2 daily applications by 34 participants who only used it in spray; while the 52 participants who chose to do both types of rinses, performed them on average 2 times as a gargle and 4 times as a spray application; on average 0.70 nasal rinse made by 64 participants; weekly number of contacts with patients diagnosed with COVID-19 or atypical pneumonia during the study period: (0–860, 169 on average) in the experimental group and (0 to 729, 146 on average) in the control group; percentage of individuals who presented symptoms of respiratory tract infection (RTI’s) at some point during the study; 18.4% in the experimental group and 35.8% in the control group; no adverse reactions were reported by performing mouthwash and nose rinse with AgNPs.
Pearson’s Chi-Square tests, the “Fisher’s exact test” and the “likelihood ratio” were run to check the statistical difference between the experimental and control groups. If the use of AgNPs had not been effective and 1) the protection measures used in the General Tijuana Hospital were very effective, the same low contagion level (1.8%) would have been presented in both groups; 2) if the protection measures were not effective, the same high contagion level (28.2%) would have been presented in both groups. Results were significant at 99% reliability for both one-tailed and two-tailed tests verified the difference in group results. The predictive model based on the experimental data reports that the odds of a participant who is treated with the AgNPs are 22 times higher than those of a participant who is not treated. The logistic model predicts that if all participants had mouthwashes and nasal rinses, the probability of success (they did not become infected with SARS-CoV-2) would be 84.8%. Table 3 shows the good fit of the data to the model taking into account that the probability of non-contagion depends on whether or not performed AgNPs solution, but the number of mouthwashes and nasal rinse on average per day (gargling and nasal rinse, spray application or combined) was also taken into account.
Table 3. Results of the logistic regression of SARS-CoV-2 infection between control and experimental groups.
| Variables | B (SE) | Exp (B) | Wald | Sig. | R2 Cox & Snell/Nagelkerke |
|---|---|---|---|---|---|
| a) SARS-CoV-2 infection between control group and experimental group with AgNPs mouthwash | |||||
| Constant | .934 (.205) | 2.545 | 20.682 | .000 | |
| Mouthwash and nose rinse with AgNPs (Gargles) | 3.091 (.742) | 22.000 | 17.33 | .000 | |
| .149 / .259 | |||||
| b) SARS-CoV-2 infection between control group and experimental group with combined mouthwash with AgNPs (gargle and spray) | |||||
| Constant | 3.280 (.732) | 26.58 | 20.058 | .000 | |
| Mouthwash and nose rinse with AgNPs (Gargles) | 4.692 (1.465) | 109.065 | 10.259 | .001 | |
| Mouthwash with AgNPs (Gargles and spray) | -.190 (.109) | .827 | 3.032 | .082 | |
| .158 / .276 | |||||
B (SE): B is the same linear regression value b that we need to substitute to obtain the ordinate at the origin (Constant) and the slope of the line of the variable, in parentheses is the Standard Error (SE). Exp (B): Odds Ratio, is an indicator of the change in the ratios, as a result of a change in the predictor. Wald: Statistical test analogous to the t-statistics test in linear regression to see the contribution of the predictive variables. Wald = b / SEb. Sig: Level of significance. R2: Goodness of fit of the model to the logistic curve with the Cox & Snell/Nagelkerke methods.
The dichotomous independent variable (participated in the experiment or not) is highly significant according to the value of the Wald statistic. The interesting thing about the logistic regression is the statistic Exp (B), which in this case tells us that for each person who “’DOESN’T become infected” with SARS-CoV-2 in the control group, there are 22 people “NOT INFECTED” when they perform mouthwash and nose rinses with AgNPs in the experimental group in similar environments or under similar conditions. To find out whether the number of times mouthwashes and nasal rinse is performed with AgNPs per day has an effect, the quantitative variable was added to the dichotomous variables of the previous model where the average number of mouthwashes and nasal rinse was combined as in our sample. It was 2 gargle mouthwashes, 1 nasal rinse, and 2 spray mouthwashes. When we assume that 4 mouthwashes were performed daily (2 gargle and 2 spray) and 1 nasal rinse, the model improves with respect to only saying that the person participated in the experiment without considering the number of applications. The EXP (B) Statistic rises from 22 to 109 when considering mouthwash in both gargles and spray. That is, for each person who becomes infected with SARS-CoV-2 by performing 4 mouthwashes (2 gargles and 2 spray) and 1 nasal rinse, per day 109 people will not be infected.
Discussion
Silver nanoparticles are known to have a powerful antimicrobial effect and there have been several studies that also demonstrated their antiviral effect. The mechanism by which AgNPs interact with viruses is not yet clear, but it has been proposed that AgNPs, as well as other metallic nanoparticles, interfere with the structural proteins of the virus by inhibiting their ability to bind with cell receptors or bind to genetic material of viruses by inhibiting their replication [25]. Since the nanoparticles used in these experiments are mixed with hydrolyzed collagen, a compound that stabilizes nanoparticles and reduces its toxicity in cell culture [26], we were able to assess higher concentrations than those used in other articles. Jeremiah et al. showed that in Vero E6 cells AgNPs alone are toxic at concentrations of 100 ppm. In contrast, we observed that collagen AgNPs are not toxic up to concentrations of 0.03% (312 ppm). Despite this fact, the toxicity of silver nanoparticles in cell culture narrows the range of concentrations at which we can study therapeutic effects against viral infections in vitro. Taking this limitation into account we could observe a clear dose-dependent inhibitory effect over SARS-CoV-2 infectivity.
To our knowledge, this study is the first experimental trial where AgNPs are applied as a mouthwash and nose rinse solution for the prevention of SARS-CoV-2 infection in health care personnel working with COVID-19 diagnosed patients. The study was carried out in a prospective, controlled and randomized way for 9 weeks in the period of pandemic by COVID-19 from April to June 2020 in Tijuana Baja California, Mexico. The characterization of the two groups showed no significant statistical differences in gender, occupation and comorbidities related to the complication of SARS-CoV-2 infection. However, a statistically significant difference was identified in variables such as: age (p = 0.004), average age 35 in the experimental group and average age 33 in the control group; anti-influenza vaccination (p = 0.000), (66%) in the experimental group and (86%) in the control group, even though, vaccination against influenza virus reduces morbidity/mortality among medical personnel exclusively against influenza virus, but does not have any antigenic similarity with SARS-CoV-2 virus, as an example in this study, the control group reported a higher percentage of participants vaccinated against influenza and a higher percentage of infection by SARS-Cov2; Body Mass Index (p = 0.000), (25.95) in the experimental group and (28.93) in the control group, both groups classifying within the 25–29 Overweight level; frequency of daily gargles before the study (p = 0.000), (0.95); frequency of daily handwashing before the study (p = 0.000), (6.3) in the experimental group and (8.9) in the control group, the experimental group had the lowest number of COVID-19 infections, being the group that washed their hands less frequently, this indicates that AgNPs contributed to mitigating the SARS-CoV-2 infection in the experimental group, consistent handwashing has previously been recognized as a protective factor against SARS and influenza [27], in addition, most respiratory pathogens are spread by direct contact, droplets, and fomites [28]; the most important statistically significant difference, the incidence of SARS-CoV-2 infection was significantly lower in the experimental group (1.8%) than the control group (28.2%) (p = 0.000); and finally, the report of symptoms related to the SARS-CoV-2 infection, there was a statistically significant difference (p = 0.003) reported by the experimental group (18.4%), while in the control group (35.8%).
These results corroborate the relevance of oral and nasal hygiene with AgNPs in the prevention of respiratory tract infection to minimize the risk of contagion by COVID-19 in health personnel. Scientific evidence supports preventive oral and nasal health regimens, including the use of mouthwashes, gargles, and nasal washes, as important components of SARS-CoV-2 infection control practices [29–31]. Using a logistic regression, the strong relationship between the fact of mouthwashes and nose rinse in the health personnel and the mitigation of the SARS-CoV-2 contagion with an efficiency of 84.8% is proved. The results provide clinical evidence to confirm the prevention of SARS-CoV-2 infection in health personnel who performed mouthwash and nose rinse solution with AgNPs. In the midst of the global pandemic, the results of in vitro infection experiments (effect of AgNPs on virus infectivity and determination of the 50% inhibitory concentration) and ARGOVIT® registration as an oral and nasal hygiene product in Russia since 2015, and with antimicrobial action for oral and nasal hygiene since 2019, were decisive to apply the AgNPs in vivo in the oral and nasal cavity to confirm its effects against SARS-CoV-2. The present study also showed that no harmful side effects were observed in the 114 participants who used AgNPs as a mouthwash and nose rinse solution for 9 weeks.
The relevance of mouthwash and nasal rinses as a prevention strategy for mitigating SARS-CoV-2 infections among health personnel also extends to other high-risk areas such as dental procedures, in these cases, specific protocols are suggested to avoid COVID-19 contamination. The oropharynx and nasopharynx are the initial entry sites where SARS-CoV-2 replicates and can produce a viral load of 1.2 × 108 infectious copies / per mL [32]. The odontologist works with a high risk of exposure during routine dental procedures. In addition, the use of different devices that generate a high amount of aerosols (rotary instruments and ultrasounds), which can be inhaled through the respiratory route, these aerosols can also carry viral particles that come into contact with the conjunctiva and also cause infection, on the other hand these residues can be deposited on different surfaces, on the work clothes, masks, hands and clothes of the patient. Dental cabinets are considered a possible source of contamination and nosocomial infection [33]. Contamination by nasal excretions can carry thousands of infectious particles, since a higher viral load has been demonstrated in the nasal cavity compared to the oral cavity [34]. Recent reports indicate that total anosmia or partial loss of the sense of smell are early markers of SARS-CoV-2 infection. This phenomenon can be caused by the expression of the receptors of the transmembrane protein of the angiotensin converting enzyme II (ACE2) and by a type II transmembrane serine protease (TMPRSS2), responsible for the entry of the virus into nasopharyngeal cells and present direct damage secondary to SARS-CoV-2 viral replication in olfactory receptor neurons (ORNs) located in the olfactory epithelium [35]. These results justify making changes to the international recommendations [33] on new dental clinical practices and the prevention of SARS-CoV-2. Since the dental clinic procedure requires that the patient, after passing the sanitary filter, has to perform a mouth rinse (on average 30 minutes before the consultation), followed by a second mouth rinse when seated in the dental chair. At no time do international recommendations [33] speak of nasal washes, when it has been demonstrated that by nasal exhalation the spread of SARS-CoV-2 to health personnel can occur. The evidence presented in this study suggests that the application of mouthwashes and nose rinse can significantly reduce the viral load in these areas, to reduce transmission, in addition to the use of personal protective equipment by healthcare personnel.
The lack of efficient and specific SARS-CoV-2 antiviral therapies could be due in large part to the underuse of nanotechnologies [36], non-pharmaceutical therapies based on nanotechnology applications are needed as preventive measures, to mitigate the nosocomial transmission of SARS-CoV-2 among health personnel. We present a new intervention strategy that uses AgNPs as a mouthwash and nasal rinse solution that can reduce the spread, transmission and/or pathogenicity of SARS-CoV-2 associated with COVID-19. Due to the frequent generation of aerosols by direct treatment with patients with COVID-19, the associated risk of virus transmission was very low among health personnel who performed mouthwashes and nasal washes with AgNPs. The antiviral effect of mouthwashes and nose rinse with AgNPs can decrease the viral load in the oral and nasal cavity, as well as inhibit the proliferation of the virus and temporarily reduce the risk of transmission. These results were demonstrated in the clinical field of the health personnel of the Tijuana General Hospital.
Conclusions
This prospective randomized study demonstrates that mouthwash and nose rinse with AgNPs is effective in decreasing SARS-CoV-2 infection rate. To our knowledge, this study is the first experimental in vitro e in vivo trial where AgNPs as mouthwash and nasal rinse solution are applied for SARS-CoV-2 contagion prevention. The results of this investigation are revealing and encouraging, due to the fact that the health personnel exposed to an excessive viral load due to the number of COVID-19 patients attended (169 patients attended on average per week per person) were not infected, which is attributed to rinsing with AgNPs. Proved its inhibitory effect on SARS-CoV-2 infectivity in vitro it is inferred that the use of AgNPs as a mouthwash and nose rinse will be very useful as a prophylactic for the prevention of SARS-CoV-2, not only for health care personnel, but also as additional protection for the general population.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors of this study would like to thank the staff of General Tijuana Hospital for their invaluable participation in this research during the extremely difficult period of the COVID-19 pandemic. The health care personnel are under intense workout during this pandemic. The authors would also like to thank CONACyT “International Network of Bionanotechnology with an Impact on Biomedicine, Food and Biosafety” and Tomsk Polytechnic University for their important collaboration in the field of fundamental and applied research on nanomaterials in nanomedicine.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
Funded studies A. Pestryakov Development Program "Priority 2030" Tomsk Polytechnic University https://tpu.ru/en The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Archived: WHO Timeline—COVID-19. In: Archived: WHO Timeline—COVID-19 [Internet]. [cited 30 Jul 2020]. https://www.who.int/news-room/detail/27-04-2020-who-timeline--covid-19?gclid=CjwKCAjw34n5BRA9EiwA2u9k385WKHmODGM3irOwTWLJfwg96XUtxDPUzllPZWhrRxp5qAOCYmJMchoCEmwQAvD_BwE
- 2.World Health Organization. Coronavirus disease (COVID-19) pandemic. In: Coronavirus disease (COVID-19) pandemic. [Internet]. 2020. http://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 3.Centers for Disease Control and Prevention. Symptoms of Coronavirus. 2020. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- 4.Lan FY, Wei CF, Hsu YT, Christiani DC, Kales SN. Work-related COVID-19 transmission in six Asian countries/areas: A follow-up study. PLoS One. 2020;15: 1–11. doi: 10.1371/journal.pone.0233588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong SCY, Kwong RTS, Wu TC, Chan JWM, Chu MY, Lee SY, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105: 119–127. doi: 10.1016/j.jhin.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Making MOF, Zhu N, Zhang D, Wang W, Li XW, Yang B, et al. Personal protective equipment for COVID-19. N Engl J Med. 2020;13: 19–21. [Google Scholar]
- 7.Wee LE, Hsieh JYC, Phua GC, Tan Y, Conceicao EP, Wijaya L, et al. Respiratory surveillance wards as a strategy to reduce nosocomial transmission of COVID-19 through early detection: The experience of a tertiary-care hospital in Singapore. Infect Control Hosp Epidemiol. 2020;41: 820–825. doi: 10.1017/ice.2020.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho HJ, Zhang ZX, Huang Z, Aung AH, Lim WY, Chow A. Use of a real-time locating system for contact tracing of health care workers during the COVID-19 pandemic at an infectious disease center in singapore: Validation study. J Med Internet Res. 2020;22. doi: 10.2196/19437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64. doi: 10.1128/AAC.00819-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SJ, Yu KM, Kim Y Il, Kim SM, Kim EH, Kim SG, et al. Antiviral efficacies of FDA-approved drugs against SARS-COV-2 infection in ferrets. MBio. 2020;11: 1–10. doi: 10.1128/mBio.01114-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet (London, England). 2021;397: 671–681. doi: 10.1016/S0140-6736(21)00234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396: 479–488. doi: 10.1016/S0140-6736(20)31605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396: 467–478. doi: 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N Engl J Med. 2020;383: 1920–1931. doi: 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. 2020;383: 2320–2332. doi: 10.1056/NEJMoa2026920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science (80-). 2003;302: 276–278. doi: 10.1126/science.1087139 [DOI] [PubMed] [Google Scholar]
- 17.Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367: 1814–1820. doi: 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 18.Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl). 2020;133: 1015–1024. doi: 10.1097/CM9.0000000000000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yip MS, Cheung CY, Li PH, Bruzzone R, Peiris JSM, Jaume M. Investigation of Antibody-Dependent Enhancement (ADE) of SARS coronavirus infection and its role in pathogenesis of SARS. BMC Proc. 2011;5: P80. doi: 10.1186/1753-6561-5-S8-P80 [DOI] [PubMed] [Google Scholar]
- 20.Jaume M, Yip MS, Cheung CY, Leung HL, Li PH, Kien F, et al. Anti-Severe Acute Respiratory Syndrome Coronavirus Spike Antibodies Trigger Infection of Human Immune Cells via a pH- and Cysteine Protease-Independent Fc R Pathway. J Virol. 2011;85: 10582–10597. doi: 10.1128/JVI.00671-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Wo J, Shao J, Zhu H, Wu N, Li M, et al. SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J Clin Virol. 2003;28: 239–244. doi: 10.1016/s1386-6532(03)00195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202: 415–424. doi: 10.1084/jem.20050828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa-meza AR, Álvarez-sánchez AR, Romo-quiñonez CR, Barraza A, Magallón-barajas FJ, Chávez-sánchez A, et al. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol. 2019;84: 1083–1089. doi: 10.1016/j.fsi.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 24.Borrego B, Lorenzo G, Mota-Morales JD, Almanza-Reyes H, Mateos F, López-Gil E, et al. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomedicine Nanotechnology, Biol Med. 2016;12. doi: 10.1016/j.nano.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 25.Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N, et al. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomater (Basel, Switzerland). 2020;10. doi: 10.3390/nano10081566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeremiah SS, Miyakawa K, Morita T, Yamaoka Y, Ryo A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem Biophys Res Commun. 2020;533: 195–200. doi: 10.1016/j.bbrc.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CDC. Handwashing | CDC. In: Handwashing [Internet]. [cited 12 Sep 2020]. https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/handwashing.html
- 28.Schwartz J, King C-C, Yen M-Y. Protecting Healthcare Workers During the Coronavirus Disease 2019 (COVID-19) Outbreak: Lessons From Taiwan’s Severe Acute Respiratory Syndrome Response. Clin Infect Dis. 2020;71: 858–860. doi: 10.1093/cid/ciaa255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casale M, Rinaldi V, Sabatino L, Moffa A, Ciccozzi M. Could nasal irrigation and oral rinse reduce the risk for COVID-19 infection? International journal of immunopathology and pharmacology. England; 2020. p. 2058738420941757. doi: 10.1177/2058738420941757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S, Sharma N, Singh U, Singh T, Mangal DK, Singh V. Nasopharyngeal wash in preventing and treating upper respiratory tract infections : Could it prevent COVID-19? 2020. doi: 10.4103/lungindia.lungindia_241_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgardo F. Can a Nasal / Oral / Ocular Spray Inactivate and Prevent SARS-CoV-2 infection? A Can a Nasal / Oral / Ocular Spray Inactivate and Prevent SARS-CoV-2 infection? A hypothesis. Int J Odontostomat. 2020; 2–5. [Google Scholar]
- 32.To KK-W, Tsang OT-Y, Yip CC-Y, Chan K-H, Wu T-C, Chan JM-C, et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin Infect Dis. 2020;71: 841–843. doi: 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12: 9. doi: 10.1038/s41368-020-0075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MM, Parab SR, Paranjape M. Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic. Am J Otolaryngol. 2020;41: 102618.: 10.1016/j.amjoto.2020.102618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butowt R, Bilinska K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem Neurosci. 2020; 3–6. doi: 10.1021/acschemneuro.9b00426 [DOI] [PubMed] [Google Scholar]
- 36.Uskoković V. Why have nanotechnologies been underutilized in the global uprising against the coronavirus pandemic? Nanomedicine (Lond). 2020;15: 1719–1734. doi: 10.2217/nnm-2020-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.